Abstract
Rice is the main food crops with the higher capacity for cadmium (Cd) uptake, necessitating the urgent need for remediation measures to address Cd in paddy soil. Reasonable agronomic methods are convenient and favorable for fixing the issue. In this study, a pot experiment was employed to evaluate the effects of two foliar (NaH2PO4, SDP; KH2PO4, PDP) and two solid phosphate fertilizers (double-superphosphate, DSP; calcium-magnesium phosphate, CMP) on uptake and remobilization of Cd in rice plants under the low-P and rich-Cd soil. The results revealed that these four phosphorus fertilizer significantly down-regulated the relative expression of OsNRAMP5 involved in Cd absorption, while up-regulated OsPCS1 expression and increased distribution of Cd into the cell wall in roots. Furthermore, phosphorus fertilizer resulted in a significant decrease in the relative expression of OsLCT1 in stems and OsLCD in leaves, decreased the transfer factor of Cd from shoots to grains, and ulterior reduced the Cd accumulation in three protein components of globulin, albumin, and glutelin, making the average Cd concentration of brown rice decreased by 82.96%. These results comprehensively indicate that in situations with similar soil backgrounds, the recommended application of solid CMP and foliar PDP can alleviate the toxicity of Cd by reducing its absorption and remobilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The overuse of pesticides and fertilizers, coupled with the indiscriminate discharge of industrial and mining sewage, has resulted in the increasingly grave issue of heavy metal pollution in farmland (Peera et al. 2023). Cadmium (Cd), one of these inorganic pollutants, has been a cause for serious concern due to its highly water-soluble and fat-soluble nature, which can lead to its accumulation in vegetation and potentially impact the quality and safety of agricultural produce, subsequently posing a risk to public health (Chen et al. 2020). Rice is a staple cereal crop with the highest Cd uptake capacity. Previous studies have shown that the average Cd concentration in rice grains is significantly markedly greater than that in wheat and other cereals (Zeng et al. 2021; Zhao et al. 2021). Elevated soil Cd concentrations may affect the amino acid metabolism of rice grains, leading to increased prolamin levels and decreased albumin and globulin concentrations (Zeng et al. 2021). The outer layer of brown rice is the main storage site of albumin and globulin. Therefore, the protein components in milled rice are mainly composed of nutrient-rich glutelin and taste-reducing prolamin (Teixeira et al. 2021). Moreover, high soil Cd concentration leads to a decrease in the breakdown value of rice-starch spectrum. However, it significantly increases the amylose content as well as its final viscosity and setback value (Huang et al. 2021). Cooking and eating quality of rice depend largely on the amount of amylose. Better flavor quality rice generally has relatively larger breakdown value and smaller setback value (Amagliani et al. 2016). Thus, stressing soil with Cd will have an adverse effect on the nutritious and gastronomic properties of paddy grains.
Based on the physiological and metabolic characteristics of Cd, experiments were conducted to determine how Cd levels can be reduced in rice plants through improved breeding, soil remediation, and agronomic regulation. The most cost-effective, efficient, and successful method among these is agronomic regulation. This process leverages cation competition and antagonistic effects to both control Cd uptake and minimize Cd accumulation in edible parts of the plant (Xiao et al. 2021). Some researchers have shown that foliar spraying of phosphorus (P), zinc (Zn), and silicon (Si) nutrition effectively dropped the amount of Cd in brown rice while ensuring food safety (Farooq et al. 2022; Barman and Kundu 2023).
Phosphorus promotes carbohydrate and plays a crucial role in the material and energy metabolism of plants (Dissanayaka et al. 2018). The utilization of phosphate fertilizers enhances the soil’s physical and chemical properties, amplifies heavy metal dispersal in root cell walls, competes with heavy metal transporters, and consequentially restrains heavy metal absorption by roots (Jiang et al. 2014; Zhao et al. 2020). Furthermore, phosphorus can facilitate the creation of immovable phosphate compounds and protein connective substances within cells, impeding the passage of heavy metals (Fu et al. 2011; Siebers et al. 2013). Phosphate fertilizers commonly used in paddy production include water-soluble fertilizers of potassium dihydrogen phosphate (PDP), sodium dihydrogen phosphate (SDP), and solid fertilizers of double superphosphate (DSP) and calcium magnesium phosphate (CMP) (Gong et al. 2022). However, the effects of various phosphate fertilizers on Cd accumulation in rice plants and the changes in grain chemical composition remain uncertain under Cd stress. To address this, we examined Cd concentration, subcellular distribution, antioxidant enzyme activity, gene expression, and their response to phosphate fertilizer in different rice sections under Cd stress. Furthermore, a study was conducted to investigate the impact of phosphorus on the accumulation of starches, proteins, and other elements in rice grains. The ultimate goal was to formulate an agronomic strategy that would enhance the nutritional and edible quality of rice while simultaneously combating heavy metal stress.
Materials and methods
Plant material and experiment design
A pot experiment using Nanjing 9108 japonica rice as the material was conducted at the Crop Production Experimental Center of Huaiyin Institute of Technology, Jiangsu Province, China. Three weeks after sowing the rice seeds, seedlings with uniform growth were transplanted into experimental pots, with five seedlings per pot. Before this experiment, we also conducted a previous pot experiment to observe the effects of water management on wheat growth under Cd stress with the conventional fertilization conditions (before wheat sowing, the soil was added with CdCl2·2.5H2O solution as Cd source; the soil was obtained from the 0–20 cm surface layer in the rice cultivation region of Huaiyin District, Huai’an City, Jiangsu Province, China, with a latitude of 33.64°N and a longitude of 118.90°E). Therefore, in order to analyze the mechanism of Cd impact on rice-wheat rotation system, the soil used in this experiment was the soil retained after wheat harvest. Prior to loading the soil into the test pots, it was centrally stacked, dried, and screened to remove wheat roots and small stones. In each experimental pot, 15 kg of sieved, dry soil, along with 0.8 g of urea and 1.2 g of K2O basal fertilizers (with no phosphate fertilizer), was applied. The soil’s physical and chemical properties utilized in this research are displayed in Table 1. As per the Soil Environmental Quality Risk Control Standard (GB 15618–2018, 5.5 < pH ≤ 6.5, 0.4 mg kg−1), it suggests the potential for soil contamination with Cd.
The experiment comprised five treatments with three replicates: CK, no phosphate fertilizer was applied, only foliar deionized water spray; SDP, 100 mL pot−1 of NaH2PO4 (50 mmol L−1) was sprayed at the booting stage and early filling stage (September 2, 2021) of rice, respectively; PDP, 100 mL pot−1 of KH2PO4 (50 mmol L−1) was sprayed in the same manner as SDP; DSP, double superphosphate (0.1 g kg−1, P2O5/soil, w/w) was evenly added into the each pot soil before rice transplanting; CMP, calcium magnesium phosphate (0.1 g kg−1, P2O5/soil, w/w) was evenly added as the same way of DSP. Meanwhile, an appropriate amount of 0.02% (v/v) Tween 20 (surfactant) was added to the SDP and PDP solutions to enhance the absorption and utilization of phosphate fertilizer by the rice plants. Additionally, an equal volume of a mixture of Tween 20 and deionized water was sprayed on the CK, DSP, and CMP treatments.
At the mature stage of rice growth (November 3, 2021), dry plant samples with similar characteristics were chosen for analysis. The roots, stems, leaf sheaths, leaves, glumes, and brown rice of the plant samples were separated, and the brown rice (100 g) was ground into two components: milled rice and aleurone layer with a stainless steel grinder (model JLGJ3, Zhejiang, China). The samples underwent a thorough washing with deionized water followed by heating in an oven at 105 °C for an hour. After that, the samples were dried at 70 °C until reaching a constant weight. Subsequently, the samples were crushed and screened to analyze their chemical elements and grain storage components. Additionally, this experiment collected fresh samples from the same parts of paddy plants during the early flowering stage (September 7). After separation, the fresh sample is immediately frozen and stored in liquid nitrogen tank for further investigation of the expression levels of Cd-related genes, Cd subcellular components, superoxide dismutase (SOD) activity, as well as the contents of malondialdehyde (MDA), phytochelatins (PCs), and glutathione (GSH).
Heavy metal and essential element concentrations in plant or soil samples
Paddy plant samples were digested with 10 mL of a mixed acid solution composed of HNO3 and HClO4 in a 3:1 ratio, while soil samples were digested with a solution of HNO3–HClO4–HF–HCl. The available Cd content in soil was extracted using DTPA. According to our early research work, inductively coupled plasma mass spectrometry (ICP-MS) was used to determine the concentrations of Cd, Zn, and other elements in plants and soil samples (Wang et al. 2023). Total As of rice grains was measured using the hydrogen-generation atomic-fluorescence spectrometer (WHG-690A, Beijing Hanshi Instruments Co., China) as described by Shi et al. (2015).
The translocation factor (Tf) of Cd between various organs in rice plants was calculated according to Huang et al. (2021).
Symbols such as Cd soil and Cd roots indicate the concentration of Cd in paddy field soil and different parts of rice plants (mg kg−1, DW).
Calculation of Cd related intake and risk factors
Daily intake of heavy metals (DIM, mg kg−1 day−1) = (C × IR)/BW (US EPA 2004).
Health risk index (HRI) = DIM/RFD (Cui et al. 2005).
C is the concentration of Cd in milled rice; IR is the ingestion rate of rice (mg daily−1, calculated as 300 g per day for adults) (Majumdar et al. 2020); BW is the body weight (kg, calculated based on an average weight of 60 kg); RFD is the oral reference dose (calculated as 1.00E − 03 of Cd) (WHO/FAO 2013). RFD represents the degree of harm to human health from consuming food contaminated with Cd. If the HRI < 1, people can safely consume it (Cui et al. 2005).
Starch and protein component contents in paddy grains and the Cd concentrations in four protein components
The total starch content of rice grains was measured by polarimetry. The amylose content was determined by iodine colorimetry of Zhu et al. (2021). The difference between the total starch content and amylose content corresponds to the amylopectin content. The each protein component content in rice grains was analyzed with reference to the extraction method of Dos Reis et al. (2020). Refer to the study method of Wei et al. (2017) to separate the protein components. The sifted paddy flour was soaked in n-hexane and dried to obtain defatted rice flour. Defatted rice flour (15 g) was extracted with ultrapure water, sodium chloride (NaCl, 5%), and sodium hydroxide solution (NaOH, 0.1 mol L−1), respectively. After centrifugation (10,000 r/min, 15 min), the supernatant was added with acetone (4 ℃) and freeze drying to collected three protein components (albumin, globulin, and glutelin). Another equivalent amount of defatted rice flour was soaked and extracted using 70% ethanol. The supernatant was then concentrated in a nitrogen evaporator to collect the prolamin, following the same centrifugation conditions as used in the previous protein components. The concentration of Cd in the four protein components was determined with the same method used for the plant samples above.
Subcellular fractions of Cd, content of PCs, GSH, MDA, and SOD activity determination in shoots and roots of rice plants
According to our previous research method (Wang et al. 2023), fresh rice shoots (including leaves, stems-sheaths) and roots samples were extracted by adding precooling extraction buffer solution of Tris–HCl (50 mM, pH 7.5), sucrose (250 mM), and DL-dithioerythritol (1 mM). Subsequently, the samples underwent two rounds of centrifugal separation, leading to the isolation of F1 (cell wall), F2 (cell organelle), and F3 (soluble) fractions. Fresh rice shoots and roots (0.2 g) were accurately weighed, pulverized using liquid nitrogen, and extracted by adding 2 mL of a liquid mixture consisting of the extraction buffer (0.1% trifluoroacetic acid (TFA) and 6.3 mmol L−1 DTPA). The contents of GSH and NPT (total non-protein thiol) in supernatant after centrifugation (12,000 × g, 4 ℃, 10 min) were determined by the kit (JianCheng, Nanjing) according to the instructions. The PC content was calculated based on the difference between NPT and GSH values (Huang et al. 2021). The SOD activity and MDA content were determined by spectrophotometry according to the kit of Nanjing JianCheng Institute of Biological Engineering (Nanjing, China). Fresh shoot and root samples were pulverized in liquid nitrogen, and normalized with 10 mL of 0.1 M phosphate buffer (pH 7.8). The centrifuged supernatant was utilized to determine SOD and MDA (Zhen et al. 2021).
Gene expression analysis
Total RNA was isolated from the stems, top three leaves, and roots of paddy plants at the early flowering period (September 7, 2021) using the FastPure Universal Plant Total RNA Lsolation Kit. The cDNA was synthesized with the Oligo dT Primer or dNTP mixture. The primer sequences used are listed in Table S1, and β-actin was used as the internal control. The qRT-PCR was performed using a FQD-48A (A4) machine (parameters: 30 s at 95 ℃, followed by 40 cycles of 5 s at 95 ℃, 20 s at 60 ℃, and 15 s at 72 ℃). All the reactions were carried out in triplicate (technical replicates) for each biological replicate (three for each treatment). The 2−△△CT method was calculated to quantify gene expression (Chakrabarti et al. 2002).
Statistical analysis
The experimental data were statistically analyzed using Excel 2010 (Microsoft, Redmond, WA) and SPSS 17.0 (Statistical Product and Service Solutions, IBM). A one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (P < 0.05) was carried out to test the significance of treatments. Additionally, multivariate analysis was conducted with principal component analysis (PCA) with the Graphpad Prism software. And spearman’s correlation analysis was used to uncover the relationships among the different indicators.
Results
Plant height, dry weight, yield parameters of rice, and soil bioavailable Cd concentrations under different treatments
The use of four different types of phosphate fertilizers substantially increased the height of rice plants. Specifically, PDP proved to be the most effective (72.53 cm, Fig. 1b). Furthermore, the dry weight of the shoots and roots significantly increased under the treatment of both PDP and CMP. Notably, the application of these four phosphate fertilizers led to a significant increase in dry matter accumulation and overall plant growth, ultimately resulting in a higher yield of grain. The maximum yield was achieved under PDP and CMP treatments, specifically 21.79 g per plant and 20.64 g per plant, respectively (Fig. 1a–d). In addition, after rice harvest, compared with CK, soil available Cd concentrations under each phosphate fertilizer treatment showed a decreasing trend, and CMP treatment reached a significant level (Table S2).
Effects of phosphate fertilizer application on plant growth (a), plant height (b), plant dry weight (c), and yield (d) of rice plants. CK, no phosphate fertilizer was applied, only foliar deionized water spray; SDP, sprayed NaH2PO4 (50 mmol L−1); PDP, sprayed KH2PO4 (50 mmol L−1); DSP, added double superphosphate (0.1 g kg−1, P2O5/soil w/w) into soil; CMP, added calcium magnesium phosphate (0.1 g kg−1, P2O5/soil w/w) into soil. Data are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate significant differences among different treatments (Duncan’s multiple range test, P < 0.05)
Cd concentration in in different parts of rice plant
The Cd concentration in rice plants is the highest in roots, followed by leaf sheaths, stems, and leaves, and lowest in four grain parts (Fig. 2). Overall, different phosphate fertilizer treatments efficiently lower the Cd concentration in vegetative organs, and the largest reduction was observed in CMP treatment. It is worth noting that the aleurone layer of grains exhibited the highest Cd concentration, while the milled rice had the lowest. All phosphate fertilizer treatments, whether applied to the soil or foliage, significantly reduced the concentration of Cd in both brown and milled rice, and the lowest reduction was noted under CMP treatment. Additionally, SDP and CMP treatments had a substantial effect on reducing the concentration of Cd in the rice aleurone layer, with the lowest values being 0.638 mg kg−1 and 0.671 mg kg−1, respectively (Fig. 2). Furthermore, the four phosphate fertilizer treatments were observed to significantly decrease daily metal intake (DIM) and health risk index (HRI) when compared to CK. Each of the HRI indicators has decreased below 1, with CMP exhibiting the lowest HRI of 0.214, indicating an 80.77% decrease in comparison to CK (Table S4).
The Cd concentration in different organs of rice plants at maturity under four phosphate fertilizer application. Data are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate significant differences among different treatments (Duncan’s multiple range test, P < 0.05)
Translocation factor of Cd between different organs
The translocation factor (Tf) values of Cd between various plant organs are presented Table S3. Among them, the values of Tf soil—roots was the highest, followed by the Tf roots-stems,-sheaths,-leaves,-glumes, and the lowest was the Tf stems, sheaths, leaves, glumes-grains. Phosphate fertilizer treatments significantly affected Tf soil-roots, with CMP treatment causing a decline of 60.38%. However, PDP, DSP, and CMP significantly increased the values of Tf roots-stems, -sheaths, -leaves. Furthermore, all treatments markedly reduced the Cd remobilization and translocation in the four values of Tf aboveground-grains and Tf roots-glumes (Table S3).
Subcellular distribution of Cd, PCs, and GSH content, MDA, and SOD accumulation in shoots and roots
Figure 3 shows the subcellular distribution of Cd in rice shoots and roots. The order of subcellular distribution of Cd was highest in the cell wall, followed by soluble fraction, and lowest in cell organelles (Fig. 3a). As compared with the CK, the four phosphate fertilizer treatments significantly increased the distribution of Cd in the cell wall of rice shoots and roots, while decreased the accumulation of Cd in soluble fraction. For instance, the treatment with CMP led to a maximum Cd proportion in the cell wall of roots, recorded as 74.89%, while minimizing its soluble fraction to 15.48%. Furthermore, rice shoot cell organelles experienced a significant increase in Cd proportion under PDP and CMP treatments, reaching 18.51% and 17.80%, respectively. However, the distribution of Cd in root cell organelles demonstrates a significant downward trend under the various treatments, particularly in the case of CMP treatment, where it dropped to 9.63% (Fig. 3a).
The subcellular distribution of Cd (a), PCs and GSH content (b), and MDA and SOD accumulation (c) in shoots and roots at early filling stage under four phosphate fertilizer application. Cell wall (F1), organelle (F2), and soluble (F3) fractions. Data are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate significant differences among different treatments (Duncan’s multiple range test, P < 0.05)
In current study, the content of GSH was higher than that of PCs in shoots, but markedly lower than that of PCs in roots. The accumulation of both PCs and GSH in paddy shoots reached its peak following the PDP treatment. Moreover, the four phosphate fertilizers considerably increased the synthesis of PCs and GSH in roots, with CMP displaying a stronger influence. Furthermore, CMP markedly increased PCs content by 32.96% and GSH by 120.47% in roots. Furthermore, there was a higher increase in accumulation of these two substances in rice shoots when treated by two foliar fertilizers as compared to two solid fertilizers. However, the trend was opposite in the roots (Fig. 3b). The content of MDA in shoots and roots decreased significantly with each treatment. Conversely, SOD activity exhibited a reduction in shoots and a considerable increase in roots, and was notably higher in roots than in shoots (Fig. 3c).
Gene expression quantification
The relative expressions of OsLCT1, OsPCS1, OsLCD, and OsNRAMP5 involved in Cd metabolism were analyzed to comprehend the molecular and physiological mechanisms of P in alleviating Cd stress in paddy plants (Fig. 4). Compared with the control, the four phosphate fertilizers significantly down-regulated the OsLCT1 expression level in stems and OsLCD expression in leaves, but markedly up-regulated the OsPCS1 expression level in the three organs (Fig. 4a–d, f). Furthermore, under Cd stress, the presence of phosphate fertilizer resulted in a significant reduction of the expression of OsNRAMP5 in the roots of rice. Of these, the greatest effects were observed with DSP and CMP, which resulted in a decrease of 45.41% and 34.86%, respectively (Fig. 4e).
The gene relative expression of OsLCT1 (a), OsPCS1 (b, d, f), OsLCD (c), and OsNRAMP5 (e) of paddy plants at early flowering period (September 7, 2021) under four phosphate fertilizer applications. Data are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate significant differences among different treatments (Duncan’s multiple range test, P < 0.05)
Starch and protein component content in paddy grains and the Cd concentrations in four protein components
Starch and protein are two important storage components in rice grains. Different treatments did not have a significant impact on the total starch and amylose levels in the grains. However, the use of phosphate treatments significantly heightened the amylopectin levels in contrast to CK (Fig. 5a). The grain albumin content increased significantly under SDP treatment, but decreased markedly under CMP treatment. The four phosphate fertilizer treatments seemed to have little influence on the globulin and prolamin content. Furthermore, these treatments significantly increased glutelin content, leading to a significant raise in the content of total protein, and reaching a higher level under DSP and CMP treatments (Fig. 5b). Furthermore, the concentrations of Cd in the four rice protein components are ranked from highest to lowest as globulin, albumin, glutelin, and gliadin. This suggests that Cd potentially binds with the globulin and albumin components to a greater extent. Notably, the use of phosphate fertilizers significantly reduced the amount of Cd in all four protein fractions, particularly in globulin, albumin, and glutelin (Fig. 5c).
The starch (a) and protein components (b) contents and the Cd concentrations in four protein components (c) of grains under four phosphate fertilizer applications. Data are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate significant differences among different treatments (Duncan’s multiple range test, P < 0.05)
Concentration of heavy metals and essential elements in grains
Compared with CK, four phosphate fertilizers enhanced the Cr accumulation in grains, but none of them reached a significant level (Fig. 6). And these treatments decreased the concentration of As in grain, in which PDP and CMP reached a significant level. Moreover, the Pb concentration in rice grain was not apparently affected by these treatments (Fig. 6a). In terms of microelements, different treatments mainly had significant effects on Zn, Fe, and Mn. Compared with the other three phosphate fertilizer treatments, PDP significantly increased the grain Zn concentration, but there no remarkable difference between PDP and CK. Both Fe and Mn exhibited the same behavior, demonstrating a significant upward trend under various phosphate fertilization procedures, with the increase in solid phosphate fertilizer being greater than that of foliar spraying (Fig. 6b). When it comes to the macro-elements, Ca was significantly improved mainly by the two solid phosphate fertilizer treatments (DSP and CMP). Under the PDP treatment, the grain K concentration was significantly increased to the highest, reaching 2738 mg kg−1. And these four phosphate fertilizer treatments markedly promoted the increase of grain Mg concentration, and the CMP treatment had the greatest effect (Fig. 6c).
The concentration of heavy metals (a), micro (b), and macro essential (c) elements in rice grains under four phosphate fertilizer applications. Data are expressed as the average ± SD (n = 3). Different lowercase letters above each bar indicate significant differences among different treatments (Duncan’s multiple range test, P < 0.05)
Correlation matrix and principal component analysis (PCA)
The principal component analysis (PCA) results showed that the first two principal components explained 91% of the data variation. According to the projection of cos2 on variables, PC1 is explained by the shoot OsPCS1 expression, shoot PCs and GSH content, and subcellular distribution of Cd in shoots cell wall (F1) and organelles (F2). PC2 is mainly explained by indicators such as prolamin-Cd content, leaves OsLCD expression, and roots SOD activity. The GSH and PC content, root OsPCS1 expression, SOD activity, and the shoot OsPCS1 expression have a promoting effect on the formation of PC3 (Fig. S1).
Correlation analysis shows that the concentration of Cd in brown rice and milled rice is mainly positively correlated with the subcellular distribution of Cd in soluble components, MDA content, SOD activity, albumin-Cd, prolamin-Cd, and the expression of OsLCD in leaves. There was a significant negative correlation with the subcellular distribution of Cd in the cell wall components of root, GSH content, SOD activity in the stem, and the expression of OSPCS1 in the roots and leaves (Fig. S2).
Discussion
Effects of phosphate fertilizer application on plant height, dry weight, and yield parameters of rice
The application of chemical fertilizers is crucial for enhancing and stabilizing rice yield. NPK, which comprises three essential nutritive elements, is particularly significant in the growth and development of rice (Phares and Akaba 2022). This study showed that four kinds of phosphate fertilizer treatments all promoted the increase of rice plant height and dry matter under Cd stress, thus improving rice yield (Fig. 1). Plant performance and yield are better under PDP and CMP compared to SDP and DSP, indicating that K, Ca, and Mg elements in these fertilizers may provide superior nutrition for rice plants under Cd stress. In addition, in current study, the available phosphorus content (Olsen-P) in text soil is 13.13 mg kg−1, which belongs to the middle low level. Under this soil condition, the application of phosphate fertilizer can help to raise the yield of rice and other crops (Gong et al. 2022). Therefore, when managing varied Cd contaminated soils, adding suitable macronutrients or micronutrients according to soil characteristics can produce dual benefits of decreasing Cd stress and enhancing yield.
Effects of phosphate fertilizer application on remobilization and concentration of Cd in rice vegetative organs
Root uptake and redistribution of vegetative organs post-anthesis is essential for the accumulation of Cd in grain (Huang et al. 2017). Firstly, in current study, Cd is mainly stored in the cell wall fraction in shoots and roots (Fig. 3a), which has been consistent with numerous existing research findings (Zhong et al. 2022). In contrast to CK, the other treatments promoted the proportion of Cd in cell wall of shoots and roots, and decreased the distribution of Cd in soluble fraction, indicating that these applications promoted Cd binding to the cell wall under Cd stress (Fig. 3a). Polysaccharides and proteins play a role in Cd fixation, either by uptake or precipitation through their active groups, thus decreasing Cd transportation to the grain (Wei et al. 2023). In addition, OsLCD and OsLCT1 were specifically expressed in vascular tissues of rice leaves and stem nodes, and were involved in Cd accumulation in rice (Shimo et al. 2011; Tian et al. 2019). The results of this study showed that the application of phosphate fertilizer significantly down-regulated the expressions of these two genes in leaves and stems, leading to a reduction of Cd redistribution to paddy grains (Fig. 4).
Secondly, synthesizing sulfhydryl compounds is of vital importance for plants to cope with heavy metal stress. PCs are a type of metal sulfhydryl protein with a unique structure that is synthesized based on GSH. PCs aid in the sequestration of heavy metals in cellular vacuoles, thereby reducing toxicity (Huang et al. 2021). In these physiological processes, OsPCs (including OsPCS1 and OsPCS2) have essential regulatory roles (Das et al. 2017; Huang et al. 2021). Our experiment showed that application of phosphate fertilizer up-regulated the OsPCS1 genes in rice plants and promoted the PCs and GSH synthesis and accumulation in shoots and roots (Fig. 3b), which is similar to the research results of Pál et al. (2017). Furthermore, this research showed that GSH content was higher than PCs in shoots and lower in roots (Fig. 3b), suggesting that these two substances may exhibit tissue specificity in heavy metal detoxification (Wang et al. 2021b).
Finally, the subcellular distribution and physiological characteristics of Cd in vegetative organs of rice plants affect the accumulation of Cd in these organs. In this study, it was found that the concentration of Cd in roots was significantly higher than that in shoots at maturity, which was in agreement with the results of previous studies (Huang et al. 2021). In addition, the phosphate fertilizer treatments significantly decreased the concentration, Tf soil-roots, and Tf aboveground-grains of Cd in different vegetative organs, among which CMP treatment decreased the most (Fig. 2; Table S3). It is widely recognized that Cd competes with Ca, Zn, and other transporters in rice, resulting in an antagonistic effect with these cations (Zhen et al. 2021). In this study, relative gene expression analysis showed that the gene OsNRAMP5 involved in Cd uptake in roots was significantly down-regulated under each treatment, especially in CMP treatment. These findings suggest that the use of phosphate fertilizer primarily decreases Cd absorption in rice roots, thereby affecting transportation and remobilization to grains.
Effects of phosphate fertilizer application on the concentration of Cd and other elements in rice grains
This study showed that the Cd concentration in aleurone layer, brown rice, and milled rice decreased significantly to a safe level under various phosphate fertilizer treatments, which may be the common result of the above absorption and remobilization. Phosphorus fertilizer treatment resulted in a significant reduction of the daily intake of metal (DIM) and health risk index (HRI) in milled rice. The application of these treatments resulted in a reduction in HRI indicators below 1, effectively mitigating the toxic hazards to human health caused by Cd (Table S4).
For other heavy metal elements, this study found that except PDP, the other three phosphate fertilizers increased the Cr concentration in grain, but the difference was not significant and far below the safe value (1 mg kg−1). In addition, each phosphate fertilizer treatment reduced the concentration of As, but had no significant effect on Pb (Fig. 6a). Note that P and As belong to group VA in the Periodic Table. Foliar phosphate fertilizer can hinder arsenic uptake in rice through competition among transporters, leading to diminished root-stem transport and stem-grain remobilization, ultimately reducing arsenic levels in rice grains (Jiang et al. 2014). Xie et al. (2018) showed that the application of Pb, Cd, Cr, and Cu significantly affected the accumulation of Cd, Cr, and Cu in grains, indicating that there was a synergistic or antagonistic mechanism among heavy metal elements. Therefore, it is necessary to deeply study the absorption, transportation, and remobilization of heavy metals in rice plants.
For macronutrient elements, in this study, two kinds of solid phosphate fertilizers (DSP and CMP) significantly increased grain Ca concentration. And the PDP and CPM treatments markedly enhanced the concentration of K and Mg in grains (Fig. 6c). Prior research has indicated that CMP fertilizers promoted the absorption of Ca and Mg in root systems, resulting in an increase of their contents in paddy soil. The summarized results indicate that applying phosphate fertilizer might enhance the absorption of other vital elements due to their varied abundance (Wang et al. 2021a). In addition, for micronutrient elements, different phosphate fertilizer treatments had no effect on Cu, but significantly increased the concentrations of Fe and Mn in grains. Compared to the CK control group, the PDP treatment resulted in an increase in grain Zn concentration, while the other three phosphate treatments did not significantly contribute to grain Zn accumulation (Fig. 6b). At present, reports on the effects of phosphate fertilizer on microelements concentrations in crops are not consistent, particularly with regard to Zn in grains. Most studies have shown that exogenous phosphorus supply reduced Zn uptake by roots and inhibited Zn transport into paddy grains (Zhang et al. 2017; Su et al. 2018). However, some researchers found that phosphorus had a slight synergistic effect on Zn concentration in grains (Naeem et al. 2018). The above differences may be caused by changes in the texture or physical and chemical properties of text soil (Hagh et al. 2016; Zhang et al. 2021). In addition, phosphorus exists mainly in the form of phytic acid in rice grains, and phytic acid can chelate with cations such as Zn, affecting human nutrition absorption (Yamaji et al. 2017). Thence, the effect of P on Zn was shown in soil availability, root absorption, and grain chelation. Consequently, further research is necessary to understand the P–Zn interaction mechanism in rice, taking into account the determination of different soil phosphorus levels.
Effects of phosphate fertilizer application on the starch and protein component contents and the Cd concentrations in four protein components
Starch makes up around 90% of rice’s dry weight and is a vital biochemical component for determining rice quality (Amagliani et al. 2016). Consumers tend to prefer medium- to low-amylose rice, which is softer and bonds more easily due to its lower amylose content. Amylopectin found in rice can increase its stickiness and sweetness, thus improving its palatability (Amagliani et al. 2016; Zhang et al. 2018). Previous research has indicated a close correlation between mineral elements and starch quality, particularly in the context of managing nitrogen, phosphorus, and potassium fertilizers (Zhang et al. 2018). The results of this study showed that four kinds of phosphate fertilizer treatments decreased the amylose content of grains, but significantly enhanced the amylopectin content (Fig. 5a). Like starch, the storage protein in rice endosperm is additionally a vital indicator to evaluate the nutrition and taste quality of rice (Baxter et al. 2004). Research shows that most Cd binds to protein in grain with sulfamino acids playing a key role in this process (Wei et al. 2017). Recent studies revealed a significant increase in glutelin content under various treatments, resulting in a higher level of overall protein (Fig. 5b). Glutelin is rich in amino acids, which have a vital catalytic role in improving the nutritional quality of rice (Teixeira et al. 2021). Coordinating the accumulation of amylopectin and glutelin in paddy grains, the application of P fertilizer could potentially mitigate the decline in rice quality under Cd stress.
Moreover, in this research, the Cd concentrations in protein components of globulin, albumin, and glutelin are higher than in prolamin (Fig. 5c). However, consistent with the aleurone layer, albumin and globulin were largely stored in the outer layer of unpolished rice and were mostly grinded off in the milling process (Dos Reis et al. 2020). Accordingly, this indicates that Cd is likely to be closely associated with these two protein fractions in the aleurone layer (Wei et al. 2017). The study demonstrated that the use of phosphate fertilizer significantly reduced Cd concentration in four protein fractions, particularly in globulin, albumin, and glutelin. Similarly to grains, Cd primarily binds with proteins and thiol compounds in the phloem of crop plant leaves and stems’ vegetative organs (Gu et al. 2023). Hence, further research is required to investigate the physiological process of Cd binding and remobilization characteristics in vegetative organs’ impact on grain Cd accumulation.
Effect mechanism of foliar and soil phosphate fertilizer on Cd accumulation in rice plants
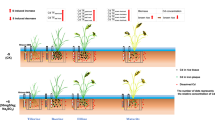
In this study, four types of foliar and solid phosphate fertilizers were used to study the effect of phosphate fertilizer on Cd accumulation in rice plants under the background of low-P and rich-Cd soil. The effects of these two phosphate fertilizers on the Cd accumulation model in rice may exhibit dissimilar mechanisms. Foliar phosphate fertilizer significantly augmented the accumulation of PCs and GSH in rice shoots, in addition to substantially enhancing the expression of OsPCS1 in shoots when compared to solid phosphate fertilizer (Figs. 3 and 4). These results suggest that foliar phosphate fertilizer may alleviate the effect of Cd stress by inhibiting root uptake and reducing retransfer form shoots to grains. For the solid phosphate fertilizer treatment, there was a substantial increase in root PC and GSH contents, along with a significant reduction in OsNRAMP5 expression in roots (Figs. 3 and 4). It is suggested that solid phosphate fertilizer may prevent the transport of Cd to aboveground mainly by inhibiting absorption and increasing root fixation (Figs. 7, S1, and S2). In addition, the amount of phosphate fertilizer used in this study is converted according to the conventional amount of local farmers (P2O5, 150 kg hm−2). Therefore, solid phosphate fertilizer is applied to the soil as a base fertilizer at one time. In addition, the yield of PDP and CMP was better among the four kinds of phosphate fertilizers. Hence, in cases where the soil background is similar, CMP can be used preferentially as a base fertilizer, while PDP can be used as a quick and convenient remedy.
Conclusions
This study clarified the response to different kinds of phosphate fertilizer (foliar fertilizers of SDP and PDP, solid application of DSP and CMP) on the root absorption, vegetative organ redistribution, and grain storage of Cd in paddy plants under low-P and rich-Cd soil, and revealed the effect of P on the chemical components of starch and protein in rice grains. First of all, phosphate fertilizer treatment decreased the absorption of Cd by down-regulating the expression level of OsNRAMP5, and promoted the synthesis of chelate PC-Cd and the regionalization of Cd to vacuole and cell wall in roots. Second, phosphate fertilizer treatments increased the distribution Cd in the cell wall of shoots, thus reducing the remobilization of Cd from source organs to grains. Finally, the application of the four phosphate fertilizer resulted in a significant reduction of Cd accumulation in the globulin, albumin, and glutelin protein components, thus contributing greatly to the decrease of Cd concentration in the grain to a safe level. The study demonstrates that foliar or soil application of phosphorus effectively regulates the metabolic processes of Cd absorption, remobilization, and grain accumulation in rice, which plays a vital role in mitigating heavy metal stress and enhancing nutritional quality (Figs. 7, S1, and S2). Furthermore, under the background of low-P and rich-Cd soil, CMP can be used preferentially as a base fertilizer, while PDP can be used as a quick and convenient remedy. Thence, appropriate micronutrients or micronutrients can be added according to different soil backgrounds to achieve the dual effects of alleviating Cd stress and increasing yield.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Amagliani L, O’Regan J, Kelly AL, O’Mahony JA (2016) Chemistry, structure, functionality and applications of rice starch. Cereal Sci 70:291–300
Barman F, Kundu R (2023) Foliar application of selenium affecting pollen viability, grain chalkiness, and transporter genes in cadmium accumulating rice cultivar: a pot study. Chemosphere 313:137538
Baxter G, Blanchard C, Zhao J (2004) Effects of prolamin on the textural and pasting properties of rice flour and starch. Cereal Sci 40(3):205–211
Chakrabarti SK, James JC, Mirmira RG (2002) Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. Biol Chem 277:13286–13293
Chen J, Huang XY, Salt DE, Zhao FJ (2020) Mutation in OsCADT1 enhances cadmium tolerance and enriches selenium in rice grain. New Phytol 226(3):838–850
Cui Y, Zhu YG, Zhai R, Huang Y, Qin Y, Liang J (2005) Exposure to metal mixtures and human health impacts in a contaminated area in Nanning, China. Environ Int 31:784–790
Das N, Bhattacharya S, Bhattacharyya S, Maiti MK (2017) Identification of alternatively spliced transcripts of rice phytochelatin synthase 2 gene OsPCS2 involved in mitigation of cadmium and arsenic stresses. Plant Mol Biol 94(1):167–183
Dissanayaka DMSB, Plaxton WC, Lambers H, Siebers M, Marambe B, Wasaki J (2018) Molecular mechanisms underpinning phosphorus-use efficiency in rice. Plant Cell Environ 41(7):1483–1496
Dos Reis AR, Boleta EHM, Alves CZ, Cotrim MF, Barbosa JZ, Silva VM, Porto RL, Lanza MGDB, Lavres J, Gomes MHF, Carvalho HWP (2020) Selenium toxicity in upland field-grown rice: seed physiology responses and nutrient distribution using the μ-XRF technique. Ecotoxicol Environ Saf 190:110147
EPA A (2004) Risk assessment guidance for superfund. In: Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment), vol 5. EPA/540/R/99
Farooq MU, Ishaaq I, Barutcular C, Skalicky M, Maqbool R, Rastogi A, Hussain S, Allakhverdiev SI, Zhu JQ (2022) Mitigation effects of selenium on accumulation of cadmium and morpho-physiological properties in rice varieties. Plant Physiol Bioch 170:1–13
Fu X, Dou C, Chen Y, Chen X, Shi J, Yu M, Xu J (2011) Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. Hazard Mater 186:103–107
Gong H, Meng F, Wang G, Hartmann TE, Feng G, Wu J, Jiao X, Zhang F (2022) Toward the sustainable use of mineral phosphorus fertilizers for crop production in China: from primary resource demand to final agricultural use. Sci Total Environ 804:150183
Gu T, Qi Z, Chen S, Yan J, Fang Z, Wang J, Ji G (2023) Dual-function DEFENSIN 8 mediates phloem cadmium unloading and accumulation in rice grains. Plant Physiol 191:515–527
Hagh ED, Mirshekari B, Ardakani MR, Farahvash F, Rejali F (2016) Optimizing phosphorus use in sustainable maize cropping via mycorrhizal inoculation. Plant Nutr 39:1348–1356
Huang GX, Ding CF, Guo FY, Li XG, Zhou ZG, Zhang TL, Wang XX (2017) The role of node restriction on cadmium accumulation in the brown rice of 12 Chinese rice (Oryza sativa L.) cultivars. Agr Food Chem 65(47):10157–10164
Huang H, Li M, Rizwan M, Dai Z, Yuan Y, Hossain MM, Cao M, Xiong S, Tu S (2021) Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. Hazard Mater 401:123393
Jiang W, Hou Q, Yang Z, Zhong C, Zheng G, Yang Z, Li J (2014) Evaluation of potential effects of soil available phosphorus on soil arsenic availability and paddy rice inorganic arsenic content. Environ Pollut 188:159–165
Majumdar S, Sachdev S, Kundu R (2020) Salicylic acid mediated reduction in grain cadmium accumulation and amelioration of toxicity in Oryza sativa L. cv Bandana. Ecotox Environ Safe 205:111167
Naeem A, Aslam M, Lodhi A (2018) Improved potassium nutrition retrieves phosphorus-induced decrease in zinc uptake and grain zinc concentration of wheat. Sci Food Agric 98:4351–4356
Pál M, Csávás G, Szalai G, Tímea O, Khalil R, Yordanova R, Gella G, Birinyia Z, Németha E, Janda T (2017) Polyamines may influence phytochelatin synthesis during Cd stress in rice. Hazard Mater 340:272–280
Peera Sheikh Kulsum PG, Khanam R, Das S, Nayak AK, Tack FMG, Meers E, Vithanage M, Shahid M, Kumar A, Chakraborty S, Bhattacharya T, Biswas JK (2023) A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ Res 220:115098
Phares CA, Akaba S (2022) Co-application of compost or inorganic NPK fertilizer with biochar influenced soil quality, grain yield and net income of rice. Integ Agr 21(12):3600–3610
Shi GL, Zhu S, Bai SN, Xia Y, Lou LQ, Cai QS (2015) The transportation and accumulation of arsenic, cadmium, and phosphorus in 12 wheat cultivars and their relationships with each other. Hazard Mater 299:94–102
Shimo H, Ishimaru Y, An G, Yamakawa T, Nakanishi H, Nishizawa NK (2011) Low cadmium (LCD), a novel gene related to cadmium tolerance and accumulation in rice. Exp Bot 62(15):5727–5734
Siebers N, Siangliw M, Tongcumpou C (2013) Cadmium uptake and subcellular distribution in rice plants as affected by phosphorus: soil and hydroponic experiments. Soil Sci Plant Nut 13(4):833–844
Su D, Zhou L, Zhao Q, Pan G, Cheng F (2018) Different phosphorus supplies altered the accumulations and quantitative distributions of phytic acid, zinc, and iron in rice (Oryza sativa L.) grains. Agric Food Chem 66(7):1601–1611
Teixeira LS, Pimenta TM, Brito FA, Malheiros RS, Arruda RS, Araújo WL, Ribeiro DM (2021) Selenium uptake and grain nutritional quality are affected by nitrogen fertilization in rice (Oryza sativa L.). Plant Cell Rep 40(5):871–880
Tian S, Liang S, Qiao K, Wang F, Zhang Y, Chai T (2019) Co-expression of multiple heavy metal transporters changes the translocation, accumulation, and potential oxidative stress of Cd and Zn in rice (Oryza sativa). Hazard Mater 380:120853
Wang C, Huang Y, Zhang C, Zhang Y, Yuan K, Xue W, Liu Y, Liu Y, Liu Z (2021a) Inhibition effects of long-term calcium-magnesia phosphate fertilizer application on Cd uptake in rice: regulation of the iron-nitrogen coupling cycle driven by the soil microbial community. Hazard Mater 416:125916
Wang K, Linghu J, Kong L, Huang S, Wang Q, Li H, Wan Y (2021b) Comparative responses of cadmium accumulation and subcellular distribution in wheat and rice supplied with selenite or selenate. Environ Sci Pollut R 28:45075–45086
Wang Z, Li Y, Liu M, Yang Y, Wang R, Chen S, Liu Z, Yan F, Chen X, Bi J, Dong Z, Wang F (2023) Alleviating effects of zinc and 24-epibrassionlide on cadmium accumulation in rice plants under nitrogen application. Chemosphere 313:137650
Wei S, Guo B, Feng L, Jiang T, Li M, Wei Y (2017) Cadmium distribution and characteristics of dadmium-binding proteins in rice (Oryza sativa L.) kernel. Food Sci Technol Res 23(5):661–668
Wei H, Li Y, Yan J, Peng S, Wei S, Yin Y, Li K, Cheng X (2023) Root cell wall remodeling: a way for exopolysaccharides to mitigate cadmium toxicity in rice seedling. Hazard Mater 443:130186
WHO/FAO (2013) Guidelines for the safe use of wastewater and food stuff: volume 2: No1 14.Wastewater Use in Agriculture, World Health Organization, Geneva, p 988
Xiao ZX, Peng M, Mei YC, Tan L, Liang YC (2021) Effect of organosilicone and mineral silicon fertilizers on chemical forms of cadmium and lead in soil and their accumulation in rice. Environ Pollut 283:117107
Xie L, Hao P, Cheng Y, Ahmed IM, Cao F (2018) Effect of combined application of lead, cadmium, chromium and copper on grain, leaf and stem heavy metal contents at different growth stages in rice. Ecotox Environ Safe 162:71–76
Yamaji N, Takemoto Y, Miyaji T, Mitani-Ueno N, Yoshida KT, Ma JF (2017) Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 541(7635):92–95
Zeng T, Fang B, Huang F, Dai L, Tang Z, Tian JL, Cao GD, Meng XL, Liu YC, Lei B, Lu MH, Cai ZW (2021) Mass spectrometry-based metabolomics investigation on two different indica rice grains (Oryza sativa L.) under cadmium stress. Food Chem 343:128472
Zhang W, Liu D, Liu Y, Chen X, Zou C (2017) Overuse of phosphorus fertilizer reduces the grain and flour protein contents and zinc bioavailability of winter wheat (Triticum aestivum L.). Agric Food Chem 65:1473–1482
Zhang H, Yu C, Hou D, Liu H, Zhang H, Tao R, Cai H, Gu J, Liu L, Zhang Z, Wang Z, Yang J (2018) Changes in mineral elements and starch quality of grains during the improvement of japonica rice cultivars. Sci Food Agr 98(1):122–133
Zhang W, Zhang W, Wang X, Liu D, Zou C, Chen X (2021) Quantitative evaluation of the grain zinc in cereal crops caused by phosphorus fertilization A meta-analysis. Agron Sustain Dev 41(1):1–12
Zhao Y, Zhang C, Wang C, Huang Y, Liu Z (2020) Increasing phosphate inhibits cadmium uptake in plants and promotes synthesis of amino acids in grains of rice. Environ Pollut 257:113496
Zhao FJ, Tang Z, Song JJ, Huang XY, Wang P (2021) Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol Plant 15(1):27–44
Zhen S, Shuai H, Xu C, Lv GH, Zhu XD, Zhang Q, Zhu QH, Núñez-Delgado A, Conde-Cid M, Zhou YY, Huang DY (2021) Foliar application of Zn reduces Cd accumulation in grains of late rice by regulating the antioxidant system, enhancing Cd chelation onto cell wall of leaves, and inhibiting Cd translocation in rice. Sci Total Environ 770:145302
Zhong S, Li X, Li F, Huang Y, Liu T, Yin H, Qiao J, Chen G, Huang F (2022) Cadmium uptake and transport processes in rice revealed by stable isotope fractionation and Cd-related gene expression. Sci Total Environ 806:150633
Zhu D, Fang C, Qian Z, Guo B, Huo Z (2021) Differences in starch structure, physicochemical properties and texture characteristics in superior and inferior grains of rice varieties with different amylose contents. Food Hydrocolloid 110:106170
Funding
This work was funded by the Basic Science (Natural Science) Research Project of Higher Education Institutions of Jiangsu Province (23KJB210001), the Talent Introduction Research Project of Huaiyin Institute of Technology (Z301B19520), the College Student Practice Innovation Program of Jiangsu Province of China (202311049440YJ), and the Joint Project of Industry-University-Research (Jie Bang Gua Shuai) of Jiangsu Province of China (BY2021601).
Author information
Authors and Affiliations
Contributions
Zunxin Wang: writing—review and editing, supervision, project administration, and funding acquisition. Yang Li: conceptualization, formal analysis, investigation, data curation, writing—original draft, and visualization. Mingsong Liu: investigation and formal analysis. Huicong Wang, Chunhui Li, and Ying Zhang: investigation. Chuanlan Fu, Yuxiu Ye, and Xinhong Chen: conceptualization, formal analysis, and investigation. Zhiyao Dong: supervision and project administration. Feibing Wang: supervision, project administration, conceptualization, formal analysis, and investigation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have given their consent to publish this research article.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Liu, M., Wang, H. et al. Effects of different phosphorus fertilizers on cadmium absorption and accumulation in rice under low-phosphorus and rich-cadmium soil. Environ Sci Pollut Res 31, 11898–11911 (2024). https://doi.org/10.1007/s11356-024-31986-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-31986-y