Abstract
Purpose
Mercury (Hg) accumulation and transfer in soil ecosystems has been altered on local, regional, and even global scales, and their environmental risk has increasingly been a concern to the public and the scientific community.
Materials and methods
A county level region in Zhangjiagang County, the Yangtze River Delta (YRD) region of China and a factory with Hg-contaminated wastewater discharging within the region were selected to study the accumulation, bioavailability, and transfer of Hg from different sources in soils and crops under rapid industrialization, urbanization, and intensive agricultural activities. Regional soil samples close to and away from factories and local soil and crop samples around a typical factory were collected in the YRD region of China. Soil and crop Hg and basic soil properties were examined.
Results and discussion
Significant soil Hg accumulation was found in soils away from factories regardless of Cambosols (Entisols) and Anthrosols (Inceptisols), while the mobile HCl-extractable Hg (HCl-Hg) were greater in soils closer to factories due to a decrease and increase in soil pH and organic matter. A high level of soil total Hg (T-Hg) was found around the factory, and soil and crop Hg accumulation in the vicinity of the factory was localized with an exponential decrease as distance away from the wastewater discharge outlet increased. Although Hg accumulated in these soils, the T-Hg levels at only a few sampling sites in acidic Anthrosols area were found to exceed the second most stringent critical value of Chinese Environmental Quality Standards for Soils.
Conclusions
Considering the cessation of Hg-containing agrochemicals and limitation of effects of industrial activities on Hg accumulation, more attention should be paid to the changes in soil properties and crop rotations than controlling the pathways of Hg entering soils because the current environmental risk is mobilization of accumulated soil Hg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rapid progress of economic and industrial development, increased loading of mercury (Hg) in the environment and the potential public health risk associated with dietary intake has increasingly become a concern (Matschullat and Filho 2001; Biester et al. 2002a, b; Rémy et al. 2003; Feng et al. 2006; Zhang and Wong 2007). Mercury accumulation in some agricultural soils has occurred as a result of uncontrolled discharge and emissions from rapidly expanding industrial area and agricultural uses of Hg-containing chemicals (Wang and Mou 1999; Wang and Stuanes 2003; Yin 2009). Although Chinese government has forbidden the use of Hg-containing fertilizer and pesticides since the 1970s (Yin 2009), Hg-containing chemicals historically used may still be accumulated in soils as an immobile heavy metal. Moreover, industrial discharges have aggravated the environmental threat of soil Hg due to rapid industrialization (Zhang et al. 2001; Biester et al. 2002a, b; Wang and Stuanes 2003; Schröder et al. 2010) and urbanization (Rodrigues et al. 2006; Qian et al. 2009) over the past few decades.
Compared to the Hg accumulation in soils of mining areas (Tomiyasu et al. 2003; Liu et al. 2004; Fernández-Martínez et al. 2005; Appleton et al. 2006; Fernández-Martínez et al. 2006; Feng et al. 2006; Loredo et al. 2006), the extent of Hg accumulation in soils of industrializing areas probably is not too much intense. However, heavy metal like Hg transfer from soil to crop or water depends not only on its accumulation but also on soil properties such as soil pH (Randall et al. 2004; Jing et al. 2007; Yang et al. 2007), Eh (Chen 1996; Randall et al. 2004), organic matter (OM) (Jing et al. 2007; Yang et al. 2007; Qian et al. 2009), and residue time (Grimaldi et al. 2008). With the rapid urbanization and industrialization in YRD region as an area with long history of agriculture, many factories were built and scattered in the agricultural lands. Meanwhile, intensive farming has been conducted due to the rising demand on agricultural products from urban areas (Huang et al. 2006). These activities have significantly changed soil properties (Darilek et al. 2009). The degree and extent of Hg accumulation in soils under such complicated situations remain unclear. The transfer and effect of accumulated Hg in soils on environmental components and their influencing factors still need to be clarified. The specific objectives of this study are: (1) to determine the spatial distribution of Hg accumulation and availability in soil and Hg in staple crops with different sources and their affecting factors using Zhangjiagang County, Yangtze River Delta region, China as a case study area; and (2) to assess the transfer of Hg in the agro-ecosystem and the potential Hg contamination threat to the environment. These data can serve as a basis for planning management strategies to achieve better environmental quality in similar areas.
2 Materials and methods
2.1 Study area
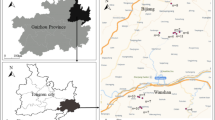
The study area, Zhangjiagang County, is situated on a flat alluvial plain in the YRD region (31°43′−32°01′N, 120°22′−120°49′E) and covers a total terrestrial area of 799 km2 with an arable land area of 409 km2 (Fig. 1a) and a population of 0.88 million in 2006. With a north sub-tropical monsoon climate, Zhangjiagang County has a mean annual temperature and precipitation of 15.2°C and 1,039.3 mm, respectively. The main soil types in the study area can be divided into two soil orders, Anthrosols (Inceptisols) and Cambosols (Entisols), according to Chinese Soil Taxonomy (CRGCST 2001) (see Fig. 1a). Anthrosols, which are the dominant order on the plains of the southern part of the county, were developed from lacustrine deposits on alluvium, with a loamy clay texture (Soil Survey Office of Zhangjiagang County 1984). Rotation of rice (Oryza sativa L.) and wheat (Triticum aestivum L.) has always been the dominant planting system. Cambosols occupy the northern part of the county along the Yangtze River and were developed from neo-alluvium parent materials with a sandy loam texture and contain free carbonates (5.7%). The cropping system on this soil is predominantly rice–wheat as well, although historically cotton–wheat was more common. Soil pH for Cambosols was higher than Anthorosols, whereas soil OM of the former was lower than that of the latter.
Since the 1980s, Zhangjiagang County has become one of the quickest economically developing areas in the Yangtze River Delta region, China, which, according to Chen et al. (2001), is 1% of China's land area but accounts for 16% of its gross domestic product (GDP). With an over 60% share in the county's total GDP, common industries in the county include metallurgical, chemical, electroplating, printing and dyeing, and paper-making.
2.2 Sample collection and preparation
Within the county, a total of 547 topsoil (0–20 cm) samples were collected throughout the county based on soil types, land use, and spatial homogeneity, of which 386 samples were randomly collected on agricultural land 500 m away from the factories and 161 samples were collected within about 50–100 m of waste discharging factories (factory locations with pollutant discharge were provided by the local environmental authority) (see Fig. 1a). The sampling design was developed to focus on possible loadings and sources of Hg from industrial areas. Thus, the whole collected soil samples were divided into two groups, away from factories and close to factories, to better elucidate the sources and environmental risk of soil mercury in the industrial region. The locations of the sampling sites are shown in Fig. 1a. Meanwhile, 56 rice samples were collected at selected soil sampling sites in harvest season. When soil and rice sampling, six to eight topsoil sub-samples or rice grain were gathered in a 0.17-ha area and then pooled and mixed. Samples were put in plastic sampling bags and brought back to the lab for analysis. All sample sites were recorded using a hand-held global position system receiver. Related information such as land-use history, vegetation, soil types, and present and potential pollutant sources were also recorded in detail.
After soil T-Hg was determined, an outlier of the highest soil T-Hg was found in southern part of the county (see Fig. 1b). According to our survey, a chemical factory located at the outlier site. A focused sampling scheme, including soil and crop (rice and wheat) sampling, was conducted around the factory in order to evaluate the effect of increased Hg in soils, its spatial distribution, and crop Hg uptake. The factory is located next to a tributary where treated wastewater is discharged annually. According to investigation, farmers near the factory often found that their fields was flooded in rice-growing season and even used the tributary water for irrigation in dry season. Narrow paths across croplands are used to transport fertilizers and crop harvests using carts. No vehicle roads are close enough to the area to affect soil Hg levels. Another tributary running along the southern edge of the cropland is farther removed from the factory and is not affected by its wastewater. The local area studied was 0.20 km2. The factory occupied ∼0.02 km2 of the area and the cropland occupied ∼0.13 km2 of the area. Established in 1993, the factory mainly produces chemicals such as active dyes. From 2004, the factory has begun producing dimethylacetamide with current production of ∼6,000 t year−1.
Soil samples were collected from sites at 20, 50, 100, 200, 300, 400, and 600 m from the wastewater outlet. Samples were taken as described above. Two soil profiles (see Fig. 1b) were exhumed and observed. Samples of soil profiles were collected from the various genetic horizons following morphological observation and description.
Wheat and rice were sampled near soil sampling sites (see Fig. 1b) when crops were in the mature stage. Similarly, the crops were randomly sampled within 10 m2, pooled, packed into cloth bags, and brought back to the lab for analysis.
2.3 Sample treatment and chemical analysis
Soils were air-dried at room temperature and ground to pass a 2-mm sieve. A small portion (50 g) was further ground with an agate mortar to pass a 0.149-mm sieve. Crop grains were thoroughly washed and rinsed using deionized water for several times. Wheat grain was air-dried at room temperature and directly ground to pass a 0.5-mm sieve. Rice grain was air-dried at room temperature and ground to pass a 0.5-mm sieve after shelling. All samples were stored at room temperature until analysis.
Soil pH (<2-mm) was measured in a 1:2.5 soil–water paste mixture using a glass electrode pH meter (PHS-3C, Shanghai, China). Soil OM was determined using the Walkley–Black method and soil samples with <0.149-mm particle size (Nelson and Sommers 1996). Randomly selected 57 soil samples were extracted using 1 M HCl solution as a test index of Hg availability in soils (HCl-Hg) according to modified methods from Yin et al. (2000) and Fernández-Martínez et al. (2005). Increased acidic extractant was used to increase the amount of Hg extracted from the soil to improve accuracy. In this procedure, approximately 1.0 g of 2 mm sieved soil was weighed and placed in centrifuge tubes, followed by the addition of 10 mL of extractant. The centrifuge tubes were sealed, shaken for 30 min, and then centrifuged at 4,500 rpm for 3 min. The supernatants were filtered through ashless filter paper. The Hg concentration in the supernatant was determined using atomic fluorescence spectroscopy (AFS) (AF-160A, Beijing Rayleigh Analytical Instrument Co., Ltd., China).
Total Hg (T-Hg) in the soil was determined using the GB/T 17136–1997 method (SEPAC 1997). Briefly, 1.0 g of 0.149 mm sieved soil was weighed into a 150-mL Erlenmeyer flask and wetted with a few drops of deionized water. A total of 5 mL of concentrated HNO3 and 5 mL of concentrated H2SO4 were added. After intense reaction ceased, 10 mL of deionized water and 10 mL of 0.13 M KMnO4 were carefully added. The flask was covered with a small glass funnel, digested on an electric hot plate at 90°C for 1 h. During the digestion, more KMnO4 solution was added if the purple color faded. The solution was cooled after digestion. A solution of NH2OH.HCl was added until the purple color faded before determination of Hg using AFS. A standard soil material (GBW07403) from the Institute of Geophysical and Geochemical Exploration, Chinese Geological Survey was used to quality control and the average T-Hg concentration was 57 ± 6 μg kg−1 (n = 3), which is comparable to the certified values of 60 ± 6 μg kg−1. The precision obtained from replicate analysis was more than 95%. The results demonstrated that the pretreatment procedures could quantitatively recover Hg from the soil samples.
The Hg fractionations of the soils from profile horizons were analyzed in triplicate based on Kingston's method and previous studies (Han et al. 2003; Fernández-Martínez et al. 2005; Fernández-Martínez et al. 2006). This fractionation scheme includes a sequential extraction process of different operationally defined fractions such as mobile Hg (M-Hg), semi-mobile Hg (SM-Hg), and non-mobile Hg (NM-Hg) and provides detailed information about the mobility of Hg in samples. Briefly, 1 g of soil was sequentially extracted with a 2% (v/v) HCl + 10% (v/v) ethanol solution as the M-Hg, with 33% (v/v) HNO3 as the SM-Hg, and with 1:6:7 (v/v/v) HCl:HNO3:H2O solution as the NM-Hg. All obtained extractants were filtered through 0.45-μm cellulose paper. The M-Hg fraction was brought to volume (50 mL) with 0.5 M HNO3, whereas SM-Hg and NM-Hg fractions were brought to volume with ultrapure distilled and deionized water.
For wheat and rice, 1.0 g of sample was digested in 10 mL of concentrated HNO3 in a 150-mL Erlenmeyer flask covered with a watch glass overnight. The digestion was continued on an electric hot plate at 90°C for 3 h, and the watch glasses were removed. After the solution volume had evaporated to a volume of 2–3 mL and cooled, 5 mL of concentrated HNO3 was added and the sample was digested again until almost void of moisture. Residue was dissolved in 5 mL HCl (50%), rinsed with deionized water along the wall of Erlenmeyer flask after cooling, filtered into a 25-mL volumetric flask, and brought to volume with deionized water. The Hg in solution was analyzed using AFS. Quality control was performed using poplar leaves as standard reference materials GBW 07604 (GSV-3). Poplar leaves contained 25 ± 4 ug kg−1 (n = 6) compared with the certified values of 26 ± 3 ug kg−1. The precision obtained from replicate analysis was more than 95%, within the range of the certified Hg concentration.
2.4 Statistical analysis
Analysis of variation was employed to determine the effects of soil types and sampling methods on soil HCl-Hg, T-Hg, and rice Hg. Regression analysis was performed to determine the relationships between Hg in crops and soils, HCl-Hg and T-Hg in the soils, and soil properties. All statistical analysis was conducted using SPSS (version 13.0). Spatial distribution maps were constructed with inverse distance weighting interpolation methods using GIS software (ArcGIS version 8.3) because of abnormal data distribution of soil total, HCl-extractable Hg, and crop Hg.
3 Results
3.1 Spatial distribution of regional soil Hg and soil properties
3.1.1 Soil total Hg
With a mean of 140 μg kg−1, the soil T-Hg concentrations of the county varied from 4 to 1,510 μg kg−1. There was a significant difference in soil T-Hg between Cambosols and Anthrosols (p < 0.05) (Table 1), which was also shown in the frequency and spatial distribution patterns of soil T-Hg (Figs. 2 and 3a). For frequency distribution of the data, the coefficient of variance of soil T-Hg in Cambosols (62%) was larger than that in Anthrosols (46%) (one outlier affected by the factory was excluded from the statistical analysis), although their positive skews and kurtosis were close (see Fig. 2). Interestingly, two frequency peaks of soil T-Hg were shown in respective soil order, in particular, Cambosols. With respect to spatial distribution, high levels of soil T-Hg were mainly distributed in the southern part of the county (see Fig. 3a). In the northern Cambosols area,77.8% of soil T-Hg concentrations in this area were below the natural background value (150 μg kg−1) of the Chinese Environmental Quality Standards for Soil (CEQS) (SEPAC 1995). However, in the southern Anthrosol area, soils with less than 150 μg kg−1 T-Hg were limited, while more than half of the soil area had T-Hg concentrations of 150–300 μg kg−1. The areas with high concentrations (>300 μg kg−1) scattered in the southern Anthrosols areas (see Fig. 3a).
Sampling site also influenced the soil T-Hg (see Table 1) concentrations significantly. The average soil T-Hg concentration was significantly higher (p < 0.05) for sampling sites away from factories than those of the sites close to factories. However, the highest concentration of soil T-Hg appeared in the sites close to the factory where sampling was more robust (see Fig. 1b).
Compared with the sampling sites away from factories, soils close to factories had relatively lower pH and higher OM (see Table 1).
3.1.2 Soil HCl-extractable Hg
Hydrochloric acid-extractable Hg (HCl-Hg) concentrations in the soil ranged from 14–84 μg kg−1, with a mean of 39 μg kg−1. Similar to soil T-Hg, soil order had a significant effect on HCl-Hg, resulting in a lower soil HCl-Hg in Cambosols than in Anthrosols (see Table 1). Likewise, a similar spatial distribution trend of soil HCl-Hg was also found (see Fig. 3b). However, the influence of sampling sites on HCl-Hg was different. The HCl-Hg concentrations for sampling sites close to factories were significantly greater than those of the sites away from factories (p < 0.05) (see Table 1).
3.1.3 Rice Hg
Concentrations of Hg in rice (rice Hg) ranged from 5 to 50 μg kg−1, with a mean of 13 μg kg−1. With HCl-Hg, soil order and sampling site had significant effects on rice Hg, resulting in higher rice Hg in Anthrosols at the sites close to factories than in Cambosols at the sites away from factories (p < 0.05) (see Table 1). Spatially, the distribution of rice Hg was similar to those of T-Hg and HCl-Hg, showing the spatial effect of soil order and higher rice Hg in Anthrosols area than Cambosols area (see Fig. 3c).
3.2 Spatial distribution of soil Hg close to factory
3.2.1 Horizontal distribution of soil Hg
Soil T-Hg and HCl-Hg concentrations ranged from 129 to 2,240 μg kg−1 and 16 to 312 μg kg−1, respectively. As for spatial distribution, the highest T-Hg and HCl-Hg concentrations were found in the southwest of the wastewater outlet within about 50 m away from the outlet and T-Hg and HCl-Hg concentrations decreased as the distance from the outlet increased (Fig. 4a). This was similar to the pattern of Hg concentrations in soils and snow around chlor-alkali plants described by Inácio et al. (1998) and Jernelov and Wallin (1973). A significant exponential relationship was obtained when HCl-Hg and T-Hg were plotted against distance (see Fig. 4b). The horizontal distributions of Hg concentrations were consistent with that of in mining areas through atmospheric transportation reported by Feng et al. (2006). However, the degree of influence by wastewater was limited compared to atmospheric pathways.
Surface soils close to the factories were acidic with pH ranging from 4.9 to 6.2. Soils were more acidic (pH < 5.5) near the wastewater outlet. Likewise, soil pH increased with increasing the distance from wastewater outlet.
3.2.2 Vertical distribution of soil Hg
In the soil profile near the outlet, the uppermost soil horizon (0–15 cm) had high T-Hg and HCl-Hg concentrations, with averages of 2,791 and 105 μg kg−1, respectively, then rapidly decreased with depth. The lowest T-Hg and HCl-Hg concentration (104 and 24 μg kg−1) was below 50 cm in the profile (Table 2). Vertical distribution of Hg concentrations was consistent with the pattern in wastewater irrigation soils in Taiyuan, China (Zhang et al. 2001) and the distribution of soils surrounding a chlor-alkali plant at Estarreja, Portugal (Inácio et al. 1998). However, it differed from atmospheric source models, which showed the greatest Hg concentrations in a limited surface soil (Wang and Mou 1999; Tomiyasu et al. 2003; Liu et al. 2004; Feng et al. 2006). Although there was a minor accumulation of T-Hg in surface soil profiles away from the outlet, the level was much lower than those near the outlet and the T-Hg concentrations below 40 cm of two profiles were similar (see Table 2).
Soil pH and OM in profiles showed certain trends as well. There was lower soil pH and greater soil OM in the uppermost soil horizons compared to subsoils (see Table 2).
3.2.3 Vertical distribution of soil Hg fractions
The soil profiles near the factory under the influence of the industrial activities exhibited more complex distribution patterns, with the M-Hg fraction of T-Hg ranged from 0.2% to 3.9%, SM-Hg 67–96%, and NM-Hg 4–32%. As such, the percentages of M-Hg and SM-Hg were higher in the topsoils and then decreased with depth. Conversely, the levels of NM-Hg were lower in the topsoils and increased with depth (see Table 2).
For soil profiles away from the outlet, variations in all fractions of soil Hg were small, although slight decreases with depth for M-Hg and SM-Hg and a slight increase for NM-Hg were found (Fig. 5). The percentage of the M-Hg fraction only varied from 0.6% to 1.4%, SM-Hg 82–87%, and NM-Hg 12–17%.
3.3 Hg in crops nearby the factory
Mercury concentrations in wheat ranged from 0.5 to 5.5 μg kg−1 with a mean of 2.8 μg kg−1 and a median of 2.5 μg kg−1. Mercury concentrations in rice ranged from 4.0 to 29.5 μg kg−1 with a mean of 13.3 μg kg−1 and a median of 13.3 μg kg−1, which was nearly five times higher than those of wheat grains. These results were consistent with the range of Hg concentrations reported in a gold mining area (Taylor et al. 2005) and a zinc smelting area (Feng et al. 2006).
There were variable trends in the distribution between wheat and rice Hg in close proximity to the wastewater outlet (see Fig. 5a). No significant relationship between wheat Hg and the distance from the wastewater outlet was found, indicating that Hg uptake by wheat was not influenced by soil Hg. As for rice, high Hg concentrations (>15 μg kg−1) were found within 200 m of the wastewater outlet and Hg concentrations decreased with the distance from the outlet (see Fig. 5b). Significant exponential relationships were also found when rice Hg concentrations were plotted against distance to the outlet.
4 Discussion
4.1 Regional risk assessment of soil and rice Hg accumulation
Soil Hg was significantly accumulated in both Cambosols and Anthrosols, which was indicated by another peak with higher levels of soil T-Hg than the dominant peak in the frequency histograms (see Fig. 2). Compared to the critical value of the CEQS (SEPAC 1995), no soil T-Hg concentrations in the Cambosols area exceeded the second most stringent critical value (500 μg kg−1 when pH > 6.5). In comparison with total Hg concentrations in soils from other areas, the mean concentration in our study area was significant lower than the level reported for urban soil samples from Beijing, China (range 22–9, 400; median 260 μg kg−1) (Chen et al. 2010), the agricultural soils near a petrochemical complex in Guangzhou, China (range 28–2, 400; mean 610 μg kg−1) (Li et al. 2009), and the former industrialized urban areas of Beijing, China (range 220–76, 270; mean 4, 850 μg kg−1) (Luo et al. 2009). In the acidic Anthrosols area, soil Hg at six sampling sites exceeded the critical value of the CEQS (300 μg kg−1 when pH < 6.5). Of those sites, five sites were located away from factories and only one site was close to a chemical factory (see Figs. 1b and 2a). Historically, Hg-containing pesticides for cotton and rice were widely used in the county (Yin 2009). The soil Hg away from factories likely originated from such agricultural activities. As for industrial sources of soil Hg, the accumulation may be localized as evidenced by lower soil T-Hg at the sites close to factories than those of the sites away from factories (see Table 1) despite a very high level of soil T-Hg close to the factory's wastewater outlet (see Figs. 1b and 2a).
Although Hg-containing pesticides has been prohibited since the 1970s in China (Yin 2009) and the risk of Hg accumulation in the county is limited (only 1% of sampling sites exceeded the critical value of the CEQS), changes in soil pH and OM in the studied area may increase the environmental risk. According to Darilek et al. (2009), soil pH in Anthrosols decreased and soil OM increased in both Anthrosols and Cambosols over the past 24 years in the county due to intensive agricultural and industrial activities. A stepwise regression of soil HCl-Hg or rice Hg against soil T-Hg, pH, and OM has confirmed that the r 2 (0.38 or 0.44) of the stepwise regression had a significant increase compared to the r 2 (0.27 or 0.21) of the regression between soil HCl-Hg or rice Hg and T-Hg (Table 3). This indicated that HCl-Hg or Hg uptake by rice increased with decreasing soil pH and increasing soil OM even if the soil T-Hg concentration remains unchanged (see Table 3). This effect was highlighted by higher soil HCl-Hg or rice Hg at the sites close to factories than the sites away from factories. The influence of sampling sites on soil HCl-Hg or rice Hg was different from that on soil T-Hg, which was significantly influenced by lower soil pH and higher OM at the sites closer to factories than those away from factories (see Table 1). Due to this effect, rice Hg concentrations in five sampling sites of Anthrosols exceeded the critical value of Hg in crops (20 μg Hg kg−1) according to the maximum allowable levels of contaminants in Chinese foods (MALCF) (GB2762-2005) (MHSA-PRC 2005) (see Fig. 3c).
Another noteworthy trend is that more and more conventional cropland has been converted to vegetable planting, a more intensive utilization of the cropland than conventional crop planting. This is being driven by increasing industrialization and urbanization in this region (Huang et al. 2006). With such changes in land use, soil pH often decreases significantly (Huang et al. 2006; Darilek et al. 2009). When land is converted to vegetable production, soil pH and HCl-Hg should be monitored to ensure increased Hg uptake by crops does not pose a health risk.
4.2 Risk assessment of soil Hg accumulation by industrial activities
Hg accumulation in the soils at the industrial site was not ubiquitous because soil Hg in the samples close to factories was significantly lower than those away from factories (see Table 1). Only one Hg extraordinary industrial site was found in the whole county, indicating a localized Hg accumulation of this source. Exponential decreases in soil Hg with distance to the outlet indicated that the accumulated range was spatially limited (see Fig. 4). However, accumulated levels of soil T-Hg and HCl-Hg were so high that part of the rice Hg concentrations exceeded the Hg MALCF (20 μg Hg kg−1) (Figs. 5 and 6). Therefore, effective measurements should be conducted to reduce Hg in the wastewater and control discharges to surface waters.
In addition to the effects of Hg accumulation and soil pH decreases by industrial activities, high mobility of Hg freshly entering the soils was also an important affecting factor. More M-Hg and SM-Hg fractions in topsoil close to the discharge outlet (see Table 2) support this conclusion. This may partially explain why the soil HCl-Hg at the sites close to factories was higher than those of the sites away from factories even though the soil T-Hg at former sites was lower than those of at latter sites (see Table 1). Probably, the accumulated Hg from Hg-containing pesticides before the 1970s was aged and existed in the soils for a long time (Grimaldi et al. 2008).
Distribution of Hg in the soil profile revealed that Hg could migrate downward to some extent. Although peak soil T-Hg existed at topsoil, a plow pan at 15–25 cm acted as a barrier limiting the downward movement of Hg (Duan 2004; Poissant and Beauvais 2001) and providing favorable reduction conditions (Liao 1989; Chen 1996; Helena et al. 2006), high levels of soil T-Hg did not dissipate until a depth of 50 cm in the soil profile (see Table 2). Considering the shallow water table (85 cm) (Soil Survey Office of Zhangjiagang County 1984) in the study area, Hg accumulation in soil may also affect groundwater quality. Zhang et al. (2001) found that Hg migrated downward because of irrigation of Hg-contaminated wastewater and led to pollution of shallow water tables. If groundwater Hg pollution occurred and the water was used for human consumption, human health could be compromised.
4.3 Risk assessment of crop Hg
Mercury concentrations in wheat samples did not exceed the MALCF level while those of 2 out of 15 rice samples exceeded the MALCF level (see Figs. 5 and 6), indicating some Hg pollution in rice. Concentrations of Hg in rice were five times higher than that of wheat, suggesting a stronger Hg uptake by rice than wheat. This may be due to reducing conditions present in water logged fields supporting rice production, which also increased the bioavailability of Hg, and thus, uptake by rice (Chen 1996) or direct Hg uptake from wastewater. Statistically significant linear relationships between rice Hg and soil HCl-Hg and T-Hg (see Fig. 6) indicated that Hg uptake by rice was related to soil Hg availability to a certain extent. In most previous experiments where Hg was sequestered from the atmosphere, no significant correlation can be detected between rice and soil Hg concentrations in mining areas (Appleton et al. 2006; Silvia et al. 2006). This was most likely because atmospheric Hg uptake occurs through the stoma, which conceals the relationship between soil Hg availability and Hg uptake by crops. However, our results were consistent with those from mine-tailing areas and sediment studies with high levels of Hg (Silvia et al. 2006). On the other hand, the 1-year monitoring Hg amounts from atmospheric deposition at a few possible sites in the studied area (data not shown) were also definitely lower than those in the area where Hg was sequestered from the atmosphere (Appleton et al. 2006; Silvia et al. 2006). Those evidences can at least rule out that direct rice uptake of Hg from the air was a predominant pollution mechanism.
At present, there is no universally recognized soil extraction method for Hg bioavailability. The extraction solution, 10% ethanol + 2% HCl (Fernández-Martínez et al. 2005) and 0.3 M HCl (He et al. 2005) for acid soil in most of mining areas have been used to extract Hg from soils in mining areas as a test for available Hg. In many areas, however, soil Hg concentrations are much lower than those of mining areas but higher than the critical value of the CEQS. Low Hg concentrations in extracts are difficult to be determined and affect the predictability of plant available Hg in these areas. In this study, an attempt to use a more acidic, stronger extraction solution of 1 M HCl increased the extractable Hg and accounted for >20% of total soil Hg (see Table 1). The inclusion of soil pH and OM with soil T-Hg in a stepwise multiple regression produced a higher correlation coefficient (see Table 3), showing that HCl-Hg is more sensitive to changes in soil properties than soil T-Hg. Likewise, Hg uptake by crop depends on not only soil T-Hg but also soil properties (Jing et al. 2007). For rice Hg in our results, the inclusion of soil pH and OM with soil T-Hg in a stepwise regression also produced a higher correlation coefficient (see Table 3), verifying the effect of soil properties on rice Hg. Thus, soil HCl-Hg appears to be better for predicting Hg uptake by crops. In fact, the prediction of soil HCl-Hg on Hg uptake by rice is better than that of T-Hg either at regional level with r 2 increasing from 0.21 (T-Hg vs. rice Hg) to 0.46 (HCl-Hg vs. rice Hg) (see Table 3) or at point level with r 2 increasing from 0.51 to 0.57 according to the regressions of soil HCl-Hg and T-Hg against rice Hg (see Fig. 6). Of course, the reliability and applicability of 1 M HCl-Hg as a soil available Hg extraction deserves further studies and needs to be validated, even though this extractable Hg is suitable for a wide range of pH (5.4–8.0) and OM (8.6–31.5 g kg−1) in our case.
5 Conclusions
Historical application of Hg-containing pesticides and herbicides and recent industrial activities have resulted in Hg accumulation and/or mobility. Consequently, the possibility of enhanced Hg uptake by crops has increased due to contemporary changes in soil properties. Therefore, soil Hg availability should be closely monitored when soil property changes.
Even though soil Hg levels are high due to industrial activity, the affected area is limited. Downward migration of Hg in soils with high level near factory was found even if a plow-pan in the soil profile blocked the migration to a certain degree, which may affect the quality of groundwater. Utilization of soils with high levels of Hg would be considered to conduct remediation if possible. Strategies to adjust soil properties or crop rotations with a weaker absorbency of hazardous elements would also be taken into account in all areas in order to mitigate the possible threat to Hg uptake by crop even human health.
Using a more acidic extraction solution for Hg in soils as a proxy for bioavailable Hg estimation was advantageous and sensitive to changes of soil pH and OM, being more suitable for the soils with low T-Hg concentrations. However, its reliability and applicability still need further study for validation.
References
Appleton JD, Weeks JM, Calvez JPS, Beinhoff C (2006) Impacts of mercury contaminated mining waste on soil quality, crops, bivalves, and fish in the Naboc River area, Mindanao, Philippines. Sci Total Environ 354:198–211
Biester H, Müller G, Scholer HF (2002a) Estimating distribution and retention of mercury in three different soils contaminated by emissions from chlor-alkali plants; part I. Sci Total Environ 284:177–189
Biester H, Müller G, Scholer HF (2002b) Binding and mobility of mercury in soils contaminated by emissions from chlor-alkali plants. Sci Total Environ 284:191–203
Chen HM (1996) Heavy metal pollution in soil-plant system. Science, Beijing (in Chinese)
Chen X, Xia X, Wu S, Wang F, Guo X (2010) Mercury in urban soils with various types of land use in Beijing, China. Environ Pollut 158:48–54
Chen ZL, Xu SY, Xu QX, Hu XF, Yu LZ (2001) Pollution patterns and modulating counter measures of surface water environment in the Yangtze River Delta. Resources and Environment in theYangtze Basin 10:353–359 (in Chinese with English abstract)
Cooperative Research Group on Chinese Soil Taxonomy (CRGCST) (2001) Chinese soil taxonomy. Science, Beijing
Darilek JL, Huang B, Wang ZG, Qi YB, Zhao YC, Sun WX, Gu ZQ, Shi XZ (2009) Changes in soil fertility parameters and the environmental effects in a rapidly developing region of China. Agric Ecosyst Environ 129:286–292
Duan YH (2004) Distribution and affecting factors of mercury and cadmium in soil profiles of sewage irrigation area. Environ Protect 12:35–37 (in Chinese with English abstract)
Feng XB, Li GH, Qiu GL (2006) A preliminary study on Hg contamination to the environment from artisanal zinc smelting using indigenous methods in Hezhang County, Guizhou, China: part 2. Mercury contaminations to soil and crop. Sci Total Environ 368:47–55
Fernández-Martínez R, Loredo J, Ordóǖez A, Rucandio MI (2005) Distribution and mobility of Hg in soils from an old mining area in Mieres, Asturias (Spain). Sci Total Environ 346:200–212
Fernández-Martínez R, Loredo J, Ordóǖez A, Rucandio MI (2006) Physicochemical characterization and Hg speciation of particle-size soil fractions from an abandoned mining area in Mieres, Asturias (Spain). Environ Pollut 142:217–226
Grimaldi C, Grimaldi M, Guedron S (2008) Mercury distribution in tropical soil profiles related to origin of mercury and soil processes. Sci Total Environ 401:121–129
Han Y, Kingston HM, Boylan HM, Rahman GMM, Shah S, Richter RC, Link DD, Bhandari S (2003) Speciation of mercury in soil and sediment by selective solvent and acid extraction. Anal Bioanal Chem 375:428–436
He HL, Ni ZM, Li B, Yang HX (2005) Trace Elemental Speciation in Environmental Samples II. speciation of As, Hg, Cd, Sn, Pb, Se and Cr. Rock and Mineral. Analysis 24:118–128 (in Chinese with English abstract)
Helena ELP, Hermínio ANJ, Cláudia LVL, CW RCS, Walter B (2006) Quantification and speciation of mercury in soils from the Tripuí Ecological Station, Minas Gerais, Brazil. Sci Total Environ 368:69–78
Huang B, Shi XZ, Yu DS, Öborn I, Paglla BK, TF WHJ, Sun WX, Sinclair FL (2006) Environmental assessment of small-scale vegetable farming systems in peri-urban areas of the Yangtze River Delta Region, China. Agric Ecosyst Environ 112:391–402
Inácio MM, Pereira V, Pinto MS (1998) Mercury contamination in sandy soils surrounding an industrial emission source (Estarreja, Portugal). Geoderma 85:325–339
Jernelov A, Wallin T (1973) Air-borne mercury fall-out on snow around five Swedish chloralkali plants. Atmos Environ 7:209–214
Jing YD, He ZL, Yang XE (2007) Effects of pH, organic acids, and competitive cations on mercury desorption in soils. Chemosphere 69:1662–1669
Li J, Lu Y, Yin W, Gan H, Zhang C, Deng X, Lian J (2009) Distribution of heavy metals in agricultural soils near a petrochemical complex in Guangzhou, China. Environ Monit Assess 153:365–375
Liao BJ (1989) Harm of trace heavy metal element pollution and transfer in environment. Science, Beijing
Liu L, Qu LY, Luo Y, Hu P, La L (2004) Influence on soil and crop around an Hg smelting. Guizhou Environ Protect Sci Technol 3:43–45 (in Chinese)
Loredo J, Ordonez A, Alvarez R (2006) Environmental impact of toxic metals and metalloids from the Munon Cimero mercury-mining area (Asturias, Spain). J Hazard Mater 136:455–467
Luo W, Lu Y, Wang B, Tong X, Wang G, Shi Y, Wang T, Giesy JP (2009) Distribution and sources of mercury in soils from former industrialized urban areas of Beijing, China. Environ Monit Assess 158:507–517
Matschullat J, Filho SR (2001) Mercury and arsenic, heavy metals and pesticides, what about soil and sediment research in Brazil? Journal of Soils and Sediments 1:199–200
Ministry of Health and Standardization Administration of the People's Republic of China (MHSA-PRC) (2005) Maximum levels of contaminants in foods (GB 2762–2005). Available in: http://www.ziq.gov.cn/portal/webfiles/web/file/12275082059288029.pdf Accessed on: 24 Nov. 2008
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL (ed) Methods of soil analysis, part 3, chemical methods-SSSA Book Series no. 5, Madison, Wisconsin, pp 961–1010
Poissant L, Beauvais C (2001) Surface exchange of mercury in the Baie St-Francois wetland under wet and dry conditions. The 6th International Conference on Mercury as a Global Pollutant, Minamata, Japan
Qian JP, Zhang L, Chen HZ, Hou M, Niu YF, Xu ZP, Liu HL (2009) Distribution of mercury pollution and its sources in soils and vegetables in Guilin area, China. Bull Environ Contam Toxicol 83:920–925
Randall PM, Chattopadhyay S, Ickes J (2004) Influence of pH and oxidation-reduction potential (Eh) on the dissolution of mercury-containing mine wastes from the Sulphur Bank Mercury Mine. Miner Metall Process 21:93–98
Rémy S, Prudent P, Hissler C, Probst JL, Krempp G (2003) Total mercury concentrations in an industrialized catchments, the Thur River basin: geochemical background level and contamination factors. Chemosphere 52:635–644
Rodrigues S, Pereira ME, Duarte AC, Ajmone-Marsan F, Davidson CM, Grčman H, Hossack I, Hursthouse AS, Ljung K, Martini C, Otabbong E, Reinoso R, Ruiz-Cortés E, Urquhart GJ, Vrščaj B (2006) Mercury in urban soils: a comparison of local spatial variability in six European cities. Sci Total Environ 368:926–936
Schröder W, Holy M, Pesch R, Harmens H, Ilyin I, Steinnes E, Alber R, Aleksiayenak Y, Blum O, Coşkun M, Dam M, De Temmerman L, Frolova M, Frontasyeva M, Gonzalez Miqueo L, Grodzińska K, Jeran Z, Korzekwa S, Krmar M, Kubin E, Kvietkus K, Leblond S, Liiv S, Magnússon S, Maňkovská B, Piispanen J, Rühling Å, Santamaria J, Spiric Z, Suchara I, Thöni L, Urumov V, Yurukova L, Zechmeister HG (2010) Are cadmium, lead and mercury concentrations in mosses across Europe primarily determined by atmospheric deposition of these metals? J Soils Sediments 10(8):1572–1584
State Environment Protection Administration of China (SEPAC) (1995) Environmental Quality Standards for Soils. Available at: http://english.mep.gov.cn/standards_reports/standards/Soil/Quality_Standard3/200710/ t20071024_111882.htm/GB15618-1995.doc. Accessed on: 01 March 1996
State Environment Protection Administration of China (SEPAC) 1997 Soil quality - Determination of total mercury-Cold atomic absorption spectrophotometer. Available in: http://www.zhb.gov.cn/tech/hjbz/bzwb/ trhj/trjcgfffbz/199805/t19980501_82033.htm/GB/T 17136–1997.pdf. Accessed on: 01 May 1998
Silvia GE, Saulo RF, Roberto CVB, Christian B (2006) Evolution of mercury pollution in cultivated and wild plants from two small communities of the Tapajós gold mining reserve, Pará State, Brazil. Sci Total Environ 368:424–433
Soil Survey Office of Zhangjiagang County (1984) Soil of Zhangjiagang County in Jiangsu Province. Suzhou: Agricultural office of Suzhou City, Soil Survey Office in Jiangsu Province
Taylor H, Appleton JD, Lister R, Smith B, Chitamweba D, Mkumbo O, Machiwa JF, Tesha AL, Beinhoff C (2005) Environmental assessment of mercury contamination from the Rwamagasa artisanal gold mining centre, Geita District, Tanzania. Sci Total Environ 343:111–133
Tomiyasu T, Okada M, Imura R, Sakamoto H (2003) Vertical variations in the concentration of Hg in soils around Sakurajima Volcano, Southern Kyishu, Japan. Sci Total Environ 304:221–230
Wang DY, Mou SS (1999) An investigation on the effect of atmospheric mercury on its accumulation in the soil-plant system in acid precipitation area. Acta Ecol Sin 19:140–144 (in Chinese with English abstract)
Wang H, Stuanes AO (2003) Heavy metal pollution in air–water–soil–plant system of Zhuzhou City, Hunan Province, China. Water Air Soil Pollut 147:79–107
Yang YK, Zhang C, Shi XJ, Lin T, Wang DY (2007) Effect of organic matter and pH on mercury release from soils. J Environ Sci 19:1349–1354
Yin J, Liu WJ, Xie JZ, Xiao CB (2000) The study on extraction conditions and extractants of soil available Cd. Hg. J Agric Univ Hebei 23:25–28 (in Chinese with English abstract)
Yin YM (2009) Key periods in the development of China pesticide industry-For China 60 Anniversary. Chem Ind 27:13–22 (in Chinese with English abstract)
Zhang NM, Zhang SP, Wu PW (2001) Soil Hg pollution and its ecological effect in the waste water irrigation area of Taiyuan. Chin J Soil Sci 32:95–96 (in Chinese with English abstract)
Zhang L, Wong MH (2007) Environment mercury contamination in China: sources and impacts. Environ Internat 33:108–121
Acknowledgments
The authors are grateful for the funding from the National Natural Science Foundation of China (40773075, 41071299) and the Special Research Foundation of the Public Natural Resource Management Department from Ministry of Environmental Protection of China (201109018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Galina Machulla
Rights and permissions
About this article
Cite this article
Huang, B., Wang, M., Yan, L. et al. Accumulation, transfer, and environmental risk of soil mercury in a rapidly industrializing region of the Yangtze River Delta, China. J Soils Sediments 11, 607–618 (2011). https://doi.org/10.1007/s11368-011-0341-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0341-8