Abstract
In this study, health risk of human exposure to organohalogenated pollutants (OHPs) through milk consumption was determined. Conventionally produced, unprocessed cow’s milk samples taken from Konya District, in Turkey, and 15 different brand ultra-high-temperature (UHT) processed cow’s milk samples taken from supermarkets of Turkey were analyzed for organochlorine pesticides (OCPs, α-, β-, γ-, and δ-HCHs, p,p’-DDE, p,p’-DDD, and p,p’-DDT, heptachlor, heptachlor epoxide, endosulfan I, endosulfan II, endosulfan sulfate, endrin, endrin aldehyde, endrin ketone, aldrin + dieldrin, methoxychlor), polychlorinated biphenyls (PCBs, PCB 28, 52, 101, 153, 138, and 180), and polybrominated diphenyl ethers (PBDEs, PBDE 47, 99, 100, 153, and 154 congeners). Estimated daily intake (EDI) values calculated for both adults and children consuming raw or UHT milk were determined to exceed maximum residue limits (MRLs) set for γ-HCH, ∑Heptachlor, and endrin. EDI values also exceeded admissible daily intake (ADI) values given for ∑HCH, ∑Heptachlor, ∑Endrin aldrin + dieldrin, and ∑PCBs. p,p’-DDT/p,p’-DDE ratio was 1 or higher for 66% of the milk samples, which is an indication of sustaining illegal use of DDT. A health risk is determined for dietary intake of OHPs via consumption of milk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the prohibition or restriction of their use, organohalogenated pollutants (OHPs) are still found in environmental matrixes as a result of their persistent and lipophilic nature (Erdoğrul et al. 2004). Most OHPs have very low solubility in water and limited vapor pressure. They are resistant to microbial degradation due to their high lipophilic properties (UNEP 2001; Kaushik and Kaushik 2007). Since they can dissolve in the oil, they can accumulate in the food chain, fatty tissues, and cells. Most pesticides cause cancer, heart disease, and Alzheimer’s and Parkinson’s diseases in human (Dhananjayan and Ravichandran 2018). Some organochlorine pesticides (OCPs) are classified as endocrine disrupting chemicals (Nassar et al. 2016). Polychlorinated biphenyls (PCBs) have been widely used as heat transfer and hydraulic fluids in electrical transformers and capacitors worldwide for many years due to their fire resistance and thermal stability (Janle et al. 2007). Since the 1970s, they have been banned, and aimed to be completely disposed of by the Stockholm Convention worldwide. However, due to their physicochemical properties, it is still possible to determine 7 PCB congeners (PCB 28, 52, 101, 118, 138, 153, 180) everywhere (Tremolada et al. 2014; Yeo et al. 2004). PCBs cause immune system disorders, behavioral changes, and reproduction problems (Grossman 2013). Polybrominated diphenyl ethers (PBDEs) have a similar structure to PCBs, exhibit chemical stability, strong lipophilicity, and persistency in environmental matrixes, and enter food chain (D’Silva et al. 2004; Schecter et al. 2008). PBDEs are commercially available in predominantly penta, octa, and deca formulations. These formulations differ from each other with 209 PBDE congeners (Lorber 2008). Since the 1960s, PBDEs have been used as flame retardants for thermoplastics used in electrical appliances, televisions, computer circuit boards, housings, and building materials. PBDEs are found in home and business furnishings, in foams and upholstery, and in the interior of cars, buses, trucks, and aircraft (WHO 1994; Erdoğrul et al. 2004). PBDEs can impair liver and thyroid function and cause neurotoxicity (Costa and Giordano 2007). PBDEs are on the persistent organic pollutants list of the Stockholm Convention (UNEP 2010).
Several studies have been conducted demonstrating the occurrence of OHPs in various foodstuffs, such as aquatic organisms, chicken, and egg (Sun et al. 2018; Zheng et al. 2015; Domingo 2014). OHPs were also widely analyzed in human milk samples (Leng et al. 2009; Behrooz et al. 2009; Meironyte et al. 1999).
Milk and dairy products represent a considerable part of total human dietary because of their nutritional benefits, low cost, and availability. Milk is necessary for protein, fat, and essential minerals especially for babies, schoolchildren, and old age people (Davies et al. 1986). However, contamination of routinely consumed foods with persistent micropollutants might pose a risk on human health. Milk and dairy products are fatty foods which make them ideal liquids for dissolving lipophilic micropollutants. Maximum residue limits (MRLs) were established for OHPs contamination of these foodstuffs by regulations. Admissible daily intakes (ADIs) were determined for OCPs and PCBs by international agencies.
There are studies demonstrating the OHPs contamination of cow’s milk; 196 cow’s milk samples from the Greek market were analyzed for DDT and its metabolites. At least one DDT metabolite or isomer was detected in 97.4% of milk samples, not exceeding European Union maximum residue limit. In the study, non-carcinogenic and carcinogenic risk effects were determined to be maximum for 1 to 3 years old children, consuming 250 mL milk for age 1 and 400 mL milk for age 3 (Tsakiris et al. 2015). Aslam et al. (2013) reported lindane contamination exceeding maximum residue limit for 50% of milk samples taken from Delhi City, India.
In the raw milk samples, ΣPBDEs (PBDE 28, 47, 100, 99, 154, 153, 183) were determined between 0.16 and 0.32 ng/g lipid wt. in Korea (Kim et al. 2013), 0.203 ng/g lipid wt. in Swiss (Grümping et al. 2006), and 0.407 ng/g lipid wt. in Ireland (Paepke et al. 2009). PBDEs concentrations have been increased in many environmental matrixes, such as foodstuff, human tissue, sewage sludge, and atmosphere, in the course of time (Schecter et al. 2010; Wang et al. 2007).
By nearly 17 million ton production, Turkey is among the top ten countries in the world ranking in the production of milk (https://www.worldatlas.com/articles/top-cows-milk-producing-countries-in-the-world.html). Hence, local products are in the market. Studies on contamination of milk are focused on human milk contamination in Turkey (Erdoğrul et al. 2004; Cok et al. 2003). There are a few studies in the literature about animal milk contamination in the country. Bulut et al. (2011) determined OCPs in 150 samples of sheep’s, cow’s, and buffalo’s milk produced in Afyonkarahisar Province, which is a close city to our sampling site, Konya Province. Total OCPs level for 21 pesticide types were reported to be 243.81 ng/mL, 151.02 ng/mL, and 133.38 ng/mL for sheep’s, cow’s, and buffalo’s milks, respectively. Kara et al. (1999) examined some OCPs residues in 24 commercial milk samples from the farms in Konya District in the 1995–1996 period. DDT, BHC, endosulfan compounds, aldrin, and dieldrin were detected in almost all samples. 87.5% of the samples were found to be contaminated with one or more BHC isomers, and 26.1% of the samples were contaminated with DDT and its metabolites. There is no study in the literature about PCBs or PBDEs residues in milk samples in Turkey.
The present study reports the level of OCPs, PCBs, and PBDEs contamination of commercially available raw and UHT cow’s milk samples. The authors aimed to compare the results with literature and determine OHPs exposure of population live in Turkey by dietary intake. EDI values were calculated both for adults and children to evaluate the dimension of risk.
Material and methods
Cow’s milk samples
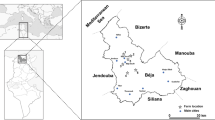
Fifteen samples of raw milk and 15 commercial brands of UHT milk were randomly collected from the market in Turkey. Analyzed milks were packed in facilities located in Sakarya, Denizli, Bursa, Izmir, Balikesir, Tokat, Konya, and Istanbul cities in Turkey. Raw milk samples were obtained from 13 farms in Konya District, Turkey. A map of the sampling points is given in Fig. 1. One liter of samples was collected from the milk storage tanks and stored in plastic bottles under 4 °C until analysis.
Standards and chemicals
Investigated compounds including α-, β-, γ-, and δ-HCHs, p,p’-DDE, p,p’-DDD, and p,p’-DDT, heptachlor, heptachlor epoxide, endosulfan I, endosulfan II, endosulfan sulfate, endrin, endrin aldehyde, endrin ketone, aldrin + dieldrin, and methoxychlor from OCPs, PCB 28, 52, 101, 153, 138, and 180, and PBDE 47, 99, 100, 153, and 154 congeners, internal standards; PCB 209 and tetrachloro-m-xylene were purchased from Accustandard Co (New Haven, CT, USA). All solvents used were of analytical grade. Acetone, n-hexane, dichloromethane, diethyl ether, iso-octane, anhydrous sodium sulfate, silica gel, and florisil were obtained from Merck Co (Darmstadt, Germany).
Extraction and clean-up
The extraction and clean-up procedures of OHPs in raw and UHT cow milk described by Johansen et al. (1994) and Ozcan et al. (2011) were used with minor modifications. Milk samples were subjected to vortex extraction and column chromatography clean-up following by GC/μ-ECD analysis (EPA 8081 B). Milk samples were homogenized via shaking for 5 min before sample processing. Two milliliters of formic acid was added to 2 g of milk sample prior to vortex extraction. Then, 2 × 3 mL n-hexane/dichloromethane (1:1 v:v) was added to the vortex shaker for 2 min of vortexing. The extract was cleaned by passing through a 30 cm long, 1 cm inner diameter column. The column was filled with 1 g of 5% deactivated silica, 1 g of 5% deactivated fluorosil, and 1 g of anhydrous sodium sulfate, by putting sodium sulfate at the top (Method 3620 C). The analytes were eluted with 15 mL n-hexane followed by 10 mL of dichloromethane. The eluents were concentrated to 1–2 mL using an evaporator at 40 °C, 335 mbar, and a gentle nitrogen stream. The remaining residues were dissolved by 1 mL n-hexane before analyses.
Determination of lipid content of milk samples
Lipid determination of milk samples was determined according to procedure described by Erdoğrul et al. (2004). One gram of milk sample was extracted with 2 × 3 mL of n-hexane/diethylether (1/1, v/v) solvent mixture for 2 min in a vortex mixer. Then, solvent phases were combined in an empty flask of known weight. The solvent was completely removed with a rotary evaporator and nitrogen gas, and the final weight was measured to determine the oil content.
GC/μ-ECD analysis
Analyses of OCPs, PCBs, and PBDEs were performed with GC/μ-ECD equipped with HP-5, 5% phenylmethyl siloxane fused silica capillary column (30-m length, 0.32-mm ID, and 0.25-mm film thickness) (Agilent Technologies, CA, USA). 99.999% pure helium was used as carrier gas. For OCPs analysis, a split/splitless injector was set at 280 °C in splitless mode (purge delay 1 min, purge flow 30.1 mL/min). Detector temperature was adjusted to 320 °C. Column temperature was set at 60 °C, first ramped to 160 °C with 40 °C/min, then ramped to 300 °C with 5 °C/min for 5 min. The flow rate of carrier gas was 2.5 mL/min. For PCBs analysis, the split/splitless injector was set at 280 °C in splitless mode (purge delay 1 min, purge flow 30.1 mL/min). Detector temperature was adjusted to 335 °C. Column temperature was set at 70 °C, first ramped to 160 °C with 30 °C/min, then ramped to 200 °C with 3 °C/min, then ramped to 280 °C with 8 °C/min for 10 min. The flow rate of carrier gas was 1.9 mL/min. For PBDEs analysis, the split/splitless injector was set at 285 °C in splitless mode (purge delay 1 min, purge flow 30.1 mL/min). Detector temperature was adjusted to 300 °C. Column temperature was set at 100 °C, first ramped to 250 °C with 30 °C/min, then ramped to 300 °C with 5 °C/min for 8 min. The flow rate of carrier gas was 2.5 mL/min. All injections were performed with the Agilent 7683 B Series automatic injector.
Quality assurance
The internal standard method was used for the quantification of the compounds. PCB 209 and tetrachloro-m-xylene were used as internal standard and added to all standard solutions at 0.1 ng/mL. Calibration curves were drawn using standard solutions of OCPs (0.01–10 ng/mL), PCBs (0.001–1 ng/mL), and PBDEs (0.001–10 ng/mL). Linearity of the compounds was higher than 0.997. Limit of detection (LOD) and limit of quantification (LOQ) were determined as the concentration corresponding to signal-to-noise ratios of 3 and 10, respectively. While LOD for OCPs, PCBs, and PBDEs were determined between 0.003–0.63 ng/g lipid wt., 0.12–0.34 ng/g lipid wt., and 0.41–0.82 ng/g lipid wt., respectively, LOQ for OCPs, PCBs, and PBDEs were ranged between 0.01–2.11 ng/g lipid wt., 0.41–1.15 ng/g lipid wt., and 1.36–2.72 ng/g lipid wt., respectively. Recovery experiments to determine the accuracy and precision of the used method were performed by spiking 10 ng/mL OCPs, PCBs, and PBDEs into blank samples. The mean recovery values were ranged from 70 to 109% for OCPs, 80 to 96% for PCBs, and 80 to 96% for PBDEs. The precision of the compounds as indicated by the RSD values was determined below 10 for all compounds.
Statistical analysis and estimation of daily intake
Concentrations of pollutants in milk samples are summarized using arithmetic means with minimum and maximum values. Contamination significance of raw and UHT cow’s milk was evaluated using t test, in Microsoft Excel with Analyse-it program (p values at 5% significance level). Non detected data assumed to be half the method limit of detection for calculation of p value. A significant difference among raw and UHT cow’s milk samples was defined for p < 0.05.
Estimated daily intakes (EDI) were calculated with Eq. 1. While Cmilk is the maximum pollutant concentration in the milk sample (μg/g lipid wt.), Clipid is the percentage of lipid in the milk. For the risk assessment, the average adult body weight was taken as 73 kg and the child body weight as 32.7 kg (Jan et al. 2010). Health risk can be determined by the ratio of EDI to admissible daily intake (ADI) that is referred to target hazardous quotient (THQ). THQ is calculated using Eq. 2. The sum of THQ values gives a mixture of total OHPs constituents of milk samples, named as hazard index (HI) calculated using Eq. 3. THQ and HI values less than one point mean that people are unlikely having toxic effects (Yu et al. 2016).
Results and discussion
Lipid content of milk samples
The minimum lipid content is 3.0 g/100 mL, the maximum lipid content is 3.87 g/100 mL, and the average lipid content is 3.51 g/100 mL for raw cow’s milk samples. For UHT cow’s milk samples, the minimum lipid content is 1.5 g/100 mL, the maximum lipid content is 3.40 g/100 mL, and the average lipid content is 2.71 g/100 mL.
Concentrations of OCPs, PCBs, and PBDEs in milk samples
Concentrations of OCPs, PCBs, and PBDEs quantified in raw and UHT cow’s milk samples are given in Table 1. In the raw milk samples, the minimum, the maximum, and the mean total OCPs concentrations were 26.79 ng/g lipid wt., 348.66 ng/g lipid wt., and 141.98 ng/g lipid wt., respectively. While δ-HCH, heptachlor, and p,p’-DDT compounds were quantified in 87% of the samples, heptachlor epoxide, p,p-DDD, endrin aldehyde, and endosulfan sulfate compounds were quantified in 93% of the samples. The rest of the pesticides were found in all raw milk samples. In the UHT milk samples, the minimum, the maximum, and the mean total OCPs concentrations were 96.67 ng/g lipid wt., 200 ng/g lipid wt., and 154.76 ng/g lipid wt., respectively. While β-HCH was quantified in 67% of the samples, methoxychlor was found in 53% of the samples. The rest of the pesticides were found in all UHT milk samples.
Contamination route of cow’s milk with OHPs is mainly comes from contaminated animal feed. Air, soil, and water matrixes are other contamination routes of cow’s milk with OHPs. Environmental pollutants enter the animals’ food chain by dust inhalation, contaminated water consumption, etc. (Gremmels 2012). OHPs are reported in urban air of Konya City by the authors of this study (Ozcan and Aydin 2009). Air is an important route for contamination of other environmental matrixes. Besides, OHPs are also found in human milk samples in Konya City, which reveals spread out of these environmental pollutants (Ozcan et al. 2011).
The origin of the milk is also an important subject to evaluate contamination route. While raw milk samples were collected from Konya District, UHT milk samples were collected from the market, selling packed milks collected and processed in different facilities located in several cities. Similar contamination levels for these two groups reveal a widespread of environmental contaminants.
Commercial DDT is a mixture of several isomers. The p,p’-DDT isomer is found in 77% of commercial DDT. p,p’-DDD and p,p’-DDE are the most important metabolites resulting from the environmental degradation of DDT (Santos et al. 2015). Half-life of DDT in the soil is 2–15 years while it goes up to 150 years in aquatic environment (Agency for Toxic Substances and Disease Registry 1994; HSDB 2017). p,p’-DDT/p,p’-DDE ratio is an indicator of application time for DDT. p,p’-DDT/p,p’-DDE ratio was found in the range of 1.00–4.99 in raw cow milk samples, and in the range of 0.50–3.50 in UHT milk samples. In case p,p’-DDT/p,p’-DDE ratio is below 1, it indicates previous use; above 1 ratio is an indication of current use (Ozcan and Aydin 2009). Sixty-six percent of the milk samples has the p,p’-DDT/p,p’-DDE ratio of 1 or higher, which is an indication of recent use. Bulut et al. (2011) also reported p,p’-DDT in higher concentrations (min-max, 13.19–28.92 ng/mL) when compared with p,p’-DDD (min-max, 0.36–2.68 ng/mL) or p,p’-DDE (not determined), which reveals a new application in Afyon City near to Konya City. DDT was banned in Turkey in 1985. The predominant detection of the p,p’-DDT isomer in milk samples is due to the fact that it is persistent and lipophilic, besides it is an indication of an illegal use.
Some of the investigated OCPs are in the list of prohibited pesticides in Turkish Food Codex, Maximum Residue Limits (MRLs) Regulation (Annex-3 Part-1 2016). A number of samples exceeding the MRLs are given in Table 1. Contamination significance of raw and UHT cow’s milk was evaluated by comparing means of two groups. p values are given in Table 1. Among analyzed parameters, α-HCH (p = 0.0009, f = 17.5), δ-HCH (p ≤ 0.0001, f = 29.61), endosulfan I (p = 0.0018, f = 14.75), dieldrin (p = 0.0238, f = 6.43), endrin aldehyde (p = 0.0135, f = 7.98), PBDE 99 (p = 0.0037, f = 9.94), PBDE 154 (p = 0.0009, f = 17.5), PCB 28 (p = 0.0017, f = 14.96), PCB 52 (p = 0.0461, f = 4.79), and PCB 101 (p = 0.0005, f = 20.72) had significant difference (p < 0.05). UHT cow’s milk samples were determined to have higher contamination of these OHPs except PBDE 99 and PBDE 154. When the sum of the OHP groups was compared for raw and UHT cow’s milk samples, contamination levels are close to each other for ∑HCH (raw cow’s milk = 63.6 ng/g lipid wt., UHT cow’s milk = 68.07 ng/g lipid wt.), ∑DDT (raw cow’s milk = 12.22 ng/g lipid wt., UHT cow’s milk = 11.49 ng/g lipid wt.), ∑Heptachlor (raw cow’s milk = 11.71 ng/g lipid wt., UHT cow’s milk = 12.98 ng/g lipid wt.), ∑Endosulfan (raw cow’s milk = 15.49 ng/g lipid wt., UHT cow’s milk = 16.97 ng/g lipid wt.), ∑Endrin (raw cow’s milk = 28.42 ng/g lipid wt., UHT cow’s milk = 30.79 ng/g lipid wt.), and ∑OCP (raw cow’s milk = 141.98 ng/g lipid wt., UHT cow’s milk = 154.76 ng/g lipid wt.). However, ∑PCB is nearly two times higher for (raw cow’s milk = 24.41 ng/g lipid wt., UHT cow’s milk = 55.76 ng/g lipid wt.). Since sampling groups are different for raw and UHT cow’s milk in our study, it is not possible to make a direct conclusion about the effect of UHT process on analyzed OHPs. While some studies in the literature reveal a very little or no effect of heat treatment on OHPs content of UHT milk (Molochnikov et al. 1970), some others put forth a degradation effect of UHT process on OHPs (Heck et al. 2007).
While only one raw milk sample determined to exceed MRL given for ∑DDT, nearly all of the samples exceeded the MRLs given for γ-HCH, ∑Heptachlor, and endrin. In similar literature studies, held in different parts of the world, quantitative amounts of OCPs were recorded in milk samples (Table 2). Our results are comparable with these studies, but quite high amount of ∑DDT were recorded in China (1270 ng/g lipid wt.) and in Spain (538 ng/g lipid wt.) (Zhong et al. 2003; Martinez et al. 1997). When compared with our study, ∑Heptachlor was determined in higher concentration, in the range of 13–536 ng/g lipid in Spain by Martinez et al. (1997).
In the raw milk samples, the minimum, the maximum, and the mean total PCBs concentrations were 10.59 ng/g lipid wt., 35.91 ng/g lipid wt., and 24.41 ng/g lipid wt., respectively. In the UHT milk samples, the minimum, the maximum, and the mean total PCBs concentrations were 21.63 ng/g lipid wt., 101.33 ng/g lipid wt., and 55.76 ng/g lipid wt., respectively. UHT milk samples were determined to contain nearly two times higher concentration of PCB congeners. PCB 52 and PCB 28 were dominant congeners for both raw and UHT milk samples. The use of PCBs was restricted to closed systems after 1993 and it was banned in 1996 in Turkey (Cok et al. 2003). Electronic waste recycling activities play a major role in the release of PCBs into the environment (Gioia et al. 2014). Predominantly, detected congeners were PCB 28, PCB 18, and PCB 138 in breast milk samples taken nearby an electronic waste disposal site in Gana (Asamoah et al. 2018). Low-chlorine congeners, such as PCB 28, may be more easily exposed to respiration due to natural volatile structures than high-chlorine congeners. In this study, PCB 28 was identified as the predominant congener in milk samples. Our findings put forth that milk samples taken for this study contain higher amount of PCBs, for nearly all congeners, when compared with the literature (Table 3). PCB 28, PCB 52, PCB 101, PCB 138, and PCB 180 congeners were found in higher concentrations in this study when compared findings of the studies in France (Costera et al. 2006), Kazakhistan (Konuspayeva et al. 2011), Italy (Lorenzi et al. 2016), and Belgium (Petro et al. 2010). PCB 153 was determined in higher concentration range in Belgium (0.6–10.9 ng/g lipid wt.) when compared with findings of our study (0.99–6.67 ng/g lipid wt.).
In the raw milk samples, the minimum, the maximum, and the mean total PBDEs concentrations were < dl, 20.22 ng/g lipid wt., and 9.51 ng/g lipid wt., respectively. PBDE 153 and PBDE 154 congeners were predominantly detected in these samples. In the UHT milk samples, the minimum, the maximum, and the mean total PBDEs were < dl, 20.0 ng/g lipid wt., and 6.99 ng/g lipid wt., respectively. PBDE 47 was dominant congener for UHT milk samples.
PBDE 47 dominantly quantified in UHT milk samples and PBDE 153 and PBDE 154 congeners dominantly quantified in raw milk samples are commonly produced congeners in the widely used penta-BDE commercial mixture (Zhang et al. 2017; Lorber 2008). Guo et al. (2016) and Siddique et al. (2012) dominantly found PBDE 47 congener in mother’s milk samples. Geyer et al. (2004) reported that the half-lives of the PBDE 47, PBDE 153, and PBDE 154 congeners in the human body were 1.8 years, 6.5 years, and 3.3 years, respectively. These congeners, found in dairy products, are consumed by adults and children, and stay in the human body for a long time. Our findings put forth that milk samples taken for this study contain higher amount of PBDEs, when compared with the literature (Table 4). Maximum PBDEs concentrations were found in higher values in this study when compared findings of the studies in Korea (Kim et al. 2013), USA (Schecter et al. 2006), Italy (Martellini et al. 2016), and Belgium (Poma et al. 2016).
Risk assessment
We assessed exposure to investigated pollutants through the diet. The amounts of daily consumptions for adults and children were taken from Hacettepe University Food and Nutrition Guide which is specific to Turkey (2004). The recommended amount of milk in this guide is 200 mL per day. For adults, the recommended daily portion is 3 per day (3 × 200 mL = 600 mL) while it is 3.5 portions per day for children (3.5 × 200 mL = 700 mL). Calculated EDI, THQ, and HI values are given in Table 5. While EDI values calculated for ∑Heptachlor, aldrin + dieldrin, and PCBs were higher for UHT milk samples, EDI values calculated for the rest of the compounds were higher for raw milk samples. According to our findings, none of the calculated EDI values exceeded ADI set for ∑DDT, ∑Endosulfan, or methoxychlor by European Food Safety (2010). However, all of the EDI values exceeded ADI set for ∑HCH, ∑Heptachlor, ∑Endrin aldrin + dieldrin, and ∑PCBs. THQ and HI values exceeding one are indications of health risk. Since children consume higher portion of milk and have lower body weight, EDI, THQ, and HI values are higher for them. Consumption of contaminated milk poses higher risk for children when compared with results.
Conclusions
The present study shows that both unprocessed raw milk and UHT milk samples in the market contain quantitative amounts of OHPs. UHT cow’s milk samples were determined to have significantly higher contamination of α-HCH, δ-HCH, endosulfan I, dieldrin, endrin aldehyde, PCB 28, and PCB 101. Even only one sample exceeded MRL set by DDT, dominant detection of p,p’-DDT isomer is an indication of continuing illegal use. Nearly all of the samples exceeded the MRLs given in Turkish regulations for γ-HCH, ∑Heptachlor, and endrin. EDI values calculated for adults and children consuming raw or UHT milk exceeded ADI set for ∑HCH, ∑Heptachlor, ∑Endrin aldrin + dieldrin, and ∑PCBs. The presence of these pollutants, especially in the dairy products consumed by vulnerable human groups, brings health risk problems. The use of PCBs and PBDEs is restricted or prohibited under the Stockholm Convention, signed also by Turkey. Continuing detection of these pollutant groups is a result of their persistent nature in different compartments of environment. There is a necessity to define contamination route of milk and take precautions by following these toxic compounds.
References
Abou Donia MA, Abou-Arab AAK, Enb A, El-Senaity MH, Abd Rabou NS (2010) Chemical composition of raw milk and the accumulation of pesticide residues in milk products. Glob Vet 4(1):6–14
Asamoah A, Essumang DK, Muff J, Kucheryavskiy SV, Søgaard GE (2018) Assessment of PCBs and exposure risk to infants in breast milk of primiparae and multiparae mothers in an electronic waste hot spot and non-hot spot areas in Ghana. Sci Total Environ 612:1473–1479
Aslam M, Rais S, Alam M (2013) Quantification of organochlorine pesticide residues in the buffalo milk samples of Delhi City, India. J Environ Prot 4:964–974
Avancini RM, Silva IS, Simões Rosa AC, Novaes Sarcinelli P, Alexandra de Mesquita S (2013) Organochlorine compounds in bovine milk from the state of Mato Grosso do Sul- Brazil. Chemosphere 90:2408–2413
Behrooz RD, Sari AE, Bahramifar N, Ghasempouri SM (2009) Organochlorine pesticide and polychlorinated biphenyl residues in human milk from the Southern Coast of Caspian Sea, Iran. Chemosphere 74:931–937
Bulut S, Akkaya L, Gök V, Konuk M (2011) Organochlorine pesticide (OCP) residues in cow’s, buffalo’s, and sheep’s milk from Afyonkarahisar region, Turkey. Environ Monit Assess 181:555–562
Cok I, Görücü E, Şatiroğlu MH, Demircigil GÇ (2003) Polychlorinated biphenl (PCB) levels in human milk samples from Turkish mothers. Bull Environ Contam Toxicol 70:41–45
Costa LG, Giordano G (2007) Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. NeuroToxicology 28:1047–1067
Costera A, Feidt C, Marchand P, Le Bizec B, Rychen G (2006) PCDD/F and PCB transfer to milk in goats exposed to a long-term intake of contaminated hay. Chemosphere 64:650–657
D’Silva K, Fernandes A, Rose M (2004) Brominated organic micropollutants – igniting the flame retardant issue Crit Rev. Environ Sci Technol 34:14–207
Darko G, Acquaah SO (2008) Levels of organochlorine pesticides residues in dairy products in Kumasi, Ghana. Chemosphere 71:294–298
Davies JF, Freed VH, Whittemore FW (1986) An agro medical approach to pesticide management. University of Miami School of Medicine. Coral Gables, FL
Dhananjayan V, Ravichandran B (2018) Occupational health risk of farmers exposed to pesticides in agricultural activities. Curr Opin in Env Sci & Health 4:31–37
Domingo JL (2014) Health risks of human exposure to chemical contaminants through egg consumption: a review. Food Res Int 56:159–165
Ejobi F, Kanja LW, Kyule MN, Muller P, Nyeko JHP, Latigo AAR (1994) Organochlorine pesticide residues in cow’s milk in Uganda. Bull Environ Contam Toxicol 56:551–557
Erdoğrul O, Covaci A, Kurtul N, Schepens P (2004) Levels of organohalogenated persistent pollutants in human milk from Kahramanmaras Region, Turkey. Environ Int 30:659–666
European Food Safety (2010) Guidance document for the use of the concise European food consumption database in exposure assesment 438, pp. 1-54. http://www.efsa.europe.eu/cs/BlobServer/General/Coincise_databssment_and_annexes,3.pdf?ssbinary=true. Accessed 5 June 2018
Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald TA (2004) Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd 66:3820–3825
Gioia R, Akindele AJ, Adebusoye SA, Asante KA, Tanabe S, Buekens A, Sasco AJ (2014) Polychlorinated biphenyls (PCBs) in Africa: a review of environmental levels. Environ Sci Pollut Res 21(10):6278–6289
Gremmels JF (2012) Animal feed contamination, effects on livestock and food safety, Woodhead Publishing Series in Food Science, Technology and Nutrition, Pages 1-10
Grossman E (2013) Nonlegacy PCBs: pigment manufacturing by-products get a second look. Environ Health Perspect 121:A86–A93. https://doi.org/10.1289/ehp.121-a86
Grümping R, Petersen M, Kuchen A, Tlustos C (2006) Levels of polybrominated diphenyl ethers in Swiss and Irish cow’s milk. Organohalogen Compd 68:2147–2150
Guo W, Holden A, Smith SC, Gephart R, Petreas M, Park JS (2016) PBDE levels in breast milk are decreasing in California. Chemosphere 150:505–513
Hazardous Substances Data Bank (HSDB), https://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?. Accessed 05 May 2017
Heck MC, Santos JS, Bogusz Tunior S, Costabeber I, Emmanelli T (2007) Estimation of children exposure to organochlorine compounds through milk in Rio Grando Do Sul, Brazil. Food Chem 102:288–294
Jan FA, Ishaq M, Khan S, Ihsanullah I, Ahmad I, Shakirullah M (2010) A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil. J Hazard Mater 179:612–621
Janle E, Sojka J, Jackson GS, Lachcik P, Einstien JA Santerre CR (2007) Measuring transfer of 14C-PCB from maternal diet to milk in a goat model using an accelerator mass spectrometer (AMS). Nucl Instrum Methods Phys Res B 259:758–762
JMPR, The Joint FAO/WHO Meeting on Pesticide Residues (2017) http://apps.who.int/pesticide-residues-jmpr-database. Accessed 02 June 2018. Accessed 02 June 2018
Johansen RH, Becher G, Polder A, Skaare JU (1994) Congener-specific determination of PCBs and OCPs in human milk from Norwegian mothers living in Oslo. J Toxicol Environ Health 42:157–171
Kampire E, Kiremire BT, Nyanzi SA, Kishimba M (2011) Organochlorine pesticide in fresh and pasteurized cow’s milk from Kampala markets. Chemosphere 84:923–927
Kara H, Aktümsek A, Nizamlıoğlu F (1999) Some organochlorine pesticide residues in commercial milk Konya-Region/Turkey. Fresenius Environ Bull 8(5-6):257–263
Kaushik P, Kaushik G (2007) An assessment of structure and toxicity correlation in organochlorine pesticides. J Hazard Mater 143:102–111
Kim DG, Kim M, Jang JH, Bong YH, Kim JH (2013) Monitoring of environmental contaminants in raw bovine milk and estimates of dietary intakes of children in South Korea. Chemosphere 93:561–566
Konuspayeva G, Faye B, De Pauw E, Focant JF (2011) Levels and trends of PCDD/Fs and PCBs in camel milk (Camelus bactrianus and Camelus dromedarius) from Kazakhstan. Chemosphere 85:351–360
Leng JH, Kayama F, Wang PY, Nakamura M, Nakata T, Wang Y (2009) Levels of persistent organic pollutants in human milk in two Chinese coastal cities, Tianjin and Yantai: influence of fish consumption. Chemosphere 75(5):634–639
Lorber M (2008) Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol 18:2–19
Lorenzi V, Ghidini S, Angelone B, Ferretti E, Menotta S, Fedrizzi G, Varisco G, Foschini S, Diegoli G, Bertocchi L (2016) Three years of monitoring of PCDD/F, DL-PCB and NDL-PCB residues in bovine milk from Lombardy and Emilia Romagna regions (Italy): contamination levels and human exposure assessment. Food Control 68:45–54
Martellini T, Diletti G, Scortichini G, Lolini M, Lanciotti E, Katsoyiannis A, Cincinelli A (2016) Occurrence of polybrominated diphenyl ethers (PBDEs) in foodstuffs in Italy and implications for human exposure. Food Chem Toxicol 89:32–38
Martinez MP, Angulo R, Pozo R, Jodral M (1997) Organochlorine pesticides in pasteurized milk and associated health risks. Food Chem Toxicol 35:621–624
Meironyte D, Bergman A, Noren K (1999) Polybrominated diphenyl ethers in Swedish human milk: a time-related trend study, 1972-1997. J Toxicol Environ Health Part A 62:101–118
Molochnikov VV, Mochalov VI, Davydova IR, Serevrennikova VA (1970) Excretion of chloro-organic pesticides with milk. Veterinariia 1:29–32
Nag SK, Raikwar MK (2008) Organochlorine pesticide residues in bovine milk. Bull Environ Contam Toxicol 80:5–9
Nassar AMK, Salim YM, Malhat FM (2016) Assessment of pesticide residues in human blood and effects of occupational exposure on hematological and hormonal qualities. Pak J Biol Sci 19:95–105
Ozcan S, Aydin ME (2009) Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and organochlorine pesticides in urban air of Konya, Turkey. Atmos Res 93:715–722
Ozcan S, Tor A, Aydin ME (2011) Levels of organohalogenated pollutants in human milk samples from Konya City, Turkey. Clean 39(10):978–983
Paepke O, Petersen M, Opel M, Lohmann N, Fürst P (2009) Polybrominated diphenyl ethers (PBDEs) in food samples from Germany. Organohalogen Compd 71:1498–1502
Pardio VT, Waliszewski KN, Landin LA, Bautista RG (2003) Organochlorine pesticide residue in cow’s milk from a tropical region of Mexico. Food Addit Contam 20:259–269
Petro EML, Covaci A, Leroy JLMR, Dirtu AC, De Coen W, Bols PEJ (2010) Occurrence of endocrine disrupting compounds in tissues and body fluids of Belgian dairy cows and its implications for the use of the cow as a model to study endocrine disruption. Sci Total Environ 408:5423–5428
Poma G, Malarvannan G, Voorspoels S, Symons N, Malysheva SV, Loco JV, Covaci A (2016) Determination of halogenated flame retardants in food: optimization and validation of a method based on a two-step clean-up and gas chromatographyemass spectrometry. Food Control 65:168–176
Republic of Turkey Ministry of Food, Agriculture and Livestock (2016) Turkish food codex maximum residue limits of pesticides regulation
Santos JSD, Schwanz TG, Coelho AN, Heck-Marques MC, Mexia MM, Emanuelli T, Costabeber I (2015) Estimated daily intake of organochlorine pesticides from dairy products in Brazil. Food Control 53:23–28
Schecter A, Päpke O, Harris TR, Tung KC, Musumba A, Olson J, Birnbaum L (2006) olybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. Food and estimated PBDE dietary intake by age and sex. Environ Health Perspect 114:10
Schecter A, Harris TR, Shah N, Musumba A, Päpke O (2008) Brominated flame retardants in US food. Mol Nutr Food Res 52:266–272
Schecter A, Colacino J, Patel K, Kannan K, Yun SH, Haffner D, Harris TR, Birnbaum L (2010) Polybrominated diphenyl ether levels in foodstuffs collected from three locations from the United States. Toxicol Appl Pharmacol 243:217–224
Siddique S, Xian Q, Abdelouahab N, Takser L, Phillips SP, Feng YL, Wang B, Zhu J (2012) Levels of dechlorane plus and polybrominated diphenylethers in human milk in two Canadian cities. Environ Int 39:50–55
Specific food and nutrition book in Turkey (2015) Hacettepe University, Faculty of Health Sciences. Department of Nutrition and Dietetics, Ankara
Sun R, Luo X, Li QX, Wang T, Zheng X, Peng P, Mai B (2018) Legacy and emerging organohalogenated contaminants in wild edible aquatic organisms: implications for bioaccumulation and human exposure. Sci Total Environ 616–617:38–45
Toxicology profile for 4,4′-DDT, 4,4′-DDE, 4,4′-DDD (1994); U. S. Department of Human Health &Human Services, Agency for Toxic Substances and Disease Registry 1994. 16.
Tremolada P, Guazzoni N, Parolini M, Rossaro B, Bignazzi MM, Binelli A (2014) Predicting PCB concentrations in cow milk: validation of a fugacity model in high-mountain pasture conditions. Sci Total Environ 487:471–480
Tsakiris IN, Goumenou M, Tzatzarakis MN, Alegakis AK, Tsitsimpikou C, Ozcagli E, Vynias D, Tsatsakis AM (2015) Risk assessment for children exposed to DDT residues in various milk types from the Greek market. Food Chem Toxicol 75:156–165
UNEP (2001) Final Act of the Conference of Plenipotentiaries on the Stockholm Convention on Persistent Organic Pollutants. United Nations Environment Programme, Stockholm, Sweden. http://www.pops.int/documents/meetings/dipcon/meetingdoclisten.htm
UNEP (2010) The nine new POPs; an introduction to the nine chemicals added to the Stockholm Convention by the Conference of the Parties at its Fourth Meeting. United Nations Environment Programme, Geneva
Van Oostdam J, Gilman A, Dewailly E, Usher P, Wheatley B, Kuhnlein H, Loring E, Muckle G, Myles E, Receveur O, Tracy B, Gill U, Kalhok S (2005) Human health implications of environmental contaminants in Arctic Canada: a review. Sci Total Environ 351-352:165–246
Wang Y, Jiang G, Lam PKS, Li A (2007) Polybrominated diphenyl ether in the east Asian environment: a critical review. Environ Int 33:963–973
WHO J (1994) Brominated diphenyl ethers. IPCS environmental health criteria, vol 162. World Health Organisation, Geneva
Yeo H, Choi M, Chun M, Kim T, Cho K, Sunwoo Y (2004) Concentration characteristics of atmospheric PCBs for urban and rural area, Korea. Sci Total Environ 324(1–3):261–270
Yu R, Liu Q, Liu J, Wang Q, Wang Y (2016) Concentrations of organophosphorus pesticides in fresh vegetables and related human health risk assessment in Changchun. Northeast China. Food Control 60:353–360
Zhang L, Yin S, Zhao Y, Shi Z, Li J, Wu Y (2017) Polybrominated diphenyl ethers and indicator polychlorinated biphenyls in human milk from China under the Stockholm Convention. Chemosphere 189:32–38
Zheng X, Luo X, Zheng J, Zeng Y, Mai B (2015) Contaminant sources, gastrointestinal absorption, and tissue distribution of organohalogenated pollutants in chicken from an e-waste site. Sci Total Environ 505:1003–1010
Zhong W, Xu D, Chai Z, Mao X (2003) 2001 Survey of organochlorine pesticide in retail milk from Beijing. PR China. Food Addit Contam 20:254–258
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aydin, S., Aydin, M.E., Beduk, F. et al. Organohalogenated pollutants in raw and UHT cow’s milk from Turkey: a risk assessment of dietary intake. Environ Sci Pollut Res 26, 12788–12797 (2019). https://doi.org/10.1007/s11356-019-04617-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04617-0