Abstract
We determined organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in raw bovine milk taken directly from the milking equipment for different animals and various farms in Béja region, which is one of the largest milk producing areas in Tunisia. All milk samples were contaminated with one or more pesticides and exhibited measurable concentrations of some PCB congeners. The residue levels are generally marked by the predominance of dichlorodiphenyltrichloroethanes (DDTs) (median: 17.60 ng g−1 fat), followed by hexachlorobenzene (HCB) (median: 14.31 ng g−1 fat), PCBs (median: 4.71 ng g−1 fat), and hexachlorocyclohexanes (HCHs) (median: 0.77 ng g−1 fat). DDT/DDE ratios across the samples vary between 0.09 to 32.24 and exhibit the hypothesis of sustaining illegal use of the banned pesticide. The historical or recent use of OCPs and possible emission sources of PCBs identified near studied farms may lead to increased levels of these contaminants in produced milk. The estimated daily intake (EDI) values were several orders of magnitude below the tolerable daily intake (TDI) fixed by FAO/WHO, though recorded concentration in some samples exceeded the maximum residue limits (MRLs) fixed by the EU, especially for OCPs. According to health risk assessments, consumption of raw milk did not pose an obvious cancer risk or other health problems for local inhabitants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
PCBs and OCPs are two groups of persistent organic pollutants (POPs) that have been widely considered over the last several decades. The origin of their occurrence in the environment as well as their main physicochemical properties are well known and documented (Fiedler 2003; Robertson and Hansen 2015). Like other POPs, they are characterized by high lipophilicity, chemical persistence, and high bioaccumulation capacity through the food web, with a high risk of causing adverse effects on human health and the environment (Carpenter 2013; Harrad 2012; Rose and Fernandes 2013).
Between the 1940s and 1980s, OCPs and PCBs were extensively used in Tunisia for agricultural, industrial, and sanitary purposes. The legal import has been prohibited since 1984 and 1986, respectively, for OCPs (dieldrin, heptachlor, DDT, aldrin, chlordane, endrin, and toxaphene) and PCBs. The Stockholm Convention was signed by our country in 2001 and became legally binding in 2004. The existing situation is characterized by relatively high quantities (1407 tons estimated in 2003) of old stockpiles of obsolete pesticides, including principally HCH and DDT, and a large number of transformers or equipment containing PCBs are still used or presently stored in unsatisfactory conditions and dispersed through the country in 132 sites (ANGED 2010; APEK 2005). Moreover, forbidden pesticides are still in demand because of their low cost and effectiveness against pests, and investigations have revealed that compounds are still being illegally imported from nearby countries where their usage is not prohibited (USDA 2014). A number of studies, carried out in Tunisia, have documented the contamination levels of organochlorine compounds (OCs) in various environmental samples in Tunisia, including sediment (Barhoumi et al. 2016; Derouiche et al. 2004), water (Necibi and Mzoughi 2020), fish (Ben Ameur et al. 2013a), atmosphere (Barhoumi et al. 2018), and in human samples, like breast milk (Ben Hassine et al. 2012; Ennaceur et al. 2007, 2008), blood (Ben Hassine et al. 2014), and adipose tissue (Achour et al. 2017).
It is known that the main route of human exposition to OCs is through food intake, with a contribution of more than 90% of the total exposure, and fat foods of animal origin (fish, meat, milk, and dairy products) present approximately 80% of dietary exposure (Djien Liem et al. 2000). Among the studies carried out on human adipose tissues and breast milk collected in the coastal area (Bizerte region, Tunisia) have shown a clear correlation between the frequency of fish consumption and the levels of PCBs (Achour et al. 2017; Ennaceur et al. 2008). Therefore, a national survey is required to examine the intake of OCs due to dietary differences, with a strong focus on the more specific populations living in agricultural and rural zones, who consume a large amount of milk and dairy products compared to the coastal regions.
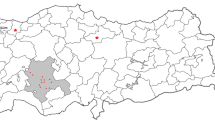
The Béja governorate is located in the northwest of Tunisia and has 306,300 inhabitants (a little less than 3% of the national population). It is characterized by a high annual amount of rainfall (between 350 and 1000 mm/year) and fertile soils. It is a territory with very appreciable natural resources, mountainous areas, grassy valleys, and green plains. Its agricultural land represents more than 91% of its area (Atlas Numérique du Gouvernorat de Béja 2016). The economic activity is focused essentially on agriculture, and placed it among the first governorates in the agronomic production of the country (Agence de promotion de l’industrie et de l’innovation 2017). Farming sheep and cattle are well developed here, which contributes to the increase in production and consumption of typical dairy products. This region produces 12.5% of Tunisia’s cow milk, making it one of the largest milk production areas. The domestic market receives 20% of this production, while 8% is transformed into fresh milk-based products using the traditional process (LACTIMED 2015).
Milk and dairy products have been recognized as among vital components of a healthy and balanced human diet (Park 2009). These fat animal foods are a large part daily diet of the inhabitants in this region. For 83% of distributors surveyed during the study carried out by local agro-clusters for typical and innovative Mediterranean dairy products: LACTIMED (2015), drinking milk continues to be the largest-selling dairy product, followed by yogurt and cheese, which represents a small proportion of these sales. Moreover, most residents of the study area buy these foods from specialized shops (creameries), which are made from raw milk delivered from local farms. Consequently, bovine raw milk is probably one of the major food products potentially responsible for human exposure in this area and can significantly contribute to the dietary intake of the OCs and can cause chronic and acute toxicity.
Regular surveys and monitoring programs of these chemicals attached with risk assessments have been carried out and reported in many developed countries worldwide, and this is done in order to protect consumer health, improve the management of agricultural resources, and minimize economic losses (DAFF 2022; EFSA 2012; US-FDA 2022). But unfortunately, data on the state of contamination of milk with persistent organochlorine pollutants and the health risk assessment related to it consumption was not well considered, and this may be due to the paucity of data and lack of interest on the institutional level. To the best of our knowledge, the present work is the first study investigating the levels of OCPs and PCBs in raw milk collected in this region. The only available Tunisian data is published by Sabbah and Bouguerra (1997), who measured the levels of OCPs in milk samples. Therefore, the current study was designed to (i) screen the status of OCP and PCB residues in bovine fresh raw milk collected from local farms in Béja region (ii) monitor the possible variation pattern across the various studied farms (iii) assess the human dietary exposure to OCPs and PCBs in the study area and (iv) estimate cancer and non-cancer health risks due to the consumption of the milk.
Materials and Methods
Sample collection and storage
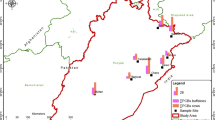
The cow milk samples were collected in late autumn 2016 from six farms located throughout Béja Governorate (Fig. 1). Organized sampling fiches were completed for each sample, including characteristics of dairy cows, water for drinking, and fodder. In addition, geographic data and anthropogenic activities around the farms were also collected to search possible for relationships between the contamination levels of milk and identifiable exposure factors.
Their herds were composed of more than 90% are Holstein-Friesians, including Holstein and Black Pied cows born in Tunisia. In farms 1–3, dairy cows are housed in free stalls, while in farms 4 to 6, tie-stall housing is used. Livestock feed consists of fodder (hay, straw, silage, and greenery) produced on their own land or purchased from the same region, supplemented with concentrate feeds (industrial and/or farmers). The farmers store the animal feed in open-sided sheds or in the open air. Water from uncontrolled wells is mostly consumed by cows in addition to municipal water.
Three farms (1, 2, and 5) are located in the north of Béja City (over 100 thousand inhabitants), near an industrial zone within a radius of 5 km. According to Atlas Numérique du Gouvernorat de Béja (2016), this industrial site had 97 small units, where the food industry represents 46.4% of the total number of companies installed. There are other industries, including textiles, leather, chemicals, and electronics. Located in southwestern Beja City, farm 3 is close to a dump of old electrical and mechanical equipment and is influenced by another industrial unit that produces steam. Farm 6 is located in an agricultural area specializing in seed production and close to a municipal landfill and incinerator situated in El Maagoula, located a few kilometers to the southeast of Béja City. Farm 4 is far from urban and industrial sources of pollution but is influenced by agricultural inputs.
A total of 30 bovine milk samples (five per farm and one per animal) were taken directly from the milking equipment, placed in analytical-grade glass containers of 100 mL capacity with teflon-lined caps, kept cooled until they reached the laboratory, then frozen and stored at – 20 °C until chemical analysis.
Chemicals and analytes
Standard of selected pesticides (HCB, β-HCH, γ-HCH, p,p′-DDE, p,p′ -DDD, and p,p′-DDT) were purchased from Polyscience Corporation Analytical Standards (Niles, IL, USA). Individual standard solutions of each compound were prepared in hexane at 1000 mg mL−1 except β-HCH which is dissolved in acetone. The PCB analytical mixture solution was obtained from Supelco (CIL, USA) containing twelve congeners (PCB 18, − 28, − 31, − 52, − 44, − 101, − 149, − 118, − 153, − 138, − 180, and − 194) at 10 mg mL−1 in heptane. PCB 15 and − 209, for use as surrogate standards, as well as internal standard of PCB 155, were purchased by Cluzeau Info Labo (Sainte-Foy La Grande, France, purity 99%) and dilute in hexane to suitable concentrations. The working standard solutions were obtained by further dilution in n-hexane to appropriate concentration levels. All of these solutions are stored in dark at 4 °C ad left for 1 h at ambient temperature before use.
The organic solvents used were pesticide quality and were obtained from Fluka (Buchs, Switzerland). Sulfuric acid and sodium oxalate were purchased from Biotechnica (Tunis, Tunisia). High-purity florisil adsorbent (60–100 mech) was obtained from Fluka, activated at 650 °C for 8 h, cooled, and then heated at 130 °C for 5 h and preserved in desiccators until use. Anhydrous sodium sulfate (analytical grade, Fluka) was baked at 300 °C and stored in an oven at 130 °C. All glassware was washed with detergent, rinsed with water, heated overnight at 300 °C, and rinsed with hexane before use.
Sample preparation and instrumental analysis
The stored milk samples were left to defrost at ambient temperature and were then shaken vigorously for 5 min. Ten milliliters of milk sample was taken, spiked with a known concentration of PCB 15 and − 209 as surrogates, and OCs were extracted according to the method described by (Moreno Frias et al. 2004) with some modifications. Initially, 1 mL of methanol and 0.1 g of sodium oxalate were added to each aliquot of 10 mL sample and vortexing for 1 min. Ten milliliters of n-hexane:diethyl ether (1:1 v/v) was added to the mixture, agitated for 2 min, and centrifuged for 5 min at 2500 rpm. The organic phase was collected, and the aqueous phase was extracted twice with n-hexane: diethyl ether (1:1 v/v). The hexane layers are combined in the same flask, filtered on anhydrous sodium sulfate, and then evaporated to 5 mL. One milliliter of this solution was taken for fat content determination using gravimetric method. The remaining extracts were first purified using 2 mL of sulfuric acid and then with adsorption chromatography according to the method (Ennaceur et al. 2008). A glass column (0.5 cm i.d.) containing a wad of silane-treated glass wool at the bottom, filled with 2 g of activated florisil, and topped with 1 g of anhydrous sodium sulfate. The organochlorine compounds were eluted with 30 mL of n-hexane:dichloromethane (9:1 v/v). The eluted was evaporated to near dryness, fortified with internal standard (PCB 155) and the final volume was adjusted to 0.5 mL in n-hexane.
A Hewlett-Packard 6890 plus gas chromatograph equipped with 63Ni electron capture detector (GC-ECD) operated by HP Chemstation software was used for target analytes determination. The final sample extract (1 µL) was injected in the pulsed-splitless mode (0.6 min) onto SLB5-MS (30 m × 0.25 mm i.d. × 0.25 µm thickness) with hydrogen as carrier gas at a flow rate (constant flow) of 0.9 mL min−1. The temperatures of injector and detector were set at 250 and 300 °C, respectively. The oven temperature program was 100 °C (1 min) to 200 °C at 10 °C min−1, increased to 220 °C at 1 °C min−1, then ramped to 280 °C at 5 °C min−1 and held for 10 min. A second SPB-608 fused silica column 30 m × 0.32 mm ID × 0.25 µm film thickness was used for confirmation of detected analytes.
Statistical analysis
Database management and statistical analysis were performed with Microsoft Excel (version 16.04339) and XLSTAT (version 2016.02.27444; Addinsoft). Data under detection limits were set to zero for statistical analysis. According to the Shapiro–Wilk test (p < 0.05) and even after the log, square root, or cube root, the data significantly deviated from normal distribution, thus nonparametric statistics were used. To characterize the distribution of examined contaminants in analyzed milk samples, concentrations are expressed with a median, the 25th, 75th, and 95th percentiles, range, and frequency of detection. The values of mean and standard deviation are also given as indicator values for comparison purposes. All p-values below 0.05 were considered statistically significant.
Quality control
Identification of examined OCs in all samples was confirmed; their concentrations were measured using surrogate standards (to monitor analyte recovery) and internal standards (to correct for injection errors) and were expressed on a fat weight basis unless otherwise specified.
The procedures of quantification and validation were performed following some parameters of the CEAEQ method MA. 400—BPCHR 1.0 (CEAEQ 2015). A complete quantitative calibration for all target analytes (concentrations ranging between 1 to 200 ng mL−1) was conducted, and randomly selected single calibration points were checked before, in the middle, and at the end of analyzing each series of samples. The linearity was verified, and the deviation of the average relative response factors (RRF) of each compound was less than 15%. To ensure the quality of the data, procedural blanks were regularly performed after every ten samples to check for interferences or contamination from solvent, glassware, equipment, and instrumentation. No quantifiable amounts of target compounds were detected in the blanks. The average surrogate recovery in spiked milk samples at two fortification levels in triplicate (1 et 0.1 ng mL−1) was higher than 75% with relative standard deviations (RSD) lower than 10%. Relative recoveries of PCB congeners ranged between 88 and 102%, and for OCP, they were 91–105%. Inter- and intra-day variability was calculated by analyzing fortified samples within the same day (repeatability) and on different days (intermediate precision), respectively, and were always < 15%. The limit of detection (LOD) and quantification were estimated as the concentration of analyte in the sample, producing a peak with a signal-to-noise ratio (S/N) of 3/1 and 10/1, respectively. The corresponding LOD and LOQ for individual OCPs and PCBs were 0.01 to 0.09 ng g−1 fat and 0.02 to 0.24 ng g−1 fat.
Estimation of dietary intake and risk assessment
Human health risk assessment is the process of determining if exposure to a chemical, at any dose, could cause an increase in the incidence of adverse health impacts. It can be assessed using a variety of approaches. The most widely used are estimation of daily intake (EDI) and comparison with the tolerable/acceptable daily intake (TDI/ADI) fixed in many guidelines, and application of the model derived from US-EPA to estimate the carcinogenic and noncarcinogenic risks.
The estimated daily intakes (EDI) were calculated by the following equation (Pardio et al. 2003) (1):
where EDI is the estimated daily intake (ng kg−1 day−1), DCR is the daily consumption rate (g d−1), Cfat is the fraction of fat in each sample CA is the 50th percentile concentration of target compounds (ng g−1 fat) in samples (or 95th percentile concentration for high exposure), and BW is the mean body weight for adults (kg). The consumption data are founded from the consumption database of the National Institute of Nutrition and Food Technology (2013). About 300 g of milk are consumed daily by an adult weighing 70 kg. To evaluate the risk of exposure to the examined contaminants via raw milk consumption were assessed according to the approaches described by US-EPA (US-ATSDR 2022). Noncarcinogenic health risks were evaluated by calculating hazard quotient (HQ) using Eq. (2):
where EDI is the estimated daily intake and RfD is recommended reference dose (US-EPA 2005).
To assess the overall potential effects posed by the different OCs, the HQs calculated for OCPs and PCBs were summed and expressed as the hazard index (HI) with Eq. (3):
In cases where HI values did not exceed one, it was assumed that no chronic risk was likely to occur.
From the US-EPA (1997) exposure factor handbook, Lifetime Cancer Risk (LCR) is calculated as follows:
where CSF is the slope factor of the contaminant via oral exposure route CSF.
The total cancer risk can be calculated from:
where EDIi is the estimated daily intake of compound/group of compounds and CSFi is the corresponding value of the cancer slope factor.
US-EPA uses a risk level of 10−6 as the point at which risk management decisions can be considered. Risk management decisions most frequently used are in the total cancer risk range of 10−6–10−4.
Results and discussion
Residue levels and contamination status
The global result of OCP and PCB concentrations in raw bovine milk samples are summarized in Table 1 and expressed in nanograms per gram of extracted fat (ng g−1 fat). All samples studied were positive for one or more residues. The highest detection frequencies were obtained in the following descending order of PCBs > DDTs > HCB = HCHs. These results show ubiquitous contamination by these chemicals in this food item and possible accumulation and health implications for the consumers, despite their prohibition of use in Tunisia before 1990. As regards the residue levels of OCs, the analyzed kinds of milk are generally marked by the predominance of DDTs, followed by HCB, PCBs, and HCHs. The results are consistent with those of recent studies in Tunisia finding DDT and HCB predominant in human biological media, including breast milk (Ben Hassine et al. 2012), blood (Ben Hassine et al. 2014), and adipose tissues (Achour et al. 2017).
Organochlorine pesticides
All milk samples were contaminated with one or more pesticides. The total OCP concentrations ranged from 0.87 to 141.08 ng g−1 fat, with a median value of 35.20 ng g−1 fat. The relative contribution of the different pesticides detected compared to the sum of pesticides in contaminated samples of milk is presented in Fig. 2. The median concentration of DDTs was 17.60 ng g−1 fat, which contributes approximately 53% to the sum-OCPs. The frequency of detection of DDT and its metabolite following this order in the investigated matrix: DDE > DDD > DDT. p,p’-DDE was found to be the major organochlorine contaminant (median 14.45 ng g−1 fat), detected in 73.3% of samples and accounted for about 58% of total DDTs. p,p’-DDT was detected only in six milk samples, with concentrations ranging from nd to 39.5 ng g−1 fat and represented 17% of DDTs. It is well-known that the concentration ratio of p,p’-DDT/p,p’-DDE could be used to distinguish between current and historical exposure to DDT (Ahlborg et al. 1995). DDT/DDE ratios below one indicate high persistence in the environment and ongoing biomagnification, while those above one indicate both increasing exposure and ongoing biomagnification to DDT (Ozcan and Aydin 2009). This ratio across the samples, when the two chemicals were detected, vary between 0.09 and 32.24, suggesting the presence of a continuing source of use of DDT, despite the lengthy period of time since this pesticide was banned in Tunisia (1981) (APEK 2005). Moreover, the presence of p,p’-DDT alone in two samples with levels of the two principal metabolites (DDE and DDD) at sublimit of detection may confirm this result. This finding was also deducted in recent studies that measured these compounds in human adipose tissue (Achour et al. 2017), human milk (Ben Hassine et al. 2012), and sediments collected from Bizerte, the neighboring of Béja region (Barhoumi et al. 2014). The authors suggest that illegal usage in some suburban agriculture and leakage from the old stockpiles deposited under unsatisfactory conditions may be responsible. Despite these findings cited above, the mean concentration of p,p’-DDE and p,p’-DDT reported by Sabbah and Bouguerra (1997) in Tunisia (89.6 and 6 ng g−1 fat, respectively) could be an indication of declining contamination status.
When comparing the sum of DDT residues recorded in this study (mean: 25.75 ng g−1 fat) with the most recent international studies, the data show that were significantly lower than Ethiopia (mean: 269–477 ng g−1 fat) (Gebremichael et al. 2013), Egypt (mean: 223 ng g−1 fat) (Abou Donia et al. 2010), India (mean: 156.77 ng g−1 fat) (Aslam et al. 2013) and Uganda (mean: 52 ng g−1 fat) (Kampire et al. 2011), comparable with those in raw milk samples from Mexico (mean: 26.48 ng g−1 fat) (Pardío Sedas et al. 2021), Jordan (mean: 27 ng g−1 fat) (Salem et al. 2009) and Pakistan (mean: 24.82 ng g−1 fat) (Fazal et al. 2022), but higher than DDTs levels in Poland (mean: 9.20 ng g−1 fat) (Pietrzak-Fiećko 2018), Turkey (mean: 12.22 ng g−1 fat) (Aydin et al. 2019), Mexico (mean: 0.44–2.44 ng g–1 fat) (Gutierrez et al. 2012) and China (mean: 2–3 ng g−1 fat) (Zhang et al. 2017).
As the second most predominant OCP, HCB was detected in 76.7% of milk samples and accounted for approximately 33% of total OCPs with a median value of 14.31 ng g−1 fat (Fig. 2). This current finding indicates that HCB contamination sources still exist in this area and we cannot eliminate the hypothesis of a secret use of this chemical in the agriculture as a fungicidal treatment for seeds and olive tree (Sabbah and Bouguerra 1997). In addition, the amount of HCB may be attributed not only to its prior legal or illegal use but also to its release from industrial processes at high temperatures, such as industrial processes, incineration, and water or wastewater treatment (Thomsen et al. 2009; Van Birgelen 1998). It may also exist as an impurity in several formulations of pesticides or chlorinated solvents (Erdogrul et al. 2005). This present result was also observed in a recent study in Tunisia, which recognized that HCB was among the most concentrated OCP after the p,p’-DDE in many humans (Achour et al. 2017; Ben Hassine et al. 2012, 2014) and environmental samples (Barhoumi et al. 2014; Ben Ameur et al. 2013a, b). On comparing our results with those obtained by Sabbah and Bouguerra (1997) during 1994 in Tunisia, we observed that contamination by HCB has fallen about four times in milk. Furthermore, the comparison of HCB amount (mean: 16.34 ng g−1 fat) in raw milk from Béja region with worldwide studies shows that is relatively higher than those reported from Brazil (mean: 0.52 ng g−1 fat) (Avancini et al. 2013), Turkey (mean: 0.30 ng g−1 fat) (Bulut et al. 2011), Tibet (mean: 1.00 ng g−1 fat) (Pan et al. 2014), and Spain (median: 2.22–3.67 ng g−1 fat) (Luzardo et al. 2012) but extremely lower than those reported from Egypt (mean: 150 ng g−1 fat) (Abou Donia et al. 2010).
For HCHs, the γ- and β-isomers were detected in 56.6 and 40% of the collected raw milk, respectively. It was shown that this group of contaminants has the lowest concentration (median: 0.77 ng g−1 fat; mean: 6.88 ng g−1 fat) and accounts for about 14% of total OCPs. This is due to their lower potential of bioaccumulation (Loganathan and Kannan 1994) and the rapid evaporation to the atmosphere after their application (Ramesh et al. 1991). Among the HCHs measured, γ-HCH was more abundant isomer in the analyzed samples. When compared with data from similar studies around the world, concentrations of HCHs (mean: 6.88 ng g−1 fat) in raw cow milk in Beja were similar to those in Tibet (mean: 4.46 ng g−1 fat) (Pan et al. 2014) and were slightly lower than those in Uganda (mean: 26 ng g−1 fat) (Kampire et al. 2011) and were much lower than those in Turkey (mean: 63.6 ng g−1 fat) (Aydin et al. 2019), Jordan (mean: 83 ng g−1 fat) (Salem et al. 2009), Romania (mean: 66.3–131.0 ng g−1 fat) (Rusu et al. 2016), Egypt (mean: 165 ng g−1 fat) (Elsharkawy 2015), and India (mean: 349.65 ng g−1 fat) (Aslam et al. 2013).
Polychlorinated biphenyls
We found that all milk samples exhibited measurable concentrations of some target PCB congeners except PCB 18, 28/31, and 52. As seen in Table 1, PCBs (defined as the sum of all detected congeners) concentrations varied between 2.19 and 42.66 ng g−1 fat with a median value of 4.71 ng g−1 fat. The distribution profiles of individual PCB congeners in the analyzed samples are presented in Fig. 3. The contamination status is marked by the predominance of PCBs 153, − 138, and − 180 and account for approximately 79% of total PCBs detected in the milk samples. The dominance of these non-dioxin-like congeners (ndl-PCB) is related to their high lipophilicity, highly resistant to metabolic attack, and their abundance in commercial mixtures. Indeed, it has been generally considered that congeners having chlorine atoms in positions 2, 4, and 5 on one or both nuclei or having no vicinal hydrogen atoms in meta-para position are poorly metabolized (De Voogt 1996). This contamination profile of raw milk collected in Béja region is similar to those reported by EFSA (2012) in different European countries for milk and dairy products. This European agency recommended the use of six ndl-PCB indicators (PCB 28, − 52, − 101, − 138, − 153, and − 180) to monitor these chemicals in food of animal origin and in human tissue. It has been proposed that ndl-PCBs indicators represent around 50% of all the PCB congeners that can be quantified in these matrices. The sum of the six ndl-PCB concentrations recorded in this study ranged from 1.49 to 31.25 ng g−1 fat, with a median of 4.04 ng g−1 fat, respectively. This level was numerically similar to the levels in raw bovine milk samples collected from different countries in Europe, such as Belgium (median: 4.72 ng g−1 fat) (Cimenci et al. 2013), Italia (median: 6.74 ng g−1 fat) (Lorenzi et al. 2016) and France (median: 3.0 ng g−1 fat) (Arnich et al. 2009). Moreover, it has been recognized that PCB 118 (dioxin-like PCB: dl-PCB) was among the most commonly detected and quantified congeners in milk (EFSA 2012). In our study, it is detected in 80% of samples with concentrations ranging from nd to 5.24 ng g−1 fat and contributes approximately 3% of the PCBs.
Comparison of contamination profile between farms
Contamination of milk with persistent organic pollutants is multifactorial: it is dependent on environmental factors, factors specific to the farming system (fodder and potentially contaminated soil, lactation stage, and health status of the herd), and the ability of the animals to metabolize and biotransform the pollutants ingested. Many studies demonstrate that dairy ruminants are exposed to OCs mainly via ingestion of contaminated fodder or soil (Rychen et al. 2014, 2008). It has been revealed that pasture can be polluted from the deposition of contaminants from the atmosphere to both the vegetation and soil (Thomas et al. 1998). Moreover, the high levels of pollutants such as PCBs and OCPs can be a consequence of the formulation of animal feeds, particularly if they contain fishmeal (Jacobs et al. 2004). The distribution of total concentration of HCB, HCHs DDTs, and PCBs in raw milk samples collected in the different investigated farms are presented in Fig. 4. The highest total HCB values were found in milk samples collected in farms 5 (132.56 ng g−1 fat) and 6 (136.78 ng g−1 fat). It may be due to the historical use of this pesticide in this area and also a possible increase, as mentioned before, through waste incineration near El Maâgoula, located a few kilometers southeast of Béja City. The other farms display similar contents of total HCB in the milk samples, and varied from 42.87 to 69.51 ng g−1 fat. The high levels of total DDTs were detected on farms 1, 2, and 3. These levels ranged from 154.17 to 191.49 ng g−1 fat. These breeding units are surrounded by fields that are used to cultivate cereals, and marked by a historical input of pesticides and exposure of animals during grazing may explain these high levels. The minimum values of total HCHs were recorded in a farm situated in rural area (farm 4: 2.74 ng g−1 fat; farm 6: 5.01 ng g−1 fat), while the other farms followed almost an equal trend of HCHs distribution ranging from 39.51 to 64.95 ng g−1 fat. The high values might result from recent use in the urban zone against heat lice and scabies or as vector control by veterinarians in these studied farms (Li 1999). The relatively high concentrations of PCBs are observed in farm 3 (86.10 ng g−1 fat) and could relate local anthropogenic sources especially the municipal garbage landfill and the old industrial unit using boilers for the generation of steam. There was no important difference between the total PCB levels reported in milk samples taken from farms 1, 2, and 5, which may be impacted by industrial and urban activities, and those from farm 4, which is located in a rural area. It is noteworthy that despite the presence of possible sources of contamination close to the studied farms, the Kruskal–Wallis analysis showed that there are no statistically significant differences in the amounts of HCB, DDTs, HCHs, and PCBs in the different farms (p > 0.05). This may be due to the small sample size or similar cattle feeding system, but the historical use, volatilization, as well as redistribution of these persistent pollutants in the different compartments of the environment over the years may explain also this result. Additionally, atmospheric transportation from neighboring regions, such as Bizerte and Grand Tunis, could also contribute to this finding. Barhoumi et al. (2018) have proven that there are relatively high levels of HCB, γ-HCH, p,p’-DDE, p,p’-DDT, and PCBs in the aerosols of Bizerte. These areas are highly populated and characterized by large-scale industrial activity. Furthermore, old pesticide stockpiles and transformers containing PCBs are still being used or are being stored in unsatisfactory conditions in these regions (UNEP 2007).
Tolerance limits and health risk assessment
The obtained residues of OCs were firstly compared to the strict maximum residual limits (MRLs) fixed by the European Union (EU) (Commission Regulation (EU) 2017; EFSA 2012). It should be noted that exceeding the MRLs is not legal for trade but does not necessarily imply a risk to health. The percentage of raw milk samples exceeding the limit was 20% for β-HCH and 13.3% for lindane (Table 1). DDT residues exceeded the MRL in only four samples, and HCB exceeded its MRL in 60% of samples. For PCBs, the European Community fixed two threshold values, one for non-dioxin-like (sum of the six ndl-PCB indicators) and the other for PCB-like (dl-PCB). For non-dioxin-like congeners, the LMR is fixed at 40 ng g−1 fat for the sum of the six ndl-PCB indicators in milk. In this study, the determined levels of this group ranged between 2.67 and 31.25 ng g−1 fat and were lowering the recommended limit.
To understand the magnitude of exposure and possible health risk associated with OCs via raw milk consumption, the estimated daily intakes (EDI) were calculated and compared with tolerable/acceptable daily intake (TDI/ADI) and EPA toxicity references (RfD and CSF). The upper values of EDI based on 95th percentile measured concentrations of DDTs and lindane were far below the ADIs set in many guidelines (JMPR-FAO/WHO 2003; US-EPA 1996; Van Oostdam et al. 1999) (Table 2). The 95th EDI value calculated for the HCB is 25 and 40 times lower than the ADI value established, respectively, by EFSA (EFSA 2006) and Health Canada (Van Oostdam et al. 1999). For ndl-PCB indicators, estimated 95th EDI value is lower than the TDI of 10 ng kg−1 day−1 fixed by the Netherlands National Institute for Public Health and the Environment (RIVM) and adopted by EFSA (EFSA 2005).
In addition, EDI data of OCs associated with milk intake for adults are not available in Tunisia, and obtained values were compared with those measured in fish (Ben Ameur et al. 2013a) and mullet (Ben Ameur et al. 2013b) collected from Bizerte region (North of Tunisia). The EDI values in our study for HCB and DDTs were much higher (3–4 orders of magnitude), for PCBs were lower (2–4 orders of magnitude), and comparable for lindane than those obtained for the fish species. The intake of fatty foods (fish, dairy, and meat) might play a crucial role in OCPs and PCBs exposure levels. For several socio-economic reasons and frequency of consumption, the EDI obtained for raw milk may be the major part of the total daily intake of these contaminants for the local population. If we regard the obtained data, we can generally assume that global intake of OCs stays always lower than recommended ADI, indicating that there is no potential health risk associated with these foods’ consumption in Tunisia.
On the other hand, the comparison with worldwide studies shows that obtained EDI based on 95th percentile value for DDTs (14.55 ng kg−1 day−1) were lower than those reported EDI in milk samples from Turkey (4780 ng kg−1 day−1) (Aydin et al. 2019), Iran (150 ng kg−1 day−1) (Bayat et al. 2011), Ethiopia (79 ng kg−1 day−1) (Gebremichael et al. 2013), Egypt (46 ng kg−1 day−1) (Abou Donia et al. 2010), India (35 ng kg−1 day−1) (Nag and Raikwar 2008), Iran (26 ng kg−1 day−1) (Ashnagar et al. 2009), similar to those from Poland (9 ng kg−1 day−1) (Radzymińska et al. 2008) and Romania (3 ng kg−1 day−1) (Georgescu et al. 2011). The EDIs of HCB and lindane were with respective values of 2.45 and 0.09 ng kg−1 day−1 were lower than those obtained in Iran (HCB: 90 ng kg−1 day−1; lindane: 111 ng kg−1 day−1) (Bayat et al. 2011) and higher than in Spain (HCB: 0.53 ng kg−1 day−1; lindane: 0.01 ng kg−1 day−1) (Luzardo et al. 2012). Concerning EDI for PCBs, our central tendency based on the median (0.81 ng kg−1 day−1) was lower than estimated in other countries, such as Turkey (1140 ng kg−1 day−1) (Aydin et al. 2019), Iran (260 ng kg−1 day−1) (Bayat et al. 2011), close to Italy (0.78 ng kg−1 day−1) (Lorenzi et al. 2016) and higher than Belgium (0.1 ng kg−1 day−1) (Cimenci et al. 2013).
The health risk assessments for dietary exposure to OCs by raw milk consumption for the local inhabitants were calculated using EPA approach (Table 2). As for the noncancer risks, the values of hazard quotient (HQ) for PCBs and OCPs based on their 50th and 95th percentile concentrations were lower than 0.040 and 0.237, respectively. The cumulative risk assessment (HI) remains less than unity even if using the higher exposure levels, indicating that the exposure to OCPs and PCBs via raw milk intake is unlikely to pose any noncarcinogenic effects to consumers. In contrast, the lifetime cancer risks (LCR) of examined compounds were all exceed one in one million except for lindane with median concentration. The total cancer risks (TCR) associated with milk consumption based on the 50th and 95th percentile levels were 6.68 and 31.90, respectively. The obtained results indicate that the intake of these compounds by only the studied food item can cause carcinogenic effects in the human body. In addition, the contribution of LCR to TCR based on central tendency reflected highest share of HCB (59%) followed by PCBs (24%), and DDTs (15%). The results indicate that these OCs may be of particular concern. This finding is also mentioned by Ben Ameur et al. (2013a), who studied health risk assessments related to these contaminants via consuming fish collected in the north of Tunisia. Moreover, we can be seen a disagreement between the EPA and WHO approaches (EDI/ADI), which also covers carcinogenic effects, concerning the possible cancer risk. This supports further the idea that a risk level of 10−6 is a highly conservative approach (Vogt et al. 2012). The highest value in our study is well below the EPA threshold of 10−4, which indicates a relatively low cancer risk (US-EPA 2009), even though it is necessary to monitor the milk as well as feeds and fodder intended for animal consumption regularly.
Conclusion
Located in the northwest of Tunisia, Béja is ranked among the first governorates in agricultural production, and it is one of the largest milk production areas. Raw milk is among the most accessible and frequent sources of animal fatty food for local inhabitants. In order to evaluate the contamination status with lipophilic contaminants such as OCPs and PCBs and to assess the potential health risks related to its consumption, raw bovine milk was collected from the milking equipment for different animals and various farms located in the studied region.
All analyzed samples showed measurable residue of examined OCs, and the contamination profile is marked by the predominance of DDTs, followed by HCB, PCBs, and HCHs. Comparison of the level of OCPs in our study with the only data available in Tunisia for more than 20 years shows a decreasing trend of these prohibited chemicals in cow milk samples. However, the presence of p,p’-DDT in some samples supported the hypothesis of an active and continuous source of its illegal use in this region. This finding is in accordance with recent studies investigated in Tunisia that measured these compounds in human biological media. The contamination status of raw milk with PCBs is marked by the predominance of congeners − 153, − 138, and − 180, and the recorded concentrations and accumulation patterns are similar to those that are found in European countries. The results indicate that historical or recent use of OCPs and possible sources of emission of PCBs in the vicinity of the studied farms may contribute to the increase in levels of these contaminants in produced milk, despite the fact that there are no statistical significant differences.
The dietary intake levels of examined OCPs and PCBs by local consumers of row milk were far below the acceptable or tolerable daily intake (TDI/ADI) established by many international guidelines, despite the fact that in some case level of OCPs exceeded the strict maximum residual limits (MRLs) fixed by the European Union (EU). The results of the health-risk assessment (carcinogenic and noncarcinogenic effects) revealed that there is no indication of potential health risks associated with this food consumption in Béja region, even if using a high level of contamination.
Therefore, these pollutants should be preferentially managed as priority contaminants in Tunisia, and is a necessity to define the contamination routes and increase the efforts to reduce human exposure through food consumption. Thus, it remains necessary to launch a national program to monitor these contaminants in a large number of different food products of animal origins, drinks, potable water, aerosol, and house dust to clarify the variation of human exposure, accumulation levels, and exposure sources across the most representative region of our country.
Data availability
Data will be made available upon request.
References
Abou Donia MA, Abou-Arab AAK, Enb A, El-Senaity MH, Abd-Rabou NS (2010) Chemical composition of raw milk and the accumulation of pesticide residues in milk products. Glob Vet 4:6–14
Achour A, Derouiche A, Barhoumi B, Kort B, Cherif D, Bouabdallah S, Sakly M, Rhouma KB, Touil S, Driss MR (2017) Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue from northern Tunisia: current extent of contamination and contributions of socio-demographic characteristics and dietary habits. Environ Res 156:635–643. https://doi.org/10.1016/j.envres.2017.04.021
Agence de promotion de l’industrie et de l’innovation (APII) (2017) Monographies sectorielles et régionales – Statistiques de l’industrie tunisienne. Gouvernorat de Béja. http://www.tunisieindustrie.nat.tn/fr/home.asp. Accessed 2022
Ahlborg UG, Lipworth L, Titus-Ernstoff L, Hsieh C-C, Hanberg A, Baron J, Trichopoulos D, Adami H-O (1995) Organochlorine compounds in relation to breast cancer, endometrial cancer, and endometriosis: an assessment of the biological and epidemiological evidence. Crit Rev Toxicol 25:463–531. https://doi.org/10.3109/10408449509017924
ANGED (2010) Agence nationale de gestion des dechets: etude d’impact cadre environnemental et social du projet de gestion et elimination des PCBs & d’amelioration de la gestion des dechets d’activites sanitaires en tunisie. http://www.anged.nat.tn/user_files/rapport-annuel-2010437.pdf. Accessed 2018
APEK (2005) Tunisia country situation report. Association for environmental protection in Kerkennah, Tunisia. http://www.ipen.org. Accessed 2018
Arnich N, Tard A, Leblanc JC, Le Bizec B, Narbonne JF, Maximilien R (2009) Dietary intake of non-dioxin-like PCBs (NDL-PCBs) in France, impact of maximum levels in some foodstuffs. Regul Toxicol Pharmacol 54:287–293. https://doi.org/10.1016/j.yrtph.2009.05.010
Ashnagar A, Naseri NG, Farmad MC (2009) Determination of organochlorine pesticide residues in cow’s milk marketed in Ahwaz city of Iran. Int J PharmTech Res 1:247–251
Aslam M, Rais S, Alam M (2013) Quantification of organochlorine pesticide residues in the buffalo milk samples of Delhi City, India. J Environ Prot 04:964–974. https://doi.org/10.4236/jep.2013.49111
ATSDR (2022) Calculating hazard quotients and cancer risk estimates. https://www.atsdr.cdc.gov/pha-guidance/conducting_scientific_evaluations/epcs_and_exposure_calculations/hazardquotients_cancerrisk.html#CommonNonCancerGuidelines. Accessed 2022
ATSDR (2002) Toxicological Profiles Web Version Toxicological Profile for Polychlorinated Biphenyls (PCBs). CRC Press
Atlas Numérique du Gouvernorat de Béja (2016) In: Ministère de l’Equipement de l’Habitat et de l’Aménagement du Territoire: la Direction Générale de l’Aménagement du Territoire (Hrsg). http://www.mehat.gov.tn/fileadmin/user_upload/Amenagement_Territoire/AtlasGouvernoratBejaFr.pdf. Accessed 2021
Australian milk residue analysis survey (2022)Annual Repport 2021-22. https://www.agriculture.gov.au/sites/default/files/documents/amra-ar-2021-22.pdf. Accessed 2022
Avancini RM, Silva IS, Rosa AC, SarcinelliPde N, de Mesquita SA (2013) Organochlorine compounds in bovine milk from the state of Mato Grosso do Sul-Brazil. Chemosphere 90:2408–2413. https://doi.org/10.1016/j.chemosphere.2012.10.069
Aydin S, Aydin ME, Beduk F, Ulvi A (2019) Organohalogenated pollutants in raw and UHT cow’s milk from Turkey: a risk assessment of dietary intake. Environ Sci Pollut Res Int 26:12788–12797. https://doi.org/10.1007/s11356-019-04617-0
Barhoumi B, LeMenach K, Devier MH, El megdiche Y, Hammami B, Ben Ameur W, Ben Hassine S, Cachot J, Budzinski H, Driss MR (2014) Distribution and ecological risk of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in surface sediments from the Bizerte lagoon, Tunisia. Environ Sci Pollut Res Int 21:6290–302. https://doi.org/10.1007/s11356-013-1709-7
Barhoumi B, Jouili S, Elbarhoumi A, Derouiche A, El Megdiche Y, Bouabdallah S, Touil S, Driss MR (2016) Organochlorines in the surface sediments of the Bahiret el Bibane lagoon (Tunisia, Southwestern Mediterranean Sea). Chem Ecol 32:884–897. https://doi.org/10.1080/02757540.2016.1195817
Barhoumi B, Castro-Jimenez J, Guigue C, Goutx M, Sempere R, Derouiche A, Achour A, Touil S, Driss MR, Tedetti M (2018) Levels and risk assessment of hydrocarbons and organochlorines in aerosols from a North African coastal city (Bizerte, Tunisia). Environ Pollut 240:422–431. https://doi.org/10.1016/j.envpol.2018.04.109
Bayat S, Esmaili Sari A, Bahramifar N, Younesi H, DahmardeBehrooz R (2011) Survey of organochlorine pesticides and polychlorinated biphenyls in commercial pasteurized milk in Iran. Environ Monit Assess 175:469–474. https://doi.org/10.1007/s10661-010-1544-y
Ben Ameur W, El Megdiche Y, Eljarrat E, Ben Hassine S, Badreddine B, Souad T, Bechir H, Barcelo D, Driss MR (2013a) Organochlorine and organobromine compounds in a benthic fish (Solea solea) from Bizerte Lagoon (northern Tunisia): implications for human exposure. Ecotoxicol Environ Saf 88:55–64. https://doi.org/10.1016/j.ecoenv.2012.10.021
Ben Ameur W, Trabelsi S, El Megdiche Y, Hassine SB, Barhoumi B, Hammami B, Eljarrat E, Barcelo D, Driss MR (2013b) Concentration of polychlorinated biphenyls and organochlorine pesticides in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) from Bizerte Lagoon (Northern Tunisia). Chemosphere 90:2372–2380. https://doi.org/10.1016/j.chemosphere.2012.10.028
Ben Hassine S, Ben Ameur W, Gandoura N, Driss MR (2012) Determination of chlorinated pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human milk from Bizerte (Tunisia) in 2010. Chemosphere 89:369–377. https://doi.org/10.1016/j.chemosphere.2012.05.035
Ben Hassine S, Hammami B, Ben Ameur W, El Megdiche Y, Barhoumi B, El Abidi R, Driss MR (2014) Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and their relation with age, gender, and BMI for the general population of Bizerte, Tunisia. Environ Sci Pollut Res Int 21:6303–6313. https://doi.org/10.1007/s11356-013-1480-9
Bulut S, Akkaya L, Gok V, Konuk M (2011) Organochlorine pesticide (OCP) residues in cow’s, buffalo’s, and sheep’s milk from Afyonkarahisar region, Turkey. Environ Monit Assess 181:555–562. https://doi.org/10.1007/s10661-010-1849-x
Carpenter DO (2013) Effects of persistent and bioactive organic pollutants on human health. John Wiley & Sons
Centre d'Expertise en Analyse Environnementale du Québec (2015) Détermination des biphényles polychlorés (congénères): dosage par chromatographie en phase gazeuse Couplé à un spectromètre de masse à haute résolution, Méthode MA. 400 – BPCHR 1.0. Ministère du Développement durable, de l’Environnement et des Pacs du Québec. https://www.ceaeq.gouv.qc.ca/methodes/pdf/MA400BPCHR10.pdf. Accessed 2016
Cimenci O, Vandevijvere S, Goscinny S, Van Den Bergh MA, Hanot V, Vinkx C, Bolle F, Van Loco J (2013) Dietary exposure of the Belgian adult population to non-dioxin-like PCBs. Food Chem Toxicol 59:670–679. https://doi.org/10.1016/j.fct.2013.06.020
Commission Regulation (EU) (2017) Legislation (L 150/1): amending Annexes II, III and V to regulation (EC) no 396/2005 of the European Parliament and of the council as regards maximum residue levels for fluopyram; hexachlorocyclohexane (HCH), alpha-isomer; hexachlorocyclohexane (HCH), beta-isomer; hexachlorocyclohexane (HCH), sum of isomers, except the gamma isomer; lindane (hexachlorocyclohexane (HCH), gamma-isomer); nicotine and profenofos in or on certain product. Off J Eur Union 60:1–37
De Voogt P (1996) Ecotoxicology of chlorinated aromatic hydrocarbons. Issues Environ Sci Technol 6:89–112
Derouiche A, Sanda Y, Driss M (2004) Polychlorinated biphenyls in sediments from Bizerte lagoon, Tunisia. Bull Environ Contam Toxicol 73:810–817. https://doi.org/10.1007/s00128-004-0499-5
DjienLiem A, Furst P, Rappe C (2000) Exposure of populations to dioxins and related compounds. Food Addit Contam 17:241–259
EFSA (2005) Opinion of the scientific panel on contaminants in the food chain [CONTAM] related to the presence of non dioxin-like polychlorinated biphenyls (PCB) in feed and food. EFSA J 284:1–137
EFSA (2006) Opinion of the scientific panel on contaminants in the food chain (CONTAM) related to hexachlorobenzene as undesirable substance in animal feed. EFSA J 402:1–49. https://doi.org/10.2903/j.efsa.2006.402
EFSA (2012) Update of the monitoring of levels of dioxins and PCBs in food and feed. EFSA J 10:2832. https://doi.org/10.2903/j.efsa.2012.2832
Elsharkawy EE (2015) Organochlorine and organophosphorus pesticide residues in raw buffalo milk from agroindustrial areas in Assiut, Egypt. J Dairy Vet Animal Res 2. https://doi.org/10.15406/jdvar.2015.02.00049
Ennaceur S, Gandoura N, Driss MR (2007) Organochlorine pesticide residues in human milk of mothers living in northern Tunisia. Bull Environ Contam Toxicol 78:325–329. https://doi.org/10.1007/s00128-007-9185-8
Ennaceur S, Gandoura N, Driss MR (2008) Distribution of polychlorinated biphenyls and organochlorine pesticides in human breast milk from various locations in Tunisia: levels of contamination, influencing factors, and infant risk assessment. Environ Res 108:86–93. https://doi.org/10.1016/j.envres.2008.05.005
Erdogrul Ö, Covaci A, Schepens P (2005) Levels of organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in fish species from Kahramanmaras, Turkey. Environ Int 31:703–711. https://doi.org/10.1016/j.envint.2005.01.002
Fazal A, Ismail A, Naeem I, Oliveira CA, Shaukat S, Saleem MU, Saima S, Nasir U, Alam A, Aslam Z (2022) Exposure assessment of selected pesticide residues using occurrence data in foods and serum samples in Pakistan. Food Sci Technol 42. https://doi.org/10.1590/fst.01222
Fiedler H (2003) Persistent organic pollutants. Springer, Berlin Heidelberg
Gebremichael S, Birhanu T, Tessema DA (2013) Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia. Chemosphere 90:1652–1657. https://doi.org/10.1016/j.chemosphere.2012.09.008
Georgescu B, Georgescu C, Dărăban S, Tania M (2011) Assessment of persistant organic pollutants acting as endocrine disruptor chemicals in animal fat, cow milk and lacteous sub-products from Cluj County, Romania. Animal Biology and Animal Husbandry 3:1–9
Gutierrez R, Ruiz JL, Ortiz R, Vega S, Schettino B, Yamazaki A, de Lourdes RM (2012) Organochlorine pesticide residues in bovine milk from organic farms in Chiapas, Mexico. Bull Environ Contam Toxicol 89:882–887. https://doi.org/10.1007/s00128-012-0764-y
Harrad S (2012) Persistent organic pollutants: environmental behaviour and pathways of human exposure. Springer, US
Jacobs MN, Covaci A, Gheorghe A, Schepens P (2004) Time trend investigation of PCBs, PBDEs, and organochlorine pesticides in selected n− 3 polyunsaturated fatty acid rich dietary fish oil and vegetable oil supplements; nutritional relevance for human essential n− 3 fatty acid requirements. J Agric Food Chem 52:1780–1788
Joint FAO and World Health Organization (2003) Inventory of IPCS and other WHO pesticide evaluations and summary of toxicological evaluations performed by the Joint Meeting on Pesticide Residues (JMPR): evaluations through 2002, World Health Organization
Kampire E, Kiremire BT, Nyanzi SA, Kishimba M (2011) Organochlorine pesticide in fresh and pasteurized cow’s milk from Kampala markets. Chemosphere 84:923–927. https://doi.org/10.1016/j.chemosphere.2011.06.011
LACTIMED (2015) Developing the typical dairy products of Bizerte and Beja: diagnosis and local strategy. https://anima.coop/wp-content/uploads/publications/lac_diagnosisreport_tunisia_en_final.pdf. Accessed 2020
Li Y-F (1999) Global technical hexachlorocyclohexane usage and its contamination consequences in the environment: from 1948 to 1997. Sci Total Environ 232:121–158
Loganathan BG, Kannan K (1994) Global organochlorine contamination trends: an overview. Ambio 23:187–191
Lorenzi V, Ghidini S, Angelone B, Ferretti E, Menotta S, Fedrizzi G, Varisco G, Foschini S, Diegoli G, Bertocchi L (2016) Three years of monitoring of PCDD/F, DL-PCB and NDL-PCB residues in bovine milk from Lombardy and Emilia Romagna regions (Italy): contamination levels and human exposure assessment. Food Control 68:45–54. https://doi.org/10.1016/j.foodcont.2016.03.034
Luzardo OP, Almeida-Gonzalez M, Henriquez-Hernandez LA, Zumbado M, Alvarez-Leon EE, Boada LD (2012) Polychlorobiphenyls and organochlorine pesticides in conventional and organic brands of milk: occurrence and dietary intake in the population of the Canary Islands (Spain). Chemosphere 88:307–315. https://doi.org/10.1016/j.chemosphere.2012.03.002
Moreno Frias M, Jimenez Torres M, GarridoFrenich A, Martinez Vidal JL, Olea-Serrano F, Olea N (2004) Determination of organochlorine compounds in human biological samples by GC-MS/MS. Biomed Chromatogr 18:102–111. https://doi.org/10.1002/bmc.300
Nag SK, Raikwar MK (2008) Organochlorine pesticide residues in bovine milk. Bull Environ Contam Toxicol 80:5–9
National Institute of Nutrition and Food Technology (2013) http://www.institutdenutrition.rns.tn/. Accessed 2018
Necibi M, Mzoughi N (2020) Determination of organochlorine pesticides in the surface water from Medjerda river, Tunisia. Int J Environ Anal Chem 1–12. https://doi.org/10.1080/03067319.2020.1849668
Ozcan S, Aydin ME (2009) Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and organochlorine pesticides in urban air of Konya, Turkey. Atmos Res 93:715–722. https://doi.org/10.1016/j.atmosres.2009.02.012
Pan J, Gai N, Tang H, Chen S, Chen D, Lu G, Yang Y (2014) Organochlorine pesticides and polychlorinated biphenyls in grass, yak muscle, liver, and milk in Ruoergai high altitude prairie, the eastern edge of Qinghai-Tibet Plateau. Sci Total Environ 491:131–137. https://doi.org/10.1016/j.scitotenv.2014.03.074
Pardío Sedas VT, López Hernández KM, Flores Primo A, Uscanga Serrano R (2021) Effect of pasteurization on the concentration of dichlorodiphenyltrichloroethane (DDT) and hexachlorocyclohexane (HCH) in bovine milk. Revista mexicana de ciencias pecuarias 12 318–336. https://doi.org/10.22319/rmcp.v12i2.5483
Pardio VT, Waliszewski KN, Landin LA, Bautista RG (2003) Organochlorine pesticide residues in cow’s milk from a tropical region of Mexico. Food Addit Contam 20:259–269. https://doi.org/10.1080/0265203021000046207
Park YW (2009) Bioactive components in milk and dairy products. John Wiley & Sons
Pietrzak-Fiećko R (2018) Relationship between the content of chlorinated hydrocarbons and fatty acid composition of milk fat. J Vet Res 62:71–78. https://doi.org/10.2478/jvetres-2018-0010
Radzymińska M, Smoczyński S, Kopeć M (2008) Persistent organochlorine pesticide, lead, cadmium, nitrate (V) and nitrate (III) in Polish milk and dairy products. Polish Journal of Environmental Studies 17:95–100
Ramesh A, Tanabe S, Murase H, Subramanian A, Tatsukawa R (1991) Distribution and behaviour of persistent organochlorine insecticides in paddy soil and sediments in the tropical environment: a case study in South India. Environ Pollut 74:293–307. https://doi.org/10.1016/0269-7491(91)90077-A
Robertson LW, Hansen LG (2015) PCBs: recent advances in environmental toxicology and health effects. University Press of Kentucky. Lexington
Rose M, Fernandes A (2013) Persistent organic pollutants and toxic metals in foods. Elsevier Science
Rusu L, Harja M, Suteu D, Dabija A, Favier L (2016) Pesticide residues contamination of milk and dairy products. A case study: Bacau district area, Romania. J Environ Prot Ecol 17:1229–1241
Rychen G, Jurjanz S, Toussaint H, Feidt C (2008) Dairy ruminant exposure to persistent organic pollutants and excretion to milk. Animal 2:312–323. https://doi.org/10.1017/S1751731107001139
Rychen G, Jurjanz S, Fournier A, Toussaint H, Feidt C (2014) Exposure of ruminants to persistent organic pollutants and potential of decontamination. Environ Sci Pollut Res Int 21:6440–6447. https://doi.org/10.1007/s11356-013-1882-8
Sabbah S, Bouguerra M (1997) Organochlorine pesticides in cow’s milk from Tunisia. Fresenius Environ Bull 6:359–364
Salem NM, Ahmad R, Estaitieh H (2009) Organochlorine pesticide residues in dairy products in Jordan. Chemosphere 77:673–678. https://doi.org/10.1016/j.chemosphere.2009.07.045
Thomas GO, Smith K, Sweetman AJ, Jones KC (1998) Further studies of the air–pasture transfer of polychlorinated biphenyls. Environ Pollut 102:119–128
Thomsen M, Nielsen O-K, Illerup J (2009) Unintentional formation and emission of the persistent organic pollutants HCB and PCBs in the Nordic countries: documentation of existing informtion regarding sources and emissions to air, water and soil, with focus on reporting obligations according to the Stockholm Convention, The UNECE POP protocol, and PRTR registers. Tema Nord 518: 1–139
United Nations Environment Program (2007) Plan d'action national de la tunisie pour la mise en œuvre de la convention de stockholm sur les polluants organiques persistants. https://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-NIP-Tunisia-1.French.pdf. Accessed 2016
US-EPA (1996) EPA Integrated Risk Information System (IRIS) electronic database. US Environmental Protection Agency, Washington, DC
US-EPA (1997) Environmental protection agency. Exposure Factors Handbook Volume 1. USEPA Office of Research and Development. EPA/600/P-95/002FA, Washington, DC
US-EPA (2005) Guidelines for carcinogen risk assessment US environmental protection agency. EPA/630/P-03/001F, Washington, DC
US-EPA (2009) Risk Assessment guidance for superfund volume I: human health evaluation manual. EPA-540-R-070–002 Washington, DC
US-FDA (2022) FDA total diet study (TDS). https://www.fda.gov/food/fda-total-diet-study-tds/fda-total-diet-study-tds-1991-2017. Accessed 2022
USDA Foreign Agricultural Service (2014) Tunisia food and agricultural import regulations and standards-narrative.TS1407. https://agriexchange.apeda.gov.in/IR_Standards/Import_Regulation/FoodandAgriculturalImportRegulationsandStandardsNarrativeTunisTunisia9192014%202.pdf. Accessed 2018
Van Birgelen A (1998) Hexachlorobenzene as a possible major contributor to the dioxin activity of human milk. Environ Health Perspect 106:683–688. https://doi.org/10.1289/ehp.106-1533492
Van Oostdam J, Gilman A, Dewailly E, Usher P, Wheatley B, Kuhnlein H, Neve S, Walker J, Tracy B, Feeley M (1999) Human health implications of environmental contaminants in Arctic Canada: a review. Sci Total Environ 230:1–82. https://doi.org/10.1016/s0048-9697(99)00036-4
Vogt R, Bennett D, Cassady D, Frost J, Ritz B, Hertz-Picciotto I (2012) Cancer and non-cancer health effects from food contaminant exposures for children and adults in California: a risk assessment. Environ Health 11:83. https://doi.org/10.1186/1476-069X-11-83
Zhang Q, Xia Z, Wu M, Wang L, Yang H (2017) Human health risk assessment of DDTs and HCHs through dietary exposure in Nanjing, China. Chemosphere 177:211–216. https://doi.org/10.1016/j.chemosphere.2017.03.003
Acknowledgements
Our gratitude goes out to the editors and reviewers who provided comments and suggestions that helped improve the manuscript.
Funding
This study was supported by the Tunisian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Abdelkader Derouiche and Amani Achour. The first draft of the manuscript was written by Abdelkader Derouiche, and Mohamed Ridha Driss commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Consent to participate is not applicable.
Consent for publication
The participant has consented to the submission of the study to the journal.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Derouiche, A., Achour, A. & Driss, M.R. Organochlorine pesticides and polychlorinated biphenyls in raw bovine milk from various dairy farms in Beja, Tunisia: contamination status, dietary intake, and health risk assessment for the consumers. Environ Sci Pollut Res 30, 65427–65439 (2023). https://doi.org/10.1007/s11356-023-26976-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26976-5