Abstract
Sarcopenia is the age-related loss of skeletal muscle mass and strength, attributable in part to muscle fibre loss. We are currently unable to prevent fibre loss because we do not know what causes it. To provide a platform from which to better understand the causes of muscle fibre death we have quantified fibre loss in several muscles of aged C57Bl/6J mice. Comparison of muscle fibre numbers on dystrophin-immunostained transverse tissue sections at 6 months of age with those at 24 months shows a significant fibre loss in extensor digitorum longus and soleus, but not in sternomastoid or cleidomastoid muscles. The muscles of the elderly mice were mostly lighter than their younger counterparts, but fibres in the elderly muscles were of about the same cross-sectional area. This study shows that the contribution of fibre death to sarcopenia is highly variable and that there is no consistent pattern of age-related fibre loss between skeletal muscles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As mammals age they progressively lose muscle mass and strength, a phenomenon known as sarcopenia (Doherty 2003; Edstrom et al. 2007; Evans 1995; Jones et al. 2009). The consequences of progressive weakness are many and varied and together are major drivers for dependence and mortality in our ageing society (Baumgartner et al. 1998). Ill health, frailty, and dependency together present an enormous financial burden on society, recently estimated at $12-26B per annum in the US alone (Janssen et al. 2004). The biological changes that underpin sarcopenia are complex and still poorly understood, but can be divided into two broad overlapping categories: changes that promote muscle atrophy; and changes that promote loss of muscle fibres (Alway and Siu 2008; Dirks and Leeuwenburgh 2002; Dupont-Versteegden 2005; Edstrom et al. 2007; Buford et al. 2010; Marzetti and Leeuwenburgh 2006; Lang et al. 2010). The drivers for atrophy and fibre loss are not necessarily separate, but the current study has examination of muscle fibre loss as its primary focus.
Examination of the nature and extent of muscle fibre loss is not trivial and has resisted elucidation for several reasons. First, cell death is typically studied by investigation of the appearance of markers of apoptosis, such as TUNEL. However, the syncytial nature of skeletal muscle fibres makes interpretation of appearance of nuclear apoptotic markers problematic since each fibre contains several hundred nuclei. Because nuclear number is known to vary up or down under a variety of conditions (Allen et al. 1995) it seems clear that the loss of a single, or small number of nuclei within a fibre does not necessarily signal the death of the whole fibre. Second, studies in which apoptotic markers have been employed on muscle transverse sections are complicated by difficulties in establishing whether apoptotic profiles represent myonuclei or those of another cell type present within muscle tissue. Gundersen and colleagues (Gundersen and Bruusgaard 2008) have recently addressed this issue and have argued that apoptotic profiles on sections of muscles undergoing induced atrophy are rarely myonuclei. Further, nuclei in denervated muscle with the ultrastructural hallmarks of imminent death rarely appear positive for apoptotic markers (Borisov and Carlson 2000), suggesting a non-apoptotic form of cell death (Kroemer and Martin 2005). Third, skeletal muscles vary widely in fibre type composition, architecture, and usage, and whether the extent of any fibre loss varies as a function of these parameters is not well established. Studies using human subjects have shown that the features of age-related decline in muscle performance vary between genders and between muscle groups (Lynch et al. 1999), so a more direct quantitative analysis of fibre loss in a variety of muscles would be welcome.
Several authors have investigated muscle fibre death by using methods that do not rely on assays for apoptosis. This has been done primarily by counting muscle fibre profiles on muscle transverse sections. This approach is not as technically straightforward as it may seem, since the plane of section and the method of identifying muscle fibres must both be carefully controlled and correlated with prior knowledge of muscle architecture. Not surprisingly, such counts have produced an inconsistent picture. The classical work of Lexell (Lexell et al. 1988) is often cited as evidence for age-related loss of fibres in humans. This study examined fibre number in sections of post-mortem male vastus lateralis, prompting the authors to conclude that progressive fibre loss was likely from about age 25, but the same data were more recently re-interpreted to support an alternate timeline in which fibres were lost from about age 50 (Faulkner et al. 2007). In lab animals, Rowe (1969) described fibre loss in male mouse Biceps brachii (but not in female), female (not male) Extensor digitorum longus (EDL), female (not male) soleus, and no fibre loss in tibialis anterior or sternomastoid of either gender. Hooper (1981) later described fibre loss in male mouse tibialis anterior and biceps brachii, but not sternomastoid. In the rat Eddinger et al. (1985) found no change in male EDL and a small decline in soleus fibre number, Alnaqeeb and Goldpsink (1987) described a big decrease in EDL but no change in soleus fibre number and Daw et al. (1988) described a decrease in both soleus and EDL fibre number in male rats. Thus, whether muscle fibres actually die, the extent and timing of any fibre loss, and the variability of fibre loss between muscles is clouded by inconsistency of outcome between muscles, species, genders, and investigators.
Of particular interest is the possible variability of fibre loss between different muscles. If fibre loss is significant in some muscles and absent in others of the same animal, then fibre loss is more likely to be driven primarily by factors that vary by muscle location, usage, architecture etc., rather than by systemic influences which might be expected to manifest uniformly throughout the body. Evidence of variation in timing or extent of fibre loss between muscles could therefore provide important clues to the primary drivers for age-related muscle fibre death, could generate new models in which these drivers could be tested or manipulated in vivo, and might ultimately lead to the development of new strategies for therapeutic intervention.
To contribute to these issues, here we have sought to quantify and describe muscle fibre loss in several muscles of different types, architectures, and locations in both male and female C57Bl/6J mice, with a view to its reliable quantification and to provide a platform for future studies in which we will examine candidate drivers for age-related fibre loss.
Methods
Animals
All experiments were done using C57Bl/6J mice from the University of Otago breeding colony. We chose this strain because it is widely used in lab studies investigating nerve and muscle ageing. According to lifespan data available from Jackson Labs and prior publications (Rowlatt et al. 1976), C57Bl/6J mice have an average lifespan of just over 2 years (http://research.jax.org/faculty/harrison/ger1vLifespan1.html), with 50% survival at 28 months and with 24 months being recommended as the upper age limit for reliable study. In terms of lifespan equivalence, 24 month mice are approximately equivalent to 70 year old humans. We used both male and female mice, as mature young (4–6 months) and elderly (22–26 months) adults.
Female mice were group-housed, and males were held in isolation to prevent fighting. Animals had access to a simple environmental enrichment device, they were fed standard mouse chow with free access to food and water, and were held on a 12:12 light:dark cycle. All mice used in this study were free of detectable pathological change when examined post mortem.
We included both male and female mice in this study, and treated the datasets separately because studies on both lab animal and human subjects have shown significant variation between males and females in the features of sarcopenia (Janssen et al. 2000; Lionikas et al. 2006; Rowe 1969).
All procedures were reviewed and had the prior approval of the University of Otago Animal Ethics Committee.
Tissue preparation
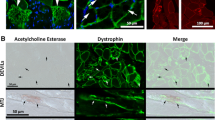
Animals were anaesthetised by intraperitoneal injection of a cocktail of Ketamine and Xylazine (87/13 mg/kg, respectively in sterile saline). When lack of corneal and foot-pinch withdrawal reflexes indicated that animals were fully insentient they were transcardially perfused with a bolus of warm heparinised saline followed by a solution of fresh 2% paraformaldehyde in 0.1 M phosphate buffer. Carcasses were skinned and eviscerated before being stored overnight at 4°C in 0.5% paraformaldehyde in 0.1 M phosphate buffer. Individual skeletal muscles were identified and carefully removed from both hindlimbs (soleus and extensor digitorum longus, EDL) and from both sides of the ventral midline in the neck region (sternomastoid and cleidomastoid). Muscles were gently cleaned of excess connective tissue, blotted dry, and weighed. After weighing they were cryoprotected by overnight immersion in 0.1 M phosphate buffer containing 20% sucrose. Muscles were oriented under a dissecting microscope and trimmed to the distal extreme of the motor nerve endplate region at one end. The endplate region had been revealed in each case in pilot experiments by whole-mount staining for Acetyl Cholinesterase (Karnovsky and Roots 1964). The muscles were then embedded with the trimmed surface upwards in a capsule of OCT compound and snap frozen by partial immersion of the capsule in melting isopentane cooled in a bath of liquid nitrogen. This procedure ensured that in all cases sectioning started at the beginning of the end-plate region and subsequently proceeded through that zone ensuring that all sections came from the middle third of the muscle where every fibre would be expected to have a profile. Specimens were stored at −75°C until needed.
Immunohistochemistry
Transverse frozen sections of skeletal muscles were cut at 10 μm in a Leica CM1850 cryostat. Sections were picked up on Vectabond™-coated glass microscope slides, air dried for 30 min, and washed in phosphate buffered saline (PBS). Sections were then processed using a standard citrate buffer or Tris antigen-retrieval protocol. Dystrophin was detected by overnight incubation at 4°C with anti-dystrophin polyclonal primary antibody (AB15277, Sapphire Bioscience Pty Ltd) diluted 1:500 in immunodiluent, myosin isoforms were identified by use of primary antibodies to fast (MY32, Sigma, 1:1,000) and slow [NOQ7.1.1A, 1:50: (Harris et al. 1989)] myosin isoforms. After thorough washing we detected the primary antibodies by 4 h room temperature incubation with 1:1,000 AlexaFluor™594- or AlexaFluor™488-conjugated anti-species secondary antibody (Invitrogen). Slides were washed and coverslipped in VectaShield™.
Imaging and image analysis
Specimens were examined with an Olympus BX-50 upright compound microscope equipped for epifluorescence, and photographed with a SPOT-RT Slider cooled CCD camera (Diagnostic Instruments, Sterling Heights, MI, USA). Photomontages were made in Adobe Photoshop CS, and an image processing routine was developed using FoveaPro (Reindeer Graphics, Asheville, NC, USA) in which the muscle fibers were defined by a ring of dystrophin immunostaining with each then being thresholded and automatically counted and measured. The software was calibrated for use with the microscope and camera system, so measurements for muscle fibre area were delivered in square microns.
Statistics
Significance of age-related variation in muscle wet weight, fibre number, and fibre cross-sectional area was determined by comparison of 6-month with 24-month data for each muscle and each gender. Two-tailed unpaired t-tests were done using Excel.
Results
Muscle mass
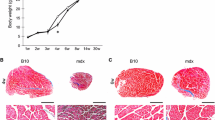
Comparison of mean weights at 6 and 24 months for each muscle in some cases showed an age-related decline in muscle mass, whilst in others there was no significant change (Table 1). EDL showed greatest loss of mass when comparing these two time points, losing 17% in the females and 28% in the males. The cleidomastoid showed no significant decline in either males or females. The elderly male soleus was significantly lighter (27%) than in young males, though in the females there was no significant age-related change. By contrast, the female sternomastoid showed a modest but significant loss of weight (7%), but the male muscle did not. This lack of consistency of weight loss between muscles and genders is similar to the results reported by previous authors. Rowe (1969) described age-related loss of mass in male mouse EDL and sternomastoid, but not in soleus, and not in the female muscles. Brooks and Faulkner (Brooks and Faulkner 1988) reported a decline in the mass of the male EDL when comparing young with old, but no decline in soleus when comparing the same age groups.
Several authors have shown that rodents grow and increase in body and muscle weight for much of their lives, before entering a period of decline over the last third of their normal lifespan (Brooks and Faulkner 1988; Shavlakadze et al. 2010; Hamrick et al. 2006; Reynolds et al. 2002; Rowlatt et al. 1976). Without data from intervening timepoints we cannot determine whether the muscles that weigh the same at 24 months as they did at 6 months have lost mass, since it is likely that they continued to increase in mass between about 6 and 18 months and would have undergone a decline thereafter. Indeed, previous authors have shown significant decline in muscle mass when comparing elderly with middle aged, but not when comparing elderly with young (Brooks and Faulkner 1988).
Dystrophin immunostaining
Specimens were well fixed and sectioned cleanly. Dystrophin-immunostaining revealed discrete rings that demarcated muscle fibre profiles enabling straightforward fibre counting and digital measurement of individual fibre cross sectional area (Fig. 1).
a Micrograph of a portion of a dystrophin-immunostained transverse section of 6-month male mouse EDL muscle. b The same panel after image processing, in which dystrophin rings were used to demarcate fibre boundaries and to facilitate fibre counting and cross sectional area measurements. Scale bar 50 μm
Muscle fibre counts
Photomontages of Dystrophin-immunostained sections were processed to generate thresholded binary images in which the dystrophin ring delineated the outer boundary of each fibre. The dystrophin-enclosed area was then filled to permit accurate cross-sectional area measurements (Fig. 1). The FoveaPro “measure all features” routine was then used to deliver fibre counts and measures of cross sectional area for each fibre profile in the image. At least two sections from each muscle were counted, the average of which was used as the value for that muscle. Between 5 and 13 individual muscles were counted for each muscle, gender, and age.
Female mice
Significant decrease in fibre number between 6 and 24 months was seen in the two hindlimb muscles, a 21% decline in EDL and 10% in soleus. There was no evidence of any significant fibre loss in either of the two neck muscles, sternomastoid or cleidomastoid (Table 2).
Male mice
The result for male mice was qualitatively the same as for females, a significant decline in fibre number was seen in both EDL (10%) and soleus (12%), but we found no significant fibre loss in either sternomastoid or cleidomastoid (Table 2).
Fibre atrophy
Loss of muscle mass could occur by loss of muscle fibres, by decrease in cross-sectional area of surviving fibres, by shift in fibre size distributions, or by loss of non-muscle tissue. Measurements of wet weight showed that muscle mass generally decreased between 6 and 24 months, and fibre counts showed that a loss of fibres was a feature of some muscles, but not others. Therefore, to determine whether surviving fibres underwent a change in cross-sectional area we took measures of area of each fibre profile for all fibres in four examples of each muscle and compared them between age groups (Table 3).
Female mice
For each of the four muscles included in the study we found a trend towards an age-related increase in the average cross sectional area of fibres within the muscle, but in no case did the difference between age groups reach statistical significance. To be certain this result was not masking any shift in fibre size distributions we made frequency distribution plots for each muscle (not shown). All muscles showed a unimodal distribution of fibre areas at both ages, and the nonsignificant increase in average fibre area was due to a slight rightward shift in the fibre size distributions.
Male mice
Fibre measurements on the muscles of male mice yielded a picture that was similar to that described above for female mice, with no significant age-related change in average fibre area for EDL, soleus, or sternomastoid. The exception was the cleidomastoid, which showed a significant 24% increase in fibre area (Table 3). In this case the age comparison (not shown) showed that the increase was due to a decrease in the proportion of very small fibres and an apparent hypertrophy amongst the mid-large fibres.
Myofibre type transformation
To further inform the issue of fibre atrophy and fibre loss we wanted to know whether changes in fibre number or size distribution correlated with any change in numbers or proportions of fast/slow fibre types within the muscles. We used antibodies that recognize slow type I myosin (NOQ7.1.1A) and fast type II isoforms (MY32) (Harris et al. 1989) on sections of EDL, soleus, and sternomastoid muscles.
Results of the analysis of fibre type counts and measurements appear in Table 4 and images of typical outcomes appear in Fig. 2. For the two predominantly fast muscles (EDL and sternomastoid) there was no overall change in proportion of fast myosin-containing fibres or in percentage of muscle cross-sectional area occupied by fast fibres, in either male or female mice. The proportion of slow fibres decreased with age in both muscles, and this was largely due to a decrease in the proportions of double-stained fibres (fibres that were positive to antibodies to both fast and slow myosin isoforms).
Micrographs of immunostained transverse sections of soleus muscles from a young (6 month, a, b) and an elderly (24 month, c, d) male mouse. a and c Stained for slow MHC and dystrophin. b and d Adjacent sections to those shown in a and c stained for fast MHC and dystrophin. Area enclosed by the white square in d is enlarged as an inset in c and d. White arrow heads in insets in c and d point to fibres that are immunopositive for both fast and slow MHC. Scale bar = 200 μm for main panels
The situation was different for the slower soleus muscle. In young mice of both genders soleus was about 70% fast. In elderly females this fell to 44% with a commensurate increase of the percentage of slow fibres from 34 to 57%. Similarly, the percentage of cross sectional area occupied by slow myosin-containing fibres increased from 36 to 57%. The transformation was not quite as profound amongst the males, with fast fibre percentage dropping from 72 to 65% and slow fibre proportion increasing from 30 to 38% and a slight increase in the proportion of double-stained fibres (Fig. 2c, d). In this case the percentage of cross sectional area occupied by slow fibres did not change.
Discussion
The main purpose of this study was to accurately document age-related decline in muscle fibre number in the mouse. We considered it important to reliably establish the nature and extent of fibre loss in both genders of a single widely-used strain of lab mice because previous studies using common lab rodents have delivered variable outcomes (Alnaqeeb and Goldspink 1987; Daw et al. 1988; Eddinger et al. 1985; Hooper 1981; Rowe 1969). Future studies aimed at discovery of the factors that drive fibre loss need a stable platform from which to investigate the cellular changes that contribute to fibre death.
One striking feature of the present work is that both leg muscles showed significant fibre loss, whilst neither of the neck muscles did. This variability in fibre loss between muscles is interesting because of what it says about sarcopenia, and because of the opportunity it presents to evaluate causal relationships between age and fibre death. Sarcopenia is widely considered to derive both from loss of muscle fibres and atrophy of surviving fibres. The present result shows that the contribution of fibre death is not equal amongst all muscles. This is an important observation for several reasons. First, it provides a basis for future investigation and comparison of candidate drivers of sarcopenia between muscles that lose fibres and those that do not, within the same animals. Second, it potentially explains variability in candidate sarcopenic drivers that differ between muscles. Third, it suggests that factors that promote loss of whole muscle fibres may vary as a function of factors such as muscle location, architecture, and usage pattern. Finally, it suggests that all muscles are not equally susceptible to candidate whole body or systemic drivers of fibre death, such as changes in hormone status.
Cell death is commonly studied by demonstration of markers of apoptosis, many studies have identified apoptotic nuclei in elderly muscle and apoptosis is widely implicated in sarcopenia (Degens 2007; Dirks and Leeuwenburgh 2002; Dupont-Versteegden 2005, 2006; Marzetti et al. 2010; Marzetti and Leeuwenburgh 2006). Interpretation of the relationship between nuclear apoptosis and loss of whole multinucleate muscle fibres is problematic. For instance, if whole fibres were dying by apoptosis one might expect to see aligned chains of apoptotic myonuclei in elderly muscles. We are aware of one study that makes reference to aligned apoptotic myonuclei during development (Trachtenberg 1998), but none describing such a finding in elderly muscle. Furthermore, studies that have investigated change in number of nuclei and nuclear volume with age have not described fibres with very low nuclear number (Allen et al. 1999; Brack et al. 2005) as would be expected if whole fibres were being lost by a mechanism involving gradual depletion of nuclei through apoptosis.
This analysis raises the prospect that whole cells are being lost by cell death mechanisms other than classical nuclear apoptosis, and several possibilities exist. First is inflammation, previous publications have investigated the presence and potential role of systemic inflammation and inflammatory cytokines in age-related muscle wasting (Degens 2010), and recent work has specifically examined the potential for impaired inflammatory response as a factor in reduced regenerative capability of elderly muscle (Shavlakadze et al. 2010). Second is non-apoptotic death, employing signalling pathways in which standard assays of apoptotic cell death may not reveal dying cells or nuclei where they are present (Kroemer and Martin 2005; Borisov and Carlson 2000). Mechanisms involving whole fibre death via lysosomal or autophagic pathways, and linkages between death and autophagocytosis are gaining support as increasingly interesting and viable candidates for investigation in muscle wasting scenarios (Eisenberg-Lerner et al. 2009; Wohlgemuth et al. 2010; Gaugler et al. 2011). Clearly, there is need to examine senescent muscles for evidence of activation of non-apoptotic cell death pathways that might mediate loss of whole fibres.
In contrast to the typical picture of sarcopenic muscle as one of reduced mass and cross-sectional area, we failed to detect significant fibre atrophy and the nonsignificant trend was generally towards a slight hypertrophy. Fibre cross-sectional area is likely to vary with muscle weight, and like muscle weight (as described above), area may have increased to a peak at about 15–18 months, and undergone a decline thereafter. So, in this case the insignificant change in muscle fibre area may reflect the comparison between young and old, rather than a complete lack of atrophy. Other parameters that may impact on mass and whole muscle cross sectional area such as fat and connective tissue are also likely to vary with age, usage, predominant fibre type, and muscle architecture, but were not measured in this study.
Many factors are considered to contribute to sarcopenia, these include but are not limited to: protein synthesis and proteolysis, metabolic changes, oxidative stress, satellite cell dysfunction, motor nerve terminal change/withdrawal, autocrine dysfunction, and paracrine dysfunction (Edstrom et al. 2007). The current observation of varied fibre loss between muscles suggests that whatever drives fibre loss, it does not manifest equally in all muscles prompting us to ask what potential drivers of sarcopenia might vary between muscles? One candidate variable that might account for differential fibre loss between muscles is fibre type. Shift in muscle fibre type distribution in old age is a classical hallmark of sarcopenic muscle (Lexell and Downham 1991), and different fibre types appear to be differentially susceptible to myonuclear loss in atrophy (Allen et al. 1999). Examination of fibre loss in some classical fast/slow muscle pairs might shed light on this issue, and to that end we included the stereotypical fast (EDL) and slow (soleus) muscles in this study. Previous studies have also examined fibre loss in these muscles, but the results have been equivocal. Rowe (1969) described fibre loss in both EDL and soleus of female but not male mice. In the rat Eddinger et al. (1985) found no change in male EDL and a small decline in soleus fibre number, whereas Alnaqeeb and Goldspink (1987) described a big decrease in rat EDL fibre number but no change in soleus. Using C57Bl/6J mice we have here described a clear and significant loss of fibres in both the fast EDL and the relatively slow soleus of mice of both genders. We also quantified muscle fibre number in two predominantly fast neck muscles but in neither did we detect significant age-related decline in fibre number. In the case of our C57Bl/6J mice, then, it would appear that fibre type is not a significant factor correlating with age-related muscle fibre death or survival.
Progressive transformation and abnormal clumping of fibre type is a widely described feature of muscle ageing, and is often attributed to repeated denervation/reinnervation cycles thought to occur in consequence of progressive loss of lower motoneurons (Luff 1998; Lexell and Downham 1991; Lexell 1995; Campbell et al. 1973). We did not attempt to quantify fibre type clumping or distributions, but we did observe age-related change in fibre type proportions in the muscles examined. We saw a trend to increased proportions of slow fibres only in the soleus muscle, and to a greater extent in female than in male mice. The two fast muscles we examined showed no trend towards increased proportions of slow fibres, in fact if any trend were evident it was a slight shift towards even greater fast fibre type dominance. If progressive loss of motoneurons is the motivator of these age-related changes in fibre type, then again it seems that motoneuron “type” does not predispose neurons to a greater or lesser likelihood of death. Indeed, previous authors have described increased clumping of both fast and slow fibre types (Lexell 1995; Lexell and Downham 1991) in aged human muscle.
As outlined above, the pattern of fibre loss did not correlate with fibre type which prompts us to consider an alternate explanation. Muscle location and primary usage pattern may be important factors (Hennig and Lomo 1985), potentially by influencing contraction-induced injury as a driver of repeated damage-repair cycles that might be met by variable regenerative capacity (Brooks and Faulkner 1990; Carlson and Faulkner 1989; Faulkner et al. 1995). Elderly muscles seem to be more susceptible to usage-related injury than young muscles, and recovery from damage seems to be more effective in young than old muscles, possibly due in part to the robustness of the motor input (Carlson and Faulkner 1996). If muscle location and usage pattern are factors that relate to muscle damage, and if damaged fibres are more likely to die in elderly animals, then usage patterns that result in higher levels of damage are likely to be accompanied by higher levels of fibre loss in elderly mice. In this regard, one might imagine that the leg muscles EDL and soleus experience higher loads and higher levels of the more damaging eccentric contractions than the neck muscles, sternomastoid and cleidomastoid. The proposal that usage-related damage may be a contributor to fibre loss is not new (Faulkner et al. 1995) and potentially fits with the idea (above) that fibre loss might be due to localised autophagic processes, but would need careful validation in view of the observation that exercise is the most effective current countermeasure for age-related loss of muscle mass (Marcell 2003). Whether the muscle-mass protecting benefits of exercise manifest by prevention of atrophy, cell death, or both remains to be reliably established.
The potential role of the motor nerve terminal in sarcopenia has received recent attention (Valdez et al. 2010; Jang and Van Remmen 2011; Deschenes et al. 2010), and motor nerve withdrawal and sprouting have been considered for many years to be primary drivers of progressive age-related muscle fibre type clumping (above). Evidence that denervated motor endplates are commonly visible in elderly muscle suggests that motor nerve terminal withdrawal occurs before fibre death, thereby implicating motoneuron death-induced fibre denervation as a potential cause of fibre death, rather than an effect of it. Our observation of variable fibre loss amongst the muscles of this study might therefore correlate with variable age-related motoneuron death amongst the various motor pools. Whether age-related loss of lower motoneurons varies as a function of motor pool location, muscle type, usage pattern etc. has not, to our knowledge, been investigated.
In summary, this study shows that skeletal muscle fibres are lost from some elderly mouse skeletal muscles, but not others. The variable fibre loss is interesting because it suggests that the factors that cause fibre loss must vary between different muscles and locations. It should therefore be possible to now begin to compare the features of muscles that lose fibres with those that do not with a view to identification of specific factors that are causally implicated in skeletal muscle fibre death during old age.
References
Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR (1995) Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol 78(5):1969–1976
Allen DL, Roy RR, Edgerton VR (1999) Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22(10):1350–1360
Alnaqeeb MA, Goldspink G (1987) Changes in fibre type, number and diameter in developing and ageing skeletal muscle. J Anat 153:31–45
Alway SE, Siu PM (2008) Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev 36(2):51–57
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763
Borisov AB, Carlson BM (2000) Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec 258(3):305–318
Brack AS, Bildsoe H, Hughes SM (2005) Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci 118(Pt 20):4813–4821
Brooks SV, Faulkner JA (1988) Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404:71–82
Brooks SV, Faulkner JA (1990) Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol 258(3 Pt 1):C436–C442
Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM (2010) Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Age Res Rev 9(4):369–383
Campbell MJ, McComas AJ, Petito F (1973) Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36(2):174–182
Carlson BM, Faulkner JA (1989) Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol 256(6 Pt 1):C1262–C1266
Carlson BM, Faulkner JA (1996) The regeneration of noninnervated muscle grafts and marcaine-treated muscles in young and old rats. J Gerontol A Biol Sci Med Sci 51(1):B43–B49
Daw CK, Starnes JW, White TP (1988) Muscle atrophy and hypoplasia with aging: impact of training and food restriction. J Appl Physiol 64(6):2428–2432
Degens H (2007) Age-related skeletal muscle dysfunction: causes and mechanisms. J Musculoskelet Neuronal Interact 7(3):246–252
Degens H (2010) The role of systemic inflammation in age-related muscle weakness and wasting. Scand J Med Sci Sports 20(1):28–38
Deschenes MR, Roby MA, Eason MK, Harris MB (2010) Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol 45(5):389–393
Dirks A, Leeuwenburgh C (2002) Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol 282(2):R519–R527
Doherty TJ (2003) Invited review: aging and sarcopenia. J Appl Physiol 95(4):1717–1727
Dupont-Versteegden EE (2005) Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40(6):473–481
Dupont-Versteegden EE (2006) Apoptosis in skeletal muscle and its relevance to atrophy. World J Gastroenterol 12(46):7463–7466
Eddinger TJ, Moss RL, Cassens RG (1985) Fiber number and type composition in extensor digitorum longus, soleus, and diaphragm muscles with aging in Fisher 344 rats. J Histochem Cytochem 33(10):1033–1041
Edstrom E, Altun M, Bergman E, Johnson H, Kullberg S, Ramirez-Leon V, Ulfhake B (2007) Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol Behav 92(1–2):129–135
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16(7):966–975
Evans WJ (1995) What is sarcopenia? J Gerontol A Biol Sci Med Sci 50 Spec No:5–8
Faulkner JA, Brooks SV, Zerba E (1995) Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci 50 Spec No:124–129
Faulkner JA, Larkin LM, Claflin DR, Brooks SV (2007) Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharm Physiol 34(11):1091–1096
Gaugler M, Brown A, Merrell E, DiSanto-Rose M, Rathmacher JA, Reynolds TH IV (2011) PKB signaling and atrogene expression in skeletal muscle of aged mice. J Appl Physiol 111(1):192–199
Gundersen K, Bruusgaard JC (2008) Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? J Physiol 586(Pt 11):2675–2681
Hamrick MW, Ding KH, Pennington C, Chao YJ, Wu YD, Howard B, Immel D, Borlongan C, McNeil PL, Bollag WB, Curl WW, Yu J, Isales CM (2006) Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone 39(4):845–853
Harris AJ, Fitzsimons RB, McEwan JC (1989) Neural control of the sequence of expression of myosin heavy chain isoforms in foetal mammalian muscles. Development 107:751–769
Hennig R, Lomo T (1985) Firing patterns of motor units in normal rats. Nature 314:164–166
Hooper AC (1981) Length, diameter and number of ageing skeletal muscle fibres. Gerontology 27(3):121–126
Jang YC, Van Remmen H (2011) Age-associated alterations of the neuromuscular junction. Exp Gerontol 46(2–3):193–198
Janssen I, Heymsfield SB, Wang ZM, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 89(1):81–88
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52(1):80–85
Jones TE, Stephenson KW, King JG, Knight KR, Marshall TL, Scott WB (2009) Sarcopenia—mechanisms and treatments. J Geriatr Phys Ther 32(2):39–45
Karnovsky MJ, Roots L (1964) A “direct coloring” thiocholine method for cholinesterases. J Histochem Cytochem 12:219–221
Kroemer G, Martin SJ (2005) Caspase-independent cell death. Nat Med 11(7):725–730
Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB (2010) Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporosis Int 21(4):543–559
Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No:11–16
Lexell J, Downham DY (1991) The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81(4):377–381
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84(2–3):275–294
Lionikas A, Blizard DA, Vandenbergh DJ, Stout JT, Vogler GP, McClearn GE, Larsson L (2006) Genetic determinants of weight of fast- and slow-twitch skeletal muscles in old mice. Mamm Genome 17(6):615–628
Luff AR (1998) Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann NY Acad Sci 854:92–101
Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF (1999) Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol 86(1):188–194
Marcell TJ (2003) Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci 58(10):M911–M916
Marzetti E, Leeuwenburgh C (2006) Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41(12):1234–1238
Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C (2010) Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophysica Acta 1800(3):235–244
Reynolds TH IV, Krajewski KM, Larkin LM, Reid P, Halter JB, Supiano MA, Dengel DR (2002) Effect of age on skeletal muscle proteolysis in extensor digitorum longus muscles of B6C3F1 mice. J Gerontol A Biol Sci Med Sci 57(5):B198–B201
Rowe RW (1969) The effect of senility on skeletal muscles in the mouse. Exp Gerontol 4(2):119–126
Rowlatt C, Chesterman FC, Sheriff MU (1976) Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim 10(10):419–442
Shavlakadze T, McGeachie J, Grounds MD (2010) Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology 11(3):363–376
Trachtenberg JT (1998) Fiber apoptosis in developing rat muscles is regulated by activity, neuregulin. Dev Biol 196(2):193–203
Valdez G, Tapia JC, Kang H, Clemenson GD Jr, Gage FH, Lichtman JW, Sanes JR (2010) Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci 107(33):14863–14868
Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C (2010) Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol 45(2):138–148
Acknowledgments
We thank Melanie Hutchison and Christopher Simon for their help with the immunohistochemistry and the muscle fibre counting. This work was supported by grants from the Dean of the Otago School of Medical Sciences and the Department of Physiology at the University of Otago. RA was the grateful recipient of a University of Otago Summer Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheard, P.W., Anderson, R.D. Age-related loss of muscle fibres is highly variable amongst mouse skeletal muscles. Biogerontology 13, 157–167 (2012). https://doi.org/10.1007/s10522-011-9365-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-011-9365-0