Abstract
The field of aging research has grown rapidly over the last half-century, with advancement of scientific technologies to interrogate mechanisms underlying the benefit of life-extending interventions like calorie restriction (CR). Coincident with this increase in knowledge has been the rise of obesity and type 2 diabetes (T2D), both associated with increased morbidity and mortality. Given the difficulty in practicing long-term CR, a search for compounds (CR mimetics) which could recapitulate the health and longevity benefits without requiring food intake reductions was proposed. Alpha-glucosidase inhibitors (AGIs) are compounds that function predominantly within the gastrointestinal tract to inhibit α-glucosidase and α-amylase enzymatic digestion of complex carbohydrates, delaying and decreasing monosaccharide uptake from the gut in the treatment of T2D. Acarbose, an AGI, has been shown in pre-clinical models to increase lifespan (greater longevity benefits in males), with decreased body weight gain independent of calorie intake reduction. The CR mimetic benefits of acarbose are further supported by clinical findings beyond T2D including the risk for other age-related diseases (e.g., cancer, cardiovascular). Open questions remain regarding the exclusivity of acarbose relative to other AGIs, potential off-target effects, and combination with other therapies for healthy aging and longevity extension. Given the promising results in pre-clinical models (even in the absence of T2D), a unique mechanism of action and multiple age-related reduced disease risks that have been reported with acarbose, support for clinical trials with acarbose focusing on aging-related outcomes and incorporating biological sex, age at treatment initiation, and T2D-dependence within the design is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Calorie restriction and aging
The field of aging research has grown rapidly over the last half-century, coincident with the development and advancement of scientific technologies including multiple areas of molecular biology. These advances have built upon the foundation of nutrition and physiologic research from the early 1900s to characterize more fully the nutrition-based interventions that have been shown to improve health, reduce disease, and ultimately increase lifespan in laboratory organisms. The most well-studied longevity and disease-altering intervention from research studies is the practice of calorie restriction (CR), a major player within the larger intervention toolkit of general “dietary restriction.” Despite the knowledge of expected benefit(s) from CR, the implementation of and adherence to CR at a sufficient level of restriction and duration necessary to achieve significant longevity and health benefits is improbable in the modern environment of dietary availability. This is evidenced by the contemporary rise and prevalence of obesity and Type 2 Diabetes (T2D), which are both associated with increased morbidity and mortality. Furthermore, refinement in our understanding of dietary composition and caloric content introduces possibilities for manipulating specific dietary components, and carbohydrates, in particular, to achieve benefits normally observed with traditional CR, which is known to counteract obesity and diabetes. Compounds which could recapitulate the health and longevity benefits of CR, referred to as calorie restriction mimetics (CRM), could target specific macronutrient classes and/or related signaling pathways to mimic CR without requiring food intake reductions. Herein, an update regarding the importance of carbohydrate metabolism in relation to longevity and age-related disease is provided. A specific focus on the antidiabetic agent and alpha-glucosidase inhibitor acarbose is provided, given its robust translational potential and clinical relevance.
Achieving the health benefits of CR through the use of CRMs
Of the many types of dietary restriction interventions that have been studied, CR remains the most highly utilized nutritional method to improve health, reduce disease, and increase lifespan in laboratory research models [1,2,3,4,5,6]. In practice as an intervention, CR is defined as a significant and sustained reduction in calorie provisions (most simply a proportional reduction of all macronutrient calories) to organisms below what their voluntary (ad libitum) intake would be. While the degree of calorie reduction is related to the health and longevity benefits, there is a level of restriction beyond which health and longevity benefits are lost. As diet provision approaches these greater levels of restriction, a necessary alteration in the proportions of macronutrient (protein preserving) and micronutrient supplementation becomes necessary [1, 7]. The beneficial impact of CR on health applies to many of the highest-ranked disease contributors to morbidity and mortality in the USA including cardiovascular diseases, cancers, and diabetes (specifically T2D) [8,9,10,11]. In contrast to CR, overfeeding and diet-induced obesity are shown to increase disease and mortality risk [12,13,14]. These divergent associations emphasize the need to understand the mechanisms which underlie the health benefits of CR (and conversely, the detrimental effects of diet-induced obesity and T2D) as a means to identify interventions which can mimic the health and longevity benefits of CR.
CRMs were initially proposed as a hypothetical class of compounds that could accomplish these goals and provide significant benefits through translational application [15,16,17]. Importantly, the benefits of CRMs would not rely on the need for individuals to maintain significant reductions in caloric intake, which remains a challenge in modern society as seen by the high prevalence of obesity and T2D in the modern nutrition environment [18, 19]. Furthermore, a CRM would be broad acting in the ability to slowing aging and improve general health rather than treat or prevent a single, exclusive disease focus. In light of these relationships between calorie intake and obesity/T2D, candidate CRMs were identified by focusing on compounds that showed benefit for treating obesity or T2D via their ability to recapitulate critical aspects of the physiologic responses induced by CR [16, 20,21,22,23,24]. The focus here will be on the class of T2D medications which target alpha-glucosidase inhibition, particularly acarbose [25, 26].
Research from the first half of the 1900s demonstrated the ability of CR to inhibit the outgrowth of transplanted or spontaneous cancers, highlighting the impact nutritional intervention (i.e., limitation) could have on rapidly dividing cells [27,28,29]. Subsequent longer-term studies revealed that CR reduced the rate of growth and maturation of rodents, coincident with increases in longevity [1, 7, 8]. Over the decades, this line of CR research has expanded to include “dose-response” assessments of levels of restriction, with carbohydrates identified as a select dietary component that could be reduced (relative to protein levels) to achieve CR longevity benefits even at more extreme levels of CR, achieving further delays and reductions in the magnitude and age of onset for common diseases [1, 7]. Across these studies of disease reduction and longevity extension, a physiologic and molecular signature of metabolic control (i.e., glucose reduction), hormonal regulation (insulin function), stress response, and metabolic rate reductions opened a window of knowledge allowing a view toward interventions that shared similarities with CR-induced molecular and physiologic responses, which would be predictive of health and longevity benefits [30, 31].
Parallel with these years of CR research, epidemiologic observations reported sustained and significant increases in obesity and T2D, which contributed to heightened morbidity and mortality within afflicted populations [18, 32,33,34]. Recent estimates indicate that approximately 40% of all adults in the USA have obesity [35, 36]. This trend is further exacerbated by the age-related increased risk in T2D, such that over half of the older adult population (> 65 years of age) in the USA are either prediabetic or diabetic [34]. The hyperglycemia associated with T2D is in stark contrast to the reduced glycemia present with CR and is implicated in the biological processes underlying fundamental aging mechanisms and disease phenotypes. Thus, improvements in the metabolism of glucose at the organismal and cellular level might reasonably offer the means not just to address T2D hyperglycemia as a treatment but also to intervene in fundamental glucose metabolism prior to development of an overt disease state, for the purpose of improving the aging trajectory [16, 37]. While a variety of pharmaceutical classes of compounds have been developed to treat T2D (biguanides, sulfonylureas, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, sodium-glucose linked transporters 2 inhibitors, etc.), the subsequent focus of this review will be on the alpha-glucosidase inhibitors (AGIs).
Acarbose is a candidate CRM that inhibits alpha-glucosidase activity
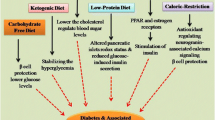
Each class of T2D medications works through distinct primary mechanisms of action, with AGIs predominantly functioning in the gastrointestinal (GI) tract [25, 26, 38, 39]. The particular AGI of interest here is acarbose, a pseudo-tetrasaccharide of bacterial origin that competitively inhibits alpha-glucosidase and alpha-amylase enzymatic digestions (Fig. 1). In doing so, acarbose delays and decreases monosaccharide cleavage from complex carbohydrates in the diet, impacting monosaccharide absorption in the gut [25, 40]. This results in a delayed and blunted postprandial glucose response, dampening the variability in blood glucose over the course of the day, particularly after meals that contain high amounts of carbohydrates [40]. In line with the health and longevity benefits observed with fiber supplementation, undigested complex carbohydrates transit further down the GI tract, resulting in an increased fiber-like effect [41,42,43,44,45,46,47]. Many of the side effects of acarbose administration (i.e., flatulence, bloating, and diarrhea) are related to its mimicry of a high-fiber diet and diminish over time or may be reduced by utilizing a dose-escalation protocol [25, 40, 48, 49].
The antidiabetic agent acarbose is an α-glucosidase inhibitor that provides multiple health benefits. a Acarbose is a pseudo-tetrasaccharide that competitively inhibits the enzymatic activity of α-glucosidases in the gut, thereby delaying and reducing the release of glucose monomers from complex carbohydrates. As a result, less glucose is absorbed in the upper intestines and made available for systemic use. b Due to its potent glucoregulatory ability, acarbose use promotes a wide range of health benefits, including type 2 diabetes (T2D) management, and decreased postprandial glucose (PPG). The figure was made with BioRender
Data from single-celled yeast to mammalian models have demonstrated that manipulation of the dietary amount and biochemical complexity of carbohydrates can result in significant impacts on cellular aging and organismal longevity [50,51,52,53,54]. In the budding yeast, Saccharomyces cerevisiae, which has been used extensively to study genetic and environmental/nutritional contributors to aging, reducing the starting glucose concentration in the media or increasing the complexity of sugars provided (i.e., by providing di- or tri-saccharides which are enzymatically digested, imported, and metabolized more slowly and efficiently than monosaccharides) both increase lifespan [50, 53]. Consistent with these effects, incorporation of acarbose at 0.1% by weight in a healthful laboratory diet with 66% carbohydrate by calories (21.2% protein and 12.8% fat) significantly increases overall, mean, and maximum lifespan (90th percentile) in non-diabetic male mice (HET3 strain, median: control—807 vs. acarbose—984 days, 22% increase, p < 0.0001) and to a lesser but statistically significant extent in female mice (HET3 strain, median: control—896 vs. acarbose—939 days, 5% increase, p = 0.01 relative to controls) [55, 56]. These results extend the previous observations of treatment efficacy and longevity promotion (recovery) in a model of diabetes with rats [57].
The benefits of acarbose outside T2D effects on longevity were replicated in a subsequent dose-response study using the same dietary composition formulations with 0.04%, 0.1%, and 0.25% acarbose by weight; where acarbose efficacy was shown to approach a plateau in longevity at 0.1% percent (median lifespan, males: control—830, acarbose [0.04%]—918, acarbose [0.1%]—975, acarbose [0.25%]—964 days; females: control—889, acarbose [0.04%]—887, acarbose [0.1%]—933, acarbose [0.25%]—922 days) [58]. Importantly, coincident with the longevity extension, multiple hormones implicated in lifespan regulation are altered with acarbose treatment [59]. Fasting insulin and insulin-like growth factor-1 (IGF-1) levels were lowered which are associated with lifespan extension that mimics a CR response, whereas fibroblast growth factor-21 (FGF-21) was significantly elevated, in contrast with the lowering of FGF-21 observed with CR [55]. Notably, the mechanism of action for acarbose is such that in these non-diabetic, healthy mice, blood glucose was not significantly lower under fasting conditions [55]. In contrast, acarbose does reduce blood glucose in both diabetic and non-diabetic animals during the postprandial phase [57, 58, 60,61,62,63,64]. This is in agreement with human studies where the primary benefits of acarbose glycemic control are acute following a meal, although longer-term benefits on insulin signaling and body composition may additionally contribute to T2D treatment efficacy [25, 26, 40, 48, 65,66,67]. Furthermore, no reduction in food intake was necessary for the acarbose-associated longevity benefit, despite animals having lower average body weight and body fat mass relative to untreated controls [55, 56, 58]. Reduced water intake has been reported with acarbose treatment in laboratory models of T2D, coincident with reduced blood glucose and urinary output; however, similar measures have not been routinely reported in non-diabetes focused, pre-clinical studies [64, 68]. Furthermore, the energy balance changes with acarbose treatment initiation appear to be largely explained by alterations in nutrient absorption [69,70,71,72], although comprehensive studies of energy expenditure (e.g., metabolic rate) across acute and long-term acarbose treatment are needed.
Systemic or off-target effects of acarbose
Although acarbose has low absorption kinetics with approximately 3% or less absorbed into the systemic circulation, and the primary mechanism of action of AGIs is focused in the GI tract, effects on longevity across multiple organ/systems via the small amount of absorbed acarbose or its secondary metabolites are not fully determined [40, 73]. As with other FDA-approved medications, including T2D medications, simulation work on acarbose protein binding domains and interaction modeling has highlighted additional unexpected potential interactions with “off-target” pathways (i.e., effects independent of alpha-glucosidase and alpha-amylase inhibition), which are frequently not considered when assessing acarbose effects [74]. Proposed off-target effects of interest include 4-α-glucanotransferase and maltose-binding periplasmic protein [74]. Whether mechanisms in addition to alpha-glucosidase inhibition contribute to acarbose’s effects independent of T2D amelioration remains to be determined.
Acarbose has distinct metabolomic and gut microbiome effects
In addition to improved glycemic control, acarbose may also modulate metabolism within tissues. Metabolomic profiling of liver and cecal samples from acarbose-treated (0.1% by weight) C57BL/6J mice compared with CR (40% reduction) or ad libitum feed controls uncovered a characteristic signature of amino acid and lipid metabolism [75]. Despite some level of similarity between acarbose and CR responses, unique differences exist in specific amino acids, bile acids, vitamin/cofactor, and xenobiotic compounds. For example, CR treatment resulted in a higher abundance of multiple vitamin/cofactor, dipeptide, and ketone metabolites relative to control and acarbose treatment, while responses within subpathways of metabolites were at times uniquely different with acarbose-treated females which exhibit a smaller longevity benefit, relative to acarbose-treated males and CR-treated males and females (e.g., heme, multiple gamma-glutamyl amino acids) [75]. These results reveal distinct effects of each longevity-promoting intervention at the specific doses of acarbose and CR used in this particular dietary composition (i.e., NIH-31 based 22.4% protein, 12.2% fat, and 65.4% carbohydrates by kcal) [75].
Given the bacterial origin of acarbose (Actinoplanes and Streptomyces sp.) and as might be anticipated with the change in GI digestion and absorption of complex carbohydrates, acarbose-induced shifts in the fecal microbiome have been reported [76,77,78,79,80]. These are characterized by a relatively greater prevalence of Bifidobacterium and lower Lactobacilli, likely driven by changes in nutrient substrate availability and the resulting competitive growth advantages to Bifidobacterium [76,77,78,79,80,81,82,83,84]. These microbiota shifts further contribute to metabolite differences observed with acarbose treatment, although the extent, if any, to which specific metabolites or individual bacterial species abundance further improve health or longevity outcomes is not yet determined with acarbose [75, 76, 79, 85,86,87,88,89,90,91]. Whether the secondary metabolites related to acarbose administration may have independent and beneficial roles reflective of prior research on short-chain fatty acids and ketone bodies is a further area of research opportunity [92,93,94,95,96,97,98,99,100].
Evidence for the emerging use of acarbose as an anti-cancer agent
Much like the early work with CR and cancer, the impact of acarbose on glucoregulatory control could be important for cancer control and/or treatment. While acarbose blunts postprandial glucose excursions, the overall effects on 24-h glucose levels tend to be lower, but of a potentially meaningful magnitude [25]. With the high glycolytic demand of tumors, the benefits of blunting postprandial glycemic excursions could, in theory, have implications for cellular proliferation within the tumor microenvironment. In support of this idea, a longitudinal study of over 1.3 million newly diagnosed diabetics illustrated that acarbose use for T2D management reduced the risk of colorectal cancer incidence by 27% [101]. However, a study of newly diagnosed cancer patients in Taiwan found that prior acarbose use was not associated with any change in all-cancer risk, illustrating the need for additional investigation of this question [102]. In rodent models, beneficial anti-cancer effects of acarbose were found in APC+/Min mice that are prone to developing bleeding intestinal neoplasias, which culminate in anemia and shortened lifespan. In this study acarbose use at 935 ppm resulted in decreased tumor formation and increased longevity [103]. Our own research using a pre-clinical model of renal cancer has found that acarbose administration at 0.1% in the diet (1000 ppm) can inhibit renal tumor progression, strengthen protective immune responses against tumors, and augment the efficacy of an immune-based cancer therapy, suggesting that acarbose may have clinical application in the treatment of established renal tumors (Orlandella et al., submitted).
While not the focus of the current review, the biguanide metformin is likely the most highly studied T2D medication regarding effects on cancer and longevity in rodent models [104]. It is of interest that metformin’s longevity effects in rodents appear to be somewhat strain-specific, with longevity benefits observed in cancer-prone rodent strains and the extension effect size proportionally larger with shorter-lived strains [104, 105]. In humans, metformin use is associated with a reduced incidence of colorectal cancer, similar to what has been reported with acarbose [106, 107]. In rodent models of established cancer, metformin use has been reported to directly impair tumor cell proliferation, reduce hypoxia within solid tumors, and improve protective immune responses [108]. The mechanisms of action of metformin for T2D (i.e., inhibition of mitochondrial Complex I, AMP-activated protein kinase activation, and decreased hepatic glucose production) are distinct from those of acarbose [109]. Thus, it may seem surprising that both acarbose and metformin were identified through an in vitro screening for compounds that impacted a genetic model of mitochondrial defect in iron-sulfur cluster formation defects with Friedreich’s ataxia [110]. Even more intriguing are the differential and opposing mitochondrial effects that were shown with the two agents. Specifically, acarbose increased mitochondrial function (oxygen consumption) whereas metformin had the opposite effect [110]. Whether acarbose is able to ameliorate the aging deficits observed with mitochondrial diseases or preserve mitochondrial function with advancing age is an area of future interest.
Acarbose effects on other age-related diseases
Early clinical studies supported a role for acarbose as both a treatment after T2D establishment, as well as a preventative intervention for pre-diabetes [67, 111, 112]. While these disease benefits were expected as the focus of acarbose development and use, initially more surprising may have been the cardiovascular-related benefits which have also been observed in patients receiving acarbose for T2D, including reduced cardiovascular disease, myocardial infarction, and hypertension [111, 113,114,115,116]. Emerging data suggest that postprandial glucose, triglyceride, and chylomicron responses, along with hormone improvements, may mediate some of these cardiovascular benefits [117,118,119,120]. In agreement with the increasingly recognized contribution of T2D to age-related neurologic disease risk (e.g., Alzheimer’s, Parkinson’s) [121, 122], recent studies highlight the potential reduced risk of onset or progression of dementia in subjects with T2D receiving acarbose treatment [123]. Finally, inflammatory-related diseases like arthritis have growing support for benefits from acarbose use to alleviate symptoms and reduce risk of progression in pre-clinical and clinical models [124,125,126,127,128], expanding the potential utility of acarbose treatment to multiple types of age-related disease mechanisms with human aging relevance.
AGIs beyond acarbose
In addition to acarbose, multiple pharmaceuticals within the AGI class (miglitol and voglibose) remain unreported regarding their ability to enhance longevity, exhibit anti-cancer activity, and achieve CRM status using non-diabetic laboratory models. Additionally, natural AGIs of phytochemical/botanical origin (e.g., cinnamon, tea) are being increasingly identified and experimentally shown to alter and improve glycemic control [129,130,131,132,133,134]. In fact, multiple botanically derived AGIs show equivalence and/or superiority regarding in vitro pharmacokinetics to acarbose, and combination treatment with acarbose further enhances glycemic suppression [135, 136]. However, less data are available to clarify whether botanical-derived AGIs can similarly improve long-term physiologic, metabolic, and longevity outcomes as seen with acarbose.
Summary and future studies
Additional studies of acarbose effects are needed at all levels of translational science. Existing literature supports consideration of broad health benefits by targeting glucoregulatory control in relation to co-morbidities and diseases (e.g., cardiovascular, neurodegenerative) [37], and may be informative in prioritizing study focus areas. Pre-clinical work has not fully investigated the dietary composition, strain background, and disease-status dependence of acarbose’s effects on age-related disease and longevity. The distinct mechanism of acarbose action from other proposed CRM raises the possibility that combinatorial therapies may be superior to any one intervention for addressing the complexity of biological aging across tissue types and organs. This may be particularly relevant in the context of sex-differential benefits observed with lifespan enhancing interventions (e.g., rapamycin, 17-estradiol) [55, 137,138,139]. Whether other AGIs have similar or greater anti-aging benefits to acarbose, particularly those of natural/botanical origin that are consumed in everyday diets at varying levels, remains understudied. Other T2D medication classes that also reduce postprandial glucose and improve overall glycemia like sodium-glucose-linked transporters 2 (SGLT2) inhibitors, which increases glucose excretion through urine, remain an interesting area of exploration as potential CR mimetics [140,141,142].
The AGI acarbose has a multi-decade safety profile as an FDA-approved treatment for T2D. While under-prescribed in the USA relative to metformin or other T2D medications, a growing body of pre-clinical, clinical, and epidemiologic research highlights the potential for acarbose (Fig. 1) as a stand-alone therapy and/or adjuvant treatment to address some of the most common ailments of biological aging, including age-related diseases and morbidities.
References
Weindruch R, Walford RL, The retardation of aging and disease by dietary restriction. Springfield. IL: C. C. Thomas Publisher; 1988.
Masoro EJ. Dietary restriction: current status. Aging (Milano). 2001;13(4):261–2.
Masoro EJ. Dietary restriction-induced life extension: a broadly based biological phenomenon. Biogerontology. 2006;7(3):153–5.
McCay CM. Effect of restricted feeding upon aging and chronic diseases in rats and dogs. Am J Public Health Nations Health. 1947;37(5):521–8.
McCay CM, Pope F, Lunsford W. Experimental prolongation of the life span. Bull N Y Acad Med. 1956;32(2):91–101.
Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790(10):1040–8.
Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–54.
Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52.
Everitt AV, Le Couteur DG. Life extension by calorie restriction in humans. Ann N Y Acad Sci. 2007;1114:428–33.
Fontana L. Calorie restriction and cardiometabolic health. Eur J Cardiovasc Prev Rehabil. 2008;15(1):3–9.
Fontana L. Nutrition, adiposity and health. Epidemiol Prev. 2007;31(5):290–4.
Flegal KM, Williamson DF, Pamuk ER, Rosenberg HM. Estimating deaths attributable to obesity in the United States. Am J Public Health. 2004;94(9):1486–9.
Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86(5):599–602.
Smith DL Jr, Yang Y, Nagy TR, Patki A, Vasselli JR, Zhang Y, et al. Weight cycling increases longevity compared with sustained obesity in mice. Obesity (Silver Spring). 2018;26(11):1733–9.
Ingram DK, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–23.
Ingram DK, Roth GS. Glycolytic inhibition as a strategy for developing calorie restriction mimetics. Exp Gerontol. 2011;46(2-3):148–54.
Ingram DK, Roth GS. Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev. 2015;20:46–62.
Sherwin R, Jastreboff AM. Year in diabetes 2012: the diabetes tsunami. J Clin Endocrinol Metab. 2012;97(12):4293–301.
Menke A, Rust KF, Fradkin J, Cheng YJ, Cowie CC. Associations between trends in race/ethnicity, aging, and body mass index with diabetes prevalence in the United States: a series of cross-sectional studies. Ann Intern Med. 2014;161(5):328–35.
Smith DL Jr, et al. Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci. 2010;65(5):468–74.
Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005;23(3):343–50.
Greenway F. Obesity medications and the treatment of type 2 diabetes. Diabetes Technol Ther. 1999;1(3):277–87.
Smith DL Jr, Robertson HT, Desmond RA, Nagy TR, Allison DB. No compelling evidence that sibutramine prolongs life in rodents despite providing a dose-dependent reduction in body weight. Int J Obes. 2011;35(5):652–7.
Minor RK, Smith DL Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, et al. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243(3):332–9.
Clissold SP, Edwards C. Acarbose. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1988;35(3):214–43.
Bischoff, H., Pharmacology of alpha-glucosidase inhibition. Eur J Clin Investig, 1994. 24 Suppl 3: p. 3-10.
Kritchevsky D. Caloric restriction and cancer. J Nutr Sci Vitaminol (Tokyo). 2001;47(1):13–9.
Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31(2):89–98.
Rous P. The influence of diet on transplanted and spontaneous mouse tumors. J Exp Med. 1914;20(5):433–51.
Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35(3):299–305.
Fontana L. The scientific basis of caloric restriction leading to longer life. Curr Opin Gastroenterol. 2009;25(2):144–50.
Ogden CL, Carroll MD, Flegal KM. Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin N Am. 2003;32(4):741–60 vii.
Lahey R, Khan SS. Trends in obesity and risk of cardiovascular disease. Curr Epidemiol Rep. 2018;5(3):243–51.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021–9.
Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–5.
Hales, C.M., et al., Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief, 2020(360): p. 1-8.
Brewer RA, Gibbs VK, Smith DL Jr. Targeting glucose metabolism for healthy aging. Nutr Healthy Aging. 2016;4(1):31–46.
Krause HP, Keup U, Puls W. Inhibition of disaccharide digestion in rat intestine by the alpha-glucosidase inhibitor acarbose (BAY g 5421). Digestion. 1982;23(4):232–8.
Lee SM, Bustamante SA, Koldovsky O. The effect of alpha-glucosidase inhibition on intestinal disaccharidase activity in normal and diabetic mice. Metabolism. 1983;32(8):793–9.
Balfour JA, McTavish D. Acarbose. An update of its pharmacology and therapeutic use in diabetes mellitus. Drugs. 1993;46(6):1025–54.
Jenkins DJ, et al. Scope and specificity of acarbose in slowing carbohydrate absorption in man. Diabetes. 1981;30(11):951–4.
McCulloch DK, Tattersall RB. Pharmacological fibre’--alpha-glucosidase inhibition in the management of diabetes. Diabet Med. 1984;1(3):189–90.
Jenkins DJ, Taylor RH, Nineham R, Goff DV, Bloom SR, Sarson DL, et al. Manipulation of gut hormone response to food by soluble fiber and alpha-glucosidase inhibition. Am J Gastroenterol. 1988;83(4):393–7.
Mc CCK, CC, Woodward JC, Sehgal BS. Cellulose in the diet of rats and mice. J Nutr. 1934;8(4):435–47.
Brussow H. Microbiota and healthy ageing: observational and nutritional intervention studies. Microb Biotechnol. 2013;6(4):326–34.
Tachon S, Zhou J, Keenan M, Martin R, Marco ML. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol Ecol. 2013;83(2):299–309.
Keenan MJ, Marco ML, Ingram DK, Martin RJ. Improving healthspan via changes in gut microbiota and fermentation. Age (Dordr). 2015;37(5):98.
Hollander P. Safety profile of acarbose, an alpha-glucosidase inhibitor. Drugs. 1992;44(Suppl 3):47–53.
Martin AE, Montgomery PA. Acarbose: an alpha-glucosidase inhibitor. Am J Health Syst Pharm. 1996;53(19):2277–90.
Smith DL Jr, et al. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6(5):649–62.
Troen AM, French EE, Roberts JF, Selhub J, Ordovas JM, Parnell LD, et al. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Dordr). 2007;29(1):29–39.
Choi SS. High glucose diets shorten lifespan of Caenorhabditis elegans via ectopic apoptosis induction. Nutr Res Pract. 2011;5(3):214–8.
Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14(14):2135–7.
Mlekusch W, Lamprecht M, Öttl K, Tillian M, Reibnegger G. A glucose-rich diet shortens longevity of mice. Mech Ageing Dev. 1996;92(1):43–51.
Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–82.
Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–84.
Cohen AM, Rosenmann E. Acarbose treatment and diabetic nephropathy in the Cohen diabetic rat. Horm Metab Res. 1990;22(10):511–5.
Harrison DE, Strong R, Alavez S, Astle CM, DiGiovanni J, Fernandez E, et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell. 2019;18(2):e12898.
McCarty MF, DiNicolantonio JJ. Acarbose, lente carbohydrate, and prebiotics promote metabolic health and longevity by stimulating intestinal production of GLP-1. Open Heart. 2015;2(1):e000205.
Lee SM. The effect of chronic alpha-glycosidase inhibition on diabetic nephropathy in the db/db mouse. Diabetes. 1982;31(3):249–54.
Casirola DM, Ferraris RP. alpha-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism. 2006;55(6):832–41.
Madariaga H, Lee PC, Heitlinger LA, Lebenthal E. Effects of graded alpha-glucosidase inhibition on sugar absorption in vivo. Dig Dis Sci. 1988;33(8):1020–4.
Madar Z. The effect of acarbose and miglitol (BAY-M-1099) on postprandial glucose levels following ingestion of various sources of starch by nondiabetic and streptozotocin-induced diabetic rats. J Nutr. 1989;119(12):2023–9.
Katovich MJ, Meldrum MJ, Vasselli JR. Beneficial effects of dietary acarbose in the streptozotocin-induced diabetic rat. Metabolism. 1991;40(12):1275–82.
Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med. 1994;121(12):928–35.
Coniff R, Krol A. Acarbose: a review of US clinical experience. Clin Ther. 1997;19(1):16–26.
Chiasson JL, Josse RG, Leiter LA, Mihic M, Nathan DM, Palmason C, et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care. 1996;19(11):1190–3.
Macedo CS, Silva MD, Spadella CT, Breim LC, Capeletti S, Mercadante MC, et al. Effect of long-term treatment with insulin and/or acarbose on glomerular basement membrane thickening in alloxan-diabetic rats. Braz J Med Biol Res. 1996;29(10):1329–35.
Dimitriadis G, Tessari P, Go V, Gerich J. Effects of the disaccharidase inhibitor acarbose on meal and intravenous glucose tolerance in normal man. Metabolism. 1982;31(8):841–3.
Holt PR, Atillasoy E, Lindenbaum J, Ho SB, Lupton JR, McMahon D, et al. Effects of acarbose on fecal nutrients, colonic pH, and short-chain fatty acids and rectal proliferative indices. Metabolism. 1996;45(9):1179–87.
Brooks B, Molyneaux L, Zilkens R, Ross G, Yue DK. The use of acarbose in type 2 diabetic patients in secondary failure: effects on glycaemic control and diet induced thermogenesis. Diabetes Res Clin Pract. 1998;42(3):175–80.
Chiasson JL, et al. Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia. 2004;47(6):969–75 discussion 976-7.
Ahr HJ, Boberg M, Krause HP, Maul W, Müller FO, Ploschke HJ, et al. Pharmacokinetics of acarbose. Part I: absorption, concentration in plasma, metabolism and excretion after single administration of [14C] acarbose to rats, dogs and man. Arzneimittelforschung. 1989;39(10):1254–60.
Haupt VJ, Daminelli S, Schroeder M. Drug Promiscuity in PDB: Protein Binding Site Similarity Is Key. PLoS One. 2013;8(6):e65894.
Gibbs VK, Brewer RA, Miyasaki ND, Patki A, Smith DL Jr. Sex-dependent differences in liver and gut metabolomic profiles with acarbose and calorie restriction in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2018;73(2):157–65.
Su B, Liu H, Li J, Sunli Y, Liu B, Liu D, et al. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7(5):729–39.
Zhang M, Feng R, Yang M, Qian C, Wang Z, Liu W, et al. Effects of metformin, acarbose, and sitagliptin monotherapy on gut microbiota in Zucker diabetic fatty rats. BMJ Open Diabetes Res Care. 2019;7(1):e000717.
Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8(1):1785.
Baxter NT, et al., The glucoamylase inhibitor acarbose has a diet-dependent and reversible effect on the murine gut microbiome. mSphere, 2019. 4(1).
Smith BJ, Miller RA, Ericsson AC, Harrison DC, Strong R, Schmidt TM. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019;19(1):130.
Goeke K, Drepper A, Pape H. Formation of acarbose phosphate by a cell-free extract from the acarbose producer Actinoplanes sp. J Antibiot (Tokyo). 1996;49(7):661–3.
Zhang CS, Podeschwa M, Altenbach HJ, Piepersberg W, Wehmeier UF. The acarbose-biosynthetic enzyme AcbO from Actinoplanes sp. SE 50/110 is a 2-epi-5-epi-valiolone-7-phosphate 2-epimerase. FEBS Lett. 2003;540(1-3):47–52.
Ortseifen V, Kalinowski J, Pühler A, Rückert C. The complete genome sequence of the actinobacterium Streptomyces glaucescens GLA. O (DSM 40922) carrying gene clusters for the biosynthesis of tetracenomycin C, 5’-hydroxy streptomycin, and acarbose. J Biotechnol. 2017;262:84–8.
Tan K, Tesar C, Wilton R, Jedrzejczak RP, Joachimiak A. Interaction of antidiabetic alpha-glucosidase inhibitors and gut bacteria alpha-glucosidase. Protein Sci. 2018;27(8):1498–508.
Weaver GA, Tangel CT, Krause JA, Parfitt MM, Jenkins PL, Rader JM, et al. Acarbose enhances human colonic butyrate production. J Nutr. 1997;127(5):717–23.
Kamimura H, Ogata H, Takahara H. Alpha-glucoside formation of xenobiotics by rat liver alpha-glucosidases. Drug Metab Dispos. 1992;20(2):309–15.
Wolin MJ, Miller TL, Yerry S, Zhang Y, Bank S, Weaver GA. Changes of fermentation pathways of fecal microbial communities associated with a drug treatment that increases dietary starch in the human colon. Appl Environ Microbiol. 1999;65(7):2807–12.
Yang HY, Liu SL, Ibrahim SA, Zhao L, Jiang JL, Sun WF, et al. Oral administration of live Bifidobacterium substrains isolated from healthy centenarians enhanced immune function in BALB/c mice. Nutr Res. 2009;29(4):281–9.
Komura T, Ikeda T, Yasui C, Saeki S, Nishikawa Y. Mechanism underlying prolongevity induced by bifidobacteria in Caenorhabditis elegans. Biogerontology. 2013;14(1):73–87.
Arboleya S, et al. Gut bifidobacteria populations in human health and aging. Front Microbiol. 2016;7:1204.
Yang B, et al. Diversity of gut microbiota and bifidobacterial community of chinese subjects of different ages and from different regions. Microorganisms. 2020:8(8).
Masoro EJ, Compton C, Yu BP, Bertrand H. Temporal and compositional dietary restrictions modulate age-related changes in serum lipids. J Nutr. 1983;113(4):880–92.
Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis. 2006;5:13.
Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065.
Edwards C, et al. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany NY). 2014;6(8):621–44.
Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. 2017;69(5):305–14.
Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017;26(3):547–57 e8.
Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2017;26(3):539–46 e5.
Davis RAH, Deemer SE, Bergeron JM, Little JT, Warren JL, Fisher G, et al. Dietary R, S-1,3-butanediol diacetoacetate reduces body weight and adiposity in obese mice fed a high-fat diet. FASEB J. 2019;33(2):2409–21.
Deemer SE, Davis RAH, Roberts BM, Smith DL Jr, Koutnik AP, Poff AM, et al. Exogenous dietary ketone ester decreases body weight and adiposity in mice housed at thermoneutrality. Obesity (Silver Spring). 2020;28(8):1447–55.
Tseng YH, Tsan YT, Chan WC, Sheu WHH, Chen PC. Use of an alpha-glucosidase inhibitor and the risk of colorectal cancer in patients with diabetes: a nationwide, population-based cohort study. Diabetes Care. 2015;38(11):2068–74.
Liu YC, Nguyen PA, Humayun A, Chien SC, Yang HC, Asdary RN, et al. Does long-term use of antidiabetic drugs changes cancer risk? Medicine (Baltimore). 2019;98(40):e17461.
Dodds SG, Parihar M, Javors M, Nie J, Musi N, Dave Sharp Z, et al. Acarbose improved survival for Apc(+/Min) mice. Aging Cell. 2020;19(2):e13088.
Anisimov VN. Metformin for cancer and aging prevention: is it a time to make the long story short? Oncotarget. 2015;6(37):39398–407.
Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12(22):3483–9.
Chen YC, Kok VC, Chien CH, Horng JT, Tsai JJ. Cancer risk in patients aged 30 years and above with type 2 diabetes receiving antidiabetic monotherapy: a cohort study using metformin as the comparator. Ther Clin Risk Manag. 2015;11:1315–23.
Anisimov VN. Metformin for prevention and treatment of colon cancer: a reappraisal of experimental and clinical data. Curr Drug Targets. 2016;17(4):439–46.
Zhao B, Luo J, Yu T, Zhou L, Lv H, Shang P. Anticancer mechanisms of metformin: a review of the current evidence. Life Sci. 2020;254:117717.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85.
Sahdeo S, Tomilov A, Komachi K, Iwahashi C, Datta S, Hughes O, et al. High-throughput screening of FDA-approved drugs using oxygen biosensor plates reveals secondary mitofunctional effects. Mitochondrion. 2014;17:116–25.
Chiasson, J.L., Acarbose for the prevention of diabetes, hypertension, and cardiovascular disease in subjects with impaired glucose tolerance: the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) Trial. Endocr Pract, 2006. 12 Suppl 1: p. 25-30.
DeFronzo RA, bdul-Ghani MA. Preservation of beta-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96(8):2354–66.
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–94.
Delorme S, Chiasson JL. Acarbose in the prevention of cardiovascular disease in subjects with impaired glucose tolerance and type 2 diabetes mellitus. Curr Opin Pharmacol. 2005;5(2):184–9.
Moelands SV, et al. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2018;12:CD005061.
Hsu PF, Sung SH, Cheng HM, Shin SJ, Lin KD, Chong K, et al. Cardiovascular benefits of acarbose vs sulfonylureas in patients with type 2 diabetes treated with metformin. J Clin Endocrinol Metab. 2018;103(10):3611–9.
Ogawa S, Takeuchi K, Ito S. Acarbose lowers serum triglyceride and postprandial chylomicron levels in type 2 diabetes. Diabetes Obes Metab. 2004;6(5):384–90.
Zheng MY, Yang JH, Shan CY, Zhou HT, Xu YG, Wang Y, et al. Effects of 24-week treatment with acarbose on glucagon-like peptide 1 in newly diagnosed type 2 diabetic patients: a preliminary report. Cardiovasc Diabetol. 2013;12:73.
McCarty, M.F. and J.J. DiNicolantonio, Acarbose, lente carbohydrate, and prebiotics promote metabolic health and longevity by stimulating intestinal production of GLP-1. Open Heart, 2015. 2(1): p. e000205.
Frantz S, Calvillo L, Tillmanns J, Elbing I, Dienesch C, Bischoff H, et al. Repetitive postprandial hyperglycemia increases cardiac ischemia/reperfusion injury: prevention by the alpha-glucosidase inhibitor acarbose. FASEB J. 2005;19(6):591–3.
Cardoso S, Moreira PI. Antidiabetic drugs for Alzheimer’s and Parkinson’s diseases: repurposing insulin, metformin, and thiazolidinediones. Int Rev Neurobiol. 2020;155:37–64.
Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis. 2010;20(3):723–36.
Tseng CH. Dementia risk in type 2 diabetes patients: acarbose use and its joint effects with metformin and pioglitazone. Aging Dis. 2020;11(3):658–67.
Chen HH, et al. Acarbose decreases the rheumatoid arthritis risk of diabetic patients and attenuates the incidence and severity of collagen-induced arthritis in mice. Sci Rep. 2015;5:18288.
Zhang L, et al. alpha-Glucosidase inhibitors alter gut microbiota and ameliorate collagen-induced arthritis. Front Pharmacol. 2019;10:1684.
Derosa G, Maffioli P, Ferrari I, Fogari E, D'Angelo A, Palumbo I, et al. Acarbose actions on insulin resistance and inflammatory parameters during an oral fat load. Eur J Pharmacol. 2011;651(1-3):240–50.
Mo D, Liu S, Ma H, Tian H, Yu H, Zhang X, et al. Effects of acarbose and metformin on the inflammatory state in newly diagnosed type 2 diabetes patients: a one-year randomized clinical study. Drug Des Devel Ther. 2019;13:2769–76.
Lin YC, Chen YC, Hsiao HP, Kuo CH, Chen BH, Chen YT, et al. The effects of acarbose on chemokine and cytokine production in human monocytic THP-1 cells. Hormones (Athens). 2019;18(2):179–87.
Rios JL, Francini F, Schinella GR. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015;81(12-13):975–94.
Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14(9):884–9.
Pereira ASP, et al. Evaluation of the anti-diabetic activity of some common herbs and spices: providing new insights with inverse virtual screening. Molecules. 2019:24(22).
Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108.
Hosoda K, Wang MF, Liao ML, Chuang CK, Iha M, Clevidence B, et al. Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care. 2003;26(6):1714–8.
Tang GY, et al. Health functions and related molecular mechanisms of tea components: an update review. Int J Mol Sci. 2019:20(24).
Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal alpha-glucosidase and pancreatic alpha-amylase. Plant Foods Hum Nutr. 2011;66(2):143–8.
Gao J, Xu P, Wang Y, Wang Y, Hochstetter D. Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against alpha-amylase and alpha-glucosidase in vitro. Molecules. 2013;18(9):11614–23.
Garratt M, Bower B, Garcia GG, Miller RA. Sex differences in lifespan extension with acarbose and 17-alpha estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell. 2017;16(6):1256–66.
Smith DL Jr, Nagy TR, Allison DB. Calorie restriction: what recent results suggest for the future of ageing research. Eur J Clin Investig. 2010;40(5):440–50.
Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–77.
List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. 2011;120:S20–7.
Shintani H, et al. Calorie restriction mimetics: upstream-type compounds for modulating glucose metabolism. Nutrients. 2018:10(12).
Zhang W, Welihinda A, Mechanic J, Ding H, Zhu L, Lu Y, et al. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA(1c) levels in db/db mice and prolongs the survival of stroke-prone rats. Pharmacol Res. 2011;63(4):284–93.
Funding
Funding in support of this work is derived in part by NIH grant award P30AG050886.
Author information
Authors and Affiliations
Contributions
All authors provided input and final approval of the submitted and published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Daniel L. Smith Jr has no conflicts relevant to the content of the review article. Dr. Smith’s institution, the University of Alabama at Birmingham, has received funds to support research from the National Institutes of Health, McCormick Science Institute and the Alliance for Potato Research and Education. Rachael M. Orlandella has no conflicts relevant to the content of this review article. Lyse A. Norian has no conflicts relevant to the content of the review article. In the last thirty-six months, Dr. Allison has received personal payments or promises for the same from the following: American Society for Nutrition; Alkermes, Inc.; American Statistical Association; Biofortis; California Walnut Commission; Columbia University; Fish & Richardson, P.C.; Frontiers Publishing; Henry Stewart Talks; IKEA; Indiana University; Laura and John Arnold Foundation; Johns Hopkins University; Law Offices of Ronald Marron; MD Anderson Cancer Center; Medical College of Wisconsin; National Institutes of Health (NIH); Sage Publishing; The Obesity Society; Soleno Therapeutics; Tomasik, Kotin & Kasserman LLC; University of Alabama at Birmingham; University of Miami; Nestle; WW (formerly Weight Watchers International, LLC). Donations to a foundation have been made on his behalf by the Northarvest Bean Growers Association. Dr. Allison has been an unpaid member of the International Life Sciences Institute North America Board of Trustees. Dr. Allison’s institution, Indiana University, has received funds to support his research or educational activities from the following: NIH; Eli Lilly, Alliance for Potato Research and Education; American Federation for Aging Research; Dairy Management Inc.; Herbalife; Laura and John Arnold Foundation; National Cattlemen’s Beef Association, Oxford University Press, the Sloan Foundation, The Gordan and Betty Moore Foundation, and numerous other for-profit and non-profit organizations to support the work of the School of Public Health and the university more broadly.
Disclaimer
The opinions expressed are those of the authors and do not necessarily represent those of the NIH or any other organization with which the authors are affiliated.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Smith, D.L., Orlandella, R.M., Allison, D.B. et al. Diabetes medications as potential calorie restriction mimetics—a focus on the alpha-glucosidase inhibitor acarbose. GeroScience 43, 1123–1133 (2021). https://doi.org/10.1007/s11357-020-00278-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00278-x