Abstract
Purpose
Despite the decline of metal mining in the UK during the early 20th century, a substantial legacy of heavy metal contamination persists in river channel and floodplain sediments. Poor sediment quality is likely to impede the achievement of ‘good’ chemical and ecological status for surface waters under the European Union Water Framework Directive. This paper examines the environmental legacy of the Dylife lead/zinc mine in the central Wales mining district. Leachable heavy metal concentrations in the bed sediments of the Afon Twymyn are established and the geochemical partitioning, potential mobility and bioavailability of sediment-associated heavy metals are established.
Materials and methods
Sediment samples were collected from the river bed and dry-sieved into two size fractions (<63 and 64–2,000 µm). The fractionated samples were then subjected to a sequential extraction procedure to isolate heavy metals (Pb, Zn, Cu, Cd, Fe, Mn) in three different geochemical phases. Sediment samples were then analysed for heavy metals using Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES).
Results and discussion
The bed sediment of the Afon Twymyn is grossly polluted with heavy metals. Within the vicinity of the former mine, Pb concentrations are up to 100 times greater than levels reported to have deleterious impacts on aquatic ecology. Most heavy metals exist in the most mobile easily exchangeable and carbonate-bound geochemical phases, potentially posing serious threats to ecological integrity and constituting a significant, secondary, diffuse source of pollution. Metal concentrations decrease sharply downstream of the former mine, although there is a gradual increase in the proportion of readily extractable Zn and Cd.
Conclusions
Implementation of sediment quality guidelines is important in order to achieve the aims of the Water Framework Directive. Assessments of sediment quality should include measurements of background metal concentrations, river water physico-chemistry and, most importantly, metal mobility and potential bioavailability. Uniformity of sediment guidelines throughout Europe and flexibility of targets with regard to the most heavily contaminated mine sites are recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Contaminated drainage from abandoned metal mines and mine spoil are issues of international concern that can severely impact hydrological (Grimshaw et al. 1976; Gammons et al. 2005), sedimentological (Macklin et al. 2006; Dennis et al. 2009) and ecological (Gower et al. 1994; Armitage et al. 2007) systems. The varied geology of the UK has provided many valuable natural resources, and mineral exploration has occurred since the early Bronze Age (Environment Agency 2008a). During the mid-nineteenth century, England and Wales experienced a boom in the metal mining industry and were the largest producers of lead, copper and tin in the world (Lewin and Macklin 1987). However, falling metal prices and the discovery of large metalliferous deposits in Australia, South America and the Iberian Peninsula forced the closure of most metal mines by the 1920s. An extensive legacy of the metal mining industry persists today with over 3,000 abandoned metal mines; almost half of these (1,311) are located in Wales (Environment Agency 2002; Jarvis et al. 2008).
Today, one of the principal long-term impacts of metal mining on riverine systems in England and Wales is contamination of sediment within river channels and floodplains by heavy metals (Lewin and Macklin 1987; Macklin 1996; Lord and Morgan 2003; Walling et al. 2003; Dennis et al. 2009). These sediments potentially pose a serious threat to human health and the ecological integrity of fluvial ecosystems (Neal et al. 2005; Environment Agency 2008b). The decline of metal mining has reduced the incidence of contamination, although significant quantities of contaminated sediment are still being mobilised from mine spoil heaps long after active working has ceased (Macklin et al. 2006). However, in some systems, a more significant source of diffuse contamination appears to be floodplain sediments contaminated by historical metal mining activities (Dennis et al. 2009).
Currently, there are no agreed European or UK guidelines pertaining to pollution of river bed sediments, although draft sediment environmental quality standards (SEQS) for heavy metals have recently been published for England and Wales (Environment Agency 2008a). The development of SEQS is seen as a crucial step in the management and improvement of watercourses impacted by mining activity in the UK and recognizes the importance of sediments in river ecosystems and in the transport and fate of contaminants. However, a major limitation of SEQS is likely to be their measurement of total metal concentrations in the sediment only. Heavy metals in sediments can be bound to many solid compounds (e.g. clay minerals, carbonates, Fe/Mn oxides, organic matter) and the type of bonding controls to a large degree the potential mobility and bioavailability of the metal (Calmano et al. 1993). For example, metals introduced to sediment through human activity (e.g. mining) often exist in weakly bound chemical forms as easily exchangeable ions and carbonate-bound metals which can interact easily with plants and animals (Jain 2004). Minerogenic metals or metals incorporated into the crystal lattice of sediments are considered unavailable to biota (Morillo et al. 2002). It is argued in this paper that measurements of total heavy metal concentrations provides little information on the potential mobility and bioavailability of heavy metals, and that measurement of important geochemical phases will yield information more pertinent to aquatic ecosystem health, which is the key consideration of the European Union Water Framework Directive (2000/60/EC).
This paper examines the environmental legacy of the Dylife lead/zinc mine (central Wales) through its continued impact on fluvial sediment characteristics of the Afon Twymyn and highlights the importance of incorporating measurements of sediment quality and in particular, heavy metal geochemistry, in the overall assessment of river ecosystems impacted by mining activity. The specific objectives of the study are: (1) to establish the impact of heavy metal contaminants (Pb, Zn, Cu, Cd, Fe, Mn) on the sediment quality of the Afon Twymyn; (2) to investigate the geochemical status and potential mobility of heavy metal contaminants in the bed sediments of the Afon Twymyn and (3) to discuss the implications of sediment quality guidelines for the management of mining-impacted watercourses.
2 Methods
2.1 Study site
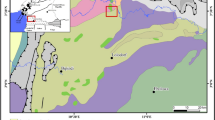
Dylife mine lies at the centre of an elevated plateau between Machynlleth and Llanidloes known as the central Wales mining district (Fig. 1). The mine was historically and industrially amongst the foremost metal mines in Wales (Bick 1977; Brown 2006), being worked principally for lead and for smaller quantities of zinc, copper and silver. Small-scale mining occurred during Roman occupation; however, the principal extraction of metalliferous ores occurred from the seventeenth century A.D. onwards. The mine site was abandoned in 1930 and currently comprises numerous spoil heaps, shafts and adits. The mine is drained by the Afon Twymyn, a tributary of the Afon Dyfi which flows into the Irish Sea at Aberdyfi. The mine has been ranked as the fifth most polluting metal mine in Wales (Environment Agency 2002).
The Afon Twymyn catchment (35 km2) lies on Upper Silurian argillaceous sediments, mainly comprised of shales, siltstones and mudstones (British Geological Survey 2007). The mudstones contain large quantities of dioctahedral mica and quartz (32%), illite (37%) and iron–magnesium chlorite (26%), with lesser amounts of feldspar, iron oxides, albite, orthoclase and rutile (Hornung et al. 1986). Igneous activity during the Caledonian (444–416 Ma) and Hercynian (390–310 Ma) mountain building periods gave rise to mineralisation. Rising hydrothermal solutions, upon meeting the shales, cooled and precipitated baryte, fluorospar and sulphides of lead, zinc, copper and iron (Evans 1987). Calcite and dolomite are rare, as are secondary minerals (Jones 1922). The mineralised faults trend east–north-east through the Ordovician and Silurian sediments (Fuge et al. 1991). The area was glacierised during the Pleistocene. Soils of the region are predominantly stagnopodzols and stagnohumic gleys (Rudeforth et al. 1984).
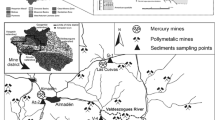
Three mineral lodes are associated with Dylife mine (Fig. 2). The Esgairgaled and Llechwedd Ddu lodes are located within the Afon Twymyn catchment. Dylife lode is located to the south of Dylife mine at Pen Dylife in the Afon Clywedog catchment. The Dylife lode trends east to west. The Esgairgaled lode trends east–north–east and unites at its western end with the Llechwedd Ddu lode which is situated between the other two lodes and trends north–east. The principal lode constituents are coarsely crystallised quartz, galena, sphalerite and chalcopyrite (Jones 1922). Secondary mineralisation on the surface of the mine spoil at Dylife generally comprises malachite, serpierite, gypsum, greenockite, hydrozincite, hemimorphite and linarite (Rust and Rust 1987). Several shafts and adits are present in the Twymyn valley (see Fig. 2). The Nant Dropyns tributary passes the Esgairgaled shaft and Pencerig adit on its left bank and Bradford’s shaft on its right bank. The Afon Twymyn passes Dylife adit and Level Goch adit on its right bank and Gwaith Gwyn adit on the left bank. Llechwedd Ddu engine shaft, Footway shaft and a collapsed stope are located on the right bank.
2.2 Sampling and analytical methods
Following a preliminary survey in April 2007, 27 sites on the Afon Twymyn were selected for analysis of bed sediment heavy metal concentrations and speciation (see Fig. 2). Sample sites were spaced more closely within the upper part of the impacted catchment, where mine workings and mine spoil may result in rapid changes in the chemical composition of sediments. Sites 20–27 represent ‘control’ sites in channels where mining activity was absent upstream of the Dylife mine. Sites 7–19 represent impacted or ‘mine’ sites situated within the boundary of the mine. Sites 1–6 are located downstream of the mine. The total length of channel sampled was 15 km with downstream sites accounting for the final 12 km.
The sampling of sites followed closely the method used in the British Geological Survey G-BASE programme (Johnson 2005). In June 2007, the upper layer of river bed sediments (c. 5–10 cm) was sampled at each site on the Afon Twymyn after removal of the heavily oxidised surface armour layer. Five random samples were taken (sediment supply permitting) from riffle locations in the middle of the channel and homogenised to form a composite sample. To minimise potential contamination, a plastic trowel was used to collect samples. Samples were double bagged in airtight polyethylene zip bags and placed in a plastic cool box for transport to the laboratory. The preparation of the samples for metal extraction tests followed the established procedure for soil samples (British Standards Institution 2006). First, samples were oven dried at 30°C for 5 days. Samples were then coned and quartered to provide representative sub-samples. These sub-samples were divided into two size fractions by sieving (<63 µm (clay/silt) and 64–2,000 µm (fine–coarse sand) in order to investigate differences in metal concentrations and geochemical partitioning between these two size fractions. A three-step sequential extraction procedure proposed by the European Union’s Standards, Measurements and Testing programme (SM&T) (Rauret et al. 1999) was utilised to extract heavy metals (Pb, Zn, Cu, Cd, Fe, Mn) in three geochemical phases: (1) acid-soluble, exchangeable and bound to carbonates; (2) reducible, bound to iron and manganese oxides; (3) oxidisable, bound to organic and sulphide compounds. This procedure was applied to the clay/silt and the sand fractions. The supernatant produced after extraction was acidified to pH 2 to prevent adsorption or precipitation of metals in storage. All solutions were analysed within a month using a Jarrell Ash ICP-AES. Each batch of samples included duplicates, blanks and a certified reference material (CRM 601) (Quevauviller et al. 1997). Detection limits (parts per million) of the Inductively Coupled Plasma (ICP) were Pb 0.1, Zn 0.01, Cu 0.03, Cd 0.02, Fe 0.06 and Mn 0.02. High precision of the ICP control standards is reported (±10%). Recovery rates for heavy metals in the CRM were between 83% and 105%.
3 Results
3.1 Longitudinal variation in leachable heavy metal concentrations (<63 and 64–2,000 µm fractions)
There is considerable longitudinal variability in leachable (summed total of three geochemical phases) metal concentrations in the bed sediment of the Afon Twymyn (Fig. 3). A similar pattern of contamination is apparent for metals in both sediment size fractions (see Fig. 3). Low levels of Pb, Zn, Cu and Cd at the control sites indicate that these are free of mine contamination (Table 1). The bed sediments are severely polluted at the Dylife mine (7–19) and concentrations of all of the metals in both size fractions, with the exception of Fe and Mn, are significantly different from those of the control sites (upstream) (Fig. 4). Pb and Zn concentrations increase progressively between sites 16 and 12 where the Afon Twymyn flows through a number of former mine workings. In the Nant Dropyns, there is a decrease in Pb and Zn concentrations in both sediment size fractions at site 17 after a progressive increase from sites 21 to 18. However, Cu and Cd concentrations continue to increase at this site and relatively high values of these metals are recorded in the sand fraction. Iron and Mn concentrations generally decrease at the mine sites. However, concentrations similar to control site values were recorded at site 16, on the Afon Twymyn, where reducing conditions predominate.
As the stream flows through the eastern part of Dylife mine (7–11), the highest leachable metal concentrations are recorded. Site 11 has the highest total metal concentration (3,521 mg/kg) and Pb concentration (2,914 mg/kg) in the silt/clay fraction of all the sites in this study. The highest total metal concentration (373 mg/kg at site 19) and single metal concentration (213 mg/kg Pb at site 7) in the sand fraction are also recorded on this reach. In general, metal concentrations begin to attenuate from site 11 to the eastern boundary of the mine, although they remain highly elevated throughout. An increase in the concentrations at site 7 may reflect the delivery of contaminated sediment by an unnamed right-bank tributary that flows through the mine spoil (see Fig. 2).
All of the heavy metals attenuate downstream of the mine (1–6). Despite this, levels of Pb, Zn, Cu and Cd in both sediment size fractions are significantly greater than those of the upstream control sites (see Fig. 4), indicating that these heavy metals remain comparatively elevated for considerable distance downstream. An initial steep drop in metal concentrations occurs between sites 7 and 6 (distance = c. 2,500 m) due to the addition of uncontaminated sediment from the scree slopes which mantle the valley sides. Metal concentrations continue to decrease, but at a lower rate. Minimum downstream metal concentrations for both sediment size fractions (except Fe) are reached at site 1, where the Afon Laen delivers inputs of uncontaminated sediment to the Afon Twymyn (see Fig. 1), there is a lack of mineralisation in the Afon Laen catchment.
Concentrations of all of the metals analysed except Pb (α = 0.08) are significantly greater in the clay/silt fraction than in the sand fraction. Considering the sum of all six metals, the percentages of metals in the clay/silt fraction at control, mine and downstream sites are 92%, 78% and 65%, respectively, and, overall, 80% of metals in samples from the Afon Twymyn system are found in the clay/silt fraction. At mine sites, the vast majority of Pb (90%), Fe (88%) and Mn (85%) are found in the clay/silt fraction.
3.2 Heavy metal geochemistry (<63 and 64–2,000 µm fractions)
At the upstream control sites, Pb, Zn, Cu and Cd in the clay/silt size fraction (<63 µm) are strongly associated with Fe/Mn oxides (Fig. 5). Relatively high proportions of Zn and Cu associated with sulphides/organics reflect the frequent occurrence of these metals in this geochemical phase (Dawson and Macklin 1998; Galan et al. 2003). Through the Dylife mine and in the clay/silt size fraction, there is a significant increase in the proportion of all four metals in the exchangeable/carbonate-bound geochemical phase. At the mine, the majority of Pb (54%), Zn (53%) and Cd (51%) are found in this phase; however, Cu (49%) is found mainly in the sulphide/organic phase. Notable increases in Pb, Zn, Cu and Cd in the sulphide/organic phase at sites 18 and 19 probably reflect sulphidic minerals in the underlying Esgairgaled lode. Downstream of the mine, the proportion of Pb (33%) in the easily exchangeable/carbonate phase decreases at the expense of the Fe/Mn oxide phase (65%) due to the chemical stability of Pb in river systems. However, the absolute concentration of Pb in the exchangeable/carbonate-bound phase is still significant. Copper initially increases in the exchangeable/carbonate (37%) and Fe/Mn oxide (48%) phases at the expense of the sulphide/organic phase (15%) (see Fig. 5). This shift is explained, perhaps, by a decrease in sulphidic and organic material downstream of the mine or by increased oxidation of sediments below the mine, forcing redistribution of Cu to more mobile geochemical phases. However, much like Pb, exchangeable/carbonate-bound Cu decreases after this initial increase due to its relatively stable chemistry (Mance et al. 1984). Downstream of the mine, the proportion of Zn (70%) and Cd (92%) in the exchangeable/carbonate phase continues to increase, reflecting the unstable nature of these two metals in river systems (Licheng and Guijiu 1996). However, although the proportions of Zn and Cd in the initial leach increases downstream of the mine, the absolute concentrations are significantly lower than those at the mine sites (see Fig. 3).
Concentration of heavy metals in the three geochemical phases of the clay/silt fraction (<63 µm) at all sample sites arranged distance (metre) above river outlet. For each metal, the ordinate scale differs for each section of the river; the abscissa scale for distances in the reach below the mine differs
The general longitudinal pattern of geochemical partitioning is similar in the sand fraction (64–2,000 µm) (Fig. 6). However, there are notably larger relative amounts in the sulphide/organic-bound phase at all sites. At the control sites, these reflect anoxic and acidic conditions in the marsh (favouring metal reduction), the presence of organic material and, possibly, the presence of naturally weathered ore minerals. At the mine site, the majority of Zn (67%) and Cd (78%) are found in the sulphide/organic fraction and the proportion of Cu (82%) in this phase has increased relative to the clay/silt fraction, reflecting the prevalence of discarded and unprocessed sphalerite and chalcopyrite. In contrast, Pb (50%) remains high in the exchangeable/carbonate-bound phase. Downstream trends in the sand fraction are similar to trends in the clay/silt fraction, with exchangeable/carbonate-bound Pb (44%) decreasing gradually, and Zn (30%) and Cd (54%) increasing (Fig. 6).
Concentration of heavy metals in the three geochemical phases of the sand fraction (<64–2,000 µm) at all sample sites arranged distance (metre) above river outlet. For each metal, the ordinate scale differs for each section of the river; the abscissa scale for distances in the reach below the mine differs
4 Discussion
4.1 The sediment quality status of the Afon Twymyn
This study has demonstrated that the bed sediments of the Afon Twymyn are grossly contaminated with heavy metals, in particular Pb. The vast majority of heavy metals were found in the clay/silt fraction. The preferential adsorption of heavy metals to smaller sediment size fractions has been demonstrated in many studies and attributed to the greater surface area per unit mass of smaller clay and silt-sized sediments (Lewin and Macklin 1987; Stone and Droppo 1996). There are currently no European Union or Environment Agency (of England and Wales) criteria for assessing the contamination of stream sediments and their potential impact on aquatic ecology. However, the Environment Agency of England and Wales recently published draft sediment quality criteria suggesting two effect levels for total heavy metals (Table Table 2) (Environment Agency 2008a). The threshold effect level (TEL) represents the value below which sediment-bound metals are not considered to be harmful to biota. The predicted effect level (PEL) represents values considered to exert a serious adverse impact on biota. Considering metals in the clay/silt fraction (<63 µm), overall, 62% of sites in the Afon Twymyn have leachable Pb concentrations above the TEL limit, the majority (45%) of ‘failures’ occurring within the vicinity of the former Dylife mine workings (see Table 2). At the mine, 41% of sites exceed the PEL, indicating the severe level of Pb contamination in the Afon Twymyn at Dylife. Downstream of the mine, 17% of sites have Pb concentrations above the TEL. As a result, these sites are anticipated to have serious deleterious impacts on aquatic ecology (Environment Agency 2008a). It must be stressed that this study has investigated leachable as opposed to total heavy metal concentrations which are specified in the draft guidelines. Therefore, greater levels of contamination of river sediments and more extensive draft guideline failures would be expected if evaluating total heavy metal concentrations.
When heavy metal concentrations are compared with data for river channel sediments in other mining-impacted rivers in the UK (Table 3), the Afon Twymyn has relatively low Zn, Cd and Cu concentrations and moderate Pb concentrations. This is partly due to the reporting of total (leachable and residual) heavy metals in these other studies. However, values are also a function of the extent of mining and mineralisation and also are related to the size of river catchments. Many of the larger river catchments of northern England (e.g. River Tees, River Swale) had far more extensive mining operations than in the Afon Twymyn, resulting in substantially greater contaminant production. Notably, the study of total metals in the Afon Twymyn by Wolfenden and Lewin (1978) recorded much higher metal concentrations than in the present study. While results from these two studies cannot be compared directly due to the different sediment size fractions measured, the large differences observed in metal concentrations are likely to be attributable to the residual geochemical phase which was not measured in the present study. As well as providing no information on the potential mobility and bioavailability of metals, measurement of total concentrations might present a false picture of the level of risk to aquatic ecosystems by including metals in the non-toxic, minerogenic form.
4.2 Heavy metal mobility and potential bioavailability
Investigations of sediment geochemistry have revealed that the vast majority of leachable sediment-bound metals in the Afon Twymyn exist in a highly mobile and potentially bioavailable state, posing serious concerns to ecological integrity. In this study, the dominant sinks for Pb, Zn and Cd in the clay/silt fraction are the most mobile, acid-soluble (easily exchangeable and carbonate bound) geochemical phases. The dominant sink for Pb in the sand fraction is also in the acid-soluble phases. Several other studies have identified Cd (Macklin and Dowsett 1989; Licheng and Guijiu 1996; Morillo et al. 2002; Jain 2004) and Zn (Morillo et al. 2002; Galan et al. 2003; Lopez-Gonzalez et al. 2006; Aleksander-Kwaterczak and Helios-Rybicka 2009) to be elevated in these phases. However, relatively few studies (Jain 2004) have recorded such high proportions of Pb in these phases, highlighting the severe level of Pb contamination at Dylife. The lack of carbonate mineralogy in the local rocks (Jones 1922) and low concentrations of base cations in the Afon Twymyn (Table 4) suggests that the majority of metals in the initial leach exist as easily exchangeable ions rather than carbonate-bound metals and can interact relatively easily with biota. The high concentrations of heavy metals in the acid-soluble phases suggest that the scenario under which these metals could be released from storage in the sediment of the Afon Twymyn is that of acidic environmental conditions (Jain 2004).
The reducible phase (Fe/Mn oxides) constituted the next largest sink for heavy metals in the clay/silt fraction, confirming metal sorption onto these metal oxides as an important attenuation mechanism. Iron and Mn oxides are well known as important metal sorbents in aquatic systems (Licheng and Guijiu 1996). However, adsorbed and co-precipitated metals could be released to the water column through reductive dissolution at low redox values or through the activity of micro-organisms in sediment (Morillo et al. 2002).
The oxidisable (sulphide/organic) phase was the largest sink for Zn, Cu and Cd in the sand fraction. At the mine site, this probably reflects the presence of zinc sulphide (sphalerite) and copper sulphide (chalcopyrite) which had been discarded (as uneconomic) in preference to galena. High proportions of Cu from the clay/silt fraction were also found in the oxidisable phase. Copper has been shown in many studies to form strong complexes with organic matter (Licheng and Guijiu 1996; Stone and Droppo 1996; Galan et al. 2003; Jain 2004). Oxidisable metals are most likely to be released from storage or redistributed to a less stable hydroxide, carbonate or easily exchangeable form under oxidising conditions (e.g. high river flows, dredging operations and drying of the riverbed) (Calmano et al. 1993; Dawson and Macklin 1998; Zoumis et al. 2001).
Several studies have noted, under changing conditions of water chemistry, the mobilisation of stored sediment-bound metals to the water column (Petersen et al. 1997; Kuwabara et al. 2000; Butler, 2009; Knott et al. 2009) and also the redistribution of metals from more stable to less stable geochemical forms (Calmano et al. 1993; Dawson and Macklin 1998; Zoumis et al. 2001). Stream/sediment pH and redox potential are known to be major controls on the mobility of metals and their partitioning between solid and dissolved phases (Younger et al. 2002). Acid drainage associated with many metal mines (Nagorski et al. 2002; Johnson 2003; Nordstrom 2003) has the capacity to release stored sediment-bound metals from their particulate phase to a more toxic, dissolved, free ion state (Wilkin 2008). Significantly acidic drainage (pH <5.5) does not appear to occur at Dylife mine, most likely due to a lack of iron sulphides, and possibly, the length of time the mine has been abandoned (Patrick Byrne, unpublished PhD thesis, Loughborough University, 2010). The circum-neutral pH of the Afon Twymyn and Dylife mine waters (see Table 4) suggests that exchangeable/carbonate-bound metals are unlikely to be released from sediment during steady flow conditions. However, it is possible that metals could be released from sediment storage during flood events. Patterns of metal flushing during flood events in the Afon Twymyn suggest two sources of contamination (Byrne et al. 2009). Peak metal concentrations before and at peak storm discharge suggests dissolution of metal sulphates on the surface of the mine spoil. Another possible source of contamination is the release of metals from river bed sediments via two mechanisms. First, disturbance and oxidation of sediments might release sulphide/organic-bound metals to the water column. Second, a drop in sediment pH accompanying oxidation might release exchangeable and carbonate-bound metals. Calmano et al. (1993) noted, in laboratory experiments, a significant fall in sediment pH (from 7.0 to 3.4) and increase in redox conditions during oxidation events in contaminated sediment. This subsequently resulted in the release of significant volumes of Zn, Cd and smaller quantities of Pb from the sediment to the water column. The level of the fall in pH was facilitated by the low acid neutralising capacity of the sediment due to the carbonate-poor mineralogy of the region, a situation also encountered at the present study site (Jones 1922). As well as releasing metals to the water column, it is also likely that the disturbance of the bed sediments in the Afon Twymyn by flood events affected the distribution of metals in geochemical phases, forcing migration of metals from more stable to less stable phases (Dawson and Macklin 1998; Zoumis et al. 2001).
The combined exchangeable, carbonate and Fe/Mn oxide phases are considered to be geochemical forms that are highly to moderately available to aquatic organisms as they can interact with organic tissue more easily than sulphide/organic and residual metals (Pierre Stecko and Bendell-Young 2000). The extremely high concentrations of Pb in very mobile geochemical phases represent a serious potential risk to the health of aquatic communities in the Afon Twymyn. By combining absolute metal concentrations (<63 µm) in this study from the exchangeable/carbonate and Fe/Mn oxide phases, metals in order of potential mobility and bioavailability are: Pb > Zn > Cu > Cd.
Metal concentrations and geochemical partitioning of floodplain sediments have not been assessed in this study, although floodplain sediments have been shown to be highly contaminated and a potential source of contamination in other mining-impacted regions of the UK (Macklin and Dowsett 1989; Dennis et al. 2009). The absence of a classic floodplain (apart from the riparian zone) at Dylife mine suggests that floodplain sediments are not a source of contamination. However, it is possible that the agriculturally important floodplain beyond the mine is significantly contaminated with heavy metals and that these metals exist in mobile geochemical phases.
4.3 Moving forward with sediment management
Water quality in England and Wales has in general improved, as has the health of aquatic ecosystems (Durance and Ormerod 2009). Even so, approximately 20% of river water quality objective failures in England and Wales are attributable to metal mine pollution (Environment Agency 2002). Furthermore, the legacy of contaminated sediments will represent a significant secondary diffuse source of pollution long after other water quality parameters have improved to acceptable levels. Therefore, contaminated sediments of mining-affected rivers will continue to pose a serious threat to ecological integrity and the achievement of ‘good’ chemical and ecological status under the European Union Water Framework Directive (WFD).
Currently, there are no agreed European or UK guidelines pertaining to pollution of river sediments. The development of sediment environmental quality standards (SEQS) is called for by the WFD. However, proposals to date have been considered technically controversial with substantial logistical problems (Crane 2003). A major difficulty stems from the nature of heavy metal pollutants. Assessment of metal toxicity is confounded by natural background concentrations, the existence of a number of chemical species, variation in pH concentrations, variations in organism tolerance/sensitivity and the fact that some heavy metals (e.g. Zn, Cu) are essential (trace) elements for organisms (Comber et al. 2008). These facts mean that metal toxicity to organisms will vary considerably between regions. Therefore, in order to classify accurately the ecological status of rivers impacted by metal mining, sediment assessments may need to be unique to each river catchment and incorporate background metal concentrations, an assessment of bioavailable fractions, and concurrent water quality measurements (including major ions) (Macklin et al. 2006; Netzband et al. 2007; Brils 2008; Förstner 2009). There is also a debate about the practicality of sediment quality measurements in routine river ecosystem monitoring because collecting and analysing sediment samples is a costly and time consuming process (Crane 2003). To compound these problems, the absence of uniformity in guidelines and in sampling and analytical methods throughout Europe results in a lack of comparability between results (Macklin et al. 2006; Brils 2008).
Recently, the importance of sediment systems in River Basin Management Plans has been accepted, following the activity of the European Sediment Network (SedNet) and an initial under-emphasis in the WFD (Förstner 2002; Förstner and Salomons 2008; Förstner 2009). An amendment to the WFD, Directive 2008/105/EC, states that it should be left to individual Member States to identify, monitor and manage sediments which may pose a risk to aquatic ecology. Currently, sediment quality is not a feature of the overall assessment of river ecosystem status in England and Wales, although draft sediment quality criteria have been published (Environment Agency 2008a). However, analysis of total heavy metals, as is proposed in the Environment Agency draft guidelines, will provide little information on the potential mobility and bioavailability of heavy metals. The discovery of high proportions of metals in mobile phases in the bed sediments of the Afon Twymyn highlights the potential risk posed to aquatic ecology and water quality. Given the results of the present study and other such geochemical investigations (Morillo et al. 2002; Galan et al. 2003; Aleksander-Kwaterczak and Helios-Rybicka 2009), should sediments of rivers draining former (and current) mining regions be assessed for leachable rather than total heavy metals? This would be a more time-consuming process. However, it is argued that the trade-off would be more useful and accurate information regarding the bioavailability of metals and the potential for metals to move beyond the mine site and to interact with different components of the river environment.
A final consideration surrounds sediment quality targets for river systems such as the Afon Twymyn which have suffered millennia of heavy metal contamination. The target of ‘good’ ecological status means that only a slight digression in water and ecological quality will be permitted from what could be expected in unmodified natural waters. In the case of most English and Welsh metal mines, which have been abandoned for almost a century, the issue is whether the river systems which drain them have in fact returned to an unmodified state, given they would naturally experience high sediment heavy metal concentrations due to local mineralisation. Clearly, as is the case with the Afon Twymyn, these river systems are grossly polluted. However, many of these sites have archaeological and sociological significance. Dylife mine is protected for its rare lichen species and is a regionally important geodiversity site for mineralogy (Brown 2006). This makes the implementation of remediation strategies/technologies difficult and controversial. Therefore, flexibility with respect to river systems with a substantial industrial legacy will be an important consideration for future sediment guidelines and River Basin Management Plans (Netzband et al. 2007).
5 Conclusions
The bed sediments of the Afon Twymyn are found to be grossly contaminated with heavy metal contaminants derived from Dylife mine. Metal concentrations in the clay/silt fraction (<63 µm) exceed draft Environment Agency of England and Wales sediment quality criteria at most sample locations. Lead is the principal contaminant and is significantly elevated for at least 12 km downstream of Dylife mine. Metal partitioning of stream sediment using the SM&T, three-step, sequential extraction procedure was successfully used to provide insight into the relative mobility and geochemical factors influencing the potential bioavailability of sediment-bound heavy metals. Investigations reveal that the vast majority of heavy metals in the sediment exist in the most mobile geochemical phases, thereby posing a serious threat to ecological integrity. A toxic risk order of Pb > Zn > Cu > Cd has been established.
The primary source of sediment contamination at Dylife mine is diffuse pollution from mine spoil. However, given the high concentration and potential mobility of sediment-bound heavy metal contaminants, the river bed sediment constitutes a major secondary diffuse source of pollution. During flood flows, it is probable that heavy metals are redistributed from more stable to less stable geochemical phases. A drop in stream and sediment pore water pH accompanying oxidation events could also release heavy metals to the water column through dissolution and desorption reactions. It is probable that metals stored in the channel margins of the Afon Twymyn exist in equally mobile geochemical phases. Similar chemical processes operating during erosion of overbank and floodplain sediments could, in the future, release heavy metals to the water column as well as introduce highly contaminated sediment into the river environment.
There is controversy regarding the development of sediment quality guidelines in the European Union due to the many logistical and technical challenges. At present, it has been left to individual Member States to define their own criteria and identify contaminant risk areas. The Environment Agency of England and Wales has begun this process with the establishment of draft sediment quality guidelines. Adoption of sediment quality guidelines in England and Wales, and eventually in the Water Framework Directive, is seen as key to protecting and improving riverine ecosystems. Assessment of metal mobility is especially important as it is the bioavailable metal phases which pose the most serious threat to the WFD target of ‘good’ ecological status. Furthermore, due to the extensive nature of contamination at some mine sites, and their historical, geological and sociological significance, flexibility with respect to targets for these sites is recommended.
References
Aleksander-Kwaterczak U, Helios-Rybicka E (2009) Contaminated sediments as a potential source of Zn, Pb, and Cd for a river system in the historical metalliferous ore mining and smelting industry area of South Poland. J Soils Sediments 9:13–22
Armitage PD, Bowes MJ, Vincent HM (2007) Long-term changes in macroinvertebrate communities of a heavy metal polluted stream: the River Nent (Cumbria, UK) after 28 years. River Res Appl 23:997–1015
Bick DE (1977) The old metal mines of mid-Wales. Part 4. West Montgomeryshire, Gloucester
Brils J (2008) Sediment monitoring and the European water framework directive. Ann Ist Super Sanita 3:218–223
British Geological Survey (2007) British regional geology: Wales. British Geological Survey, Keyworth
British Geological Survey (2000) Regional geochemistry of Wales and part of west-central England: stream sediment and soil. British Geological Survey, Keyworth
British Standards Institution (2006) BS ISO 11464:2006. Soil quality—pretreatment of samples for physico-chemical analysis. British Standards Institution, London
Brown M (2006) Dylife. The industrial and social history of a famous Welsh lead mine. Y Lolfa Cyf, Ceredigion
Butler BA (2009) Effect of pH, ionic strength, dissolved organic carbon, time, and particle size on metals release from mine drainage impacted streambed sediments. Water Res 43:1392–1402
Byrne P, Reid I, Wood PJ (2009) Short-term fluctuations in heavy metal concentrations during flood events through abandoned metal mines, with implications for aquatic ecology and mine water treatment. In: Proceedings of the International Mine Water Conference, 19–23 October 2009, International Mine Water Association, Pretoria, South Africa. CD-ROM 124-902, ISBN 978-0-09802623-5-3, http://www.imwa.info/publications/symposium_2009.php
Calmano W, Hong J, Förstner U (1993) Binding and mobilisation of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci Technol 28(8–9):223–235
Comber SDW, Merrington G, Sturdy L, Delbeke K, van Assche F (2008) Copper and zinc water quality standards under the EU Water Framework Directive: the use of a tiered approach to estimate the levels of failure. Sci Total Environ 403(1):12–22
Crane M (2003) Proposed development of sediment quality guidelines under the European Water Framework Directive: a critique. Toxicol Lett 142:195–206
Dawson EJ, Macklin MG (1998) Speciation of heavy metals on suspended sediment under high flow conditions in the River Aire, West Yorkshire, UK. Hydrol Process 12:1483–1494
Dennis IA, Coulthard TJ, Brewer P, Macklin MG (2009) The role of floodplains in attenuating contaminated sediment fluxes in formerly mined drainage basins. Earth Surf Processes Landf 34:453–466
Durance I, Ormerod SJ (2009) Trends in water quality and discharge confound long-term warming effects on river macroinvertebrates. Freshw Biol 54:388–405
Environment Agency (2002) Metal mine strategy for Wales. Environment Agency Wales, Cardiff
Environment Agency (2008a) Assessment of metal mining-contaminated river sediments in England and Wales. Environment Agency, Bristol
Environment Agency (2008b) Abandoned mines and the water environment. Environment Agency, Bristol
Evans AM (1987) An introduction to ore geology, 2nd edn. Blackwell, Oxford
Förstner U (2009) Sediments and priority substances in river basins. J Soils Sediments 9:89–93
Förstner U (2002) Sediments and the European water framework directive. J Soils Sediments 2:54
Förstner U, Salomons W (2008) Trends and challenges in sediment research 2008: the role of sediments in river basin management. J Soils Sediments 8:281–283
Fuge R, Laidlaw IMS, Perkins WT, Rogers KP (1991) The influence of acidic and mine spoil drainage on water quality in the mid-Wales area. Environ Geochem Health 13:70–75
Galan E, Gomez-Ariza JL, Gonzalez I, Fernandez-Caliani JC, Morales E, Giraldez I (2003) Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Appl Geochem 18(3):409–421
Gammons CH, Shope CL, Duaime TE (2005) A 24 h investigation of the hydrogeochemistry of baseflow and stormwater in an urban area impacted by mining: Butte, Montana. Hydrol Process 19:2737–2753
Goodyear KL, Ramsey MH, Thornton I, Rosenbaum MS (1996) Source identification of Pb–Zn contamination in the Allen Basin, Cornwall, S.W. England. Appl Geochem 11:61–68
Gower AM, Myers G, Kent M, Foulkes ME (1994) Relationships between macroinvertebrate communities and environmental variables in metal-contaminated streams in south-west England. Freshw Biol 32:199–221
Grimshaw DL, Lewin J, Fuge R (1976) Seasonal and short-term variations in the concentration and supply of dissolved zinc to polluted aquatic environments. Environ Pollut 11:1–7
Hornung M, Adamson JK, Reynolds B, Stevens PA (1986) Influence of mineral weathering and catchment hydrology on drainage water chemistry in three upland sites in England and Wales. J Geol Soc 143:627–634
Jain CK (2004) Metal fractionation study on bed sediments of the River Yamuna, India. Water Res 38:569–578
Jarvis AP, Johnston D, Mayes WM, Potter HAB (2008) Application of a new methodology for assessing the priorities for abandoned mine water pollution remediation at a national scale: results and implications from a study across England and Wales. In: Rapantova N, Hrkal Z (eds) Proceedings of the 10th International Mine Water Association Congress: mine water and the environment, 2–5 June 2008. Karlsbad, Czech Republic
Johnson CC (2005) G-BASE field procedures manual. British geological survey internal report, IR/05/097
Johnson DB (2003) Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water Air Soil Pollut 3:47–66
Jones OT (1922) Lead and zinc. The mining district of north Cardiganshire and west Montgomeryshire. British Geological Survey, London
Knott NA, Aulbury JP, Brown TH, Johnston EL (2009) Contemporary ecological threats from historical pollution sources: impacts of large-scale resuspension of contaminated sediments on sessile invertebrate recruitment. J Appl Ecol 46:770–781
Kuwabara J, Berelson W, Balistrieri L, Woods P, Topping B, Steding D, Krabben Hoeft D (2000) Benthic flux of metals and nutrients into the water column of Lake Couer d’Alene, Idaho: report of an August 1999 pilot study. US Geological Survey Water Resources Investigation 00-4132, California
Lewin J, Macklin MG (1987) Metal mining and floodplain sedimentation in Britain. In: Gardiner V (ed) International geomorphology 1986 Part I. Wiley, Chichester, pp 1009–1027
Licheng Z, Guijiu Z (1996) The species and geochemical characteristics of heavy metals in the sediments of Kangjiaxi River in the Shuikoushan Mine Area, China. Appl Geochem 11:217–222
Lopez-Gonzalez N, Borrego J, Morales JA, Carro B, Lozano-Soria O (2006) Metal fractionation in oxic sediments of an estuary affected by acid mine drainage (south-western Spain). Estuar Coast Shelf Sci 68:297–304
Lord RA, Morgan PA (2003) Metal contamination of active stream sediments in Upper Weardale, Northern Pennine Orefield, UK. Environ Geochem Health 25:95–104
Macklin MG (1996) Fluxes and storage of sediment-associated metals in floodplain systems: assessment and river basin management issues at a time of rapid environmental change. In: Anderson MG, Walling DE, Bates P (eds) Floodplain processes. Wiley, Chichester, pp 441–460
Macklin MG, Brewer PA, Hudson-Edwards KA, Bird G, Coulthard TJ, Dennis IA, Lechler PJ, Miller JR, Turner JN (2006) A geomorphological approach to the management of rivers contaminated by metal mining. Geomorphology 79:423–447
Macklin MG, Dowsett RB (1989) The chemical and physical speciation of trace elements in fine grained overbank flood sediments in the Tyne Basin, North-East England. Catena 16:135–151
Mance G, Brown VM, Yates J (1984) Proposed environmental quality standards for list II substances in water—copper. Water Research Centre Technical Report TR210, Wiltshire
Morillo J, Usero J, Gracia I (2002) Partitioning of metals in sediments from the Odiel River (Spain). Environ Int 28(4):263–271
Nagorski SA, Moore JN, Smith DB (2002) Distribution of metals in water and bed sediment in a mineral-rich watershed, Montana, USA. Mine Water Environ 2:121–136
Neal C, Whitehead PG, Jeffrey H, Neal M (2005) The water quality of the River Carnon, west Cornwall, November 1992 to March 1994: the impacts of Wheal Jane discharges. Sci Total Environ 338:23–29
Netzband A et al (2007) Sediment management: an essential element of River Basin Management Plans. J Soils Sediments 7(2):117–132
Nordstrom DK (2003) Negative pH and extremely acidic mine waters from Iron Mountain, California. Environ Sci Technol 34:254–258
Petersen W, Willer E, Williamovski C (1997) Remobilisation of trace elements from polluted anoxic sediments after resuspension in oxic water. Water Air Soil Pollut 99:515–522
Pierre Stecko JR, Bendell-Young LI (2000) Contrasting the geochemistry of suspended particulate matter and deposited sediments within an estuary. Appl Geochem 15:753–775
Quevauviller P, Rauret G, Lopez-Sanchez JF, Rubio R, Ure A, Muntau H (1997) Certification of trace metal extractable contents in a sediment reference material (CRM 601) following a three-step sequential extraction procedure. Sci Total Environ 205:223–234
Rauret G, Lopez-Sanchez JF, Sauquillo A, Rubio R, Davidson C, Ure A, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Rudeforth CC, Hartnup R, Lea JW, Thompson TRE, Wright PS (1984) Soils and their use in Wales. Soil Survey of England and Wales, Kent
Rust S, Rust D (1987) Micro minerals from Dyfngwm Mine, UK. J Mines Minerals 2:28–32
Stone M, Droppo IG (1996) Distribution of lead, copper and zinc in size-fractionated river bed sediment in two agricultural catchments of southern Ontario, Canada. Environ Pollut 93:353–362
Vivian CMG, Massie KS (1977) Trace metals in waters and sediments of the River Tawe, South Wales, in relation to local sources. Environ Pollut 14:47–61
Walling DE, Owens PN, Carter J, Leeks GJL, Lewis S, Meharg AA, Wright J (2003) Storage of sediment-associated nutrients and contaminants in river channel and floodplain systems. Appl Geochem 18:195–220
Wilkin RT (2008) Contaminant attenuation processes at mine sites. Mine Water Environ 27:251–258
Wolfenden PJ, Lewin J (1978) Distribution of metal pollutants in active stream sediments. Catena 5:67–78
Yim WWS (1981) Geochemical investigations on fluvial sediments contaminated by tin mine tailings, Cornwall, England. Environ Geol 3:245–256
Younger PL, Banwart SA, Hedin RS (2002) Mine water. Hydrology, pollution, remediation. Kluwer Academic Publishers, Dordrecht
Zoumis T, Schmidt A, Grigorova L, Calmano W (2001) Contaminants in sediments: remobilisation and demobilization. Sci Total Environ 226(1–3):195–202
Acknowledgements
PB gratefully acknowledges the support of a Loughborough University Department of Geography Scholarship. The authors would like to thank Stuart Ashby, Barry Kenny, Andy Bicket and Jonathan Lewis for their valued assistance with field sampling and laboratory analysis. Access to privately owned land in the study area was granted by Mr. Anwyl and Mr. Hales. The authors would also like to thank the three reviewers for their valuable comments and feedback.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Marcel van der Perk
Rights and permissions
About this article
Cite this article
Byrne, P., Reid, I. & Wood, P.J. Sediment geochemistry of streams draining abandoned lead/zinc mines in central Wales: the Afon Twymyn. J Soils Sediments 10, 683–697 (2010). https://doi.org/10.1007/s11368-009-0183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-009-0183-9