Abstract
The age-related decline in muscle function contributes to the movement limitations in daily life in old age. The age-related loss in muscle force is attributable to loss of myofibers, myofiber atrophy, and a reduction in specific force. The contribution of each of these determinants to muscle weakness in old age is, however, largely unknown. The objective of this study is to determine whether a loss in myofiber number, myofiber atrophy, and a reduction in specific muscle force contribute to the age-related loss of muscle force in 25-month-old mouse. Maximal isometric force of in situ m. plantaris of C57BL/6J male adult (9 months) and old (25 months) mice was determined and related to myofiber number, myofiber size, intramuscular connective tissue content, and proportion of denervated myofibers. Isometric maximal plantaris muscle force was 13 % lower in old than adult mice (0.97 ± 0.05 N vs. 0.84 ± 0.03 N; P < 0.05). M. plantaris mass of old mice was not significantly smaller than that of adult mice. There was also no significant myofiber atrophy or myofiber loss. Specific muscle force of old mice was 25 % lower than that of adult mice (0.55 ± 0.05 vs. 0.41 ± 0.03 N·mm−2, P < 0.01). In addition, with age, the proportion of type IIB myofibers decreased (43.6 vs. 38.4 %, respectively), while the connective tissue content increased (11.6 vs. 16.4 %, respectively). The age-related reduction in maximal isometric plantaris muscle force in 25-month-old male C57BL/6J mice is mainly attributable to a reduction in specific force, which is for 5 % explicable by an age-related increase in connective tissue, rather than myofiber atrophy and myofiber loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with a progressive decline in muscle power generating capacity (Runge et al. 2004) that contributes to an increased incidence of falls, a decreased independence and reduced quality of life (Kamel 2003; Roubenoff and Hughes 2000). Improvement of muscle function in the older person may alleviate many of these problems. To develop strategies to preserve, slow, or even reverse muscle weakness in advanced age, a profound understanding of the etiology of age-related muscle weakness is needed.
An important determinant of muscle power is the force generating capacity of the muscle. Muscle force generating capacity in humans can decrease as much as 60 % between the age of 30 and 80 years (Faulkner et al. 2007). Also in rodents, a 25–65 % age-related deterioration in the muscle force generating capacity has been reported (Ballak et al. 2014; Brooks and Faulkner 1988; Carter et al. 2010; Degens and Alway 2003), which is not only due to a loss of muscle mass but also a reduction in the force-generating capacity per muscle cross-sectional area, specific force (Degens et al. 2009). It could be that during the initial stages of sarcopenia a reduction in specific force precedes the loss in muscle mass.
Concomitant with the loss of muscle force generating capacity there is a loss of muscle mass. In the human m. vastus lateralis, this amounted to approximately 30 % between 40 and 80 years (Janssen et al. 2000; Klitgaard et al. 1990; Maden-Wilkinson et al. 2014; Overend et al. 1992; Rice et al. 1989; Young et al. 1984; Young et al. 1985). In rodents, at ages representative of these ages in humans, a similar age-related reduction in muscle mass is observed (Ballak et al. 2014). Typically, the age-related decrease in muscle mass is due to both a reduction in myofiber number (Lexell et al. 1988; Lushaj et al. 2008; McKiernan et al. 2004; Sato et al. 1984; Zerba et al. 1990) and (preferential type II) myofiber atrophy (Brown and Hasser 1996; Carter et al. 2010; Klitgaard et al. 1990; Larsson 1978; Tomonaga 1977; Verdijk et al. 2007). However, the loss in maximal muscle force with age is more than proportional to the decrease in muscle mass (Degens et al. 2009). This implies age-related intrinsic changes of the muscle affecting the quality of the remaining muscle tissue (Frontera et al. 2008). In fact, several studies in both humans (Frontera et al. 2008; Klein et al. 2001; Morse et al. 2005) and rodents (Degens and Alway 2003; Degens et al. 1995; Kadhiresan et al. 1996; Rice et al. 2005) have reported a reduced force per unit cross-sectional area (referred to as specific force) during aging. An increased connective tissue content (Brooks and Faulkner 1994; Lushaj et al. 2008; Overend et al. 1992), inclusion of denervated, and thus non-force-producing myofibers (Urbanchek et al. 2001), and a selective atrophy of type II myofibers that reportedly have a higher specific force than type I fibers (Larsson et al. 1997a; Yu et al. 2007), may also contribute to the age-related reduction of specific muscle force.
It thus appears that the three prime factors that contribute to the age-related muscle weakness are a reduction in myofiber number, myofiber atrophy, and a reduction in specific force. Yet, these determinants of the age-related muscle dysfunction are hitherto not evaluated collectively, especially in early stages of sarcopenia. Since the contribution of each of these determinants to the age-related changes in muscle force generating capacity cannot be investigated in humans, we used m. plantaris in adult (9 months) and old (25 months) mice. We hypothesized that skeletal muscle weakness in old age is in order of importance due to (a) a loss in muscle quantity, (b) a reduction in muscle quality.
To investigate this, we determined the maximal isometric force of mouse m. plantaris in situ. To determine the relative contribution of the three abovementioned factors, tetanic force was related to m. plantaris mass, myofiber CSA, myofiber number, and specific force. Histological analysis was also performed to determine connective tissue content and proportion of denervated, and hence non-force producing, myofibers.
Methods
Animals
Experiments were performed on eleven 9-month-old (adult) and ten 25-month-old (old) male C57BL/6J mice (Janvier, France). Mice were housed individually and kept under specific-pathogen-free conditions at 20–22 °C at a 12 h light/dark cycle. Animals were given free access to water and chow (Ssniff® S8189-S095). For the experiments described below, we analyzed histological and contractile force characteristics of the right leg. At the age of 7.5 (adult) or 23.5 (old) months, in the left leg of the mice the m. gastrocnemius and m. soleus were denervated to overload the m. plantaris muscle for 6 weeks. The effects of overload are beyond the scope of the present study.
All experiments were approved by the local animal use and care committee of the VU University Amsterdam and conformed to the guide of the Dutch Research Council for care and use of laboratory animals.
Surgery and preparation
Fifteen minutes prior to the start of the experiment, mice received a subcutaneous injection of 0.06 mL 1 % Temgesic (Reckitt Benckiser, UK) as an analgesic and were anesthetized with 4 % isoflurane, 0.1 L min−1 O2 and 0.2 L min−1 air. Subsequently, the level of anesthesia was maintained with 1.5–2.5 % isoflurane. A humidifier moistened the inhaled air to prevent dehydration due to respiration. The mice were placed on a heated pad to maintain body temperature at ∼36.5 °C.
The experiments were performed as explained previously (Degens and Alway 2003). The m. plantaris was dissected free from surrounding tissue while maintaining its innervation and blood supply. The sciatic nerve was severed and the proximal end was placed over an electrode for stimulation of the muscle. The distal tendon of the m. plantaris was dissected free and tightened with a Kevlar thread via a small steel link to a force transducer, which was mounted on the lever arm of an isovelocity measuring system. The femur was fixed by a clamp on the condyle of the femur. During the experiment, the muscle and its surrounding were kept moist at physiological temperature (34–36 °C) with a water-saturated airflow.
Experimental setup and force measurements
Figure 1 shows a diagram of the experimental setup for in situ muscle force measurements which is a modification of that described (de Haan et al. 1989). Contractions were induced by supramaximal electrical stimulation of the sciatic nerve at a constant current (2 mA; 200 μs pulse width). Optimal muscle length (ℓ o) was defined as the muscle length at which maximal tetanic isometric force was generated. To determine ℓ o, first the length at which the muscle produced maximal twitch force was assessed. To fine adjust ℓ o tetani (150 Hz, 150 ms) were applied once every 2 min. Two minutes after the last tetanus the muscle was stimulated by applying a 400-Hz, 80-ms pulse train to determine the maximal rate of force development (MRTD). Finally, the muscle was stimulated by applying a 150-Hz, 150-ms pulse trains once every second for 5 min to deplete the glycogen in fibers expressing type IIB myosin. Force and length signals (1–10 kHz) were stored on disk. At the end of the experiment, the m. plantaris was excised and weighed and the mouse was killed by cervical dislocation.
Experimental setup as used during all measurements. This is a modification of the setup used by de Haan et al. (1989). The anesthetized mouse was placed on a heated pad, while the m. plantaris was attached to the force transducer, which allowed to measure muscle force in situ
Analyses
Tissue preparation and cutting of histological sections
The m. plantaris was embedded at ℓ o in a gelatin tyrode (NaCl, 128.3 mM; KCl, 4.7 mM; MgCl2, 1.05 mM; NaH2PO4H2O, 0.42 mM; NaHCO3, 20.2 mM; EGTA, 15.0 mM; Gelatine 15 % (w/v), pH 7.2) solution and frozen in liquid nitrogen. Subsequently, m. soleus and m. gastrocnemius medialis were excised and also frozen in liquid nitrogen. All chemicals were obtained from Sigma Aldrich (The Netherlands) unless stated otherwise.
Within a month after the contraction protocol, serial cross-sections (10 μm) were cut from the midbelly of the m. plantaris using a cryostat at −20 °C. The in-gelatin tyrode-embedded sections were mounted with Tissue-Tek (Jung, Leica Microsystems, Germany) onto a specimen holder, avoiding direct contact between Tissue-Tek and the part of the muscle that would be sectioned to prevent freeze-thaw artifacts. Sections were mounted on glass slides (Menzel-Gläser, superfrost® plus, Germany), air-dried, and stored at −80 °C until further use.
Myosin heavy chain composition
Serial sections of the m. plantaris were immunohistochemically stained against type I, IIA, IIX, and IIB MHC using monoclonal antibodies BAD5 (1 μg/mL), SC-71 (1 μg/mL), 6H1 (10 μg/mL), and BF-F3 (1–10 μg/mL) (Developmental Studies Hybridoma Bank, USA), respectively. In short, sections were fixed with acetone for 10 min at 4 °C and washed in phosphate-buffered saline with 0.05 % tween (PBST) three times for 5 min. After blocking with 10 % normal swine serum for 30 min, the sections were incubated with the primary antibody for 90 min. Subsequently, sections were washed in PBST three times for 3 min and incubated in the dark with secondary antibody (Alexa 488 anti-mouse, 1:500, Life Technologies, The Netherlands) for 30 min. After washing with PBST, incubating with wheat germ agglutinin (WGA, 1:50, Life Technologies, The Netherlands) for 20 min, washing with PBST, and afterwards washing once more with PBS all in the dark, the sections were enclosed with Vectashield®-hardset mounting medium with DAPI (1.5 μg/mL; Vector Laboratories, USA). The public domain software ImageJ 1.45s (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, MD, USA) was used to assess myofiber number, type, and myofiber cross-sectional area.
Determination of denervated myofibers by PAS-staining
The glycogen content of individual myofibers was determined in 10-μm-thick sections using the periodic acid–Schiff (PAS) staining reaction (van der Laarse et al. 1992). The sections were air-dried and fixed for 5 min in 4 % formaldehyde in 20 mL, 0.2 M imidazole, and 180 mL acetone. Subsequently, sections were incubated in 44 mM periodic acid solution for 30 min at room temperature. After incubation, sections were briefly washed in 0.1 M HCl and stained with Schiff’s reagents for 25 min at room temperature.
Intramuscular connective tissue content
Intramuscular connective tissue was determined using Sirius Red. The sections were air-dried and fixed for 30 min in Bouin solution. Next, sections were washed for 10 min, before 30 min incubation in Sirius Red saturated with picric acid. Sections were dehydrated by rapidly dipping in absolute ethanol. For a better image quality, sections were cleared with xylene and mounted with DPX (Sigma Aldrich, UK). A Matlab-script (version R2012a) was used to quantify the connective tissue content per image. The RBG threshold was set at R > 140, B < 110, G < 110, to create a binary image allowing to filter all red pixels. The same threshold was applied to all images that were stained at the same time.
Statistics
To determine significant differences between age groups, Student’s t tests were used. To test for differences in fiber CSA, a two-way ANOVA was used with factors age and fiber type. Effects were considered significant at P < 0.05. Data are expressed as mean ± SEM. All calculations were performed using IBM SPSS version 20.
Results
Mice characteristics
Table 1 shows that body mass, muscle optimum length (ℓ o), myofiber number, and muscle mass/body mass ratio did not differ significantly between adult and old mice. The masses of the plantaris, gastrocnemius medialis, and soleus muscles (PGMS) combined were -8 % less in old than adult animals (P = 0.03; Table 1). Mass of m. plantaris alone was, however, not significantly reduced in the old compared to the adult group (P > 0.05; Table 1).
Age-related changes in morphology and in situ force characteristics of m. plantaris
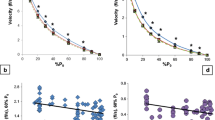
The ACSA of the m. plantaris muscle did not differ significantly between adult and old mice (Table 1). Maximal isometric force of m. plantaris of old mice was 13 % lower compared to that of adults (Fig. 2a, c; P < 0.05). Maximal tetanic force divided by muscles ACSA (specific tetanic force) of old muscles was 25 % lower than that of adult muscle (P < 0.01; Fig. 2d). Five percent of this lower specific force was explicable by the higher relative content of connective tissue in the old (16.4 vs. 11.6 % in adult) muscles (specific muscle force corrected for collagen content: adult 0.63 ± 0.06 N·mm−2; vs old 0.50 ± 0.03 N·mm−2; P =0.087). However, specific tetanic force expressed as maximal tetanic force per muscle mass did not differ significantly between adult and old muscles (Table 1).
Maximal isometric tetanus force (N) during a 150 Hz isometric tetanus plotted against time (ms) for adult and old m. plantaris of mice (a). Force (N) during a 400 Hz isometric tetanus plotted against time for adult and old m. plantaris of mice (b). Maximal force (mN) in adult mice was higher than that in old mice (*P < 0.05) (c). Specific force (N·mm−2) was less in old than adult m. plantaris (*P < 0.05). Values are mean ± SEM
Figure 3 shows examples of m. plantaris cross-sections stained specifically for type IIB myosin heavy chain. The percentage of type IIB myofibers decreased with age (43.6 vs. 38.4 % in adult and old, respectively; P < 0.05; Fig. 3c). The CSAs of type IIA, IIX, and IIB myofibers did not differ significantly between adult and old mice (Fig. 3d). Likewise, pooled CSA did not change significantly with age (Table 1).
Examples of immunofluorescent staining for type IIB myosin in an adult (a) and old (b) m. plantaris. Bar represents 500 μm. Type IIB myofiber proportion (%) in adult and old mice was significantly different (P < 0.05). Type I, IIA, IIAX, IIX, and IIXB proportions did not change significantly (c). Myofiber CSA (μm2) did not significantly decline for any fiber type (P > 0.05) (d). Note that type I myofibers were not included in this analysis, because of the low presence of type I myofibers within the m. plantaris of C57Bl/6 mice. Values are mean ± SEM
Figure 4a, b show examples of Sirius Red-stained muscle cross-sections. The percentage of collagen in the m. plantaris was higher in the old group compared to that in the adult group (16.4 vs. 11.6 %, respectively; P = 0.03; Fig. 4c). Figure 5 shows examples of serial muscle sections stained for type IIB myosin and PAS to quantify the number of denervated myofibers that express IIB myosin. The percentage of all fibers and the percentage of IIB myosin expressing myofibers that were denervated did not differ significantly between adult and old mice (Fig. 5e).
Serial sections stained for type IIB myosin and PAS of adult (a and b) and old (c and d) muscles. Bar represents 100 μm. Corresponding denervated myofibers are specified with an asterisk. Analysis revealed no significant difference between adult and old denervated myofiber count expressed as percentage of all IIB myofibers (e). Values are mean ± SEM
Maximal rate of force development (MRTD) did not significantly differ between adult and old plantaris muscles (Fig. 2b; Table 1). Also, normalized MRTD (MRTD/Fmax) did not decrease with age. However, half relaxation time of the 400 Hz contraction was significantly longer in the old than in adult muscles (P < 0.01) (Table 1), indicating that the old muscles were slower.
Discussion
The main finding of the present study is that the age-related loss in maximal force of plantaris muscle in 25-month-old male C57BL/6 mice is mainly attributable to a reduction in specific force. In contrast to our hypothesis, plantaris muscles of old mice did not show a reduction in total and functional myofiber number, nor myofiber CSA.
Age-related decline in force generating capacity is explained by loss of muscle mass and reductions in specific force
Here, we observed a 13 % lower force generating capacity in 25- compared to male 9-month-old mice. Our aim was to determine to what extent reductions in muscle mass (quantity) and specific muscle force (quality) contribute to this age-related decline in muscle force generating capacity.
Other studies in male mice have reported that the ∼25 % age-related decline in muscle force in both slow and fast hindlimb muscles is explained for 55 % by the age-related loss of muscle mass, and for ∼45 % by a reduction in specific force in 26–28-month-old mice (Brooks and Faulkner 1988; 1991; Lynch et al. 2001; Zerba et al. 1990). We did not observe a decrease in m. plantaris mass which is different from what has been reported in other studies in which a 15–26 % lower muscle mass was reported in 26–28-month-old mice (Brooks and Faulkner 1988; Jackson et al. 2011; Lynch et al. 2001; McArdle et al. 2004). The discrepancy between the effects of aging on muscle mass and maximal force shown in our study and those previously reported is unlikely related to the muscles used, as other studies observed the effects of aging in both fast and slow muscles. It is more likely that the use of older animals (26–28 months) in other studies than in our study (25 months) contributes to the discrepancy, as larger effects of aging on maximal muscle force are likely to be observed in the older the animal (Ballak et al. 2014; Brooks and Faulkner 1988; Lynch et al. 2001; McArdle et al. 2004). Indeed, Chan and Head (2010) observed that even in 20–22-month-old mice, specific force was already reduced without significant muscle atrophy. Combination of the data of these studies with slightly older and younger mice than the ones used in the present study suggests that a reduction in muscle quality precedes a reduction in muscle mass. If so, it is likely that the contribution of a loss of muscle mass to the age-related muscle weakness increases with increasing age.
Effects of aging on atrophy and loss in myofiber number
An age-related reduction in muscle mass can be the result of both a loss of myofibers and myofiber atrophy (Lexell et al. 1988; Lushaj et al. 2008). Here, no myofiber atrophy nor myofiber loss was observed. Our observations are in line with other reports in which age-related decline in myofiber CSA could not be observed in m. extensor digitorum longus and m. soleus of 24- compared to 5-month-old mice (Sheard and Anderson 2012; Zerba et al. 1990). It is possible that the age-related myofiber atrophy and loss occur only at a later age as a small decrease in myofiber number of 10–15 % has been observed in m. extensor digitorum longus and m. soleus of 24–27-month-old C57Bl/6 mice (Sheard and Anderson 2012; Zerba et al. 1990). Also, human myofiber loss has not unequivocally been reported. Recently, in m. vastus lateralis of healthy 70-year-old subjects, a decrease in muscle mass was solely explained by a decrease in type II fiber CSA, and not by a loss of myofibers (Nilwik et al. 2013). Overall, it appears that in 25-month-old male C57BL/6J mice myofiber number size are well maintained. Therefore, if these factors are involved in aging-related reduction in maximal muscle force, the impact on the decline in maximal isometric muscle force will be minor and may only become significant at later stages of the aging process.
Effects of aging on fibrosis, denervation, and myofiber type changes
The specific force (force per anatomical cross-sectional area) of 25-month-old m. plantaris was 25 % lower compared to that of 9-month-old mice. The magnitude of the decrease in specific force was similar to that reported by others (Brooks and Faulkner 1988; Lynch et al. 2001; McArdle et al. 2004). Therefore, the age-related decrease in specific force appears to be an important factor contributing to an age-related decline in muscle force.
Theoretically, the loss of specific muscle force may be the result of an increase in connective tissue content, a larger proportion of denervated myofibers, a decrease in the proportion of fast type IIB myofibers, and/or a reduced force generating capacity of individual myofibers (reduced specific force). The fraction of intramuscular connective tissue within the m. plantaris was higher in muscles from old than from adult animals. Others have also observed an increase in connective tissue content with age (Brooks and Faulkner 1994; Lushaj et al. 2008; Overend et al. 1992). When we corrected the specific force for the connective tissue content, the difference between adult and old muscles was not significant anymore. Thus, part of the reduction in specific force in muscles from old mice is attributable to an increased content of connective tissue.
Another factor that could potentially reduce specific muscle force is an age-related increase in the number of denervated myofibers as a result of motor neuron death (Hashizume et al. 1988; Ishihara et al. 1987; Larsson and Ansved 1995). Using glycogen depletion, we were able to assess the proportion of non-recruited, presumable denervated fibers. However, no significant difference in the proportion of non-recruited myofibers was observed between the age groups, and therefore the lower specific force in the older animals is not attributable to loss of functional myofibers.
The proportion of myofibers expressing type IIB myosin decreased with age. The longer half relaxation time of the 400 Hz tetanus in aged than in adult muscle fits this observed reduction in the proportion of fibers expressing type IIB myosin. It is debatable, however, how much this actually contributes to the lower specific force. It is controversial whether the specific force of type IIB fibers is higher than that of other fiber types (Bottinelli et al. 1996; Widrick et al. 1996) or not (Degens and Larsson 2007; Larsson and Moss 1993). Even if specific force of type IIB fibers was 1.41 times as high of that of other fibers (Larsson and Moss 1993; Bottinelli et al. 1996 IIB vs. I) the decrease from 44 % IIB fibers in adult to 38 % in old muscle would only cause a 2 % reduction in specific force. This is even an overestimation as the plantaris muscle of the mouse barely contains type I fibers and the differences in specific force between fast fibers, if existent, are much smaller than those reported in (Bottinelli et al. 1996) between type I and type IIB fibers. It is thus unlikely that the fiber type shift contributes to the observed age-related decline in specific force.
Finally, the observed age-related decrease in specific muscle force may be due to a loss of specific myofiber force with age (D’Antona et al. 2003; Gonzalez et al. 2000; Larsson et al. 1997a, b; Lowe et al. 2001; Thompson and Brown 1999). This reduced skeletal muscle force is possibly related to a decreased number of cross-bridges (D’Antona et al. 2003), oxidative modifications (Degens and Larsson 2007; Gilliver et al. 2010; Lowe et al. 2001) and/or an impaired excitation-contraction uncoupling (Delbono 2002; Gonzalez et al. 2000).
Muscle aging in 25-month-old mice mimics the initial stages of sarcopenia in humans
The decreases in maximal muscle force observed in this and other mice studies are rather small, compared to the effects reported at a similar relative mean life expectancy in humans (see, for review, Ballak et al. 2014). Nevertheless, the 13 % decrease in muscle strength between 9- and 25 months of age in our C57BL/6J mice is comparable to that between the age of 25–30-year-old and 60–70-year-old humans (Ballak et al. 2014).
Implications
Future studies could investigate the time course of the relative contribution of decrements in specific force and muscle mass to the loss of muscle strength. To do so, both somewhat younger and also older C57BL/6J mice (e.g., 26–30 months) should be studied, while making sure that the impact of disease is minimized. Furthermore, nutritional intake may be changed (e.g., lower protein intake) to observe whether a poor diet causes not only a lower specific muscle force and a larger loss of muscle mass at a given age, but also an accelerated rate of sarcopenia.
To counteract sarcopenia, it is essential to know the mechanisms and causes of muscle aging, especially in the initial stages of sarcopenia. The present data suggest that interventions should, at least during the early stages of sarcopenia, aim primarily to stop or reverse the age-related decline in specific muscle force. This could be achieved by resistance training that has been shown to increase specific force (Erskine et al. 2011). The impact of training may decrease with increasing age (Degens 2012; Slivka et al. 2008) and it thus appears essential to start interventions before sarcopenia takes its toll.
Conclusion
In conclusion, the age-related reductions in maximal isometric muscle force in male C57BL/6J mice were mainly attributable to a reduced specific force. Part of the reduction in specific force was due to an age-related increase in connective tissue content, but not to an increase in the percentage of denervated IIB myofibers. It is conceivable that the age-related reduction in specific force is the result of loss of specific force of individual myofibers. We did not observe an age-related decline of myofiber number and myofiber CSA. Thus, our model seems to mimic force reductions similar to those reported in the early stages of sarcopenia in humans, and it is suggested that the decline in muscle quality precedes the decline in muscle quantity with age. Therefore, interventions to constrain early sarcopenia should aim particularly to improve specific muscle force.
References
Ballak SB, Degens H, de Haan A, Jaspers RT (2014) Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res Rev 14:43–55. doi:10.1016/j.arr.2014.01.005
Bottinelli R, Canepari M, Pellegrino MA, Reggiani C (1996) Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495(Pt 2):573–586
Brooks SV, Faulkner JA (1988) Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404:71–82
Brooks SV, Faulkner JA (1991) Maximum and sustained power of extensor digitorum longus muscles from young, adult, and old mice. J Gerontol 46:B28–B33
Brooks SV, Faulkner JA (1994) Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc 26:432–439
Brown M, Hasser EM (1996) Complexity of age-related change in skeletal muscle. J Gerontol A: Biol Med Sci 51:B117–B123
Carter EE, Thomas MM, Murynka T, Rowan SL, Wright KJ, Huba E, Hepple RT (2010) Slow twitch soleus muscle is not protected from sarcopenia in senescent rats. Exp Gerontol 45:662–670. doi:10.1016/j.exger.2010.04.001
Chan S, Head SI (2010) Age- and gender-related changes in contractile properties of non-atrophied EDL muscle. PLoS One 5:e12345. doi:10.1371/journal.pone.0012345
D’Antona G et al (2003) The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552:499–511. doi:10.1113/jphysiol.2003.046276
de Haan A, Jones DA, Sargeant AJ (1989) Changes in velocity of shortening, power output and relaxation rate during fatigue of rat medial gastrocnemius muscle. Arch Eur J Physiol 413:422–428
Degens H (2012) Determinants of skeletal muscle hypertrophy and the attenuated hypertrophic response at old age. Sports Med Doping Stud S1:1–8
Degens H, Alway SE (2003) Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve 27:339–347. doi:10.1002/mus.10314
Degens H, Larsson L (2007) Application of skinned single muscle fibres to determine myofilament function in ageing and disease. J Musculoskelet Nueronal Interact 7:56–61
Degens H, Hoofd L, Binkhorst RA (1995) Specific force of the rat plantaris muscle changes with age, but not with overload. Mech Ageing Dev 78:215–219
Degens H, Erskine RM, Morse CI (2009) Disproportionate changes in skeletal muscle strength and size with resistance training and ageing. J Musculoskelet Nueronal Interact 9:123–129
Delbono O (2002) Molecular mechanisms and therapeutics of the deficit in specific force in ageing skeletal muscle. Biogerontology 3:265–270
Erskine RM, Jones DA, Maffulli N, Williams AG, Stewart CE, Degens H (2011) What causes in vivo muscle specific tension to increase following resistance training? Exp Physiol 96:145–155. doi:10.1113/expphysiol.2010.053975
Faulkner JA, Larkin LM, Claflin DR, Brooks SV (2007) Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol 34:1091–1096. doi:10.1111/j.1440-1681.2007.04752.x
Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA (2008) Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol 105:637–642. doi:10.1152/japplphysiol.90332.2008
Gilliver SF, Jones DA, Rittweger J, Degens H (2010) Effects of oxidation on the power of chemically skinned rat soleus fibres. J Musculoskelet Nueronal Interact 10:267–273
Gonzalez E, Messi ML, Delbono O (2000) The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol 178:175–183
Hashizume K, Kanda K, Burke RE (1988) Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol 269:425–430. doi:10.1002/cne.902690309
Ishihara A, Naitoh H, Katsuta S (1987) Effects of ageing on the total number of muscle fibers and motoneurons of the tibialis anterior and soleus muscles in the rat. Brain Res 435:355–358
Jackson JR, Ryan MJ, Alway SE (2011) Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A: Biol Med Sci 66:751–764. doi:10.1093/gerona/glr047
Janssen I, Heymsfield SB, Wang ZM, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 years. J Appl Physiol 89:81–88
Kadhiresan VA, Hassett CA, Faulkner JA (1996) Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol 493(Pt 2):543–552
Kamel HK (2003) Sarcopenia and aging. Nutr Rev 61:157–167
Klein CS, Rice CL, Marsh GD (2001) Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol 91:1341–1349
Klitgaard H et al (1990) Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140:41–54
Larsson L (1978) Morphological and functional characteristics of the ageing skeletal muscle in man. A cross-sectional study. Acta Physiol Scand Suppl 457:1–36
Larsson L, Ansved T (1995) Effects of ageing on the motor unit. Prog Neurobiol 45:397–458
Larsson L, Moss RL (1993) Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 472:595–614
Larsson L, Li X, Frontera WR (1997a) Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol 272:C638–C649
Larsson L, Li X, Yu F, Degens H (1997b) Age-related changes in contractile properties and expression of myosin isoforms in single skeletal muscle cells. Muscle Nerve Suppl 5:S74–S78
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84:275–294
Lowe DA, Surek JT, Thomas DD, Thompson LV (2001) Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol 280:C540–C547
Lushaj EB, Johnson JK, McKenzie D, Aiken JM (2008) Sarcopenia accelerates at advanced ages in Fisher 344xBrown Norway rats. J Gerontol A: Biol Med Sci 63:921–927
Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA (2001) Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 535:591–600
Maden-Wilkinson TM, McPhee JS, Rittweger J, Jones DA, Degens H (2014) Thigh muscle volume in relation to age, sex and femur volume. Age (Dordr) 36:383–393. doi:10.1007/s11357-013-9571-6
McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ (2004) Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J: Off Publ Fed Am Soc Exp Biol 18:355–357. doi:10.1096/fj.03-0395fje
McKiernan SH, Bua E, McGorray J, Aiken J (2004) Early-onset calorie restriction conserves fiber number in aging rat skeletal muscle. FASEB J: Off Publ Fed Am Soc Exp Biol 18:580–581. doi:10.1096/fj.03-0667fje
Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV (2005) In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol 99:1050–1055. doi:10.1152/japplphysiol.01186.2004
Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ (2013) The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48:492–498. doi:10.1016/j.exger.2013.02.012
Overend TJ, Cunningham DA, Paterson DH, Lefcoe MS (1992) Thigh composition in young and elderly men determined by computed tomography. Clin Physiol 12:629–640
Rice CL, Cunningham DA, Paterson DH, Lefcoe MS (1989) Arm and leg composition determined by computed tomography in young and elderly men. Clin Physiol 9:207–220
Rice KM, Linderman JK, Kinnard RS, Blough ER (2005) The fischer 344/NNiaHSd X brown Norway/BiNia is a better model of sarcopenia than the fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology 6:335–343. doi:10.1007/s10522-005-4808-0
Roubenoff R, Hughes VA (2000) Sarcopenia: current concepts. J Gerontol A: Biol Med Sci 55:M716–M724
Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D (2004) Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging 24:335–340
Sato T, Akatsuka H, Kito K, Tokoro Y, Tauchi H, Kato K (1984) Age changes in size and number of muscle fibers in human minor pectoral muscle. Mech Ageing Dev 28:99–109
Sheard PW, Anderson RD (2012) Age-related loss of muscle fibres is highly variable amongst mouse skeletal muscles. Biogerontology 13:157–167. doi:10.1007/s10522-011-9365-0
Slivka D, Raue U, Hollon C, Minchev K, Trappe S (2008) Single muscle fiber adaptations to resistance training in old (>80 year) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295:R273–R280. doi:10.1152/ajpregu.00093.2008
Thompson LV, Brown M (1999) Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol 86:881–886
Tomonaga M (1977) Histochemical and ultrastructural changes in senile human skeletal muscle. J Am Geriatr Soc 25:125–131
Urbanchek MG, Picken EB, Kalliainen LK, Kuzon WM Jr (2001) Specific force deficit in skeletal muscles of old rats is partially explained by the existence of denervated muscle fibers. J Gerontol A: Biol Med Sci 56:B191–B197
van der Laarse WJ, van Noort P, Diegenbach PC (1992) Calibration of quantitative histochemical methods: estimation of glycogen content of muscle fibers using the PAS reaction. Biotech Histochem: Off Publ Biol Stain Comm 67:303–308
Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ (2007) Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292:E151–E157. doi:10.1152/ajpendo.00278.2006
Widrick JJ, Trappe SW, Blaser CA, Costill DL, Fitts RH (1996) Isometric force and maximal shortening velocity of single muscle fibers from elite master runners. Am J Physiol 271:C666–C675
Young A, Stokes M, Crowe M (1984) Size and strength of the quadriceps muscles of old and young women. Eur J Clin Investig 14:282–287
Young A, Stokes M, Crowe M (1985) The size and strength of the quadriceps muscles of old and young men. Clin Physiol 5:145–154
Yu F, Hedstrom M, Cristea A, Dalen N, Larsson L (2007) Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf) 190:229–241. doi:10.1111/j.1748-1716.2007.01699.x
Zerba E, Komorowski TE, Faulkner JA (1990) Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol 258:C429–C435
Acknowledgments
This research was funded by the European Commission through MOVE-AGE, an Erasmus Mundus Joint Doctorate program (2011-2015). The authors would like to thank Guus Baan for excellent technical assistance. Furthermore, we would like to acknowledge Frank van’t Hoff and Joshua Dunnink for their support.
Disclosure statement
The authors declare no conflicts of interest, financial or otherwise. The sponsor was in no way involved in the design or execution of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
• Lower specific muscle force rather than muscle atrophy explains reduced maximal isometric force in 25- compared to 9-month-old mice.
• Part of the reduction in specific muscle force was due to an age-related increase in connective tissue content.
• No age-related decline in myofiber number nor CSA was observed.
• If early effects of aging on human muscle are similar as those on mouse plantaris muscle, interventions to constrain early sarcopenia should aim at improving specific muscle force.
About this article
Cite this article
Ballak, S.B., Degens, H., Busé-Pot, T. et al. Plantaris muscle weakness in old mice: relative contributions of changes in specific force, muscle mass, myofiber cross-sectional area, and number. AGE 36, 9726 (2014). https://doi.org/10.1007/s11357-014-9726-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-014-9726-0