Abstract
Purpose
It has been suggested that above and belowground interactions produce important feedbacks in natural ecosystems. It is necessary to study the relationships between aboveground plant functional group traits and belowground biomass and soil chemical properties in natural grasslands.
Materials and methods
In a field study, four natural alpine meadows dominated by different plant functional groups were selected. We assigned the plant species to one of two functional groups: the grasses functional group (GFG) or the forbs functional group (FFG). The aboveground GFG and FFG biomass and total belowground biomass were measured. At the same time, for each sampling quadrat, soil pH, soil organic matter (SOM), total nitrogen (TN), available nitrogen (AN), total phosphorus (TP), and available phosphorus (AP) were determined.
Results and discussion
GFG-dominated meadows had significantly higher total belowground biomass, SOM, TN, TP, AN, and AP than FFG-dominated meadows. Correlation analyses showed that total belowground biomass (to a depth of 30 cm) and soil nutrient contents were significantly and positively correlated with the GFG biomass proportion, but negatively correlated with the FFG biomass proportion.
Conclusions
There were significant positive correlations among above and belowground biomass and the soil chemical properties studied. The GFG proportion may thus be an indicator of soil chemical properties in the studied meadow types. This implies that natural increases in, or introduction of more, GFG species in FFG-dominated meadows may improve soil nutrient conditions. This study provides the basis of understanding for future studies on plant–soil interactions and feedbacks in grassland ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ecologists are becoming increasingly aware of the role of above- and belowground feedbacks in controlling ecosystem processes and properties (Robertson and Gross 1994; Van der Putten et al. 2001; De Ruiter 2002; Bardgett et al. 2005; De Deyn and Van der Putten 2005). Plant–soil feedbacks play an important role in the maintenance of both plant community structure and soil properties. The mechanisms of plant–soil feedback depend on plant species, plant functional groups, and site-specific differences in abiotic and biotic soil chemical properties (Bezemer et al. 2006). Plant functional group composition also plays an important role in determining ecosystem function variation such as decomposition and species loss in changing environments (McLaren 2008). Many recent studies have focused on understanding how plant functional groups influence ecosystem properties (Wardle and Zackrisson 2005). Plant species composition had positive feedbacks in nutrient cycle patterns in natural ecosystems, and the plant–soil feedback depended upon plant species, plant taxonomic (or functional) groups, and site-specific differences in abiotic and biotic soil chemical properties (Bezemer et al. 2006). Changes in plant composition can also alter biotic and abiotic soil characteristics (soil organic matter, soil nutrient availability, and the composition of soil microbial communities) by interspecific combined effects of feedback (Casper and Castelli 2007). These influences on soil chemical properties result in either net positive or negative feedback effects, which influence plant performance and also plant community composition. Different plant functional groups (groups of plants that have similar roles in a community, e.g., grasses, legumes) both influence plant community dynamics and determine ecosystem function (properties and processes of an ecosystem, McLaren 2006). Changes in plant functional group and species composition have significant effects on soil nutrients in grassland and impair key ecosystem processes (Wardle and Zackrisson 2005; McLaren 2008).
Soil chemical properties can also potentially affect species composition (Bever 1994) and community structure in plant communities (Kardol et al. 2006). Soil nutrient availability is crucial for understanding the consequences of above- and belowground trophic interactions for ecosystem functioning (Ettema and Wardle 2002). The strength of above–belowground linkages may therefore depend on the availability of nutrients in ecosystems (Haase et al. 2008).
Plant species in grasslands are often separated into different groups (grasses and forbs) with presumed links to ecosystem functioning and taxonomy (Aerts and Chapin 2000; Lavorel and Garnier 2002), with regard to their grazing utilization value. Some studies suggested that a feedback effect between a single species and soil characteristics might also exist (e.g., Kardol et al. 2006), but these results were usually restricted to controlled environments or greenhouse studies. Therefore, we conducted a field study to investigate relationships between two aboveground dominant functional groups and the soil chemical properties in an alpine meadow on the Qinghai–Tibetan Plateau. We hypothesized that there should be positive relationships between the grasses functional group (GFG) and both the belowground biomass and the soil chemical properties in an alpine meadow. We set out to prove this hypothesis by comparing GFG vs forbs functional group (FFG) in four different alpine meadow types.
2 Materials and methods
2.1 Study site
Four types of natural alpine meadow dominated by different species were selected at the Maqu Wetland Protection Area (33°06′ ~ 34°33′ N, 100°46′ ~ 102°29′ E) in Gansu Province, People's Republic of China, at an altitude of 3,500 m a.s.l. on the eastern Qinghai–Tibetan Plateau. The soil types were classed as felty soils. The mean annual daily air temperature was 1.2°C, ranging from −10°C in January to 11.7°C in July. Mean annual precipitation was 620 mm, mainly falling during the short, cool summer. The accumulated annual time with cloud-free solar radiation was about 2,580 h. The vegetation was that of a typical alpine meadow and was dominated by clonal Kobresia spp., Carex atrofusca, and Blysmus sinocompressus. Typically, there were 20 ~ 30 vascular plant species and 800 ~ 1,000 individual plants per square meter (Wu et al. 2009).
The four meadow types comprised B. sinocompressus–C. atrofusca meadow (B-C meadow), Kobresia tibetica–Kobresia humilis meadow (K-K meadow), Potentilla anserina–Leontopodium nanum meadow (P-L meadow), and Ligularia virgaurea–Ajania tenuifolia meadow (L-A meadow). Descriptions of the location, altitude, main species composition, and their relative cover are given in Table 1. We assigned the plant species to one of two functional groups: the GFG or the FFG. Most of the grasses and forbs were perennials. The GFG mainly contained gramineous and sedge species, which are excellent forage species for herbivores (sheep and yak), whereas the FFG mainly contained forbs and some unpalatable species, which are not preferred or are often rejected by herbivores (Wu et al. 2009). The original species of the four meadow types were all Kobresia spp. and Carex spp., but the vegetation of the meadows has diverged because of long-term grazing effects. The B-C and K-K meadows were GFG-dominated meadows, while the P-L and L-A meadows were FFG-dominated meadows. The grazing history of the four meadow types was similar, i.e., winter grazing by nomadic Tibetan herds each year from November to June until 2005. They had all undergone grazing by Tibetan sheep and yak herds of medium herd density (the approximate ratio of sheep to yaks was about 1.6:1) during the grazing season. From 2005, all meadows were fenced to exclude livestock grazing following implementation of the Natural Grassland Protection Project of China.
2.2 Experimental procedures
In each meadow, we randomly selected three 50 × 50-m plots and placed nine quadrats (0.5 × 0.5 m) diagonally in each plot (Fig. 1). A total of 108 samples were collected in early August 2008, when the mean biomass reached its peak (Wu et al. 2009). The biomass of the two functional groups (GFG and FFG) and total belowground biomass were measured. Ramets were counted, and shoots were clipped at ground level. The biomass of each functional group was determined by drying at 80°C for 48 h to constant weight. The GFG and FFG biomass proportions were calculated based on their relative proportions in the total aboveground biomass. Belowground biomass was collected from soil removed to a depth of 30 cm in a quadrat (0.25 × 0.25 m), which was located inside the corresponding quadrat (0.5 × 0.5 m) used for the community survey. Belowground roots were sieved out on a thin, plastic gauze screen (aperture, 0.5 mm) and washed with water. Small stones were removed, and the roots were dried.
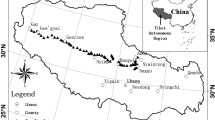
Map of the Maqu Wetland Protection Area showing the location of the four study sampling sites located in southern Gansu Province on the eastern Qinghai–Tibetan Plateau, China. Red circles are sampling sites and open squares are plant sampling quadrats. Three plots were selected at each sampling site and nine quadrats were measured in each plot. A total of 108 squares (0.5 × 0.5 m) were sampled
At the same time, from each sampling quadrat, we collected three soil samples along the diagonal to a depth of 30 cm using a bucket auger. The three soil samples taken within each separate quadrat were mixed. The nine mixed soil samples from each sampling plot were used to analyze soil characteristics. All soil samples were air-dried and then passed through a 0.14-mm sieve. Soil pH was determined using a soil–water ratio of 1:5; soil organic matter (SOM) was measured using the K2Cr2O7 method; soil total nitrogen (TN), available nitrogen (AN), total phosphorus (TP), and available phosphorus (AP) were measured using air-dried samples according to standard procedures (Agriculture Chemistry Council, Soil Science Society of China 1983). The SOM, TN, and TP were calculated as a percentage of the oven-dried (105°C) mass of the soil; AN and AP were expressed as milligrams of N or P per kilogram of soil (oven-dried).
2.3 Data analysis
Differences in total aboveground biomass, biomass by functional group, total belowground biomass, pH, SOM, TN, TP, AN, and AP were analyzed by multivariate analysis using the general linear model among plots and meadow types. Correlations among GFG and FFG biomass proportions, belowground biomass, SOM, TN, TP, AN, and AP were calculated using parametric Pearson's correlation (R) with a threshold probability of 0.01 and the log-transformed values of the data for the four meadow types. All calculations were made using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) software.
3 Results
The overall ANOVA results showed that there were significant differences in all measured parameters among the four meadow types; above- and belowground biomass, GFG biomass, and SOM also demonstrated significant differences among the study plots within each meadow type (P < 0.01). There were also significant interaction effects between the study plots and the meadow types on aboveground biomass, GFG biomass, and soil pH value (Table 2). The total aboveground biomass decreased in the order B-C meadow > K-K meadow > P-L meadow > L-A meadow (F = 4.23, P < 0.01). The GFG-dominated meadows (B-C and K-K meadows) had significantly higher total belowground biomass (Fig. 2), SOM, TN, AN, TP, and AP contents and lower pH than the FFG-dominated meadows (P-L and L-A meadows). The AN contents decreased in the sequence K-K meadow > B-C meadow > P-L meadow > L-A meadow (Fig. 3).
Mean (±standard error) of aboveground biomass (in grams per square meter, a), belowground biomass (in grams per square meter, b). Aboveground GFG biomass (in grams per square meter, c) and FFG biomass (in grams per square meter, d) for four meadow types (B-C meadow, K-K meadow, P-L meadow, and L-A meadow). Different lowercase letters inside a histogram indicate significant differences among the four meadow types (P < 0.05)
Mean (±standard error) of soil pH value (a), soil organic matter content (in percent, b), total nitrogen content (in percent, c), total phosphorus content (in percent, d), available nitrogen content (in milligrams per kilogram, e), and available phosphorus content (in milligrams per kilogram, f) for four meadow types (B-C meadow, K-K meadow, P-L meadow, and L-A meadow). Different lowercase letters inside a histogram indicate significant differences among the four meadow types (P < 0.05)
Correlation analyses showed that total belowground biomass and soil nutrient contents were all significantly and positively related to the proportion of GFG. There was also a significant positive correlation between belowground biomass and soil chemical properties studied. Additionally, there were significant positive correlations among SOM, TN, TP, AN, and AP (Table 3).
4 Discussion
We found that the biomass and proportion of GFG were positively correlated with belowground biomass and soil nutrient properties, and conversely that the biomass and proportion of FFG were negatively correlated with these parameters in alpine meadow communities. Wang et al. (2007) also found a significant positive correlation between aboveground productivity, SOM, and soil TN in K. tibetica meadows, K. humilis meadows, and Kobresia pygmaea meadows of the Qinghai–Tibetan Plateau. Furthermore, long-term fencing has been shown to increase the GFG proportion in alpine meadow communities and to improve soil chemical properties and soil organic carbon storage in an alpine swamp meadow (Wu et al. 2010a). Similarly, establishing an artificial gramineous-grassland improved soil nutrient and carbon storage in a degraded black-soil-type grassland (Wu et al. 2010b). However, grazing decreased the GFG proportion and increased the FFG proportion in alpine meadow communities, and there were lower soil nutrient contents in grazed meadows than in fenced meadows (Wu et al. 2009).
Bezemer et al. (2006) had proposed that plant–soil feedback depends upon the plant functional group. Differences in plant functional groups can create different soil biotic and abiotic microhabitats by affecting soil organic matter, soil nutrient availability, and soil microbial communities (Casper and Castelli 2007). Gastine et al. (2003) also found that the functional group identity of the plants can significantly affect root biomass and soil abiotic factors. Belowground properties may be more influenced by the identity (and litter quality) of plant functional groups than by plant species diversity. Likewise, we found that GFG-dominated meadows had higher values of total belowground biomass, SOM, TN, TP, AN, and AP than those dominated by FFG in the studied meadows. Plant productivity in terms of nutrient cycling depends on the traits of the plant species pool with respect to complementarity in nutrient supply to the soil and the acquisition of minerals and water from the soil (Eviner 2004). Graminoid species had higher productivity, which can result in greater amounts of nutrients fixed within their tissues (Harris et al. 2007), and a relatively higher proportion of belowground biomass and specific root length (Šmilauerová and Šmilauer 2006), which is related to a higher litter decomposition rate and soil nitrogen availability, than the forbs species (Moretto et al. 2001; Moretto and Distel 2002; McLaren 2006). Graminoid aboveground plant resources were returned to the soil when litter layers and roots decomposed (Bardgett and Wardle 2003). Graminoids can grow rapidly, producing readily degradable litter and root mass, further enhancing rates of nutrient cycling, and making a contribution to increases in soil fertility (reviewed in Hobbie 1992; Moretto et al. 2001; Moretto and Distel 2002).
Our results suggested that GFG species produce a positive feedback on soil nutrient properties and aboveground dominant functional groups can indicate belowground biomass conditions and soil chemical properties in grassland ecosystems. However, many other factors could also contribute to the differences in soil attributes, such as different soil substrates, soil microbial communities, herbivores and decomposers, etc. (Haase et al. 2008). These are significant because plants build up positive feedbacks in the nutrient cycle related to carbon deposition and competition with microbes for nutrients in the rhizosphere, factors that are plant species dependent (Hobbie 1992). The interaction between microbes and specific plant species alters nutrient cycles and improves plant performance or provides a competitive advantage in complex plant communities (Hawkes et al. 2005; Casper and Castelli 2007).
Our study also showed that belowground biomass had significant positive relationships with the aboveground biomass and soil nutrient properties. Differences in the quantity and quality of roots provide feedbacks that affect C and N cycling and help to maintain and even promote the fundamental differences found in N cycling in tallgrass prairies (Johnson and Matchett 2001). It suggests that belowground biomass is also an important source and sink for soil nutrients that also affect aboveground vegetation biomass in terrestrial biogeochemistry (Gordon and Jackson 2000). These observations strengthen the hypothesis that the GFG provides a significant root biomass and nutrient supply to the soil.
Positive effects of GFG species on soil chemical properties may also result in positive or negative feedback effects, which influence plant performance and plant community dynamics and composition (McLaren 2006). Huston (1996) proposed that higher soil nutrient availability improved plant community structure and productivity because higher nutrient availability favors the competitiveness of graminoid species, which have greater competitive and colonization ability over other forbs species under low grazing disturbance (Distel and Boo 1996; Moretto and Distel 1997; van der Wal et al. 2004).
Additionally, there were significant positive correlations among SOM, TN, TP, AN, and AP. These may be caused by positive feedbacks of the higher GFG species proportions in the meadow communities. Furthermore, increased N contents can boost microbial respiration and population growth, resulting in accelerated N turnover and net mineralization from decomposition of microbial residues (Mengel 1996; Ebersberger et al. 2003). The AN may also support root accelerated mineralization of SOM (Peralta and Wander 2008). Moreover, GFG-dominated meadows had lower pH values, which may impact plant rhizosphere secretion, soil acidity, and the organic acids released during the decomposition process.
5 Conclusions
In conclusion, plant functional group types that were dominated either by grasses or by forbs played an important role in determining the relationships of aboveground vegetation and belowground soil chemical properties. The GFG species induced positive feedback effects on soil nutrient accumulation and cycling in alpine meadows. These imply that natural increases in, or the introduction of more, grass species in forbs-dominated meadows may improve soil nutrient conditions since GFG-dominated meadows were associated with relatively higher soil nutrient availability than FFG-dominated meadows. Our findings suggest that the proportion of GFG may be an indicator of the soil chemical properties in these studied meadow types. Our observations may promote a better understanding of plant–soil feedbacks and soil nutrient cycling in plant communities dominated by different plant functional groups. However, a series of comprehensive research studies on specific plant species, soil interaction processes, and the feedback mechanisms at different times of the year should be conducted.
References
Aerts R, Chapin FSIII (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Agriculture Chemistry Council, Soil Science Society of China (1983) General analysis methods of soil agriculture chemistry. Science Press, Beijing
Bardgett RD, Wardle D (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK (2005) A temporal approach to linking aboveground and belowground ecology. Trend Ecol Evol 20:634–641
Bever JD (1994) Feedback between plants and their soil communities in an old field community. Ecology 75:1965–1977
Bezemer TM, Lawson CS, Hedlund K, Edwards AR, Brook AJ, Igual JM, Mortimer SR, van der Putten WH (2006) Plant species and functional group effects on abiotic and microbial soil properties and plant–soil feedback responses in two grasslands. J Ecol 94:893–904
Casper BB, Castelli JP (2007) Evaluating plant–soil feedback together with competition in a serpentine grassland. Ecol Lett 10:394–400
De Deyn GB, Van der Putten WH (2005) Linking aboveground and belowground diversity. Trend Ecol Evol 20:625–633
De Ruiter PC (2002) Ecosystem structures above- and belowground. Book review of communities and ecosystems: linking the aboveground and belowground components (D.A. Wardle, Princeton University Press). Trend Ecol Evol 17:584–585
Distel RA, Boo RM (1996) Vegetation states and transitions in temperate semiarid rangelands of Argentina. In: West NE (ed) Rangelands in a sustainable biosphere. Society for Range Management, Denver, pp 117–118
Ebersberger D, Niklaus PA, Kandeler E (2003) Long term CO2 enrichment stimulates N-mineralisation and enzyme activities in calcareous grassland. Soil Bio Biochem 35:965–972
Ettema CH, Wardle DA (2002) Spatial soil ecology. Trend Ecol Evol 17:177–183
Eviner VT (2004) Plant traits that influence ecosystem processes vary independently among species. Ecology 85:2215–2229
Gastine A, Scherer-Lorenzen M, Leadley PW (2003) No consistent effects of plant diversity on root biomass, soil biota and soil abiotic conditions in temperate grassland communities. Appl Soil Ecol 24:101–111
Gordon WS, Jackson RB (2000) Nutrient concentrations in fine roots. Ecology 81:275–280
Haase J, Moretto AS, Distel RA, Boutton TW, Boo RM (2008) Above-belowground interactions are mediated by nutrient availability. Ecology 89:3072–3081
Harris WN, Wren IF, Herman DJ, Firestone MK (2007) Fire and grazing in grasslands of the Argentine Caldenal: effects on plant and soil carbon and nitrogen. Acta Oecol 32:207–214
Hawkes CV, Wren IF, Herman DJ, Firestone MK (2005) Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol Lett 8:876–985
Hobbie SE (1992) Effects of plant species on nutrient cycling. Trend Ecol Evol 7:336–339
Huston MA (1996) Biological diversity: the coexistence of species on changing landscapes. Cambridge University Press, New York, p 681
Johnson LC, Matchett JR (2001) Fire and grazing regulate belowground processes in tallgrass prairie. Ecology 82:3377–3389
Kardol P, Bezemer TM, van der Putten WH (2006) Temporal variation in plant–soil feedback controls succession. Ecol Lett 9:1–9
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
McLaren JR (2006) Effects of plant functional groups on vegetation dynamics and ecosystem properties. Infor North 59:449–451
McLaren JR (2008) Effects of plant functional group loss on soil chemical properties in a northern grassland. The 93rd ESA Annual Meeting (August), COS 84 Ecosystem Function: Biodiversity II-3
Mengel K (1996) Turnover of organic nitrogen in soils and its availability to crops. Plant Soil 181:83–93
Moretto AS, Distel RA (1997) Competitive interactions between palatable and unpalatable grasses native to a temperate semiarid grassland of Argentina. Plant Ecol 130:155–161
Moretto AS, Distel RA (2002) Soil nitrogen availability under grasses of different palatability in a temperate semiarid rangeland of central Argentina. Austral Ecol 27:509–514
Moretto AS, Distel RA, Didone NE (2001) Decomposition and nutrient dynamic of leaf litter and roots from palatable and unpalatable grasses in a semi-arid grassland. Appl Soil Ecol 18:31–37
Peralta AL, Wander MM (2008) Soil organic matter dynamics under soybean exposed to elevated CO2. Plant Soil 303:69–81
Robertson GP, Gross KL (1994) Assessing the heterogeneity of belowground resources: quantifying pattern and scale. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants: ecophysiological processes above- and belowground. Academic Press, San Diego, pp 237–253
Šmilauerová M, Šmilauer P (2006) Co-occurring graminoid and forb species do not differ in their root morphological response to soil heterogeneity. Folia Geobot 41:121–135
Van der Putten WH, Vet LEM, Harvey JA, Wackers FL (2001) Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trend Ecol Evol 16:547–554
Van der Wal R, Bardgett RD, Harrison KA, Stien A (2004) Vertebrate herbivores and ecosystem control: cascading effects on tundra ecosystems. Ecography 27:242–252
Wang CT, Cao GM, Wang QL, Jing ZC, Ding LM, Long RJ (2007) Research on variation of species composition and biomass along environmental gradient of plant community in alpine meadow of the Qinghai–Tibetan Plateau. Sci China C Life Sci 37:585–592
Wardle DA, Zackrisson O (2005) Effects of species and functional group loss on island ecosystem properties. Nature 435:806–810
Wu GL, Du GZ, Liu ZH, Thirgood S (2009) Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai–Tibetan Plateau. Plant Soil 319:115–126
Wu GL, Liu ZH, Zhang L, Cheng JM, Hu TM (2010a) Long-term fencing improved soil properties and soil organic carbon storage in an alpine swamp meadow of western China. Plant Soil 332:331–337
Wu GL, Liu ZH, Zhang L, Hu TM, Cheng JM (2010b) Effects of artificial grassland establishment on soil nutrients and carbon properties in a black-soil-type degraded grassland. Plant Soil 333:469–479
Acknowledgments
The authors thank Robert M. Rees, Wim van der Putten, Nicholas B. Comerford, Kimberly Y. Epps, and two anonymous reviewers for their valuable comments and suggestions, and David Warrington for improving the English writing of the manuscript. We also thank Liu Zhen-Heng and other colleagues of the Maqu Grassland Station for their assistance in field investigation and soil sampling. This work is supported by the Strategic-leader Sci-Tech Projects of Chinese Academy of Sciences (XDA05050403), the “100-Talent Program” of Chinese Academy of Sciences, and the Open Funds of the State Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau China (10502-Z8-5, Z12).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Winfried Schröder
Rights and permissions
About this article
Cite this article
Wu, GL., Li, W., Shi, ZH. et al. Aboveground dominant functional group predicts belowground properties in an alpine grassland community of western China. J Soils Sediments 11, 1011–1019 (2011). https://doi.org/10.1007/s11368-011-0367-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0367-y