Abstract
Purpose
Up to date, most studies about the plant photosynthetic acclimation responses to elevated carbon dioxide (CO2) concentration have been performed in temperate areas, which are often N limited under natural conditions and with low ambient N deposition. It is unclear whether photosynthetic downregulation is alleviated with increased N availability, for example, from increased N deposition due to fossil fuel combustion in the tropics and subtropics. Awareness of plant photosynthetic responses to elevated CO2 concentration will contribute to the better understanding and prediction of future forest productivity under global change.
Materials and methods
Four tree species, Schima superba Gardn. et Champ., Ormosia pinnata (Lour.) Merr, Castanopsis hystrix AC. DC., and Acmena acuminatissima (Blume) Merr. et Perry were exposed to a factorial combination of atmospheric CO2 concentration (ambient and elevated CO2 concentration at ca. 700 μmol CO2 mol−1) and N deposition (ambient and ambient + 100 kg N ha−1 year−1) in open-top chambers in southern China for 3 years since March 2005. Light-saturated net photosynthetic rate, leaf N concentration, and tree growth of all species were measured.
Results and discussion
The CO2 treatments did not affect light-saturated net photosynthetic rate of all species grown with the high N treatment. However, S. superba grown with the low N treatment (ambient) had 23% and 47% greater net photosynthesis in the ambient CO2 concentration than those in the elevated CO2 concentration for December 2006 and November 2007 (20 and 31 months after the treatments were applied), respectively, and A. acuminatissima grown with the low N treatment had 173%, 26%, and 121% greater net photosynthesis in trees grown in the ambient CO2 concentration than those in the elevated CO2 concentration for July 2006 (16 months after the treatments), December 2006 (20 months), and November 2007 (31 months), respectively, whereas, photosynthetic acclimation was not found for C. hystrix and O. pinnata. With the photosynthetic acclimation of S. superba and A. acuminatissima, we also found that the elevated CO2 concentration decreased significantly leaf N concentration in trees of S. superba and A. acuminatissima grown with the low N treatment, respectively.
Conclusions
C. hystrix seems to be a good species for C fixation under global climate change, particularly with the rising CO2 concentration. We demonstrated that the relative responses to elevated CO2 concentration and N treatment differ among tree species and functional types in the tropical and subtropical areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Elevated atmospheric CO2 concentration has increased short-term photosynthesis in numerous plant studies (Curtis 1996; Xu and Chen 2006). In contrast, a reduction in photosynthesis with increased CO2 concentration, often termed downregulation or photosynthetic acclimation, also has been reported (Rey and Jarvis 1998; Hyvönen et al. 2007). Photosynthesis reduction appears to be brought about by end product inhibition, resulting from an imbalance in the supply and demand of carbohydrates (Jones et al. 1996; Cheng et al. 1998). Substantial production of carbohydrates induced by higher photosynthetic rates at elevated CO2 partial pressure initiates a feedback mechanism that ultimately reduces photosynthetic capacity. The lacks of sufficient “sink capacity” for incoming photosynthate lead to a negative feedback effect and downward acclimation of photosynthetic capacity.
In the long term, photosynthetic rates and tree growth responses to elevated CO2 concentration are dependent on environmental and genetic factors affecting the plant's ability to develop new sinks for C (Hyvönen et al. 2007; Cai et al. 2010). Environmental and genetic factors can affect photosynthetic capacity and thus the nature of acclimation to CO2 concentration (Wolfe et al. 1998; Robredo et al. 2010). Among all the factors which influence photosynthetic capacity, genotypic variation and N availability are the most pronounced. Acclimation is more profound for C3 plants compared to species with the C4 or CAM photosynthetic pathways (Wolfe et al. 1998; Ainsworth and Long 2005). Within the C3 species, genotypic variation in sink capacity for photosynthates is likely a very important factor in determining the magnitude and nature of acclimation responses to CO2 concentration (Wolfe et al. 1998; Ainsworth and Long 2005). In a comparison of the growth responses of 156 species to elevated CO2 concentration, it has been concluded that inherently fast-growing C3 species exhibit stronger CO2 responses than slow-growing species (Poorter 1993; Hovenden 2003). Nitrogen availability is important for leaf photosynthesis because leaf chlorophyll concentration, Rubisco (ribulose-1-5-biphosphate carboxylase), and plant growth all increase with the greater N availability (Kutik et al. 1995; Xu et al. 2002; Bondada and Syvertsen 2003; Huang et al. 2008). Downregulation of photosynthesis would occur with elevated CO2 concentration exposure due to the N limitation effect (Sefcik et al. 2006, 2007; Hyvönen et al. 2007). Plants grown with high N availability would increase light-saturated photosynthetic rate, alleviating the downregulation responses for plants grown under elevated CO2 concentration (Saxe et al. 1998; Medlyn et al. 1999; Reich et al. 2006; Sefcik et al. 2007).
In Asia, emission of reactive N increased from 14 Tg N year−1 in 1961 to 68 Tg N year−1 in 2000 and is expected to reach 105 Tg N year−1 in 2030 (Zheng et al. 2002). Currently, this leads to high atmospheric N deposition (NH +4 –N, NO −3 –N) in precipitation for some forests of southern China (30–73 kg N ha−1 year−1, Ren et al. 2000; Mo et al. 2006). The N deposition in Guangzhou City of southern China increased from 46 kg N ha−1 year−1 in 1988 to 73 kg N ha−1 year−1 in 1990 (Ren et al. 2000). High N deposition in this area leads to higher N availability in most forest ecosystems. Up to date, most studies about the downregulation responses to elevated CO2 concentration have been performed in temperate areas, which are often N limited under natural conditions and with low ambient N deposition. It is unclear whether photosynthetic downregulation is alleviated with increased N availability, for example, from increased N deposition due to fossil fuel combustion in the tropics and subtropics. It is also unclear that with the increasing N deposition how plant photosynthesis and growth respond to elevated CO2 concentration in the tropics and subtropics.
Numerous experiments were carried out to study the responses of trees species to the elevated CO2 concentration and N addition, however most of these studies just focused on one or individual species. Relative responses to elevated CO2 concentration differ among tree species and functional types within a forest ecosystem (Curtis and Wang 1998). In this study, we used open-top chambers to expose four tree species, growing with high or low N treatment, to either ambient (about 390 ppm) or elevated (about 700 ppm) CO2 concentration for 3 years. These four tree species are: Castanopsis hystrix Hook.f. & Thomson ex A. DC., Schima superba Gardn. and Champ., Acmena acuminatissima (Blume) Merr. et Perry, and Ormosia pinnata (Lour.) Merr. All these native species are distributed widely in subtropical areas. C. hystrix and S. superba are pioneer tree species and grow very fast. O. pinnata is an N-fixing species. A. acuminatissima is a native understory species, growing relatively slowly. Light-saturated photosynthetic rate, leaf N concentration, and tree growth were measured periodically for about 3 years in our study. Variations in the above parameters among the four tree species were examined. We hypothesized: (1) long-term growth in elevated CO2 concentration would lead to the downward photosynthetic acclimation in mature leaves and (2) the magnitude of downward acclimation would be different among the different species grown in the elevated CO2 concentration.
2 Materials and methods
2.1 Open-top chamber design
The experiment was carried out in 10 open-top chambers. Each cylindrical chamber had a diameter of 3 m, 3 m high above-ground section (was adjusted to 4.5 m later), and 0.7 m below-ground section. The above-ground section was wrapped with impermeable and transparent plastic sheets, leaving the top totally open. Sunlight intensity in the chamber was 97% of that in open space with no spectral change detected. Rainfall intensity and air temperature were also identical inside and outside the chambers. In the treatments with elevated CO2 concentration, CO2 was distributed in each chamber by a transparent pipe with pinholes. A fan was connected to the pipe to ensure equal distribution of CO2 in the entire chamber. The CO2 flux from the tank was controlled by a flowmeter, and the CO2 concentrations in the chambers were periodically examined using a Licor-6400 (LI-COR Inc., Lincoln, NE, USA). The open chambers used in the experiment were located in an open space where they all were exposed to full light and rainfall. The distance between elevated CO2 chambers and ambient CO2 chambers is about 150 m. We considered the prevailing wind direction. The elevated atmospheric CO2 concentration of the elevated CO2 chambers will not affect the CO2 concentration of the ambient CO2 chambers.
2.2 Experimental design
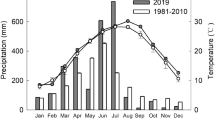
The study was carried out in Guangzhou City, Guangdong Province, China (23°20′ N and 113°30′ E). The area has a monsoon climate characterized by mean annual total solar radiation of 4,367.2–4,597.3 MJ m−2 in the visible waveband and a mean annual temperature of 21.5°C. The annual precipitation ranges from 1,600 to 1,900 mm, and the mean relative air humidity is 77%. There are two seasons, a wet/rainy season from April to September and a dry season from October to March.
In March 2005, we collected the soil from a nearby evergreen broadleaved forest. The soil was a lateritic soil and was collected as three different layers (0–20, 20–40, and 40–70 cm depth) that were homogenized separately and used to fill the below-ground section of the chambers. One to 2-year-old seedlings grown in a nursery were transplanted in the chambers with minimal damage to the roots. In order to determine responses of different tree species to elevated CO2 concentration and N over a short time frame, seedlings were planted at a high density. Each chamber was planted with 48 randomly located seedlings, eight for each of six species: C. hystrix, S. superba, A. acuminatissima, O. pinnata, Syzygium hancei Merr. et Perry, and Pinus massoniana Lambert. These species were selected because they are native and the most widely spread tree species in southern China. As trees were growing fast, one tree per species was harvested at the end of each year to avoid excess crowd in each chambers. As most seedlings of P. massoniana died in the second year of our experiment, and the seedling leaves of S. hancei are too small which could not be measured for the photosynthesis using our instrument, we only studied the other four species in our experiment.
From April 2005, the chambers were exposed to different treatments. The three chambers received an elevated CO2 concentration and high N treatment (NH4NO3 applied at 100 kg N ha−1 year−1), three chambers received the high CO2 concentration treatment (with the low N treatment: ambient without any N fertilizer application), two chambers the high N treatment (but with ambient CO2 concentration), and finally two chambers were used as a control (with the ambient CO2 concentration and the low N treatment) and did not receive the high CO2 or high N treatment. The high CO2 concentration treatments were achieved by supplying additional CO2 from a tank until a concentration of ca. 700 ppm CO2 was reached in the chambers. The high N addition treatments were achieved by spraying seedlings once a week for a total amount of NH4NO3 at 100 kg N ha−1 year−1. No other fertilizer was used. The seedlings were watered with tap water. About 600 mm extra water was applied in each chamber per year. All other chambers received the same amount of water as the control chambers. More detailed information about the experimental design has been reported previously (Liu et al. 2008, 2010).
2.3 Photosynthesis measurements
We examined the influence of CO2 exposure, N treatment, measurement month, and their interactions on leaf light-saturated net photosynthesis for the four tree species. All measurements were made between 0900 and 1200 hours and 1400 and 160 hours on sunny days in July 2005, October 2005, July 2006, December 2006, and November 2007 (3, 6, 16, 21, and 31 months after the treatments were applied), respectively. At least 2 days were chosen during each of the above measurement months. Four fully expanded leaves (the current year leaves) per tree in the middle canopy were randomly selected for measurements. Light-saturated rate of photosynthesis was measured at ambient CO2 concentration and photosynthetic active radiation of 1,200 μmol m−2 s−1 using a portable infrared gas exchange system (Licor 6400, Lincoln, NE, USA) in each chamber. Preliminary measurements showed that this light intensity was above the light-saturation point for all leaves. At each measurement, temperature and air humidity were controlled according to the environmental conditions.
2.4 Measurements of plant growth and leaf N concentration
Plant growth was measured as the increment in plant dimensions over the 3-year study period to determine how CO2 and N treatments and their interactions affected growth for each tree species. Only the growth of the trees alive until the end of the experiment was considered. Tree height and basal diameter were measured at the time of planting in early March 2005. Tree height and basal diameter were assessed for five times later: in August 2005, November 2005, May 2006, September 2007, and January 2008 (4, 7, 13, 29, and 33 months after the treatments were applied), respectively. Tree height was measured from the soil–stem surface to the tip of the apical bud, and the diameter was assessed at the soil surface.
Leaf N concentration was measured in January 2006, January 2007, and July 2007 (9, 21, and 27 months after the treatments were applied), respectively. In January 2006 and January 2007, we chose one tree per species in each chamber and harvested all the leaves on this tree and pooled for the N measurements. In July 2007, depending on the leaf sizes, about 8 to 20 mature leaves were collected per species in each chamber. Leaf samples were dried for 3 days at 70°C in an oven and then ground prior to N analysis. Foliar N concentrations were determined using the Kjeldahl method as described previously (Xu et al. 1993).
2.5 Data analysis
Data analyses were carried out using the SAS software (SAS Institute Inc., Cary, NC, USA). We chose α equal to 0.05. Variables normality and residual homocedasticity were checked. Analyzed data consisted of light-saturated rate of photosynthesis measured at common CO2 concentration, leaf N concentration, and tree basal diameter and height. Data were analyzed using the following mixed linear model:
Dependent variables = C + N + species + time + C × N + C × species + C × time + N × species + N × time + species × time + species × C × time + species × N × time + C × N × species + time × C × N.
Where C was the effect of the CO2 concentration treatments (ambient or ca.700 ppm), N the effect of the N treatments (ambient or high N addition), time the effect of the measurement months, and the interactions between the factors. Repeated measurements for a chamber were stated to follow an unstructured covariance structure. The interaction of species ×C × N × time never significantly affected any parameters, and it was subsequently removed from the model and was not displayed in Section 3.
When the effects were significant, they were further analyzed using Tukey multiple comparison test (HSD). Additionally, simple correlations were performed on the whole set of data using the Pearson correlation coefficients.
3 Results
3.1 Leaf N concentration
Leaf N concentrations primarily were affected by the species, measurement month, and CO2 and N treatments (Table 1). O. pinnata had the highest N concentration compared to the other three species (Fig. 1). For the same species, leaf N concentrations change with month. Except for O. pinnata, the other three species' N concentrations were significantly affected by the CO2 treatments. For S. superba, elevated CO2 concentration decreased significantly leaf N concentration in trees grown with the low N treatment in January and July 2007 (21 and 27 months after the treatments were initially imposed, p < 0.001) and in trees grown with the high N treatment in July 2007 (p < 0.001). For A. acuminatissima, decreased N concentrations were found in the leaves of trees grown with the low N treatments and exposed to the elevated CO2 concentration in January 2006 and July 2007 (9 and 27 months after the treatments, p < 0.0001 and p < 0.001, respectively). For C. hystrix, elevated CO2 concentration only decreased significantly leaf N concentration in trees grown with the low N treatment in January 2007. The high N treatment only increased significantly leaf N concentration in the trees of O. pinnata and C. hystrix over the 3-year period.
Leaf N concentrations of four tree species: a S. superba, b O. pinnata, c C. hystrix, and d A. acuminatissima grown in ambient and elevated CO2 concentration under high and low N treatments for three measurement months of January 2006, January 2007, and July 2007 (9, 21, and 27 months after the treatments were applied), respectively. Different letters indicate significant differences at the confidence level of p < 0.05 between elevated CO2 concentration and ambient CO2 concentration treatments but at the same N treatment
3.2 Light-saturated rate of photosynthesis
In our experiment, light-saturated rate of photosynthesis measured at common CO2 concentration was dependent on the tree species, CO2 exposure, N treatment, measurement month, and their interactions (see Table 1). Among all the factors, the species and measurement month affected the photosynthesis the most (F values are 269.75 and 218.57, respectively). Elevated CO2 concentration did not affect light-saturated net photosynthetic rate of S. superba grown under the high N treatment. However, when S. superba was grown under the low N treatment, there were 23% and 47% greater net photosynthesis in the ambient CO2 concentration than those in the elevated CO2 concentration in December 2006 and November 2007 (20 and 31 months after the treatments), respectively (Fig. 2), suggesting that photosynthetic acclimation happened. With O. pinnata grown under the high N treatment, elevated CO2 concentration treatment increased net photosynthesis by 30% and 140% in December 2006 and November 2007, respectively. With O. pinnata grown under the low N treatment, CO2 treatment significantly increased light-saturated net photosynthesis by 70%, 109%, and 142% in July 2005, October 2005, and December 2006, respectively. No photosynthetic acclimation was found for O. pinnata during the study period. For C. hystrix, only when they were grown in the low N treatment in October 2005, elevated CO2 concentration increased significantly net photosynthesis (p < 0.0001). There was also no photosynthetic acclimation for this species. For A. acuminatissima grown in the high N treatment, there was 177% greater net photosynthesis in trees grown in the elevated CO2 concentration than those in the ambient CO2 concentration in November 2007. Whereas, for A. acuminatissima grown in the low N treatment, there were 173%, 26%, and 121% greater net photosynthesis in trees grown in the ambient CO2 concentration than those in the elevated CO2 concentration in July 2006, December 2006, and November 2007, respectively (see Fig. 2). Overall, the low N treatment led to photosynthetic acclimation for species of S. superba and A. acuminatissima. Photosynthetic acclimation responded to be earlier for A. acuminatissima than for S. superba.
Net light-saturated rate of photosynthesis (A sat ) measured at common CO2 concentration of four tree species: a S. superba, b O. pinnata, c C. hystrix, and d A. acuminatissima grown in ambient and elevated CO2 concentration under high and low N treatments for five measurement months of July 2005, October 2005, July 2006, December 2006, and November 2007 (3, 6, 16, 21, and 31 months after the treatments were applied), respectively. Different letters indicate significant differences at the confidence level of p < 0.05 between elevated CO2 concentration and ambient CO2 concentration treatments but at the same N treatment
3.3 Plant growth
Tree growth shown as basal diameter and height in our experiment differed with the species (see Table 1, p < 0.0001) and generally increased with the elevated CO2 and N treatments (p < 0.0001, see Table 1). There was a significant CO2 concentration treatment by species interaction for both basal diameter and height growth (p < 0.0001, see Table 1). For S. superba, the high N treatment significantly increased the basal diameter and height growth (Figs. 3 and 4). The basal diameter and height growth of O. pinnata were not affected significantly by both the CO2 and N treatments. Elevated CO2 concentration significantly increased the growth of C. hystrix (p = 0.02 and p = 0.01, respectively, Figs. 3 and 4), which translated into greater basal diameter and height for C. hystrix grown in the elevated CO2 concentration over time (see Figs. 3 and 4). At the early growth stage, the high CO2 concentration treatment increased the height growth of A. acuminatissima grown with the high N treatment, but this early greater height growth disappeared later. This was more obvious in the trees grown with the low N treatment (see Fig. 4).
Basal diameter growth of four tree species: a S. superba, b O. pinnata, c C. hystrix, and d A. acuminatissima grown in ambient and elevated CO2 concentration under high and low N treatments for six measurement months of March 2005, August 2005, November 2005, May 2006, September 2007, and January 2008, respectively. Treatments are: CK control, NN high N, CC high CO2, CN high CO2 + high N
Tree height growth of four species: a S. superba, b O. pinnata, c C. hystrix, and d A. acuminatissima grown in ambient and elevated CO2 concentration under high and low N treatments for six measurement months of March 2005, August 2005, November 2005, May 2006, September 2007, and January 2008, respectively. Treatments are: CK control, NN high N, CC high CO2, CN high CO2 + high N
4 Discussion
Many studies have shown that elevated CO2 concentration can reduce foliar nutrient concentrations (Johnson et al. 2003, 2004; Finzi et al. 2002; Hyvönen et al. 2007). Above-ground tissue N concentrations consistently decreased with the CO2 enrichment were reported (Blank and Derner 2004; Johnson et al. 2004). The same results were obtained for some species in our experiment, especially when the species were grown with the low N treatment. The highest N concentration was found in leaves of O. pinnata as it is an N2-fixing species.
Downregulation of photosynthesis would occur with elevated CO2 concentration exposure due to the N limitation effect (Sefcik et al. 2006, 2007). Effects of elevated CO2 concentration on leaf C assimilation occurred largely through changes in leaf N level (Ellsworth et al. 2004; Hyvönen et al. 2007). In our experiment, downregulation of photosynthesis only happened in the leaves of S. superba and A. acuminatissima grown in the elevated CO2 concentration with the low N treatment. Compared to the other treatments, these two species grown in the elevated CO2 concentration with the low N treatment had lower leaf N concentrations, which supports the finding of the downregulation of photosynthesis in the subtropical environment. Sheu and Lin (1999) also reported that decreased photosynthetic rate with elevated CO2 concentration was observed in the leaves of S. superba when measured in the ambient CO2 concentration over a long-term exposure of 6 months. O. pinnata is an N fixation species. It is understandable that no photosynthetic acclimation was found for this species since greater N concentrations were shown in the leaves of this species. It has also been reported that the N-fixing ability of legumes generally enhances their responses to elevated CO2 concentration (Hebeisen et al. 1997; Lüscher et al. 1998, 2000). In the Soy FACE experiment, non-nodulating soybeans showed downregulation of photosynthesis in elevated CO2 concentration, while nodulating ones maintained the same photosynthetic capacity under both ambient and elevated CO2 concentration (Ainsworth et al. 2004). Lüscher et al. (2000) also found that under elevated CO2 concentration, effectively nodulating Medicago sativa increased harvestable biomass and N yield, while ineffectively nodulating plants were negatively affected by elevated CO2 concentration. C. hystrix is not an N fixation species, however no photosynthetic acclimation was shown for this species over time, because C. hystrix is a fast-growing species which probably has the large sink. Hovenden (2003) reported in his experiment that downregulation of photosynthesis was only in the slow-growing poplar clones. Hence, C. hystrix is a very good species for C fixation under elevated CO2 concentration.
The N treatment affected the growth of S. superba which was proved by the low N concentration found in the leaves of S. superba grown with the low N treatment. Relatively low photosynthesis rate in the leaves of S. superba grown with the low N treatment and elevated CO2 concentration in the later measurement period also supported the finding. During our measurement, O. pinnata growth was not affected by the CO2 concentration treatments, which is contrary with most of previous studies where tree growth was positively affected by elevated CO2 concentration (Xiao et al. 2005; Ceulemans et al. 1996; Sefcik et al. 2007). However, we hypothesized that the greater growth would soon be found for the O. pinnata grown with the elevated CO2 concentration as downregulation of photosynthesis was not found for this species. The N treatments did not affect O. pinnata growth, which attributed that this species was an N fixation species. The elevated CO2 concentration treatment increased significantly the growth of C. hystrix. Consistent high photosynthetic rate in the seedlings of C. hystrix grown with the elevated CO2 concentration led to consistently greater basal area and height growth of C. hystrix exposed to the high CO2 concentration. Elevated CO2 concentration had no significant effect on the growth of A. acuminatissima as photosynthetic acclimation was found in the trees grown with the elevated CO2 concentration during the earlier measurement month.
The responses of different plant species to elevated CO2 concentration varied greatly (Ceulemans et al. 1996; Wullschleger 1997; Curtis and Wang 1998; Ainsworth and Long 2005; Luo et al. 2005). It is important to incorporate as many of these sources of variation as possible into estimates of the CO2 concentration enhancement of photosynthesis because predictions of future forest productivity rely on these values. Our results showed that there were variations of leaf photosynthesis and tree growth for the four tree species in response to elevated CO2 concentration. The magnitude of photosynthetic acclimation differed between C3 functional groups in our experiment. Net light-saturated rate of photosynthesis tended to be reduced to a greater extent in the slow-growing species (A. acuminatissima) than in the fast-growing species (C. hystrix) or legume (O. pinnata). This result is consistent with the other reports (Hovenden 2003; Ainsworth and Long 2005).
Elevated CO2 concentration increased the growth of C. hystrix significantly, while the high N treatment increased the growth of S. superba. Our first hypothesis was partly supported as the long-term growth in the elevated CO2 concentration only led to the downward photosynthetic acclimation in two species. Our second hypothesis was largely supported as the magnitude of downward acclimation differed among the species grown in the elevated CO2 concentration.
Few experiments about the downregulation responses to elevated CO2 concentration have been performed in subtropical China with a high ambient N deposition. In the experiment, we found that photosynthetic downregulation still happened in two species grown with the elevated CO2 concentration under the high ambient N deposition for the 3-year study period. At the same time, the consistently high net photosynthesis was also obtained for one species grown with the elevated CO2 concentration under the high ambient N deposition. As we only studied four tree species in our experiment and no other such experiments were carried out in subtropical China, more research should be done to improve the understanding of how different tree species would respond to climate change and N deposition in terms of biogeochemical cycles and forest ecosystem productivity in the subtropical and tropical environments, particularly over longer periods (>5 years).
5 Conclusions
Four tree species, S. superba Gardn. et Champ., O. pinnata (Lour.) Merr, C. hystrix AC. DC., and A. acuminatissima (Blume) Merr. et Perry were exposed to the elevated CO2 concentration and ambient N deposition in open-top chambers for about 3 years; photosynthetic acclimation was found for S. superba and A. acuminatissima, which indicates that the long-term tree growth in the elevated CO2 concentration would lead to the downward photosynthetic acclimation in mature leaves of these species in subtropical China, even with the high ambient N deposition. However, photosynthetic acclimation was not found for the fast-growing species (C. hystrix) and the N fixation species (O. pinnata). The photosynthetic acclimation of S. superba and A. acuminatissima was attributed to the relative low leaf N concentration for these two species. The fast growth of C. hystrix exposed to the elevated CO2 concentration highlights that C. hystrix should be a good species for C fixation in response to the expected rising CO2 concentration in the future.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Ainsworth EA, Rogers A, Nelson R, Long SP (2004) Testing the ‘source–sink’ hypothesis of down-regulation of photosynthesis in elevated CO2 concentration in the field with single gene substitutions in Glycine max. Agr Forest Meteorol 122:85–94
Blank RR, Derner JD (2004) Effects of CO2 enrichment on plant-soil relationships of Lepidium latifolium. Plant Soil 262:159–167
Bondada BR, Syvertsen JP (2003) Leaf chlorophyll, net gas exchange and chloroplast ultrastructure in citrus leaves of different nitrogen status. Tree Physiol 23:553–559
Cai YF, Zhang SB, Hu H, Li SY (2010) Photosynthetic performance and acclimation of Incarvillea delavayi to water stress. Biol Plantarum 54(1):89–96
Ceulemans R, Shao BY, Jiang XN, Kalina J (1996) First-and second-year aboveground growth and productivity of two Populus hybrids grown at ambient and elevated CO2. Tree Physiol 16:61–68
Cheng SH, Moore BD, Seemann JR (1998) Effects of short-on the expression of ribulose-1, 5-and long-term elevated CO2 bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol 116:715–723
Curtis PS (1996) A meta-analysis of leaf gas exchange and nitrogen in trees grown under elevated carbon dioxide. Plant Cell Environ 19:127–137
Curtis P, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
Ellsworth D, Reich P, Naumburg E, Kochs G, Kubiske M, Smith S (2004) Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Global Change Biol 10:2121–2138
Finzi AC, DeLucia EH, Hamilton JG, Richter DD, Schlesinger WH (2002) The nitrogen budget of a pine forest under free air CO2 enrichment. Oecologia 132:567–578
Hebeisen T, Luscher A, Zanetti S, Fischer BU, Hartwig UA, Frehner M, Hendrey GR, Blum H, Nosberger J (1997) Growth response of Trifolium repens L. and Lolium perenne L. as monocultures and bi-species mixture to free air CO2 enrichment and management. Global Change Biol 3:149–160
Hovenden MJ (2003) Photosynthesis of coppicing poplar clones in a free-air CO2 enrichment (FACE) experiment in a short-rotation forest. Funct Plant Biol 30:391–400
Huang ZQ, Xu ZH, Bubb KA, Blumfield TJ (2008) Effect of mulching on the growth, foliar photosynthetic nitrogen and water use efficiency of hardwood plantations in subtropical Australia. Forest Ecol Manag 255:3447–3454
Hyvönen R, Agren GI, Linder S, Persson T, Cotrufo MF, Ekblad A, Freeman M, Grelle A, Janssens IA, Jarvis PG, Kellomäki S, Lindroth A, Loustau D, Lundmark T, Norby RJ, Oren R, Pilegaard K, Ryan MG, Sigurdsson BD, Strömgren M, Van Oijen M, Wallin G (2007) The likely impact of elevated CO2 concentration, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol 173:463–80
Johnson DW, Hungate BA, Dijkstra P, Hymus G, Hinkle CR, Stiling P (2003) The effects of elevated CO2 on nutrient distribution in a fire-adapted Scrub Oak Forest. Ecol Appl 13:1388–1399
Johnson DW, Cheng W, Joslin JD, Norby RJ, Edwards NT, Toddjr DE (2004) Effects of elevated CO2 on nutrient cycling in a sweet gum plantation. Biogeochemistry 69:379–403
Jones PG, Lloyd JC, Raines CA (1996) Glucose feeding of intact wheat leaves repressed the expression of a number of Calvin cycle genes. Plant Cell Environ 19:231–236
Kutik J, Natr L, Demmers-Derks HH, Lawlor DW (1995) Chloroplast ultrastructure of sugar beet (Betavulgaris L.) cultivated in normal and elevated CO2 concentrations with two contrasted nitrogen supplies. J Exp Bot 46:1797–1802
Liu JX, Zhang DQ, Zhou GY, Faivre-Vuillin B, Deng Q, Wang CL (2008) CO2 enrichment increases nutrient leaching from model forest ecosystems in subtropical China. Biogeosciences 5:1783–795
Liu JX, Zhou GY, Zhang DQ, Xu ZH, Duan HL, Deng Q, Zhao L (2010) Carbon dynamics in subtropical forest soil: effects of atmospheric carbon dioxide enrichment and nitrogen addition. J Soils Sediments 10:730–738
Luo ZB, Langenfeld-Heyser R, Calfapietra C, Polle A (2005) Influence of free air CO2 enrichment (EUROFACE) and nitrogen fertilisation on the anatomy of juvenile wood of three poplar species after coppicing. Trees 19:109–118
Lüscher A, Hendrey GR, Nosberger J (1998) Long-term responsiveness to free air CO2 enrichment of functional types, species and genotypes of plants from fertile permanent grassland. Oecologia 113:37–45
Lüscher A, Hartwig UA, Suter D, Nosberger J (2000) Direct evidence that symbiotic N2 fixation in fertile grassland is an important trait for a strong response of plants to elevated atmospheric CO2. Global Change Biol 6:655–662
Medlyn BE, Badeck FW, de Pury DGG, Barton CVM, Broadmeadow M, Ceulemans R, De Angelis P, Forstreuter M, Jach ME, Kellomaki S, Laitat E, Marek M, Philippot S, Rey A, Strassemeyer J, Laitinen K, Liozon R, Portier B, Roberntz P, Wang K, Jstbid PG (1999) Effects of elevated CO2 on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ 22:1475–1495
Mo JM, Brown S, Xue JH, Fang YT, Li ZA (2006) Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests of subtropical China. Plant Soil 282:135–151
Poorter H (1993) Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 104(105):77–97
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Ren R, Mi F, Bai N (2000) A chemometrics analysis on the data of precipitation chemistry of China. J Beijing Polytechnic University 26:90–95, In Chinese with English abstract
Rey A, Jarvis PG (1998) Long-term photosynthetic acclimation to increased atmospheric CO2 concentration in young birch (Betula pendula) trees. Tree Physiol 18:441–450
Robredo A, Perez-lopez U, Lacuesta M, Mena-petite A, Munoz-rueda A (2010) Influence of water stress on photosynthetic characteristics in barley plants under ambient and elevated CO2 concentrations. Biol Plantarum 54(2):285–292
Saxe H, Ellsworth DS, Heath J (1998) Tree and forest functioning in an enriched CO2 atmosphere. Tansley Review No.98. New Phytol 139:395–436
Sefcik LT, Zak DR, Ellsworth DS (2006) Photosynthetic responses to understory shade and elevated CO2 in four northern hard-wood tree species. Tree Physiol 26:1589–1599
Sefcik LT, Zak DR, Ellsworth DS (2007) Seedling survival in a northern temperate forest understory is increased by elevated atmospheric carbon dioxide and atmospheric nitrogen deposition. Global Change Biol 13:132–146
Sheu BH, Lin CK (1999) Photosynthetic response of seedlings of the sub-tropical tree Schima superba with exposure to elevated carbon dioxide and temperature. Environ Exp Bot 41:57–65
Wolfe DW, Gifford RM, Hilbert D, Luo YQ (1998) Integration of photosynthetic acclimation to CO2 at the whole-plant level. Global Change Biol 4:879–893
Wullschleger SD (1997) The potential response of terrestrial carbon storage to changes in climate and atmospheric CO2. Climatic Change 35:199–227
Xiao CW, Sun OJ, Zhou GS, Zhao JZ, Wu G (2005) Interactive effects of elevated CO2 and drought stress on leaf water potential and growth in Caragana intermedia. Trees 19:711–720
Xu ZH, Chen CR (2006) Fingerprinting global climate change and forest management within rhizosphere carbon and nutrient cycling processes. Environ Sci Pollut Res 13:293–298
Xu ZH, Saffigna PG, Myers RJK, Chapman AL (1993) Nitrogen cycling in leucaena (Leucaena leucocephala) alley cropping in semi-arid tropics I. Mineralization of nitrogen from leucaena residues. Plant Soil 148:63–72
Xu ZH, Bubb KA, Simpson JA (2002) Improved nitrogen nutrition and growth of Araucaria cunninghamii plantation from application of nitrogen fertilizer and weed control to 4-year-old stands in subtropical Australia. J Trop Forest Sci 14:213–222
Zheng X, Fu C, Xu X, Yan X, Huang Y, Chen G, Han S, Hu F (2002) The Asian nitrogen cycle case study. Ambio 31:79–87
Acknowledgments
The financial and in-kind support was received from the National Natural Science Foundation of China (Grant No. 31070439), the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant Nos. KSCX2-EW-Q-8 and KSCX2-EW-J-28 ), and the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Liu, J., Zhou, G., Xu, Z. et al. Photosynthesis acclimation, leaf nitrogen concentration, and growth of four tree species over 3 years in response to elevated carbon dioxide and nitrogen treatment in subtropical China. J Soils Sediments 11, 1155–1164 (2011). https://doi.org/10.1007/s11368-011-0398-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0398-4