Abstract
Metallic elements are ubiquitous in the natural environment and always collaborate to affect human health. The relationship of handgrip strength, a marker of functional ability or disability, with metal co-exposure remains vague. In this study, we aimed to investigate the effect of metal co-exposure on sex-specific handgrip strength. A total of 3594 participants (2296 men and 1298 women) aged 21 to 79 years recruited from Tongji Hospital were included in the present study. Urinary concentrations of 21 metals were measured by inductively coupled plasma mass spectrometer (ICP-MS). We used linear regression, restricted cubic spline (RCS) model, and weighted quantile sum (WQS) regression to evaluate the association of single metal as well as metal mixture with handgrip strength. After adjusting for important confounding factors, the results of linear regression showed that vanadium (V), zinc (Zn), arsenic (As), rubidium (Rb), cadmium (Cd), thallium (Tl), and uranium (U) were adversely associated with handgrip strength in men. The results of RCS showed a non-linear association between selenium (Se), silver (Ag), and nickel (Ni) with handgrip strength in women. The results of WQS regression revealed that metal co-exposure was inversely related to handgrip strength for men (β = -0.65, 95% CI: -0.98, -0.32). Cd was the critical metal in men (weighted 0.33). In conclusion, co-exposure to a higher level of metals is associated with lower handgrip strength, especially among men, and Cd may contribute most to the conjunct risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Handgrip strength is a strong predictor of functional ability or disability (Rantanen et al. 1999). Previous studies have demonstrated that lower handgrip strength is associated with a higher risk for all-cause mortality, cardiovascular disease, respiratory disease and cancer (Celis-Morales et al. 2018; Liu et al. 2021; Rantanen et al. 2000). As an accurate indicator of muscle mass, handgrip strength is one of the components of diagnostic criteria for Sarcopenia defined by muscle loss and muscle dysfunction (Chan et al. 2022; Nishikawa et al. 2016). In addition to age, handgrip strength is known to be affected by body mass index, smoking, physical activity, diet, or mood. Individuals with lower body weight, longer secondary time, or depression may be with lower handgrip strength (Charles et al. 2006; Stenholm et al. 2012). Except for the above factors, metals have been reported to be associated with handgrip strength (Garcia-Esquinas et al. 2020, Garcia-Esquinas et al. 2021a, Garcia-Esquinas and Rodriguez-Artalejo 2017, Gbemavo and Bouchard 2021, Khalil et al. 2014, Kim et al. 2016).
Most metals are emitted into the natural environment and widely distributed in air, soil and water through natural or anthropogenic activities (Clemens and Ma 2016, Tchounwou et al. 2012). Previous studies have shown that higher cadmium is associated with decreased handgrip strength (Garcia-Esquinas et al. 2020, 2021b). Another study exploring the association between lead, mercury, selenium and manganese with handgrip strength suggested that lead was associated with weaker handgrip strength while selenium was associated with stronger handgrip strength, but only in women (Gbemavo and Bouchard 2021). Some studies investigated the effect of dietary selenium intake on handgrip strength and observed the protection of dietary selenium to muscle function (Heath et al. 2010; Perri et al. 2020; Walsh et al. 2021). Several experimental studies demonstrated that exposure to excessive metal elements like manganese (Krishna et al. 2014), copper (Kalita et al. 2020), and uranium (Barber et al. 2007) could cause lower handgrip strength in rats via the muscle tissue damage triggered by oxidative stress and inflammation. Metals always coexist in the real world and exert effects through synergy, antagonism, or interaction (Bauer et al. 2020). However, none of the previous studies has simultaneously assessed the association between single metal as well as metal mixture exposure with handgrip strength in adults.
Therefore, in the present research, we measured 21 common metal elements in urine samples including aluminum (Al), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), selenium (Se), rubidium (Rb), strontium (Sr), silver (Ag), cadmium (Cd), cesium (Cs), barium (Ba), mercury (Hg), thallium (Tl), lead (Pb), and uranium (U). Among the 21 metals, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, and Se are essential elements, which are necessary for biological function. However, deficient or excess essential elements may induce cellular and tissue damage (Zoroddu et al. 2019). As, Cd, Hg, Tl, and Pb are considered toxic metals, which induce numerous adverse effects on living organisms, even at a lower level of exposure (Tchounwou et al. 2012; Wu et al. 2016). The rest of metals including Al, Rb, Sr, Ag, Cs, Ba, and U have no known biological function and are lumped into non-essential elements (Tchounwou et al. 2012). The detection rates of these 21 metals were all higher than 95% in urine. Our objective was to explore the association of exposure to the 21 single metal and metal mixture with handgrip strength based on a cross-sectional study among 3594 adults in Wuhan, China. We used univariate linear regression analysis and multivariate linear regression to evaluate the linear association and restricted cubic spline model to evaluate the potential non-linear association of single metal with handgrip strength. Furthermore, Weighted Quantile Sum (WQS) regression was applied to estimate the cumulative effect of multiple metals exposure on handgrip strength.
Materials and methods
Study population
In the present study, we recruited 4185 participants aged 18 to 89 years from the health management Center of Tongji Hospital between August 2018 and March 2019, Wuhan, China. Information about demographics, behavior, lifestyle, history of diseases, use of medication, and family medical history of participants was collected by trained research staff through a face-to-face interview. All participants underwent medical examinations and were required to provide urine samples.
Of the 4185 participants, we excluded those who were ≤ 20 years (n = 2) or ≥ 80 years (n = 6). Furthermore, we excluded those participants without urine samples (n = 113) or information on complete handgrip strength and its potential confounders (n = 464). There were 3 participants with outliers of handgrip strength (defined as the value lower than 25th percentile minus 3 times interquartile range (3IQR) (n = 0) or the value higher than 75th percentile plus 3IQR (n = 3)) among women being excluded. Finally, there were 3594 participants (2296 men and 1298 women) included in the present study. All participants provided written informed consent. The study protocol was approved by the Ethics Review Board of Tongji Medical College, Huazhong University of Science and Technology.
Measurement of urinary metals
Morning urine samples of participants were collected in trace element-free containers and then placed at -20 ℃ until further analysis. Before measuring the metal level, an aliquot of urine sample (500 μL) was moved to a polyethylene tube containing 20 μL of 67% HNO3 (vol/vol) and stored in a refrigerator overnight. After digestion, we diluted 0.5 ml of the sample tenfold with 1% HNO3 (vol/vol). The concentrations of 21 metals in urine, aluminum (Al), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), arsenic (As), selenium (Se), rubidium (Rb), strontium (Sr), silver (Ag), cadmium (Cd), cesium (Cs), barium (Ba), mercury (Hg), thallium (Tl), lead (Pb), and uranium (U) were determined using inductively coupled plasma mass spectrometer (ICP-MS; Agilent Technologies, 7700x, USA). For quality control, we used standard reference materials (SRM1640a, National Institute of Standards and Technology, Gaithersburg, MD, USA) and blanks (1% HNO3) every time to verify instrument performance. Moreover, we measured spiked pooled samples that were randomly selected from 200 urine samples including low and high concentrations of 21 metals to evaluate the precision and accuracy of the measurement method. Spiked recovery rates of quality control standards were 82%-125%, and intra- and inter-assay variance coefficients were less than 10%. The concentrations of urinary metal less than the limit of detection (LOD) were assigned as LOD/\(\sqrt{2}\). To provide better reliability for the estimation of individual metal exposure level, urinary metal concentrations were corrected for serum creatinine (SCR) by applying the following equation: Rc = R × 106 / (SCR × 113.12), where Rc is SCR-corrected metal concentration (μg/g creatinine), R is the uncorrected metal concentration (μg/L), SCR is serum creatinine concentration (μmol/L) (Barr et al. 2005). The corrected concentrations of urinary metals were log 10-transformed to reduce the skewness.
Measurement of handgrip strength
Handgrip strength (kg) was measured using CAMRY electronic handgrip dynamometer, model EH101 (Xiangshan Weighing Instrument Group Co., Ltd, Guangdong, China). Participants were asked to keep a standing posture and arms resting naturally to squeeze the dynamometer as hard as possible with one hand. The measurement was repeated three times with a one-minute break between measurements on the same hand to avoid repetition fatigue. We calculated the average of the largest readings from each hand as combined handgrip strength for the analysis.
Covariates assessment
We collected several variables likely to be the potential confounding factors including sex, age, education (primary school or below, middle school or high school, college or above), smoking (never, ex-smoker, current smoker), passive smoking (not exposed, exposed), alcohol consumption (never, ex-drinker, current drinker), physical activity (METs-hour/week), body mass index (BMI; < 18.5 kg/m2, 18.5 ~ 23.9 kg/m2, ≥ 24.0 kg/m2), and history of chronic diseases including hypertension (yes, no), diabetes mellitus (yes, no), cardiovascular disease (yes, no), or respiratory disease (yes, no). Individuals who smoked at least one cigarette per day over six months were defined as current smokers, those who used to smoke but stopped for at least six months were defined as former smokers, and those who smoked less than one cigarette per day or never smoked were defined as non-smokers. Individuals who drank at least once per week over six months were defined as current drinkers, those who used to drink but stopped for at least six months were defined as former drinkers, and those who drank less once per week or never drank were defined as non-drinkers. Physical activity was estimated by multiplying the time spent every week in each activity by specific METs values based on a previous study (Ng et al. 2009). Hypertension was defined as a self-reported physician diagnosis, exhibiting a systolic blood pressure (SBP) level ≥ 140 mmHg or diastolic blood pressure (DBP) level ≥ 90 mmHg, or taking antihypertensive medication. Diabetes was defined as a self-reported physician diagnosis, exhibiting fasting blood glucose (FBG) ≥ 7.0 mmol/L, or taking antidiabetic medication or insulin. Cardiovascular disease (CVD) was defined as a self-reported physician diagnosis of coronary heart disease (CHD), myocardial infarction (MI), or stroke. Respiratory disease was defined as a self-reported physician diagnosis of emphysema, chronic bronchitis, or asthma.
Statistical analysis
Considering the large difference in handgrip strength between men and women and previous studies showing sex-specific neurotoxic susceptibility to several metals (Gade et al. 2021), participants were divided into men’s group and women’s group for the statistical analysis. Continuous variables were presented as mean (SD) for normal distributed data and median (IQR) for skewed distributed data, while categorical variables were presented as numbers and percentages. We used the student t-test to compare continuous variables with normality, the Wilcoxon rank test to compare continuous variables without normality, and the Chi-square test to compare categorical variables between men and women. Pearson correlation analysis was used to calculate pairwise correlation coefficients for 21 log 10-transformed creatinine-corrected urinary metal concentrations.
We used linear regression to estimate regression coefficients (β value) and 95% confidence intervals (95% CI) of single metal and handgrip strength. The creatinine-corrected metal level was analyzed respectively as a continuous variable and rank variable categorized by quartile (Q1, Q2, Q3, Q4). Model 1 was used to estimate the association between single metal and handgrip strength without adjustment of any covariates. Model 2 was adjusted for age, physical activity, education, smoking status, passive smoking, alcohol drinking, body mass index, hypertension, diabetes, cardiovascular diseases, and respiratory diseases. We calculated P for the trend by treating the median value of each quartile as a continuous variable. The restricted cubic spline (RCS) model with a setting of 4 knots was used to explore the non-linear relationship between single metal and handgrip strength.
In addition to analyzing the effect of single metal exposure, we used weighted quartile sum (WQS) regression to estimate the co-exposure effect of 21 metals and identify the important metals. Traditional methods (e.g. linear or logistic regression) introduce variance inflation when they are subjected to environmental mixture (e.g. metal mixture) datasets with a high correlation to each other. WQS regression can keep accuracy when it is used to handle such high-dimensional data. The model was fit with 100 bootstraps to estimate the weight of each metal and then constructed the WQS index based on a weighted average of the empirical weights across the 100 bootstrap samples (Carrico et al. 2015). WQS index for each metal was constrained to add up to 1.0 and represented relative importance among the effect of metal mixture. Since WQS regression constrained the relationship between metal mixture exposure and handgrip strength into one direction, the direction was limited to negative in men and women.
All analyses were conducted with R version 4.2.0 and a P value < 0.05 was considered significant.
Results
Characteristics in participants and the metal concentrations in urine
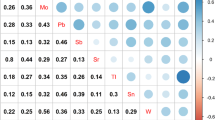
The characteristics of participants stratified by sex are shown in Table 1. The mean and standard deviation of handgrip strength was 41.26 ± 6.70 kg for men and 24.13 ± 4.37 kg for women. There was no statistical difference in age between men and women (P = 0.525). Table 2 shows the distribution of creatinine-corrected metal concentrations of men, women and all participants, which were presented as median (IQR) and geometric mean. Figure S1 presents the Pearson correlation coefficients of 21 metals. All the pairwise correlations of 21 metals were positive with each other. Table 2 shows the distribution of 21 creatinine-corrected urinary metal concentrations in all participants, men and women.
The linear and non-linear association between single metal and handgrip strength stratified by sex
The results of linear regression analysis stratified by sex are presented in Table 3. In the adjusted single-metal models, for each unit increase of log 10-transformed and creatinine-corrected urinary V, Mn, Co, Zn, As, Rb, Sr, Cd, Cs, Tl and U, handgrip strength decreased 0.83 kg, 0.64 kg, 0.78 kg, 0.82 kg, 0.75 kg, 0.95 kg, 0.74 kg, 0.94 kg, 1.16 kg, 1.08 kg and 0.81 kg in men, respectively. However, no metal was associated with handgrip strength in women. Among men’s group, a decrease of handgrip strength for the highest quartile of exposure (versus Q1) was found in urinary Al (β = -0.80; 95% CI: -1.54, -0.05), V (β = -0.92; 95% CI: -1.66, -0.17), Zn (β = -0.92; 95% CI: -1.68, -0.16), As (β = -0.89; 95% CI: -1.63, -0.14), Se (β = -0.77; 95% CI: -1.52, -0.02), Rb (β = -0.95; 95% CI: -1.69, -0.20), Cd (β = -1.29; -2.08, -0.51), Tl (β = -0.88; 95% CI: -1.62, -0.13) and U (β = -0.89; 95% CI: -1.64, -0.15) (all the P for trend < 0.05). Although in adjusted models, handgrip strength had no significant difference for women in the highest quartile versus the lowest quartile of each metal, handgrip strength in women presented an increased trend in the second quartiles versus the lowest quartiles of several metals including Se (β = 1.01; 95% CI: 0.36, 1.67), Cs (β = 0.86; 95% CI: 0.20, 1.52), Hg (β = 0.73; 95% CI: 0.07, 1.38) and Tl (β = 0.78; 95% CI: 0.12, 1.44), which suggested a potential nonlinear association between these metals and handgrip strength.
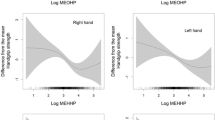
The results of restricted cubic spline (Fig. 1) demonstrated that Ni, Se and Ag had the nonlinear dose–response relationship with handgrip strength (Ni: P for nonlinear = 0.017, Se: P for nonlinear = 0.006, Ag: P for nonlinear = 0.009). Figure S2 and Fig. S3 present dose–response curves of 21 metal elements with handgrip strength among men and women, respectively.
Association between urinary metals and handgrip strength analyzed by restricted cubic spline (RCS) with 4 knots for Ni, Se, and Ag among women. Solid lines were predicted curves, shadow parts were 95% confidence intervals. Models were adjusted for age, physical activity, education, smoking status, passive smoking, alcohol drinking, body mass index, hypertension, diabetes, cardiovascular diseases, and respiratory diseases. Urinary metal concentrations were creatinine-corrected and further log10-transformed
Sex-specific associations between mixed metals exposure and handgrip strength
Figure 2 demonstrates the results of WQS regression for men and women. The results showed that the mixture of 21 metals had a negative impact on handgrip strength in men (β = -0.65, 95% CI: -0.98, -0.32). Cd as the most important metal element accounted for 33% of weights, followed by U (weight index = 0.18), As (weight index = 0.11), Sr (weight index = 0.08), Rb (weight index = 0.06), and Al (weight index = 0.05). Among the women’s group, 21 metals mixture played a negative impact on handgrip strength despite no statistical significance (β = -0.06, 95% CI: -0.36, 0.23). The results of stratified analysis by BMI (BMI ≤ 23.9 kg/m2, BMI ≥ 24.0 kg/m2) and physical activity (low-intensity physical activity; high-intensity physical activity, divided by median of METs-hour/week) were similar to the results of non-subgroup analysis and there was no significant interaction effect between BMI/physical activity and metal (data not shown).
Discussion
The results of linear regression analysis showed that increased V, Zn, As, Rb, Cd, Tl, and U were independently associated with decreased handgrip strength in men. Despite no significant linear association between single metal and handgrip strength in women, the curves of RCS model performed a nonlinear correlation between Se, Ag, and Ni with handgrip strength. The results of WQS regression analysis indicated that mixed metals exposure was inversely associated with handgrip strength among men, mainly driven by Cd.
Cd is a non-essential toxic heavy metal without known physiological function for the human body and is toxic at a low concentration (Gade et al. 2021). The result of our research that increased Cd level was associated with decreased handgrip strength agreed with several studies. Research in an elderly population observed an association between blood cadmium level and lower handgrip strength (Kim et al. 2016). Another two studies conducted among older adults also showed that higher blood Cd concentration was an independent risk factor of physical function impairment including frailty (Garcia-Esquinas et al. 2021b) and lower gait speed (Kim et al. 2018). A cross-sectional study based on NHANES among adults aged ≥ 40 years demonstrated that blood Cd and urine Cd concentrations were both negatively associated with handgrip strength (Garcia-Esquinas et al. 2020). Potential physiological mechanisms have not been clarified so far. Several in vitro studies have proposed the possible evidence that Cd exposure may disrupt cellular homeostasis in skeletal muscle via increasing cellular oxidative stress and compromising cell adhesion (Papa et al. 2014, Yano and Marcondes 2005). The alteration of cellular homeostasis in skeletal muscle can reduce muscle mass and physiology, resulting in lower handgrip strength (Derbre et al. 2014; Siparsky et al. 2013). In the present study, we found several novel inverse correlations between V, Zn, As, Rb, Tl and U with handgrip strength in men. Several animal studies in rats have found neurobehavioral impairment such as locomotor insufficient and diminution in muscle strength exerted by As (Adedara et al. 2020; Yadav et al. 2009) or V (Azeez et al. 2016; Mustapha et al. 2014) exposure. Exposure to excessive as may decrease muscle mass and even cause muscle atrophy in mice (Chen et al. 2020). In addition, Rb (Barrientos et al. 2020) and Tl (Wu et al. 2022) reported an inverse effect on muscular function. Uranium is a naturally occurring heavy metal widely spreading in the environment, individuals are exposed to uranium in several ways including water drinking, air inhaling, and food intake (Drake and Hazelwood 2005). To our knowledge, no study has explored the association between uranium exposure and handgrip strength among humans. There was only an animal study showing that acute uranium exposure might cause ambulatory activity and handgrip strength reduction (Barber et al. 2007).
We found that Se had a nonlinear dose–response association with handgrip strength among women participants. The results of RCS model indicated that Se was positively associated with handgrip strength within a relatively lower Se concentration range while such association became not significant within a relatively higher Se concentration range. A study based on NHANES investigated the association between blood Se and handgrip strength among US adults, RCS curve for women showed that handgrip strength increased steeply along with Se increasing, and then the upward curve tended to flatten out within higher Se levels (Gbemavo and Bouchard 2021). Moreover, a parallel dose–response association was revealed by a meta-analysis both in men and women (Garcia-Esquinas et al. 2021a). Those findings along with our results have suggested that handgrip strength is associated with lower selenium levels rather than higher selenium levels. Selenium is an important component of selenoproteins, and most of the known selenoproteins are antioxidant enzymes (e.g., glutathione) involved in a variety of antioxidant pathways like free radical scavenging, oxidized lipids repairing, etc. (Hariharan and Dharmaraj 2020). Selenoprotein deficiency as an indicator of selenium deficiency is associated with muscle dysfunction (e.g., muscle pain and weakness, sarcopenia) (Hariharan and Dharmaraj 2020, Rederstorff et al. 2006) and even nutritional muscular dystrophy (Orndahl et al. 1982). We detected a positive association between lower Ag concentration and handgrip strength whereas there was an inverse association between higher Ag concentration and handgrip strength. Ag is a non-essential element and rarely receives attention. Owing to scarce studies exploring the physiological function of Ag in the human body, we deduce that low concentrations of Ag protect muscle function but high concentrations of Ag damage muscle function. For Ni, performance on handgrip strength altered more significantly in higher than lower urinary Ni concentrations. Although none of the evidence indicates a possible benefit of modest Ni levels to the human body, a high level of Ni undermines human health (Genchi et al. 2020) and muscular strength (Alegre-Martinez et al. 2022).
Apart from the single metal effect on handgrip strength, we found metal mixture negatively associated with handgrip strength in men and women, despite no statistical significance in women. Potential biological mechanisms may indicate oxidative stress and inflammatory response. Some heavy metals induce excessive reactive oxygen species (ROS) accumulation and pro-inflammatory cytokines generation (Anyanwu et al. 2018; Renu et al. 2021), undermining skeletal muscle function and homeostasis by affecting lipid metabolism, protein function and DNA integrity, which causes handgrip strength decrease and muscle mass loss (Lian et al. 2022, Meng and Yu 2010).
We find a sex-specific association between metals and handgrip strength, which may owe to sexual dimorphism. A review pointed out possible reasons for sexual dimorphism including different hormonal influences, different anatomic, neurochemical, genetic, behavioral and lifestyle characteristics, different gliosis, inflammation, and immune response (Gade et al. 2021). In the present study, we did not observe a significant impairment of handgrip strength from metal mixture in women although a negative association between metal mixture and handgrip strength was observed in men. The protection of estrogen may account for such a phenomenon. Estrogen has been proven to benefit skeletal and muscle systems and it can enhance muscle mass and muscle strength (Chidi-Ogbolu and Baar 2018). Compelling studies have indicated that estrogen supplementation can maintain and improve muscular function in postmenopausal women or protect against musculoskeletal damage of aging (Javed et al. 2019; Tiidus 2011). Estrogen seems to maintain cellular homeostasis in skeletal muscle, regulate mitochondria function, and reduce oxidative damage by endoplasmic reticulum (ER)-mediated mechanism, which may offset part of sarcous damage exerted from heavy metals (Ikeda et al. 2019; Papa et al. 2014).
Some limitations in our study should be noted. First, the cross-sectional design of our study cannot infer causality between metal exposure and handgrip strength. Second, spot urinary metal levels could not fully reflect the real exposure of individuals. Finally, despite controlling important confounding factors, there were unknown and unmeasured confounders that might bias our discoveries.
Conclusion
The findings of the present study indicate that single metal exposure including V, Zn, As, Rb, Cd, Tl, and U is inversely associated with handgrip strength in men. Moreover, among men, increased metal mixture exposure is associated with decreased handgrip strength, with Cd as the most crucial metal. Among women, Ni, Se, and Ag have a non-linear association with handgrip strength. With a view to the limitations of cross-sectional design, further prospective studies should be conducted to confirm these results.
Data availability
The data are available from the corresponding author on reasonable request.
References
Adedara IA, Fabunmi AT, Ayenitaju FC, Atanda OE, Adebowale AA, Ajayi BO, Owoeye O, Rocha JBT, Farombi EO (2020) Neuroprotective mechanisms of selenium against arsenic-induced behavioral impairments in rats. Neurotoxicology 76:99–110. https://doi.org/10.1016/j.neuro.2019.10.009
Alegre-Martinez A, Martinez-Martinez MI, Rubio-Briones J, Cauli O (2022): Plasma Nickel Levels Correlate with Low Muscular Strength and Renal Function Parameters in Patients with Prostate Cancer. Diseases 10 https://doi.org/10.3390/diseases10030039
Anyanwu BO, Ezejiofor AN, Igweze ZN, Orisakwe OE (2018): Heavy Metal Mixture Exposure and Effects in Developing Nations: An Update. Toxics 6 https://doi.org/10.3390/toxics6040065
Azeez IA, Olopade F, Laperchia C, Andrioli A, Scambi I, Onwuka SK, Bentivoglio M, Olopade JO (2016) Regional Myelin and Axon Damage and Neuroinflammation in the Adult Mouse Brain After Long-Term Postnatal Vanadium Exposure. J Neuropathol Exp Neurol 75:843–854. https://doi.org/10.1093/jnen/nlw058
Barber DS, Hancock SK, McNally AM, Hinckley J, Binder E, Zimmerman K, Ehrich MF, Jortner BS (2007) Neurological effects of acute uranium exposure with and without stress. Neurotoxicology 28:1110–1119. https://doi.org/10.1016/j.neuro.2007.05.014
Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL (2005) Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113:192–200. https://doi.org/10.1289/ehp.7337
Barrientos G, Alves J, Toro V, Robles MC, Munoz D, Maynar M (2020): Association between Trace Elements and Body Composition Parameters in Endurance Runners. Int J Environ Res Public Health 17 https://doi.org/10.3390/ijerph17186563
Bauer JA, Devick KL, Bobb JF, Coull BA, Bellinger D, Benedetti C, Cagna G, Fedrighi C, Guazzetti S, Oppini M, Placidi D, Webster TF, White RF, Yang Q, Zoni S, Wright RO, Smith DR, Lucchini RG, Claus Henn B (2020) Associations of a Metal Mixture Measured in Multiple Biomarkers with IQ: Evidence from Italian Adolescents Living near Ferroalloy Industry. Environ Health Perspect 128:97002. https://doi.org/10.1289/ehp6803
Carrico C, Gennings C, Wheeler DC, Factor-Litvak P (2015) Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 20:100–120. https://doi.org/10.1007/s13253-014-0180-3
Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, Iliodromiti S, Sillars A, Graham N, Mackay DF, Pell JP, Gill JMR, Sattar N, Gray SR (2018): Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 361, k1651. https://doi.org/10.1136/bmj.k1651
Chan J, Lu YC, Yao MM, Kosik RO (2022) Correlation between hand grip strength and regional muscle mass in older Asian adults: an observational study. BMC Geriatr 22:206. https://doi.org/10.1186/s12877-022-02898-8
Charles LE, Burchfiel CM, Fekedulegn D, Kashon ML, Ross GW, Sanderson WT, Petrovitch H (2006) Occupational and other risk factors for hand-grip strength: the Honolulu-Asia Aging Study. Occup Environ Med 63:820–827. https://doi.org/10.1136/oem.2006.027813
Chen CM, Chung MN, Chiu CY, Liu SH, Lan KC (2020): Inorganic Arsenic Exposure Decreases Muscle Mass and Enhances Denervation-Induced Muscle Atrophy in Mice. Molecules 25 https://doi.org/10.3390/molecules25133057
Chidi-Ogbolu N, Baar K (2018) Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front Physiol 9:1834. https://doi.org/10.3389/fphys.2018.01834
Clemens S, Ma JF (2016) Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu Rev Plant Biol 67:489–512. https://doi.org/10.1146/annurev-arplant-043015-112301
Derbre F, Gratas-Delamarche A, Gomez-Cabrera MC, Vina J (2014) Inactivity-induced oxidative stress: a central role in age-related sarcopenia? Eur J Sport Sci 14(Suppl 1):S98-108. https://doi.org/10.1080/17461391.2011.654268
Drake PL, Hazelwood KJ (2005) Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg 49:575–585. https://doi.org/10.1093/annhyg/mei019
Gade M, Comfort N, Re DB (2021): Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ Res 201, 111558. https://doi.org/10.1016/j.envres.2021.111558
Garcia-Esquinas E, Rodriguez-Artalejo F (2017) Environmental Pollutants, Limitations in Physical Functioning, and Frailty in Older Adults. Curr Environ Health Rep 4:12–20. https://doi.org/10.1007/s40572-017-0128-1
Garcia-Esquinas E, Carrasco-Rios M, Navas-Acien A, Ortola R, Rodriguez-Artalejo F (2020): Cadmium exposure is associated with reduced grip strength in US adults. Environ Res 180, 108819. https://doi.org/10.1016/j.envres.2019.108819
Garcia-Esquinas E, Carrasco-Rios M, Ortola R, Sotos Prieto M, Perez-Gomez B, Gutierrez-Gonzalez E, Banegas JR, Queipo R, Olmedo P, Gil F, Tellez-Plaza M, Navas-Acien A, Pastor-Barriuso R, Rodriguez-Artalejo F (2021a): Selenium and impaired physical function in US and Spanish older adults. Redox Biol 38, 101819. https://doi.org/10.1016/j.redox.2020.101819
Garcia-Esquinas E, Tellez-Plaza M, Pastor-Barriuso R, Ortola R, Olmedo P, Gil F, Lopez-Garcia E, Navas-Acien A, Rodriguez-Artalejo F (2021b): Blood cadmium and physical function limitations in older adults. Environ Pollut 276, 116748. https://doi.org/10.1016/j.envpol.2021.116748
Gbemavo MCJ, Bouchard MF (2021): Concentrations of Lead, Mercury, Selenium, and Manganese in Blood and Hand Grip Strength among Adults Living in the United States (NHANES 2011–2014). Toxics 9 https://doi.org/10.3390/toxics9080189
Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A (2020): Nickel: Human Health and Environmental Toxicology. Int J Environ Res Public Health 17 https://doi.org/10.3390/ijerph17030679
Hariharan S, Dharmaraj S (2020) Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology 28:667–695. https://doi.org/10.1007/s10787-020-00690-x
Heath JC, Banna KM, Reed MN, Pesek EF, Cole N, Li J, Newland MC (2010) Dietary selenium protects against selected signs of aging and methylmercury exposure. Neurotoxicology 31:169–179. https://doi.org/10.1016/j.neuro.2010.01.003
Ikeda K, Horie-Inoue K, Inoue S (2019): Functions of estrogen and estrogen receptor signaling on skeletal muscle. The Journal of Steroid Biochemistry and Molecular Biology 191 https://doi.org/10.1016/j.jsbmb.2019.105375
Javed AA, Mayhew AJ, Shea AK, Raina P (2019): Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-analysis. JAMA Netw Open 2, e1910154. https://doi.org/10.1001/jamanetworkopen.2019.10154
Kalita J, Kumar V, Misra UK, Bora HK (2020) Movement Disorder in Copper Toxicity Rat Model: Role of Inflammation and Apoptosis in the Corpus Striatum. Neurotox Res 37:904–912. https://doi.org/10.1007/s12640-019-00140-9
Khalil N, Faulkner KA, Greenspan SL, Cauley JA, Osteoporotic Fractures in Men Research G (2014): Associations between bone mineral density, grip strength, and lead body burden in older men. J Am Geriatr Soc 62, 141-6https://doi.org/10.1111/jgs.12603
Kim J, Garcia-Esquinas E, Navas-Acien A, Choi YH (2018) Blood and urine cadmium concentrations and walking speed in middle-aged and older U.S. adults. Environ Pollut 232:97–104. https://doi.org/10.1016/j.envpol.2017.09.022
Kim KN, Lee MR, Choi YH, Lee BE, Hong YC (2016) Associations of Blood Cadmium Levels With Depression and Lower Handgrip Strength in a Community-Dwelling Elderly Population: A Repeated-Measures Panel Study. The journals of gerontology. Series a, Biol Sci Med Sci 71:1525–1530. https://doi.org/10.1093/gerona/glw119
Krishna S, Dodd CA, Hekmatyar SK, Filipov NM (2014) Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch Toxicol 88:47–64. https://doi.org/10.1007/s00204-013-1088-3
Lian D, Chen MM, Wu H, Deng S, Hu X (2022): The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants (Basel) 11 https://doi.org/10.3390/antiox11040755
Liu W, Chen R, Song C, Wang C, Chen G, Hao J, Wang Y, Yu C (2021): A Prospective Study of Grip Strength Trajectories and Incident Cardiovascular Disease. Front Cardiovasc Med 8, 705831. https://doi.org/10.3389/fcvm.2021.705831
Meng SJ, Yu LJ (2010) Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 11:1509–1526. https://doi.org/10.3390/ijms11041509
Mustapha O, Oke B, Offen N, Siren AL, Olopade J (2014) Neurobehavioral and cytotoxic effects of vanadium during oligodendrocyte maturation: a protective role for erythropoietin. Environ Toxicol Pharmacol 38:98–111. https://doi.org/10.1016/j.etap.2014.05.001
Ng SW, Norton EC, Popkin BM (2009) Why have physical activity levels declined among Chinese adults? Findings from the 1991–2006 China Health and Nutrition Surveys. Soc Sci Med 68:1305–1314. https://doi.org/10.1016/j.socscimed.2009.01.035
Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S (2016) Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 46:951–963. https://doi.org/10.1111/hepr.12774
Orndahl G, Rindby A, Selin E (1982) Myotonic dystrophy and selenium. Acta Med Scand 211:493–499. https://doi.org/10.1111/j.0954-6820.1982.tb01988.x
Papa V, Wannenes F, Crescioli C, Caporossi D, Lenzi A, Migliaccio S, Di Luigi L (2014) The environmental pollutant cadmium induces homeostasis alteration in muscle cells in vitro. J Endocrinol Invest 37:1073–1080. https://doi.org/10.1007/s40618-014-0145-y
Perri G, Mendonca N, Jagger C, Walsh J, Eastell R, Mathers JC, Hill TR (2020): Dietary Selenium Intakes and Musculoskeletal Function in Very Old Adults: Analysis of the Newcastle 85+ Study. Nutrients 12 https://doi.org/10.3390/nu12072068
Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L (1999) Midlife hand grip strength as a predictor of old age disability. JAMA 281:558–560. https://doi.org/10.1001/jama.281.6.558
Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM (2000) Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. The journals of gerontology. Series a, Biol Sci Medi Sci 55:M168–M173. https://doi.org/10.1093/gerona/55.3.m168
Rederstorff M, Krol A, Lescure A (2006) Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci 63:52–59. https://doi.org/10.1007/s00018-005-5313-y
Renu K, Chakraborty R, Myakala H, Koti R, Famurewa AC, Madhyastha H, Vellingiri B, George A, Valsala Gopalakrishnan A (2021): Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium) - induced hepatotoxicity - A review. Chemosphere 271, 129735. https://doi.org/10.1016/j.chemosphere.2021.129735
Siparsky PN, Kirkendall DT, Garrett WE (2013) Muscle Changes in Aging. Sports Health: A Multidiscip Appr 6:36–40. https://doi.org/10.1177/1941738113502296
Stenholm S, Tiainen K, Rantanen T, Sainio P, Heliovaara M, Impivaara O, Koskinen S (2012) Long-term determinants of muscle strength decline: prospective evidence from the 22-year mini-Finland follow-up survey. J Am Geriatr Soc 60:77–85. https://doi.org/10.1111/j.1532-5415.2011.03779.x
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Tiidus PM (2011) Benefits of Estrogen Replacement for Skeletal Muscle Mass and Function in Post-Menopausal Females: Evidence from Human and Animal Studies. Eur J Med 43:109–114. https://doi.org/10.5152/eajm.2011.24
Walsh JS, Jacques RM, Schomburg L, Hill TR, Mathers JC, Williams GR, Eastell R (2021) Effect of selenium supplementation on musculoskeletal health in older women: a randomised, double-blind, placebo-controlled trial. The Lancet Healthy Longevity 2:e212–e221. https://doi.org/10.1016/s2666-7568(21)00051-9
Wu M, Shu Y, Wang Y (2022) Exposure to mixture of heavy metals and muscle strength in children and adolescents: a population-based study. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-022-19916-2
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int 23:8244–8259. https://doi.org/10.1007/s11356-016-6333-x
Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, Khanna VK (2009) Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol 240:367–376. https://doi.org/10.1016/j.taap.2009.07.017
Yano C, Marcondes M (2005) Cadmium chloride-induced oxidative stress in skeletal muscle cells in vitro. Free Radical Biol Med 39:1378–1384. https://doi.org/10.1016/j.freeradbiomed.2005.07.001
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM (2019) The essential metals for humans: a brief overview. J Inorg Biochem 195:120–129. https://doi.org/10.1016/j.jinorgbio.2019.03.013
Acknowledgements
We thank all the study participants and the staffs of the Tongji Hospital.
Funding
This study was supported by the National Natural Science Foundation of China (82073660, 82003479) and China Postdoctoral Science Foundation (2019M662646, 2020T130220).
Author information
Authors and Affiliations
Contributions
Xiya Qin: formal analysis, writing- original draft, writing- review & editing. Gaojie Fan: data curation, writing- review & editing. Qing Liu: investigation, data curation. Mingyang Wu: conceptualization, data curation. Jianing Bi: investigation, data curation. Qing Fang: investigation, data curation. Zhengce Wan: resource. Yongman Lv: resource. Lulu Song: methodology, funding acquisition, supervision. Youjie Wang: funding acquisition, project administration, supervision, writing- review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
The study protocol was approved by the Ethics Review Board of Tongji Medical College, Huazhong University of Science and Technology.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, X., Song, L., Fan, G. et al. Sex-specific associations of single metal and metal mixture with handgrip strength: a cross-sectional study among Chinese adults. Environ Sci Pollut Res 30, 66585–66597 (2023). https://doi.org/10.1007/s11356-023-26926-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26926-1