Abstract
Populus × euramericana, P. alba, and P. nigra clones were exposed to ambient or elevated (about 550 ppm) CO2 concentrations under field conditions (FACE) in central Italy. After three growing seasons, the plantation was coppiced. FACE was continued and in addition, one-half of each experimental plot was fertilised with nitrogen. Growth and anatomical wood properties were analysed in secondary sprouts. In the three poplar clones, most of the growth and anatomical traits showed no uniform response pattern to elevated [CO2] or N-fertilisation. In cross-sections of young poplar stems, tension wood amounted to 2–10% of the total area and was not affected by elevated CO2. In P. nigra, N-fertilisation caused an about twofold increase in tension wood, but not in the other clones. The formation of tension wood was not related to diameter or height growth of the shoots. In P. × euramericana N-fertilisation resulted in significant reductions in fibre lengths. In all three genotypes, N-fertilisation caused significant decreases in cell wall thickness. In P. × euramericana and P. alba elevated [CO2] also caused decreases in wall thickness, but less pronounced than nitrogen. In P. nigra and P. × euramericana elevated [CO2] induced increases in vessel diameters. These results show that elevated [CO2] and N-fertilisation affect wood structural development in a clone specific manner. However, the combination of these environmental factors resulted in overall losses in cell wall area of 5–12% in all three clones suggesting that in future climate scenarios negative effects on wood quality are to be anticipated if increases in atmospheric CO2 concentration were accompanied by increased N availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest ecosystems cover 43% of the terrestrial biosphere (Melillo et al. 1993). During the past century human activities, such as combustion of fossil fuels, deforestation, wide application of nitrogen-containing fertilisers, etc., have resulted in a dramatic increase in the atmospheric CO2 concentration and enhanced nitrogen deposition (Huang et al. 1999). Increased [CO2] is expected to increase biomass accumulation and net primary productivity of forest ecosystems (Melillo et al. 1993; Wullschleger et al. 1995; Gielen and Ceulemans 2001; Calfapietra et al. 2003a). Increasing nitrogen deposition also has been observed to stimulate wood production (Brix 1981; McGuire et al. 1992, 1993).
Despite compelling evidence that these environmental factors can result in enhanced above-ground biomass and increased annual ring width (Hättenschwiler et al. 1996; Telewski et al. 1999; Yazaki et al. 2001; Ceulemans et al. 2002; Mäkinen et al. 2002), there is no clear picture how CO2 and nitrogen affect the anatomical properties of wood. For example, Atkinson and Taylor (1996) found that elevated [CO2] significantly increased both vessel number and mean vessel size of Quercus seedlings, but had no influence on the vessel number and size of Prunus seedlings. In Pinus radiata CO2 enrichment caused no differences in tracheid length, lumen diameter or wall thickness (Donaldson et al. 1987). In contrast to these results, Conroy et al. (1990) reported that tracheid wall thickness increased by 44% in Pinus radiata, whereas Yazaki et al. (2001) found reduced wall thickness in Larix sibirica under elevated [CO2]. Ceulemans et al. (2002) found that the wood of Pinus sylvestris grown at elevated [CO2] contained significantly increased numbers of tracheids, tracheid diameters, and annual ring widths, whereas wood density remained unchanged and wood strength decreased.
Although high nitrogen increased the growth rate and resulted in higher stem wood production, impacts of high nitrogen supply on wood quality were questioned (Shupe et al. 1996; Pape 1999). Several studies have shown that fertilisation enhanced tracheid lumen diameter, decreased cell wall thickness and the basic density of wood (Brolin et al. 1995; Lindström 1996). The results of Yang et al. (1988) and Dutilleul et al. (1998) suggest that increased nutrient availability might decrease fibre length; however, contradictory results were also reported (Schmidtling 1973; Zobel and Van Buijtenen 1989).
Obviously, these contrasting data demand further investigations before general conclusions about the influence of enriched [CO2] and nitrogen on wood structure and quality can be drawn (Saxe et al. 1998; Pritchard et al. 1999; Ward and Strain 1999; Ceulemans et al. 2002). With respect to these uncertainties it is important to analyse the wood of trees exposed to elevated [CO2] and additional nitrogen fertilisation under field conditions and to compare the CO2 responses in different species to find out whether the responsiveness to CO2 depends strongly on the genetic background or not.
The objective of the present study was to investigate the impact of elevated [CO2] and N-fertilisation on wood structure and quality of field-grown trees. For this purpose the study was conducted at the EUROFACE field site, where poplars have been grown under ambient and elevated [CO2] of about 550 ppm since 1999. In 2001 the trees had reached heights of 8.5–9.3 m (Calfapietra et al. 2003b) and the plantation was cut. Secondary sprouts developed from the stools in 2002. We used this experimental approach to address the following questions: do elevated [CO2] and nitrogen as single factors or in combination: (1) affect tension wood formation or is tension wood formation mainly related to growth characteristics (radial growth, height), (2) affect the structure of normal wood, and (3) affect the anatomical properties of wood elements (fibre lumen, vessel lumen, length, etc.)? Since the EUROFACE field site contains three different poplar clones (P. alba, Populus × euramericana and P. nigra) with different growth characteristics, the results of this study can furthermore contribute to elucidate whether the responsiveness of certain traits to [CO2] and nitrogen enrichment is uniform or is genotype-dependent.
Materials and methods
Site description
The study site is located in central Italy (42°22′N, 11°48′E, altitude 150 m) on 9 ha of former agricultural land. In spring 1999, following detailed soil analysis, six 30 m ×30 m experimental areas (“plots”) were selected and FACE facilities were installed in three of the plots whereas the other three plots, representing the control treatment, were left under natural conditions. The minimum distance between plots is 120 m to avoid cross-contamination between FACE and control treatments. The CO2 enrichment was realised through octagonal polyethylene rings (22 m diameter) mounted on telescopic poles. Pure CO2 (Messer Griesheim) was released through laser-drilled holes in the polyethylene rings to achieve the target [CO2] (550 μmol mol−1) inside the FACE plots. A meteorological station located at the centre of each FACE plot was used to control the release of CO2, whose amount was determined by wind direction and speed and by an algorithm developed for the FACE facility according to a 3-D gas dispersion model. The FACE system was controlled and monitored by a computer to reach the target [CO2]. In the FACE plots daytime [CO2] was 554±1.6 μmol mol−1 during the growing season of 2002 (from bud burst to leaf fall; F. Miglietta, CNR-IATA, Florence, Italy, unpublished data). A detailed description of FACE facilities was given by Miglietta et al. (2001).
Plant material and plantation layout
In spring 1999, on 9 ha of land six experimental plots with homogenous soil and microclimatic conditions were selected and on the surrounding land P. × euramericana (Dode) Guinier (clone I-214) was planted at a planting density of 5,000 trees per ha (2 m ×1 m). Each experimental plot was divided into halves by a physical resin-glass barrier (1 m deep in the soil) to provide N-fertilisation in each half plot. Each half plot was further divided into three slices (subplots), planted with one of three poplar clones, P. alba L. (2AS-11), P. × euramericana (Dode) Guinier (I-214), and P. nigra L. (Jean Pourtet), at a planting density of 10,000 trees per ha (1 m ×1 m). A detailed description of the clone properties was given by Calfapietra et al. (2001). In 2001, the trees reached heights of 8.5–9.3 m (Calfapietra et al. 2003b) and all trees were cut to the base of the stem at 5–8 cm above the ground. As a result, secondary sprouts developed from the stools in the spring of 2002. During the growing season of 2002, Navarson (Amiad, Imago, Italy), a fertiliser with a 10:3:3 NPK content (nitrogen with a 4:1 NH4+: NO3− ratio) and micro-nutrients, dissolved in 200 l tanks, was applied once per week, starting July 8, for a period of 16 weeks, through hydraulic pumps installed outside plots and a drip-irrigation system. A total amount of 212 kg N ha−1 was supplied throughout the growing season.
Sampling
In the first week of September 2002, the first harvest was achieved by the following sampling strategy: two stools were marked randomly per subplot; on each marked stool, the shoot with the second thickest diameter, measured at the height of 20 cm above the stool, was harvested. The shoot with the third thickest diameter was marked for the following harvest in March 2003. A total of 72 shoots were harvested from the six experimental plots. Each selected shoot was cut at the stool level. Its height and biomass were determined. An 8 cm long stem segment was removed from the height of 1.92 m to 2.00 m of each shoot and preserved for anatomical studies in FAE (37% formalin/glacial acetic acid/70% ethyl alcohol =5 parts/5 parts/90 parts) in wide-mouthed jars with lids. The sampling was repeated in March 2003.
Analysis of wood anatomy
Stem cross-sections (30 μm) were obtained with a sliding microtome (Reichert-Jung, Heidelberg, Germany) and mounted in 50% glycerol for microscopy. For an overview sections were viewed by fluorescence microscopy (Axioskop, Zeiss, Oberkochen, Germany) using the filter combination G365/FT395/LP420 to document autofluorescence. Sections were stained for 10 min with toluidine blue (pH 7.0, w/v=0.05%). Well-stained sections and a micrometer scale were photographed under a light microscope (Axioskop, Zeiss, Oberkochen, Germany) with a digital camera (Nikon CoolPix 990, Nikon, Tokyo, Japan) with 40× and 400× magnifications. In addition, photographs of stem cross-sections from cambium to pith were taken under a binocular (Stemi SV11, Zeiss, Oberkochen, Germany) with 6× magnification. A 2.5 cm part of the same stem segment was removed and dried at room temperature. Then its cross-sectional plane was polished, stained with zinc chloriodide (Purvis et al. 1966) which stained cellulose indicative for tension wood and photographed under a binocular with 6× magnification.
Microphotographs of normal wood were analysed by an image analysis software (analySIS 3.2, Soft Imaging System, Münster, Germany) for the following parameters: diameter of vessel and fibre lumina, thickness of double fibre wall (the wall between two adjacent fibre cells), areas of ray parenchyma cells, vessel lumina, and fibre lumina as well as the number of vessels per unit area. The unit area was set as a square-shaped area of 598,333 μm2. The percentage of cell wall area (CWA) was calculated as follows:
The xylem width was measured as the distance from the cambium to the pith. For tension wood determination, sections stained purple by zinc chloriodide in whole-stem cross-sections were measured. The percentage of tension wood area (TWA, portion of purple sections) was calculated as follows:
To determine the lengths of vessel elements and fibres, the same stem segments as those used for cross-sections were chosen. About 1 mm width of wood next to the cambium was discarded to avoid young xylem cells and the residual wood was cut longitudinally into pieces for chemical maceration in 65% nitric acid (Merck, Darmstadt, Germany) and traces of sodium chlorate (Merck, Darmstadt, Germany) after Kitin et al. (1999). After 2 h of maceration at room temperature, the materials were transferred into reaction cups and centrifuged (3,200g, 2 min, Eppendorf Centrifuge). The pellets were washed with distilled water, centrifuged again, and then preserved in 70% ethanol for further analysis. The prepared cell mixture was stained with toluidine blue (pH 7.0, w/v=0.05%) and mounted in 50% glycerol for microscopy. The short lengths of vessel elements (the short distance between the perforations) were measured after the definition of Chalk and Chattaway (1934).
Statistical analysis
To determine the main effects of species (clone), CO2 treatment (CO2) and N-fertilisation (nitrogen) on all variables, an ANOVA, i.e. a randomised-complete-block design, with species, CO2 treatment, N-fertilisation and their interactions as fixed factors and block as a random factor, was applied. All statistical tests were performed in Statgraphics (STN, St Louis, Mo., USA) using the mixed procedure and plot as a replicate. When interactions were significant, a posteriori comparison of means was done. To reduce the chance on type I errors, all P-values of these multi-comparisons were corrected by Tukey method. Data were tested for normality with the Shapiro–Wilk’s test. Differences between parameter means were considered significant when the P-value of the ANOVA F-test was less than 0.05.
Results
Growth and tension wood as affected by FACE and N-fertilisation
The heights and diameters of the second and third thickest shoot were measured in September, when the trees were still in the active phase of growth, and in March, when the trees were dormant. There were significant differences between the clones with P. × euramericana displaying tallest and thickest shoots and P. alba the shortest (Table 1). Exposure to FACE conditions caused increases in height and diameter of the selected shoots (Table 1, P-values for CO2 main effect). Nitrogen fertilisation had no significant influence as a main factor, perhaps because of the relatively short duration of the treatment (ca. 2 months before harvest in September). Only P. × euramericana shoots responded positively with respect to diameter and height growth to N-addition (Table 1, significant P-value for the interaction of clone × nitrogen).
To find out whether clone-specific growth characteristics or growth stimulation by FACE or N-fertilisation affected wood properties, we determined the occurrence of tension wood in stem cross-sections of P. alba, P. nigra and P. × euramericana. Tension wood formation was significantly dependent on the clone and in most cases lower in P. × euramericana than in the two other species (Table 1). The main factor CO2 had no and nitrogen only in March significant effects on the proportion of tension wood formed (Table 1). To test whether correlations existed between tension wood and growth parameters, we performed linear correlation analysis between shoot height or diameter and the portion of tension wood area. No significant relationships were found (R2=0.0006 for tension wood area vs diameter; R2=0.0001 for tension wood area vs height). The shoot diameters were measured 0.2 m above ground, whereas the stem sections analysed for tension wood were collected about 2 m above the ground. To investigate the possibility that the tension wood area was related to the radial growth observed 2 m above ground, correlation analysis was also conducted for tension wood area and the xylem width of the wood slice used for anatomical studies (September 2002 samples only). Again no significant correlation was observed (R2=0.0179).
Influence of FACE and N-fertilisation on structural wood composition
The three poplar clones showed significant structural differences in their normal wood composition (Table 2). The secondary xylem of P. nigra displayed larger vessel lumina and higher vessel numbers than those of P. alba and P. × euramericana (Fig. 1a–c). This accumulated to significantly larger vessel lumen areas in P. nigra than in the other two genotypes (Fig. 2). In all three poplar species, FACE significantly decreased the percentages of cell wall area compared with those grown at the ambient CO2 concentration (Fig. 2, Table 2). The decreased portion of cell wall area was mainly caused by increased fibre lumen areas in the three poplar clones (Fig. 2). Furthermore, elevated [CO2] also resulted in increased percentages of ray parenchyma areas compared with the ambient [CO2] (Fig. 2, Table 2). N-fertilisation also significantly reduced the percentages of cell wall area in comparison with shoots from unfertilised plots (Fig. 2, Table 2). These decreases were accompanied by decreases in vessel lumina but increases in fibre lumina areas (Fig. 2, Table 2). The observation that N-fertilisation had these consistent effects on wood properties was surprising since the nitrogen influence on radial or height growth was genotype-specific (Table 1).
Typical cross-sections of P. alba (A), P. × euramericana (B) and P. nigra (C) grown under ambient [CO2] in the absence of additional N-fertilisation. For more details, cross-sections of P. nigra D–G were photographed with higher magnification and insets in D–G show details of typical thickness of double fibre wall under different conditions (D: under ambient [CO2] in absence of N-fertilisation; E: under ambient [CO2] in presence of N-fertilisation; F: under elevated [CO2] in absence of N-fertilisation; G: under elevated [CO2] in presence of N-fertilisation). The sections were viewed by fluorescence microscopy. Magnifications are indicated by scale bars
Relative abundance of cell lumina and wall areas in cross-sections of secondary xylem of P. alba, P. × euramericana and P. nigra grown under either ambient (A) or elevated (E) [CO2] in the presence (H) or absence (L) of N-fertilisation. The pies correspond to fractional areas of cell walls (PCWA), vessel lumina (PVA), fibre lumina (PFA), and ray parenchyma (PRA). Data indicate means (n=18) and different letters indicate significant differences at P≤0.05
Effects of FACE and N-fertilisation on anatomical characteristics of wood
There were significant differences in vessel anatomical traits among the three poplar genotypes (Figs. 1a–c, 3, Tables 2, 3). Under most conditions P. × euramericana contained the longest vessel elements with diameters intermediate between those of P. alba (smaller) and P. nigra (larger) (Table 3). The main factors (CO2) and nitrogen also had significant influence on vessel properties (Table 3). With the exception of P. alba, FACE caused decreases in vessel element lengths and increases in vessel element diameters (Table 3). Under most conditions, nitrogen fertilisation resulted in the production of shorter vessel elements (Table 3). A notable exception was P. alba under ambient CO2, where nitrogen fertilisation resulted in about 12% longer vessels.
Vessel frequency (number mm−2) in cross-sections of P. alba (Pa), P. × euramericana (Pe) and P. nigra (Pn) grown under either ambient (A) or elevated (E) [CO2] in the presence (H) or absence (L) of N-fertilisation. Bars indicate means (±SD, n=18). Different letters indicate significant differences at P≤0.05
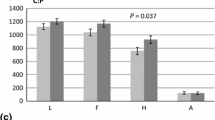
The vessel frequency in wood of P. nigra was significantly higher than in that of P. × euramericana or P. alba (Figs. 1a–c, 3). The main factors [CO2] and nitrogen had no significant influence on the vessel frequencies (Table 2). Only in P. nigra grown under ambient [CO2] nitrogen fertilisation caused increased vessel numbers, an effect which disappeared when this clone was grown under FACE conditions (Fig. 3).
The anatomical characteristics of fibres were also significantly affected by the three main factors, clone, [CO2], and nitrogen (Tables 2, 3). P. alba wood generally contained fibres with larger average lumen diameters than that of wood of P. × euramericana or P. nigra (Table 3). Growth under FACE had no effect on fibre lumen diameter of P. alba but caused significant increases in P. nigra (Table 3). Nitrogen fertilisation caused increased fibre lumina in P. alba and P. × euramericana under FACE (Table 3).
The influence of FACE or nitrogen fertilisation on fibre lengths was also strongly clone-specific. For instance, in P. × euramericana, N-fertilisation caused strong reductions in fibre lengths, whereas P. nigra showed clear increases (Table 3). In P. alba the response of fibre length to N-fertilisation was modulated by FACE (Table 3).
An important feature affecting wood density is the thickness of the fibre wall. For practical purposes, we measured the thickness of the walls between two adjacent fibre cells, denominated as “double fibre wall thickness”. This parameter was also significantly affected by clone, CO2, and nitrogen (Fig. 1d–g, Table 2). The double fibre walls of P. × euramericana and P. nigra were significantly thicker by 9.4% and 24.9%, respectively, than those of P. alba (Fig. 4). In all three poplar genotypes, FACE significantly decreased the thickness of double fibre wall compared with the ambient [CO2] (Figs. 1d–g, 4, Table 2). N-fertilisation consistently caused strong decreases of the thickness of double fibre cell walls (Figs. 1d–g, 4, Table 2).
Thickness of double fibre walls in cross-sections of P. alba (Pa), P. × euramericana (Pe) and P. nigra (Pn) grown under either ambient (A) or elevated (E) [CO2] in the presence (H) or absence (L) of N-fertilisation. The bars indicate means (±SD, n=216). Different letters indicate significant differences at P≤0.05
Discussion
Is tension wood formation affected by FACE or N-fertilisation or related to growth characteristics?
An important difference of our study compared with previous studies on tree responses to elevated [CO2] was that we analysed newly formed poplar shoots after coppicing. The number of shoots formed on stools differed between the different poplar clones (data not shown). Coppicing increased source-to-sink relationships (Hovenden 2003) probably explaining that in contrast to the previous single-stem system (Calfapietra et al. 2001, 2003a) or to other CO2-response and N-response studies (Conroy et al. 1990; Prior et al. 1997; Jach and Ceulemans 1999; Peltola et al. 2002; Günthardt-Goerg et al. 1996; Yazaki et al. 2001) FACE and N-fertilisation did not always have positive effects on individual shoot heights and diameters (Table 1). This does not imply a lack of nitrogen or FACE effects on biomass because we analysed only selected shoots for the present study.
A major question addressed in this investigation was whether FACE or N-fertilisation stimulated tension wood formation or whether tension wood production was mainly related to growth characteristics (radial growth, height) or clone-specific traits. The observation that the clones showed height and radial diameter differences as well as differences in tension wood but no correlation between these parameters (Table 1 and R2-values under Results), indicates that tension wood formation was clone-specific but not related to their typical growth characteristics. This was corroborated by the finding that the taller and, thus, probably more wind-exposed P. × euramericana shoots (2.4–5.4%) produced less tension wood than P. nigra (3.3–8.9%).
Gartner et al. (2003) induced tension wood formation experimentally by inclining the pots by 30° and found no influence of elevated [CO2] on tension wood formation in Quercus ilex. In this experimental system the highest tension wood formation was found at the stem base. In upright stems the frequency of tension wood formation at the stem base was similar to that in the middle (Gartner et al. 2003). In our study with naturally inclined or upright shoots, tension wood formation in the middle of the stem was quite variable (1.5–9.7%). This suggests that under field conditions tension wood is primarily a response to inclination angles and other forces acting by chance affecting shoots individually. Our data show that the extent of this response was determined by the genetic constitution and stimulated by N-fertilisation (Table 1). The latter observation is important because it indicates that an overabundance of nitrogen is likely to have negative effects on wood quality. The mechanisms which lead to the stimulation of tension wood production are not clear, but it is possible that the decreased wall thickness found here (Fig. 4) may render stems more flexible and, thus, more prone to tension wood formation. In conclusion, tension wood formation in poplar shoots was genotype-dependent and stimulated by nitrogen fertilisation but not related to radial and height growth.
Are the structural properties of normal wood affected by FACE or N-fertilisation?
Environmental factors, such as CO2-enrichment and N-fertilisation, can affect cell division and differentiation finally causing changes in xylem anatomy and wood structural composition. However, as outlined in the introduction, the data found in the literature give no conclusive picture as to whether and how elevated [CO2] affects xylem anatomy and tissue composition. The present study shows that CO2-enrichment and N-fertilisation under field conditions mainly increased the fraction of fibre lumen area (2–8%) and decreased the cell wall area (−3 to −8%). Decreased cell wall thickness was also found after fertilisation of conifers (Brolin et al. 1995; Lindström 1996). These observations indicate that wall thickness is regulated in a yet unknown manner by nitrogen availability in a genotype-independent manner. Cooke et al. (2003) have shown recently that gene expression in phloem versus xylem was altered within few days in response to N-fertilisation. It will be interesting to analyse in future studies expression patterns of cambial genes to find out which are regulated by N-fertilisation.
The observation that growth under elevated [CO2] also caused reductions in fibre wall thickness, which were more pronounced in poplars grown on non-fertilised plots than in those on fertilised plots (Fig. 4), is surprising and unexpected because it has been suggested that elevated [CO2] will increase the internal resources of assimilated carbon for processes such as cell wall formation (Conroy et al. 1990). Thus, in contrast to the results obtained here, increases in wall thickness would be expected. Actually, increased wall thickness was found in Pinus radiata seedlings grown under elevated [CO2] (Conroy et al. 1990). Currently, we can only speculate about the reasons for these contrasting responses to CO2-enrichment. It is possible that sink/source relationships, unknown environmental factors in the field, species-inherent features, etc., were decisive for wall thickness. But regardless of the reasons, the variable responses indicate that increased photosynthesis, which was generally found under elevated [CO2] (Medlyn et al. 1999; Hovenden 2003) is not a factor directly stimulating increased cell wall synthesis.
The consistent loss in cell wall area in response to FACE and N-fertilisation in all three genotypes is remarkable since other traits showed significant species-specific variations. It should be noted that these structural changes occurred, even though [CO2] and fertilisation effects on height or radial growth were small and not uniform between the three species (Table 1). A wider interpretation of these data may be premature because the results of this study were restricted to juvenile wood, which has anatomical properties different from mature wood (Zobel and Van Buijtenen 1989; Ceulemans et al. 2002). However, if these responses persisted and were also found in other tree species, we predict an aggravation of technological wood properties for trees in future environmental scenarios with increased availability of atmospheric CO2 and nitrogen in forest ecosystems. In conclusion, independent from the genotype elevated [CO2] and N-fertilisation had negative effects on the structural properties of juvenile poplar wood.
Is wood anatomy affected by FACE and N-fertilisation and what are possible implications?
In addition to technological wood properties, anatomical features of the wood cells are of interest. For example, vessel lumina determine the capacity for water transport and, thus, contribute to climatic adaptation of trees. In our study, where water was not limiting, CO2-enrichment resulted in wider vessel lumina. This suggests that poplars grown in the FACE system were more sensitive to drought since wider vessels make trees more prone to cavitation (Hargrave et al. 1994; Tyree et al. 1994). However, a generalisation of these results is not possible since the experimental plots were irrigated and we observed that the anatomy of the secondary xylem in poplar is responsive to water availability (Polle, unpublished data).
Vessel frequency is another important factor for water conduit in the stem. The vessel frequency decreased in P. nigra grown on fertilised plots under FACE, but not in the other clones (Fig. 3). In P. × canescens grown with CO2-enrichment in a greenhouse Gross (2002) also found diminished vessel frequencies. In other studies no influence of elevated [CO2] on the vessel frequencies (Q. ilex, Gartner et al. 2003; Prunus avium, Atkinson and Taylor 1996) or increased vessel frequencies (Q. robur, Atkinson and Taylor 1996) were found. These contrasting observations suggest that vessel frequency is influenced by strong CO2 × genotype interactions.
In conclusion, elevated [CO2] and N-fertilisation altered the dimensions of wood cells in a genotype-specific manner, whereas increased N-availability resulted in thinner fibre cell walls independent of the genotype. The combination of both factors led to anatomical alterations in the xylem structure with potentially negative effects on wood quality such as decreased density and decreased mechanical strength in addition to an increased risk of cavitation.
References
Atkinson CJ, Taylor JM (1996) Effects of elevated CO2 on stem growth, vessel area and hydraulic conductivity of oak and cherry seedlings. New Phytol 133:617–626
Brix H (1981) Effects of nitrogen fertilizer source and application rates on foliar nitrogen concentration, photosynthesis, and growth of Douglas-fir. Can J For Res 11:775–780
Brolin A, Noren A, Stähle E (1995) Wood and pulp characteristics of juvenile Norway spruce: a comparison between a forest and an agricultural stand. Tappi J 78:203–214
Calfapietra C, Gielen B, Sabatti M, Angelis PD, Mugnozza GS, Ceulemans R (2001) Growth performance of Populus exposed to “Free Air Carbon Dioxide Enrichment” during the first growing season in the POPFACE experiment. Ann For Sci 58:819–828
Calfapietra C, Gielen B, Galema ANJ, Lukac M, Angelis PD, Moscatelli MC, Ceulemans R, Mugnozza GS (2003a) Free-air CO2 enrichment (FACE) enhances biomass production in a short-rotation poplar plantation. Tree Physiol 23:805–814
Calfapietra C, Gielen B, Sabatti M, Angelis PD, Miglietta F, Mugnozza GS, Ceulemans R (2003b) Do above-ground growth dynamics of poplar change with time under CO2 enrichment? New Phytol 160:305–318
Ceulemans R, Jach ME, Velde RVD, Lin JX, Stevens M (2002) Elevated atmospheric CO2 alters wood production, wood quality and wood strength of Scots pine (Pinus sylvestris L) after three years of enrichment. Global Change Biol 8:153–162
Chalk L, Chattaway MM (1934) Measuring the length of vessel members. Trop Woods 40:19–26
Conroy JP, Milham PJ, Mazur M, Barlow EWR (1990) Growth, dry matter partitioning and wood properties of Pinus radiata D. Don. after 2 years of CO2 enrichment. Plant Cell Environ 13:329–337
Cooke JEK, Brown KA, Wu R, Davis JM (2003) Gene expression associated with N-induced shifts in resource allocation in poplar. Plant Cell Environ 26:757–770
Donaldson LA, Hollinger D, Middleton TM, Souter ED (1987) Effect of CO2 enrichment on wood structure in Pinus radiata D. Don. IAWA Bull New Ser 8:285–289
Dutilleul P, Herman M, Avella ST (1998) Growth rate effects on correlations among ring width, wood density, and mean tracheid length in Norway spruce (Picea abies). Can J For Res 28:56–68
Gartner B, Roy J, Huc R (2003) Effects of tension wood on specific conductivity and vulnerability to embolism of Quercus ilex seedlings grown at two atmospheric CO2 concentrations. Tree Physiol 23:387–395
Gielen B, Ceulemans R (2001) The likely impact of rising atmospheric CO2 on natural land and managed Populus: a literature review. Environ Pollut 115:335–358
Gross K (2002) Wachstum und Sekundärstoffwechsel von Wildtyp und transgenen Pappeln (Populus × canescens [AIT.] SM.) unter ambienten und erhöhten atmosphärischen CO2-Konzentrationen. In: 6-IV Cell wall composition under elevated CO2. Cuvillier, Göttingen, pp 1–16
Günthardt-Goerg MS, Schmutz P, Matyssek R, Bucher JB (1996) Leaf and stem structure of poplar (P. × euramericana) as influenced by O3, NO3, their combination, and different soil N supplies. Can J For Res 26:649–657
Hargrave KR, Kolb KJ, Ewers FW, Davis SD (1994) Conduit diameter and drought-induced embolism in Salvia millifera Greene (Labiatae). New Phytol 126:695–705
Hättenschwiler S, Schweingruber FH, Körner C (1996) Tree ring responses to elevated CO2 and increased N deposition in Picea abies. Plant Cell Environ 19:1369–1378
Hovenden MJ (2003) Photosynthesis of coppiced poplar clones in a free-air CO2 enrichment (FACE) experiment in a short-rotation forest. Funct Plant Biol 30:391–400
Huang Y, Eglinton G, Ineson P, Bol R, Harkness D (1999) The effects of nitrogen fertilisation and elevated CO2 on the lipid biosynthesis and carbon isotopic discrimination in birch seedlings (Betula pendula). Plant Soil 216:35–45
Jach ME, Ceulemans R (1999) Effects of elevated atmospheric CO2 on phenology, growth and crown structure of Scots pine (Pinus sylvestris) seedlings after two years of exposure in the field. Tree Physiol 19:289–300
Kitin P, Funada R, Sano Y, Beechman H, Ohtani J (1999) Variations in the lengths of fusiform cambial cells and vessel elements in Kalopanax pictus. Anal Bot 84:621–632
Lindström H (1996) Basic density in Norway spruce. I. A literature review. Wood Fiber Sci 28:15–27
Mäkinen H, Saranpää P, Linder S (2002) Wood density variation of Norway spruce in relation to nutrient optimisation and fibre dimensions. Can J For Res 32:185–194
McGuire AD, Melillo JM, Kicklighter DW, Grace AL, Moore B, Vorosmarty CJ (1992) Interactions between carbon and nitrogen dynamics in estimating net primary productivity for potential vegetation in North America. Global Biogeochem Cycles 6:101–124
McGuire AD, Joyce LA, Kicklighter DW, Melillo JM, Esser G, Vorosmarty CJ (1993) Productivity response of climax temperate forests to elevated temperature and carbon dioxide: a North American comparison between two global models. Clim Change 24:287–310
Medlyn BE, Badeck FW, De Pury DGG, Barton CVM, Broadmeadow M, Ceulemans R, De Angelis P, Forstreuter M, Jach ME, Kellomäki S, Laitat E, Marek M, Philippot S, Rey A, Strassemeyer J, Laitinen K, Liozon R, Portier B, Roberntz P, Wang K, Jarvis PG (1999) Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ 22:1475–1495
Melillo JM, McGuire AD, Kicklighter DW, Moore B III, Vorosmarty CJ, Schloss AL (1993) Global climate change and terrestrial net primary production. Nature 363:234–240
Miglietta F, Peressotti A, Vaccari FP, Zaldei A, DeAngelis P, Mugnozza GS (2001) Free-air CO2 enrichment (FACE) of a poplar plantation: the POPFACE fumigation system. New Phytol 150:465–476
Pape R (1999) Effects of thinning regime on the wood properties and stem quality of Picea abies. Scand J For Res 14:38–50
Peltola H, Kilpeläinen A, Kellomäki S (2002) Diameter growth of Scots pine (Pinus sylvestris) trees grown at elevated temperature and carbon dioxide concentration under boreal conditions. Tree Physiol 22:963–972
Prior SA, Runion GB, Mitchell RJ, Rogers HH, Amthor SJ (1997) Effects of atmospheric CO2 on longleaf pine: productivity and allocation as influenced by nitrogen and water. Tree Physiol 17:397–405
Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Global Change Biol 5:807–837
Purvis MJ, Collier DC, Walls D (1966) Some useful tests for biologically important substances. In: Laboratory techniques in botany, 2nd edn. Butterworth, London
Saxe H, Ellsworth DS, Heath J (1998) Tree and forest functioning in an enriched CO2 atmosphere. New Phytol 139:395–436
Schmidtling RC (1973) Intensive culture increases growth without affecting wood quality of young southern pines. Can J For Res 3:565–573
Shupe TF, Choong ET, Yang CH (1996) The effects of silvicultural treatments on the chemical composition of plantation-grown loblolly pine wood. Wood Fiber Sci 28:295–300
Telewski FW, Swanson RT, Strain BR, Burns JM (1999) Wood properties and ring width response to long-term atmospheric CO2 enrichment in field-grown loblolly pine (Pinus taeda L.). Plant Cell Environ 22:213–223
Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: Is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA J 15:335–360
Ward JK, Strain BR (1999) Elevated CO2 studies: past, present and future. Tree Physiol 19:211–220
Wullschleger SD, Post WM, King AW (1995) On the potential for a CO2 fertilization effect in forests: estimates of the biotic growth factor based on 58 controlled-exposure studies. In: Woodwell GM, Mackenzie FT (eds) Biotic feedbacks in the global climatic system. Oxford University Press, New York, pp 85–107
Yang RC, Wang EIC, Micko MM (1988) Effects of fertilisation on wood density and tracheid length of 70-year-old lodgepole pine in west-central Alberta. Can J For Res 18:954–956
Yazaki K, Funada R, Mori S, Maruyama Y, Abaimov A, Kayama M, Koike T (2001) Growth and annual ring structure of Larix sibirica grown at different carbon dioxide concentrations and nutrient supply rates. Tree Physiol 21:1223–1229
Zobel BJ, Van Buijtenen JP (1989) Wood variation: its causes and control. Springer, Berlin Heidelberg New York
Acknowledgements
We are grateful to the European Union (contract number: EVR1-CT-2002-40027) and the Programme “Nachwuchswissenschaftler aus auβereuropäischen Ländern nach Niedersachsen” for financial support. Christine Kettner, Gisbert Langer-Kettner, Michael Reichel, Rainer Schulz and Thomas Klein are acknowledged for their assistance with sample collection in the field.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, ZB., Langenfeld-Heyser, R., Calfapietra, C. et al. Influence of free air CO2 enrichment (EUROFACE) and nitrogen fertilisation on the anatomy of juvenile wood of three poplar species after coppicing. Trees 19, 109–118 (2005). https://doi.org/10.1007/s00468-004-0369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-004-0369-0