Abstract
Cadmium (Cd) contamination of crop plants has aroused a worldwide concern because of the threats posed to human health through accumulation in the food chains. Selenium (Se) can alleviate the Cd-induced phytotoxicity, but the relevant underlying mechanisms are not fully understood. Therefore, with wheat (Triticum aestivum L.) and rice (Oryza sativa L.) chosen as the target plants in this study, the effects of selenite or selenate on Cd accumulation and subcellular distribution were investigated through greenhouse hydroponic experiments; and simultaneously, the effects of pre-Se treatment with selenite or selenate on Cd accumulation and root-to-shoot translocation in the studied plants were also included. Results showed the addition of Se slightly changed the Cd content in plant roots in a time-dependent manner; however, with the obvious decreasing trend on the Cd transfer factor (TF), its content in plant shoots was significantly reduced by selenite or selenate in a plant species-dependent manner. At 48 h of exposure, the supplementation of selenite and selenate significantly decreased the Cd content by 40.4% and 38.0% in wheat shoots, and by 72.2% and 40.9% in rice shoots, respectively. Additionally, the order of Cd proportion distributed to the different subcellular fractions of plant tissues was as follows: cell wall > soluble cytosol > organelle, irrespective of the Se treatments or the plant species. However, selenate increased the Cd percentage in soluble cytosol of wheat shoots, while selenite increased that percentage in the cell wall of rice shoots; and the Cd proportion in soluble cytosol of the studied plant roots was significantly enhanced owing to selenite or selenate addition. Moreover, similar to the co-application, the pre-Se treatment with inorganic Se also reduced the Cd accumulation and translocation both in wheat and rice. Our results proved that the inorganic Se could decline the Cd accumulation and translocation in the crop plants, although selenite was found more effective than selenate regarding such effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a highly toxic metal that can accumulate in the agricultural fields due to the various anthropogenic activities, such as metal mining, industrial effluent discharge, atmospheric deposition, and wastewater irrigation (Rizwan et al. 2017; Shi et al. 2018); hence, soil Cd pollution has become a widespread environmental concern over large areas (Järup and Åkesson 2009; Du et al. 2013; Zhao et al. 2015). According to the national soil pollution survey report of the People’s Republic of China, Cd ranks the first among the metals and metalloids in the percentage of sampling sites (7%) that exceed the soil quality standards (MEP and MLR 2014). When Cd is present in the agricultural soils, it can readily accumulate in the edible parts of crops and then transfer to human beings through the food chains, posing potential health risks (Huang et al. 2008; Li et al. 2017). Wheat (Triticum aestivum L.; a dry-land crop) and rice (Oryza sativa L.; a wet-land crop) are the two predominant cereals that approximately fed the whole world’s population (FAO 2012; Meharg et al. 2013). Although the Cd accumulation in wheat/rice straw and grains varied greatly among the different cultivars even under the same growth conditions and metal contamination (Liu et al. 2005, 2015), both of them can accumulate a large amount of Cd mainly through their roots and subsequently transport to the aerial parts and finally accumulate in the grains (Jafarnejadi et al. 2010; Meharg et al. 2013; Arduini et al. 2014). Consuming wheat or rice that contains higher Cd has been confirmed as the principal source of dietary Cd intake for the populations (Tsukahara et al. 2003; Williams et al. 2009; Meharg et al. 2013; Rizwan et al. 2016a); thus, developing an effective agronomic strategy to reduce the Cd content in wheat and rice becomes a major environmental issue for the sustainability of agriculture production and food security (Rizwan et al. 2016a, b).
During the past decades, various chemical, physical, and biological approaches have been employed with the aim of reducing Cd uptake and accumulation in the edible parts of crops grown in the Cd-polluted soils (Rizwan et al. 2016a, b). Of all these strategies, exogenous application of nutrients such as selenium (Se) has gained great attentions to alleviate the Cd-mediated adverse effects (Lin et al. 2012; Wan et al. 2016; Yu et al. 2019). As reported in the previous studies, application of Se in plants usually alleviated Cd toxicity by reducing oxidative stress (Yu et al. 2018), improving nutrient balance and photosynthesis (Lin et al. 2012; Qin et al. 2018), and even regenerating damaged cells (Khan et al. 2015). More importantly, the Se-mediated detoxification mechanisms could be also ascribed to the inhibition of root uptake and/or root-to-shoot translocation of Cd in plants (Saidi et al. 2014; Wan et al. 2016; Yu et al. 2018). For example, Sun et al. (2016) found the Cd content in leaves, stems, and roots of cucumber (Cucumis sativus) was decreased by 43%, 26%, and 23%, respectively, when supplied with 6 mmol L–1 of selenite. In agreement with these results, Mozafariyan et al. (2014) and Wan et al. (2016) observed that the addition of selenite also reduced the Cd content, respectively, in pepper fruits and rice seedlings under hydroponic conditions. Conversely, a pot experiment conducted by Yu et al. (2017) observed that the foliar application of selenite elevated the Cd accumulation in tobacco leaves. However, selenate, irrespective of application rate, did not influence the Cd content in the grain of durum wheat cultivated in the Cd-contaminated fields (Grant et al. 2007). These inconsistent findings made the background for this study to explore the detailed impact involving in the fate of Cd in plants during inorganic Se supplementation.
In soil environments, selenite (SeO32−) and selenate (SeO42−) are the two major Se species that can be absorbed by plant roots (Terry et al. 2000; Sors et al. 2005). However, because of the different charges and transporters of Se (SeO32− and SeO42−) and Cd (Cd2+), the uptake competition for binding sites between these two elements may not occur in plant roots (Terry et al. 2000; Clemens et al. 2013). Therefore, in vivo interaction of Se and Cd is considered to be a reasonable explanation for Se impacting the Cd accumulation in plant tissues, but the relevant mechanism has not been fully studied yet. Furthermore, the available literatures in this field were mainly focused on a single plant species, such as rice (Wan et al. 2019), pak choi (Yu et al. 2019), lettuce (He et al. 2004), and cucumber (Sun et al. 2016); while the systematic information on the relationship between Se and Cd are deficient in the different plants, especially for terrestrial plants and aquatic plants, which might exhibit various responses. On the other hand, we consider the Se-mediated reduction of Cd accumulation in plant tissues is probably associated with its subcellular distribution, which may be sequestrating more Cd ions into less active subcellular fractions, such as the cell wall and vacuole (the most important compartment contributing to a soluble fraction), resulted in the limitation of the Cd movement within plants (Qiu et al. 2011; Clemens et al. 2013). Until recently, some distinguished literatures concerning the subcellular distribution of toxic metals during the Se application have been published (Feng et al. 2011; Ding et al. 2015; Yu et al. 2019), but the underlying mechanism has not been fully clarified and thus required to be investigated in depth.
Therefore, with wheat and rice chosen as the target plants in this study, the effects of selenite or selenate on Cd accumulation, translocation, and subcellular distribution were investigated by conducting the hydroponic experiments in a greenhouse. The obtained results will strengthen our understanding of the mechanism of how Se regulates the Cd accumulation in crop plants and further, provide a basis for adopting feasible measures to decline the Cd-induced human health risks.
Materials and methods
Seedlings preparation and grown condition
Seeds of wheat (Triticum aestivum L. cv. Luyuan 502, a medium Cd-accumulating wheat cultivar) and rice (Oryza sativa L. cv. Zhunliangyou 608, a medium Cd-accumulating rice cultivar) were surface-sterilized in 10% (v/v) NaClO for 20 min and then rinsed by deionized water at least three times. The sterilized seeds were germinated on the floating plastic screen in 0.5 mmol L–1 CaCl2 solution at 25 ± 2 °C in darkness. Seven days later, the healthy and uniform seedlings were selected for pre-hydroponic experiments in the PVC pots containing 2.5 L nutrient solution. The 1/5-strength Hoagland nutrient solutions were used for the wheat seedling cultivation (Huang et al. 2017; Hu et al. 2018), whose compositions were as follows: 1000 μmol L–1 Ca(NO3)2·4H2O, 100 μmol L–1 KH2PO4, 1000 μmol L–1 KNO3, 457 μmol L–1 MgSO4·7H2O, 1 μmol L–1 MnCl2, 1 μmol L–1 ZnSO4·7H2O, 0.2 μmol L–1 CuSO4·5H2O, 1 μmol L–1 (NH4)6Mo7O24·5H2O, 3 μmol L–1 H3BO3, and 60 μmol L–1 Fe(III)-EDTA, while the 1/2-strength Kimura B nutrient solutions were applied for the rice seedling growth (Huang et al. 2015; Wan et al. 2016; Camara et al. 2018), whose compositions were as follows: 183 μmol L–1 Ca(NO3)2·4H2O, 100 μmol L–1 KH2PO4, 91 μmol L–1 KNO3, 274 μmol L–1 MgSO4·7H2O, 183 μmol L–1 (NH4)2SO4, 1 μmol L–1 MnSO4·H2O, 1 μmol L–1 ZnSO4·7H2O, 0.2 μmol L–1 CuSO4·5H2O, 1 μmol L–1 (NH4)6Mo7O24·4H2O, 3 μmol L–1 H3BO3, and 60 μmol L–1 Fe(III)-EDTA. The pH of the solutions was buffered at 6.0 and 5.5 for wheat and rice, respectively, by 2 mM 2-(N-morpholino) ethanesulfonic acid monohydrate (MES) and adjusted using KOH or HCl as required. The nutrient solutions were renewed every 3 days, and the solutions for wheat seedlings were aerated continuously throughout the experiment. These seedlings were cultivated in a greenhouse under the following controlled conditions: 25 ± 4 °C/20 ± 2 °C (day/night) temperatures, 14-h photoperiod with a light intensity of 240~350 μmol (m2 s)−1, and relative humidity of 60~70%.
Experimental treatments
Experiment 1
The effects of exogenous Se on Cd accumulation and translocation, as well as the subcellular distribution in plant tissues, were studied. Four-week-old wheat and rice seedlings were exposed to the culture solution, in which Cd (5 μmol L–1 3CdSO4·8H2O) or Se (0, 5 μmol L–1 Na2SeO3 or Na2SeO4) was added and expressed as Cd, Cd+SeIV, and Cd+SeVI, respectively. Each treatment was replicated three times (two plants per pot). The plants were sampled after 3, 24, and 48 h of exposure; and the plants collected at 48 h were also used for the analysis of subcellular Cd partitioning.
Experiment 2
The effects of endogenous Se on Cd accumulation and translocation in plants were studied. Four-week-old wheat and rice seedlings were exposed to the culture solution, in which Cd (5 μmol L–1 3CdSO4·8H2O) or Se (0, 5 μmol L–1 Na2SeO3 or Na2SeO4) was added to form the following treatments: Cd, Cd+SeIV, Cd+SeVI, pre-SeIV+Cd, and pre-SeVI+Cd (plants were pre-treated with selenite or selenate for 2 days before exposed to Cd). Each treatment was replicated three times (two plants per pot). The plants were sampled after 48 h of exposure.
The harvested plants were immersed in an ice-cold desorption solution composed of 1 mmol L–1 CaSO4 for 15 min to remove the ions adhered on root surfaces, and then thoroughly washed with deionized water for three times. The separated shoots and roots of wheat and rice plants were oven-dried, weighted, powdered, and then stored in the polyethylene bags for the analysis of Cd content.
Determination of Cd content in plant tissues
The samples of plant tissues were digested by 8 mL of concentrated HNO3 (GR) using a microwave system (MARS5, CEC Corp., USA). The total Cd concentration in the digestion solutions was determined by inductively coupled plasma mass spectrometry with the instrumental detection limit of 0.01 μg L–1 for Cd (ICP-MS 7700, Agilent Technologies, USA) (Yu et al. 2018, 2019). The blank and a standard reference material (GSB-27, Chinese onion) were both included in the digestion process to verify the accuracy and precision of the sample analysis, and the recovery for GSB-27 was between 83 and 112%.
Analysis of subcellular Cd partitioning
The subcellular Cd partitioning in plant tissues was analyzed following the method described by Yu et al. (2019) with minor modifications. Briefly, 1.0 g of fresh shoots and 0.5 g of fresh roots were homogenized in 20 mL of pre-cooled extracted solution (1 mmol L–1 dithioerythritol, 250 mmol L–1 sucrose, and 50 mmol L–1 Tris buffer [pH 7.5]). The homogenate was centrifuged at 300×g for 10 min at 4 °C, and the sediment was collected as the cell wall (F1). The supernatant was then centrifuged at 20000×g for 30 min at 4 °C, and the supernatant and residue from this step were considered as the soluble cytosol (F2) and organelle (F3), respectively. Afterward, the soluble cytosol was diluted to 50 mL with 5% HNO3 (GR), while the other two fractions were digested with 8 mL HNO3 (GR) at a controlled temperature of 180 °C in the MARS5. The total Cd concentration in the different fractions was determined by ICP-MS.
Statistical analysis
All data were calculated on a dry weight (DW) basis and were presented as means ± standard error (SE) of the three independent replicates. The statistical differences among the different treatments in the same exposure time were determined by one-way analysis of variance (ANOVA); and the effects of Se treatment, exposure time, and their interaction on Cd-related variables were determined by two-way ANOVA with post hoc multiple comparisons (least significance difference, LSD, P < 0.05), which were performed using SAS 9.0.
The transfer factor for Cd from roots to shoots was calculated according to the following equation:
where CS and CR stand for the Cd content (mg kg–1 DW) in plant shoots and roots, respectively.
Results
Se reduced Cd accumulation and translocation at different exposure time
There were no significant changes in either shoot or root biomass of the crop plants in response to the different treatments at the same exposure time (Table 1). However, irrespective of the treatments or the plant species, the Cd content in shoots and roots was significantly increased with the increase of exposure time, and the Cd level in roots was obviously higher than that in shoots (Fig. 1). Moreover, the root Cd content in wheat was 1.5~3.1 times as much as that in rice, even though the shoot Cd content between wheat and rice was approximately equal (Fig. 1).
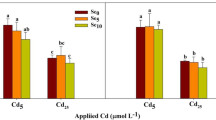
The shoot Cd content in wheat and rice were both obviously influenced by Se treatment, exposure time, and the interaction between Se treatment and exposure time (Table 2). At the initial exposure time (3 h), Se treatments had no significant impact on the Cd content in wheat or rice (Fig. 1). With the prolonged exposure time, exogenous Se significantly decreased the Cd content in plant shoots, but the effect was depended on plant species and Se forms. As for the Cd content in rice shoots, more decrease was achieved in selenite treatment than in selenate treatment; however, there was no significant difference for the two Se form amendments on the Cd content in wheat shoots. At 48 h of exposure, the addition of selenite and selenate significantly decreased the Cd content in wheat shoots by 40.4% and 38.0%, in rice shoots by 72.2% and 40.9%, respectively, when compared to Cd applied alone (Fig. 1). On the other hand, the root Cd content in rice was significantly influenced by Se treatment, exposure time, and their interaction, while its content in wheat root was only significantly affected by exposure time (Table 2); and the change of Cd in plant roots caused by Se addition was delayed and smaller. At 24 h, the application of selenite and selenate reduced the Cd level in wheat roots by 17.5% and 24.5%, respectively, although the former was not significant, while the selenate addition increased the Cd level in rice roots by 10.3%. At 48 h, the Cd level in rice roots under Cd+SeIV and Cd+SeVI treatment was 34.9% and 2.5%, respectively, lower than that in Cd alone treatment, while the corresponding differences were 5.4% and 7.6% in wheat roots, respectively (Fig. 1).
Transfer factors (TFs) of Cd from roots to shoots in wheat and rice were both significantly impacted by Se treatment, exposure time, and the interaction between them (Table 2); and the TFs in the studied crop plants were raised significantly with extending culture time (Fig. 2). It has been found that the TF of Cd in rice was 1.2~2.4 times as much as that in wheat. Relative to the Cd alone treatment, the addition of selenite significantly decreased the TF in wheat by 46.6% and 40.1% at 24 and 48 h, respectively, and the differences were even 68.6% and 56.3% in rice, respectively. In comparison, the reductions of TF induced by selenate were smaller. At 24 and 48 h of exposure, Cd+SeVI treatment reduced the TF by 31.6% and 34.5% in wheat, while by 22.3% and 38.8% in rice, respectively, which were not significant (Fig. 2).
Se affected the subcellular distribution of Cd
With respect to all the treatments, the proportions of Cd distributed to the different subcellular fractions of plant tissues were ranked as follows: cell wall > soluble cytosol > organelle. Upon exposure to Cd alone, about half of the Cd taken up was sequestrated by cell wall: 47.0% and 63.2% in shoots and roots of wheat, 38.9% and 53.4% in shoots and roots of rice (Fig. 3). However, the subcellular distribution of Cd in plants was significantly affected by inorganic Se (Fig. 3), but the extent of effects depended on the Se forms, plant species, and specific tissues. In plant shoots, neither form of Se affected the distribution pattern of Cd significantly, but the addition of selenate still decreased the Cd sequestration by the cell wall and increased that in soluble cytosol of wheat, while the exogenous selenite increased the percentage of Cd in the cell wall and decreased that in the organelle of rice (Fig. 3). By contrast, the application of selenite and selenate significantly increased the percentage of Cd in soluble cytosol in plant roots: by 33.0% and 47.2% in wheat, by 25.6% and 15.2% in rice, respectively; and the differences were all significant except for the rice in Cd+SeVI treatment. Furthermore, the distribution ratios of Cd in the cell wall of wheat roots were decreased by Se addition, which different is non-significant, while the percentage of Cd in organelle was significantly reduced by 30.6% in rice roots when treated with selenite (Fig. 3).
Se pretreatment also declined Cd accumulation and translocation
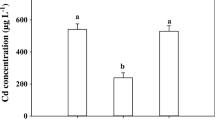
Neither Cd nor Se treatment resulted in an obvious effect on the growth of wheat or rice (data not shown). The Cd content in plant shoots was decreased by the Se pretreatment or simultaneous addition (Fig. 4). In wheat shoots, however, the change of Cd caused by the various Se treatment was consistent, in contrast to Cd-alone treatment, Cd+Se, and pre-Se+Cd treatment decreased the Cd content by 42.5~50.2% and 32.0~43.4%, respectively (Fig. 4a). In comparison, a significant difference in the decrease of Cd in rice shoots between selenite and selenate treatment was obtained, and this decrease caused by the pre-selenite treatment was smaller than that in the simultaneous selenite treatment (Fig. 4b). The Cd level in rice shoots under Cd+SeIV treatment was 85.7%, lower than that in Cd alone treatment, and the corresponding differences in pre-SeIV+Cd treatment were 47.3%. Similarly, even smaller decrease was also induced by the selenate addition, which, however, was not significant (Fig. 4b).
On the other hand, the variation of Cd caused by the Se treatment was minimal in plant roots (Fig. 4). Simultaneous addition and pretreatment of selenate significantly decrease the Cd level in wheat roots by 30.7% and 27.4%, respectively, relative to that of the treatment applied Cd alone, while the differences in selenite treatment were 23.0% and 15.7%, though not significant (Fig. 4a). As for the Cd content in rice roots, Se treatment had no significant effect on that, though slightly decreased by selenite whereas increased by selenate addition (Fig. 4b).
Both the pre-Se treatment and simultaneous addition reduced the TF of Cd in the crop plants with the distinct extents (Fig. 5). Although not significant, Cd+SeIV and pre-SeIV+Cd treatments decreased the TF of Cd in wheat by 30.6% and 42.9%, respectively, while little smaller differences were achieved in selenate treatment, which were 28.6% and 24.4%, respectively (Fig. 5a). Contrarily, the selenite application affected the TF of Cd in rice obviously, Cd+SeIV treatment decreased the TF by 83.2%; and the corresponding differences in pre-SeIV+Cd treatment were 47.2%. However, the decrease of the TF caused by selenate was smaller, which was only 32.9% and 26.7%, respectively (Fig. 5b).
Discussion
Effects of Se on Cd accumulation and translocation
Numerous researches confirmed the application of inorganic Se at a proper dose could alleviate the phytotoxicity induced by metals, including Cd and As (Wan et al. 2016; Camara et al. 2018; Wu et al. 2020). As shown in the present study, gradually reduced effects of the Se treatments on the root Cd accumulation in wheat and rice were observed with increasing of exposure time (Fig. 1). It has been proved that Se has a strong ability to combine with the metals (Zhang et al. 2012); hence, the reduced result might be ascribed to selenite or selenate chelating with Cd2+ to form CdSeO3 or CdSeO4 complexes, which are less available to plants (Badiello et al. 1996). Shanker et al. (1995) observed that the application of selenite or selenate could decrease the Cd uptake by kidney bean owing to the formation of Cd-Se complex that is unavailable to the plants, since Se shows the following reduction course in an acid soil environment: SeO42–→SeO32–→Se0→Se2– (Diplock 1993). Here, however, we conducted the experiments under hydroponic conditions, where few insoluble Cd-Se complexes were produced. Other researchers such as Cui et al. (2018) reasoned that Se caused the reduction in the Cd uptake by rice plants which is probably associated with, e.g., downregulation of the expression of OsNramp5 (natural resistance-associated macrophage protein 5) and OsLCT1 (low-affinity cation transporter 1), which are responsible for the Cd uptake and transport, respectively.
Additionally, pre-treatment with inorganic Se (namely, endogenous Se) also slightly decreased the Cd accumulation in roots, especially for wheat (Fig. 4), indicating an intracellular interaction between Se and Cd might exist. Within plant cells, Cd is often bound to S-containing ligands, such as those present in glutathione (GSH) and phytochelatins (PCs) (DalCorso et al. 2008; Yadav 2010), which plays an important role in sequestrating and detoxifying metals (Ernst et al. 2008; Yu et al. 2019). However, GSH–PCs can be widely activated by Se in various plants when exposed to the metals (Malik et al. 2012), suggesting Se has the capability of alleviating the Cd-induced toxicity in plants. It has been reported that Se and S share the common metabolic pathways in plants and probably compete with each other during the biochemical processes (Terry et al. 2000; Sors et al. 2005). Therefore, selenos (–SeH) might have similar functions to thiols (–SH) with regard to the chelation of metal ions when S was substituted with Se in Cys/SeCys residues and, finally, Cd combined with Se ligands (just like S ligands) to form the stable complexes in plant cells (Jalilehvand et al. 2012).
After being absorbed by plant roots, most of Cd accumulated in the below-ground parts, and only a small amount of it can be transported to the above-ground parts by the longitudinal translocation via the system of xylem vessels (Uraguchi et al. 2009; Wu et al. 2015), which might be a natural protective response of plants against Cd toxicity. In the current research, both the Se pretreatment and simultaneous addition dramatically decreased the shoot Cd accumulation in the studied crop plants (Figs. 1 and 4), even though such effects in rice were more obvious than in wheat. This could be attributed to the role of Se in increasing formation of Cd-thiol complexes in roots and the reduction of Cd translocation from roots to shoots (Figs. 2 and 5). Wang et al. (2014) observed that Se could enhance the development of apoplastic barriers in the plant root endodermis, which could restrict the apoplastic transport of metals. However, Pedrero et al. (2008) found the co-presence of selenite decreased the Cd accumulation in the aerial parts while increased it in the roots of broccoli cultivated in the hydroponic culture. Contrarily, in a pot experiment involving tobacco, we observed that the application of selenite in foliar method slightly increased the Cd content in leaves (Yu et al. 2017). We consider the different studied plant species might be the main reason for these contradictory results due to the different abilities in Cd response among various plant species; and the different employed experimental conditions and the applied Se forms might be other reasonable explanations for these inconsistent phenomena. In addition, a summary for the studies cited in this paper regarding the effects of Se on the fate of Cd in plants is presented in Table 3.
Effects of Se on Cd subcellular distribution
Apart from the reduction of root-to-shoot Cd translocation, the reduced effect of shoot Cd accumulation induced by Se might be also related to the subcellular distribution of Cd in plants. In this study, the addition of selenate increased the percentage of Cd in soluble cytosol of wheat shoots, while selenite increased that percentage in the cell wall of rice shoots (Fig. 3); and the proportion of Cd in soluble cytosol of the studied plant roots was significantly enhanced by selenite or selenate application (Fig. 3). Similar to the obtained results, in a hydroponic experiment using rice, we found the supply of selenite increased the distribution ratio of As in cell wall or soluble cytosol but slightly declined its proportion in the organelle (Wang et al. 2021).
In plant tissues, cell wall can join the Cd ions under the function of some functional groups and then restrain the movement of the metal ions across the cytomembrane due to its substantial mechanical rigidity and strength (Fry 1986; Cui et al. 2017); and the addition of Se improves the mechanical force of the cell wall, resulting in an increased accumulation of Cd in the plant cell wall (Cui et al. 2018). On the other hand, vacuole can act as a dominant site for binding of Cd ions, and the formed Cd–PC complexes can be sequestrated mostly in the vacuole when Cd ions were combined with GSH or PCs that contain thiol (–SH) groups (DalCorso et al. 2008), which cannot inflict further damage to the organelle (Wang et al. 2008). Se can activate PC synthase and stimulate the synthesis of PC precursor GSH (Feng and Wei 2012); hence, when plants are exposed to Cd stress, the increasing amount of Cd ions can be bound to GSH or PCs and, consequently, the formed Cd–PC complexes will be sequestrated in the vacuole of roots. Furthermore, OsHMA3 (P1B type Heavy Metal ATPase 3), a tonoplast Cd transporter, is responsible for sequestrating Cd into the vacuole of root cells in rice. It has been reported that rice cultivars possessing the loss-of-function or weak alleles of the OsHMA3 have low abilities to sequester Cd in the root vacuoles, leading to high Cd transportation to the shoots (Ueno et al. 2010; Miyadate et al. 2011). Therefore, the Se-regulated reduction in the Cd accumulation of rice shoots might be partially ascribed to the over-expression of OsHMA3 induced by Se (Cui et al. 2018), leading to the more Cd ions sequestrated into the root vacuole and eventually decreasing the Cd translocation to the rice shoots (Figs. 2 and 5). Contrarily, although a major locus of regulating Cd translocation has been identified in durum wheat, the causal gene still remains unknown (Wiebe et al. 2010); and it will be interesting to explore the other functional genes controlling the Cd translocation in wheat plants. However, further works that focused on the physiological and molecular levels are still needed before we can fully understand the mechanism of Se-mediated regulation of Cd accumulation in the crop plants.
Different impacts between selenite and selenate on the fate of Cd
In this study, selenite was found more effective than selenate in reducing the Cd accumulation and translocation in the studied crop plants, especially in rice (Figs. 1 and 2). We speculate the distinct effects between the two forms of Se might have resulted from the difference in their assimilation (Terry et al. 2000; Zhu et al. 2009). In plants, Se is metabolized through sulfur-assimilation pathways (Terry et al. 2000; Zhu et al. 2009), but selenate is first reduced to selenite, which is the rate-limiting step, and then incorporated into amino acids and proteins (Pilon-Smits et al. 1999; Terry et al. 2000). Therefore, the insufficient selenate assimilation was processed due to the relative short exposure time, and thus selenite-mediated the Cd reduction in plant shoots and its sequestration in plant roots was much earlier than selenate did (Wan et al. 2016).
Conclusion
In this study, the supply of inorganic Se did not significantly affect the root Cd content, but they dramatically reduced the shoot Cd content in both wheat and rice, and such effects were more obvious in the selenite than in the selenate applied treatment. Moreover, the pre-Se treatment with inorganic Se also declined the Cd accumulation in plant shoots. The Se-mediated reduced effects might be attributed to the inhibition of Cd translocation from roots to shoots, and also the alteration of Cd subcellular distribution in plant tissues which is reflected in the sequestration of more Cd ions in less active cell compartments. However, prior to the practical application of inorganic Se in the Cd-contaminated agricultural fields, additional researches are still required to determine whether similar antagonistic interaction of Se and Cd can be achieved in a soil-plant system.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Arduini I, Masoni A, Mariotti M, Pampana S, Ercoli L (2014) Cadmium uptake and translocation in durum wheat varieties differing in grain-Cd accumulation. Plant Soil Environ 60(1):43–49. https://doi.org/10.17221/416/2013-PSE
Badiello R, Feroci G, Fini A (1996) Interaction between trace elements: selenium and cadmium ions. J Trace Elem Med Biol 10(3):156–162. https://doi.org/10.1016/S0946-672X(96)80026-5
Camara AY, Wan YN, Yu Y, Wang Q, Li HF (2018) Effect of selenium on uptake and translocation of arsenic in rice seedlings (Oryza sativa L.). Ecotox Environ Safe 148:869–875. https://doi.org/10.1016/j.ecoenv.2017.11.064
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18(2):92–99. https://doi.org/10.1016/j.tplants.2012.08.003
Cui JH, Liu TX, Li FB, Yi JC, Liu CP, Yu HY (2017) Silica nanoparticles alleviate cadmium toxicity in rice cells: mechanisms and size effects. Environ Pollut 228:363–369. https://doi.org/10.1016/j.envpol.2017.05.014
Cui JH, Liu TX, Li YD, Li FB (2018) Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Sci Total Environ 644:602–610. https://doi.org/10.1016/j.scitotenv.2018.07.002
DalCorso G, Farinati S, Maistri S, Furini A (2008) How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50(10):1268–1280. https://doi.org/10.1111/j.1744-7909.2008.00737.x
Ding YZ, Wang RG, Guo JK, Wu FC, Xu YM, Feng RW (2015) The effect of selenium on the subcellular distribution of antimony to regulate the toxicity of antimony in paddy rice. Environ Sci Pollut R 22(7):5111–5123. https://doi.org/10.1007/s11356-014-3865-9
Diplock AT (1993) Indexes of selenium status in human populations. Am J Clin Nutr 57(2):256S–258S. https://doi.org/10.1093/ajcn/57.2.256S
Du Y, Hu XF, Wu XH, Shu Y, Jiang Y, Yan XJ (2013) Affects of mining activities on Cd pollution to the paddy soils and rice grain in Hunan province, Central South China. Environ Monit Assess 185(12):9843–9856. https://doi.org/10.1007/s10661-013-3296-y
Ernst WHO, Krauss G, Verkleij JAC, Wesenberg D (2008) Interaction of heavy metals with the sulphur metabolism in angiosperms from an ecological point of view. Plant Cell Environ 31(1):123–143. https://doi.org/10.1111/j.1365-3040.2007.01746.x
Food and Agriculture Organization of the United Nations (2012) Core Production Data Base, ProdStat
Feng RW, Wei CY (2012) Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant. Plant Soil Environ 58(3):105–110. https://doi.org/10.17221/162/2011-PSE
Feng RW, Wei CY, Tu SX, Tang SR, Wu FC (2011) Simultaneous hyperaccumulation of arsenic and antimony in Cretan brake fern: evidence of plant uptake and subcellular distributions. Microchem J 97(1):38–43. https://doi.org/10.1016/j.microc.2010.05.010
Fry SC (1986) Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol 37:165–186. https://doi.org/10.1146/annurev.pp.37.060186.001121
Grant CA, Buckley WT, Wu RG (2007) Effect of selenium fertilizer source and rate on grain yield and selenium and cadmium concentration of durum wheat. Can J Plant Sci 87(4):703–708. https://doi.org/10.4141/CJPS06060
He PP, Lv XZ, Wang GY (2004) Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ Int 30(2):167–172. https://doi.org/10.1016/S0160-4120(03)00167-3
Hu T, Li HF, Li JX, Zhao GS, Wu WL, Liu LP, Wang Q, Guo YB (2018) Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front Plant Sci 9:597. https://doi.org/10.3389/fpls.2018.00597
Huang ML, Zhou SL, Sun B, Zhao QG (2008) Heavy metals in wheat grain: assessment of potential health risk for inhabitants in Kunshan, China. Sci Total Environ 405(1−3):54–61. https://doi.org/10.1016/j.scitotenv.2008.07.004
Huang QQ, Yu Y, Wang Q, Luo Z, Jiang RF, Li HF (2015) Uptake kinetics and translocation of selenite and selenate as affected by iron plaque on root surfaces of rice seedlings. Planta 241(4):907–916. https://doi.org/10.1007/s00425-014-2227-7
Huang QQ, Wang Q, Wan YN, Yu Y, Jiang RF, Li HF (2017) Application of X-ray absorption near edge spectroscopy to the study of the effect of sulphur on selenium uptake and assimilation in wheat seedlings. Biol Plant 61(4):726–732. https://doi.org/10.1007/s10535-016-0698-z
Jafarnejadi AR, Homaee M, Sayyad G, Bybordi M (2010) Large scale spatial variability of accumulated cadmium in the wheat farm grains. Soil Sediment Contam 20(1):98–113. https://doi.org/10.1080/15320383.2011.528472
Jalilehvand F, Amini Z, Parmar K (2012) Cadmium(II) complex formation with selenourea and thiourea in solution: an XAS and 113Cd NMR study. Inorg Chem 51(20):10619–10630. https://doi.org/10.1021/ic300852t
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208. https://doi.org/10.1016/j.taap.2009.04.020
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18. https://doi.org/10.1016/j.jplph.2014.09.011
Li H, Luo N, Li YW, Cai QY, Li HY, Mo CH, Wong MH (2017) Cadmium in rice: transport mechanisms, influencing factors, and minimizing measures. Environ Pollut 224:622–630. https://doi.org/10.1016/j.envpol.2017.01.087
Lin L, Zhou WH, Dai HX, Cao FB, Zhang GP, Wu FB (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235−236:343–351. https://doi.org/10.1016/j.jhazmat.2012.08.012
Liu JG, Zhu QS, Zhang ZJ, Xu JK, Yang JC, Wong MH (2005) Variations in cadmium accumulation among rice cultivars and types and the selection of cultivars for reducing cadmium in the diet. J Sci Food Agric 85(1):147–153. https://doi.org/10.1002/jsfa.1973
Liu WT, Liang LC, Zhang X, Zhou QX (2015) Cultivar variations in cadmium and lead accumulation and distribution among 30 wheat (Triticum aestivum L.) cultivars. Environ Sci Pollut R 22(11):8432–8441. https://doi.org/10.1007/s11356-014-4017-y
Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H (2012) Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot 77:242–248. https://doi.org/10.1016/j.envexpbot.2011.12.001
Meharg AA, Norton G, Deacon C, Williams P, Adomako EE, Price A, Zhu YG, Li G, Zhao FJ, McGrath S, Villada A, Sommella A, De Silva PMCS, Brammer H, Dasgupta T, Islam MR (2013) Variation in rice cadmium related to human exposure. Environ Sci Technol 47(11):5613–5618. https://doi.org/10.1021/es400521h
Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, Katou K, Kodama I, Sakurai K, Takahashi H, Satoh-Nagasawa N, Watanabe A, Fujimura T, Akagi H (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189(1):190–199. https://doi.org/10.1111/j.1469-8137.2010.03459.x
Mozafariyan M, Shekari L, Hawrylak-Nowak B, Kamelmanesh MM (2014) Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol Trace Elem Res 160(1):97–107. https://doi.org/10.1007/s12011-014-0028-2
Pedrero Z, Madrid Y, Hartikainen H, Cámara C (2008) Protective effect of selenium in broccoli (Brassica oleracea) plants subjected to cadmium exposure. J Agric Food Chem 56(1):266–271. https://doi.org/10.1021/jf072266w
Pilon-Smits EAH, Hwang S, Mel Lytle C, Zhu YL, Tai JC, Bravo RC, Chen YC, Leustek T, Terry N (1999) Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol 119(1):123–132. https://doi.org/10.1104/pp.119.1.123
Qin XM, Nie ZJ, Liu HG, Zhao P, Qin SY, Shi ZW (2018) Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ Exp Bot 150:232–239. https://doi.org/10.1016/j.envexpbot.2018.03.024
Qiu Q, Wang YY, Yang ZY, Yuan JG (2011) Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem Toxicol 49(9):2260–2267. https://doi.org/10.1016/j.fct.2011.06.024
Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016a) Cadmium minimization in wheat: a critical review. Ecotox Environ Safe 130:43–53. https://doi.org/10.1016/j.ecoenv.2016.04.001
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016b) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23(18):17859–17879. https://doi.org/10.1007/s11356-016-6436-4
Rizwan M, Ali S, Adrees M, Ibrahim M, Tsang DCW, Zia-ur-Rehman M, Zahir ZA, Rinklebe J, Tack FMG, Ok YS (2017) A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 182:90–105. https://doi.org/10.1016/j.chemosphere.2017.05.013
Saidi I, Chtourou Y, Djebali W (2014) Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J Plant Physiol 171(5):85–91. https://doi.org/10.1016/j.jplph.2013.09.024
Shanker K, Mishra S, Srivastava S, Srivastava R, Dass S, Prakash S, Srivastava MM (1995) Effect of selenite and selenate on plant uptake of cadmium by kidney bean (Phaseolus mungo) with reference to Cd-Se interaction. Chem Speciat Bioavailab 7(3):97–100. https://doi.org/10.1080/09542299.1995.11083251
Shi TR, Ma J, Wu X, Ju TN, Lin XL, Zhang YY, Li XH, Gong YW, Hou H, Zhao L, Wu FY (2018) Inventories of heavy metal inputs and outputs to and from agricultural soils: a review. Ecotox Environ Safe 164:118–124. https://doi.org/10.1016/j.ecoenv.2018.08.016
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86(3):373–389. https://doi.org/10.1007/s11120-005-5222-9
Sun HY, Wang XY, Wang YN, Wei YY, Wang GH (2016) Alleviation of cadmium toxicity in cucumber (Cucumis sativus) seedlings by the application of selenium. Span J Agric Res 14(4):e1105. https://doi.org/10.5424/sjar/2016144-10008
Terry N, Zayed AM, de Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432. https://doi.org/10.1146/annurev.arplant.51.1.401
The Ministry of Environmental Protection (MEP), the Ministry of Land and Resources (MLR) (2014) Report on the National Soil Contamination Survey. http://www.zhb.gov.cn/gkml/hbb/qt/201404/t20140417_270670.htm. Accessed 17 Apr 2014
Tsukahara T, Ezaki T, Moriguchi J, Furuki K, Shimbo S, Matsuda-Inoguchi N, Ikeda M (2003) Rice as the most influential source of cadmium intake among general Japanese population. Sci Total Environ 305(1−3):41–51. https://doi.org/10.1016/s0048-9697(02)00475-8
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. P Natl Acad Sci USA 107(38):16500–16505. https://doi.org/10.1073/pnas.1005396107
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60(9):2677–2688. https://doi.org/10.1093/jxb/erp119
Wan YN, Yu Y, Wang Q, Qiao YH, Li HF (2016) Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotox Environ Safe 133:127–134. https://doi.org/10.1016/j.ecoenv.2016.07.001
Wan YN, Wang K, Liu Z, Yu Y, Wang Q, Li HF (2019) Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ Sci Pollut R 26(16):16220–16228. https://doi.org/10.1007/s11356-019-04975-9
Wang X, Liu YG, Zeng GM, Chai LY, Song XC, Min ZY, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62(3):389–395. https://doi.org/10.1016/j.envexpbot.2007.10.014
Wang X, Tam NF, Fu S, Ametkhan A, Ouyang Y, Ye ZH (2014) Selenium addition alters mercury uptake, bioavailability in the rhizosphere and root anatomy of rice (Oryza sativa). Ann Bot-London 114(2):271–278. https://doi.org/10.1093/aob/mcu117
Wang K, Wang YQ, Wan YN, Mi ZD, Wang QQ, Wang Q, Li HF (2021) The fate of arsenic in rice plants (Oryza sativa L.): influence of different forms of selenium. Chemosphere 264:128417. https://doi.org/10.1016/j.chemosphere.2020.128417
Wiebe K, Harris NS, Faris JD, Clarke JM, Knox RE, Taylor GJ, Pozniak CJ (2010) Targeted mapping of Cdu1, a major locus regulating grain cadmium concentration in durum wheat (Triticum turgidum L. var durum). Theor Appl Genet 121(6):1047–1058. https://doi.org/10.1007/s00122-010-1370-1
Williams PN, Lei M, Sun GX, Huang Q, Lu Y, Deacon C, Meharg AA, Zhu YG (2009) Occurrence and partitioning of cadmium, arsenic and lead in mine impacted paddy rice: Hunan, China. Environ Sci Technol 43(3):637–642. https://doi.org/10.1021/es802412r
Wu ZC, Zhao XH, Sun XC, Tan QL, Tang YF, Nie ZJ, Hu CX (2015) Xylem transport and gene expression play decisive roles in cadmium accumulation in shoots of two oilseed rape cultivars (Brassica napus). Chemosphere 119:1217–1223. https://doi.org/10.1016/j.chemosphere.2014.09.099
Wu C, Dun Y, Zhang ZJ, Li ML, Wu GQ (2020) Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotox Environ Safe 190:110091. https://doi.org/10.1016/j.ecoenv.2019.110091
Yadav SK (2010) Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76(2):167–179. https://doi.org/10.1016/j.sajb.2009.10.007
Yu Y, Wan YN, Wang Q, Li HF (2017) Effect of humic acid-based amendments with foliar application of Zn and Se on Cd accumulation in tobacco. Ecotox Environ Safe 138:286–291. https://doi.org/10.1016/j.ecoenv.2017.01.011
Yu Y, Yuan SL, Zhuang J, Wan YN, Wang Q, Zhang JS, Li HF (2018) Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotox Environ Safe 162:571–580. https://doi.org/10.1016/j.ecoenv.2018.07.041
Yu Y, Fu PN, Huang QQ, Zhang JS, Li HF (2019) Accumulation, subcellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages. Chemosphere 216:331–340. https://doi.org/10.1016/j.chemosphere.2018.10.138
Zhang H, Feng XB, Zhu JM, Sapkota A, Meng B, Yao H, Qin HB, Larssen T (2012) Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ Sci Technol 46(18):10040–10046. https://doi.org/10.1021/es302245r
Zhao FJ, Ma YB, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: Current status and mitigation strategies. Environ Sci Technol 49(2):750–759. https://doi.org/10.1021/es5047099
Zhu YG, Pilon-Smits EAH, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14(8):436–442. https://doi.org/10.1016/j.tplants.2009.06.006
Funding
This work was supported by the National Natural Science Foundation of China (No. 41907146).
Author information
Authors and Affiliations
Contributions
KW, JYLH, and YNW conceived and designed the experiments. JYLH and YNW performed the experiments. KW analyzed the data and wrote the paper with the help of LXK, SYH, and QW. HFL and YNW reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, K., Linghu, J., Kong, L. et al. Comparative responses of cadmium accumulation and subcellular distribution in wheat and rice supplied with selenite or selenate. Environ Sci Pollut Res 28, 45075–45086 (2021). https://doi.org/10.1007/s11356-021-13554-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13554-w