Abstract
Atmospheric deposition of pollutants decreases pH and increases the nutrient concentration in the surface water. To examine its impact on coastal phytoplankton composition and primary production, monthly atmospheric aerosol samples were mixed with coastal waters in the microcosm experiments. These experiments suggested that the biomass of Bacillariophyceae, Dinophyceae and Chlorophyceae were increased and primary production of the coastal waters increased by 3 to 19% due to the addition of aeolian nutrients. The increase in primary production displayed significant relation with a concentration of sulphate and nitrate in the atmospheric aerosols suggesting that both decreases in pH and fertilization enhanced primary production. The impact of acidification on primary production was found to be 22%, whereas 78% was contributed by the nutrient increase. The atmospheric pollution is increasing rapidly over the northern Indian Ocean since past two decades due to rapid industrialization. Hence, it is suggested that the impact of atmospheric pollution on the coastal ecosystem must be included in the numerical models to predict possible changes in the coastal ecosystem due to climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The transport of nutrients from continent to ocean, through atmospheric aerosols, is recognized as one of the important sources of nutrients to the surface ocean (e.g. Duce et al. 1991; Prospero et al. 1996). The deposition of atmospheric pollutants has been reported to have an impact on primary production (Duce et al. 2008; Boyd et al. 2010; Mahowald et al. 2011; Yadav et al. 2016) and produce ocean acidification (Doney et al. 2007; Hagens et al. 2013; Sarma et al. 2015; Kumari et al. 2021). The impact of deposition of atmospheric pollutants on ocean acidification is more significant in the northern hemisphere than in the south (Doney et al., 2007). The global aeolian dissolved inorganic nitrogen (DIN) is almost found to be equivalent to that of riverine sources (Jickells 1998; Boyd et al. 2010), and therefore, it may have a significant impact on surface ocean biogeochemistry.
The Bay of Bengal (BoB) is surrounded by landmass, where one-third of the world population is dwelling. As a result, BoB receives significant amounts of atmospheric pollutants throughout the year. Seasonal reversing in direction of winds (Schott and McCreary 2001) led to a change in the source of atmospheric aerosols over the BoB (Kumar et al. 2008). Higher levels and rates of increase in aerosol optical depth (AOD) had been reported over the western BoB compared to them elsewhere in the global ocean over the past two decades (Zhang and Reid 2010; Yadav et al. 2021). A significant increase in surface Chl-a associated with atmospheric aerosol deposition was reported in the BoB (Patra et al. 2007; Banerjee and Prasanna Kumar 2014; Guieu et al. 2019; Lachkar et al. 2021). Kumar et al. (2021) estimated that the dry depositional flux of DIN to the BoB is equal to that of riverine sources to the BoB. Assuming that the dry depositional nutrients are completely utilized by marine phytoplankton, it supports 3 to 33% of the primary production in the coastal BoB (Srinivas et al. 2011; Yadav et al. 2016). These estimates carry significant errors due to a lack of knowledge on depositional rates at a variable particle size of total suspended particulates (TSP). The change in N:P ratios due to the deposition of pollutants may modify phytoplankton composition (Yadav et al. 2016). On the other hand, a significant decrease in pH of surface waters was reported due to the deposition of atmospheric pollutants (Sarma et al. 2015; 2021; Sridevi and Sarma 2021; Kumari et al. 2021). The decrease in pH may influence plankton biomass and primary production in the marine environment (e.g. Biswas et al. 2012; 2017; Mackey et al. 2015; Scherner et al. 2016). The objective of this work is to examine the influence of the monthly variability in the dry depositional flux of aerosols on phytoplankton composition and primary production in the coastal BoB.
Material and methods
Study area and collection of atmospheric aerosols
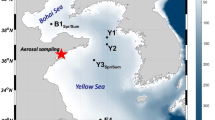
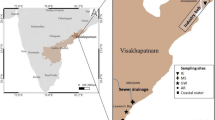
The aerosol samples were collected over the sampling location, Visakhapatnam (17.62°N and 83.22°E; Fig. 1), which is a moderate city, and harbour several industries and ports. The National Thermal Power Plant (NTPC) is located close to the (within 20 km) study region. The total suspended particulates (TSP) in the atmosphere over the study region were collected from March 2015 to February 2016 from the terrace of the CSIR-National Institute of Oceanography, Visakhapatnam building at a height of ~ 20 m above the mean sea level (Fig. 1) using a high-volume sampler (Envirotech-430) at a flow rate of 1.2 m3 min−1. The samples were collected on pre-combusted quartz fibre filters (395.5 cm2; PALLFLEX).
The map showing the study region where atmospheric aerosol samples (red circle) were collected and surface seawater was obtained for incubation experiments (blue circle) from the coastal Bay of Bengal. The weekly HYSPLIT wind trajectories during different months were also given in red (first week), blue (second week), green (third week), pink (fourth week) and grey (fifth week) to the study region
Microcosm experiments
We have conducted three microcosm experiments to examine the (1) impact of monthly variability in aerosol dissolution on phytoplankton pigment composition and primary production, (2) changes in phytoplankton composition due to acidification caused by aerosol dissolution and (3) modification in phytoplankton composition due to an increase in nitrate due to addition of aerosols.
Impact of dissolution of atmospheric aerosols on coastal water phytoplankton pigment composition and primary production
The surface seawater was collected from the coastal BoB and filtered through 200-μm mesh to remove larger zooplankton. Half of the filter (198 cm2) containing aerosols was dissolved in the 150 ml filtered nutrients-free seawater using ultrasonic agitation for 30 min. The amount of TSP in the aliquot of filter used in each month was given in Table 1. The extracted solution was filtered through a 0.7-μm GF/F filter to remove the undissolved particles. The filtered extract was then divided into three 50 ml fractions. Every 50 ml of extract was added to 20 L of coastal surface seawater in the Nalgene bottles (called “experiment”). Along with this, 20 L of coastal surface water were also incubated (without the addition of atmospheric aerosols) (called “control”). The incubation was conducted under natural sunlight, and the temperature of the incubation bottle was controlled by temperature-controlled water circulators, and bottles were stirred at regular intervals to avoid settling of planktons in the bottle. All experiments were conducted for 4 days in triplicate, and sampling was done every day for nutrients, phytoplankton pigment composition, and primary production.
Impact of acidification on coastal water phytoplankton pigment composition and primary production
Since in the above experiment both increases in nutrients and decreases in pH occurred due to the dissolution of aerosols from the different months, the net impact on primary production and pigment composition is known. To examine the role of a decrease in pH (acidification) due to the addition of aerosols on primary production and pigment composition, an additional experiment was conducted by decreasing in pH of the surface coastal water through the addition of acid. To determine the magnitude of changes in pH to be brought in the experiment, we have considered the experiments conducted by Kumari et al. (2021) on the decrease in seawater pH due to the dissolution of aerosols using the same samples used. Kumari et al. (2021) measured the pH of surface seawater, and an aliquot of aerosols was dissolved and measured pH again. The difference in pH was normalized to the weight of the aerosol added to the sample to estimate the decrease in pH due to the addition of 1 μg m−3 of aerosol added. Based on this monthly data, 0.03 to 0.06 units of pH were decreased due to the addition of 1 μg m−3 of aerosol to 20 L of seawater. Hence, pH of the coastal water in the microcosm tank was decreased from 0.03 to 0.06 by the addition of 0.1 N hydrochloric acid. One set of bottles was kept without a change in pH as a control. This experiment was conducted for 4 days in triplicate, and sampling was done every day.
Impact of increase in nitrate on coastal water phytoplankton pigment composition and primary production
Similar to the acidification experiment, to examine the impact on primary production and phytoplankton composition due to the increase in nitrate, another experiment was conducted with the addition of commercial potassium nitrate. To determine the magnitude of nitrate to be added in the experiment, an aliquot of the filter was added to the nutrient-free surface seawater, and an increase in the concentration of nitrate was measured. The increase in nitrate was then normalized to the weight of the aerosol added to the sample to estimate the increase in nitrate due to the addition of 1 μg m−3 of aerosol added. Based on this data, 0.2 to 0.8 μM of nitrate was increased due to the addition of 1 μg m−3 of aerosol to 20 L of seawater. Hence, nitrate was increased by 0.2 and 0.8 μM in the two sets of microcosm tanks. One set of bottles was kept without changes in nitrate concentration as a control. This experiment was conducted for 4 days in triplicate, and sampling was done every day.
Impact of the combined effect of the increase in nitrate and decrease in pH on phytoplankton composition
To examine the combined effect of an increase in nitrate and a decrease in pH, another experiment was conducted with an increase in nitrate by 0.2 μM along with a decrease in pH by 0.03 and an increase in nitrate by 0.8 μM along with the decrease in pH by 0.06. One set of bottles was kept with changes in neither pH nor nitrate. The experiment was conducted for 4 days in triplicate and sampling was done every day.
In vitro primary production experiments
Every day an aliquot of sample was subsampled from each bottle to 1 L Nalgene transparent bottle and spiked with 0.2 ml of 2 mM of 13C enriched sodium bicarbonate (NaH13CO3; 99%; Sigma-Aldrich, USA) and incubated in duplicate in light and dark bottles in the incubator under natural sunlight conditions. Before the addition of the spike, one set of samples was filtered through a pre-combusted (300 °C for 6 h) GF/F filter to examine the initial atomic ratio of 13/12C. Samples were incubated for 24 h and filtered through pre-combusted filters and dried at 50 °C for 12 h for further analysis.
Measurements
Nutrients
Nutrients in aerosols
Nutrients in aerosol samples were measured using ion chromatography (850 Metrohm Professional, Switzerland). A subsample of quartz fibre filter, on which aerosol samples were collected, was taken and kept in the 50 ml of Milli-Q water and sonicated for 30 min to extract nutrients into the water phase. Similarly, the blank filter was also sonicated to examine the possible source of elements through the filter and did not find a significant contribution within the limits of measurements. The extract was filtered through a 0.2-μm polycarbonate filter and injected into ion chromatography using a cation exchange column (Metrosep C4-150/4.0) and an anion exchange column (Metrosep A Supp 5 250/4.0) using a conductivity detector. The mixture of Na2CO3 (3.2 mM), NaHCO3 (1 mM), and H2SO4 (0.1 mM) was used as a mobile phase for anions, whereas HNO3 (2 mM) is used as eluent for cations. A detailed analysis of ion chromatography is given elsewhere (Yadav et al. 2016; Kumari et al. 2021). The overall precision of these measurements is < 10%.
Nutrients in seawater
The concentration of nutrients (nitrate, nitrite and phosphate, silicate) was estimated following Grasshoff et al. (1992)using a colorimetric procedure with an autoanalyzer (Ecolab, USA). The analytical precision for nitrate, nitrite, phosphate and silicate was ± 0.02, ± 0.01, ± 0.01 and ± 0.02 μM, respectively.
Pigments
The pigment retained on the filter was extracted into 5 ml of 90% acetone (HPLC grade from Merck) at − 20 °C for 12 h in amber colour bottles to prevent photodecomposition.
To break the cells, a tissue homogenizer (ULTRA-TURRAX, Germany) was used by keeping the bottles on an ice-filled tray followed by cold centrifugation at − 10 °C (Eppendorf, Germany). The extract was mixed with a 90:10 ratio of 20 mM aqueous tetra butyl ammonium acetate (AR grade, Fluka) and methanol (GC Assay 99.7% pure, Merck) to maintain a pH of 6.5. The mixture of buffer and samples was injected into a high-pressure liquid chromatograph (Agilent 1200 series) at a pressure of 200b (maximum) (Van Heukelem and Thomas 2001). The peaks of the marker pigments were identified using high purity standards from DHI Laboratory products, Denmark. The precision of the pigment analysis was ± 1%. A total of 16 pigments (peridinin, fucoxanthin, zeaxanthin, 19′-but-fucoxanthin, 19′-hexa-fucoxanthin, violaxanthin, astaxanthin, diadinoxanthin, diatoxanthin, alloxanthin, lutein, chlorophyll-a, chlorophyll-b, divinyl Chl-a, pheophytin, B-carotene) can be detected from this methodology.
Using pigment data, the relative contribution of different size classes of phytoplankton was computed (Uitz et al. 2006) using the following equation:
The percentage contribution of different size class of phytoplankton was computed as
Primary production
The filters were acid-fumigated for 12 h to remove inorganic carbon and dried again. An aliquot of the filter was packed in the tin cup and introduced to an Elemental Analyser (Isocube) coupled to an isotope ratio mass spectrometer (IRMS; M/s Elementar, Germany) to measure the content and the atomic ratio of organic carbon on the filter. The primary production was computed following Hama et al. (1983), and the precision of the measurement was estimated to be 5%.
Air mass back trajectories (AMBT)
The monthly mean AMBT were derived using the GDAS database of the National Oceanic and Atmospheric Administration (NOAA) Air Resources Laboratory and the Hybrid Single-Particle Lagrangian Integrated Trajectory Model (HYSPLIT program, version 4.0) at the height of 500 m (Draxler, 2002). The AMBTs were calculated for 7 days before our aerosol sample collection.
Results
Air mass back trajectories (AMBT) and composition of nutrients in the aerosols
The AMBT suggested that aerosols were brought to the study region from marine origin during March–April, whereas terrestrial aerosols from west or southwest from June to September and northeast or northwest from October to February (Fig. 1). The concentration of TSP over the study region varied between 20 and 98 µg m−3 with higher concentration during January and lower concentration during June and increased again with minima during September (Fig. 2a). The concentration of soluble nitrate and sulphate did not follow TSP as higher soluble nitrate (3.7 to 11.4 μg m−3) was observed from August to November (Fig. 3b). The concentration of phosphate was significantly higher during January followed by December (0.1 to 0.2 μg m−3), whereas it was very low from February to April (0.005 to 0.01 μg m−3) and under detection limits during other months (Fig. 2c). Higher soluble sulphate concentration (6.1 to 22.1 μg m−3) was found from September to November than in other months (Fig. 2d).
Changes in nutrients concentration after addition of atmospheric aerosol
The half of the quartz filter (198 cm2) was used for the experiment. The aerosols in this piece of the filter were extracted into the 150 ml of water, and each 50 ml was added to one microcosm experiment for each month. Therefore, each microcosm bottle received 3.54 to 16.19 μg of aerosols each month (Table 1). The concentration of nitrate and phosphate in the coastal water was 0.48 ± 0.08 and 0.11 ± 0.02 μM, respectively, with an N:P ratio of 4.3 ± 0.5. After the addition of atmospheric aerosols, the concentration of nitrate in the incubated seawater increased by 0.03 to 1.05 μM with a rise of 6 to 219% from that of seawater (Table 1). A higher increase in nitrate was observed during September (219%) and the least during March (6%; Table 1). Similarly, the concentration of phosphate was increased by 0.0 to 0.09 μM (0 to 87%; Table 1) with a high increase during March (87%), but it was ~ 5% during April, July, August, and December (Table 1). After the addition, the N:P ratios increased by 0.4 to 9.5 from that of 4.3. The decrease in N:P ratio was observed during March due to higher phosphate than nitrate addition by aerosols. The mean increase in N/P ratio was ~ 7.0 ± 2 after the addition of aerosols, which is still lower than the Redfield ratio of 16.
Response of phytoplankton biomass and pigment composition to aerosol dissolution
We targeted 16 pigments for analysis; however, the significant concentrations of only 8 pigments (peridinin, fucoxanthin, 19′HF, 19′BF, zeaxanthin, alloxanthin, Chl-b, Div-Chl-a) were detected in the samples. The concentration of peridinin and fucoxanthin was higher in the control during January to May and December, whereas a higher concentration of zeaxanthin, Chl-b and Div-Chl-a were observed from June to November (Fig. 3a). With reference to the increase in nutrient concentrations, a significant increase in peridinin and fucoxanthin (8.4–102.6 ng/l and 6.9 to 194.0 ng/l, respectively) was observed (Table 2). The increase in peridinin was more from July to December (> 60 ng/l) and all months, except March, for fucoxanthin (> 75 ng/l) due to the addition of aerosols compared to control. Similarly, a significant increase in Chl-b was observed from June to November, whereas Div-Chl-a was noticed throughout the year with a maximum during September (75–200 ng/l) compared to control (Fig. 3b). A significant decrease in zeaxanthin and alloxanthin was observed during the entire study period and between June and November for 19′HF from that of control (Fig. 3c). An increase in Chl-a from 0.4 to 6.4 μg/l was observed compared to control with a higher increase during September (Table 2). The percentage of increase in peridinin and fucoxanthin was higher from January to May and December by up to 2400%, whereas it was 250–500% from June to November from that of control (Fig. 3d). The increase in Div-Chl-a and Chl-a was higher (> 250%) during January, September and December. The increase in Chl-b and Alloxanthin varied between 50 and 150%, whereas it was low (< 50%) in the case of zeaxanthin than control (Fig. 3d). The increase in pigment concentration displayed a significant linear relationship with an increase in nitrate concentrations, and this relationship was insignificant for zeaxanthin, 19′HF and 19′BF (Table 3). Similarly, the N:P ratios in the sample, after the addition of atmospheric aerosols, displayed a linear relationship with all pigments, except for Chl-b, 19′HF and alloxanthin (Table 3).
The contribution of microplankton to the total phytoplankton biomass varied between 40 and 68% in the surface water which is increased to 44–74% after the addition of atmospheric aerosols than control (Fig. 4). A higher increase in microplankton contribution was observed from July to November (by 17–30%) and lower during other periods (< 10%) than control. The contribution of nanoplankton was decreased during the entire study period (by 5 to 10%) except in March, April and December when it was increased by 5–10% compared to control. Picoplankton was decreased during the entire study period by 5 to 23%, except during April, when it was increased by 17% than control (Fig. 4).
Response of primary production to nutrient enrichment due to addition of atmospheric aerosols
Primary production in the control during January to April and December was 16.2 ± 3 mgC m−3 d−1, whereas it was 39.2 ± 6 mgC m−3 d−1 from June to November. After the addition of aerosols, primary production increased by 0.6 to 7.6 mgC m−3 d−1 (3 to ~ 19%) from that of control (Table 2). The higher rate of increase was noticed during September (~ 19%) and the least during June (3%). The rate of increase in primary production displayed linear relation with concentrations of nitrate (r2 = 0.89; p < 0.001), phosphate (r2 = 0.43; p < 0.01) and sulphate (r2 = 0.68; p < 0.001) in the atmospheric aerosols (Fig. 5), and similar relationship was observed between increase in nitrate and phosphate, after the addition of aerosols from that of control, and increase in primary production.
The addition of 1 μg m−3 of aerosols in 20L of seawater decreased pH by 0.03 to 0.06 (Fig. 6a) and displayed linear relation with an increase (r2 = 0.45; p < 0.001; Fig. 6b). A linear relationship was also observed between an increase in primary production to sulphate concentration in aerosols added (Fig. 5c).
Role of decrease in pH on phytoplankton biomass, pigment composition and primary production
An insignificant difference in response of pigment composition relative to control was observed between the decrease in pH by 0.03 and 0.06; hence, mean response was discussed. In the control, relatively higher peridinin, fucoxanthin and Chl-b (20–30 ng/l) were observed compared to other pigments. After a change in pH from 0.03 to 0.06, the concentration of fucoxanthin and Chl-b increased by 14.5 and 36.1 ng/l, respectively, with an increase of 47 and 109% than control (Table 4). Chl-a increased by 0.4 μg/l (17%) from that of control. The increase in nitrate by 0.2 μM increased fucoxanthin, Chl-b and Chl-a by 11.7, 5 ng/l and 0.6 μg/l, respectively, whereas peridinin and zeaxanthin decreased from that of control (Table 4). In contrast, an increase in nitrate by 0.8 μM, increased peridinin, fucoxanthin, Chl-b and Chl-a were found, but a decrease in Zeaxanthin was noticed than control. The mixture of a decrease in pH by 0.03 and an increase in nitrate by 0.2 μM enhanced fucoxanthin, Chl-b and Chl-a by 23%, 15% and 13%, respectively, but the other pigments were decreased, whereas a decrease in pH by 0.06 and increase in nitrate by 0.8 μM enhanced concentration of fucoxanthin (22%), Chl-b (100%), peridinin (10%) and Chl-a (38%; Table 4) than control. The decrease in pH by 0.03 and 0.06 decreased the contribution of microplankton and nanoplankton by 7% and 9%, respectively, whereas picoplankton contribution increased by 17% than control. The addition of nitrate (both 0.2 and 0.8 μM) enhanced microplankton contribution by 7%, whereas an increase in picoplankton contribution was noticed (7%) in the case of low nitrate. The decrease in pH and increase in nitrate concentration enhanced microplankton contribution by up to 30%, but a decrease in nano- and picoplankton was noticed compared to control (Fig. 7).
Variability in percentage of the contribution of a microplankton, b nanoplankton and c picoplankton in the control (blue bars) and experimental (red bars) due to change in pH and addition of nitrate and combination of both in the experimental mesocosm tank. The variability in their contribution within triplicate is shown as vertical bars
The primary production in the control was 11.4 mgC m−3 d−1 and a decrease in pH by 0.03 to 0.06 increased production by 2.5 mgC m−3 d−1 (by 22%) from that of control with an insignificant difference between the range of pH decreased. The addition of low and high nitrate increase enhanced primary production by 1.1 and 5.7 mgC m−3 d−1 (by 10 and 50%), respectively, than control (Table 3). The increase in both nitrate and decrease in pH together enhanced primary production by 3.8 and 8.6 mgC m−3 d−1 (33 to 75%) from that of control.
Discussion
Response of coastal phytoplankton pigment composition to the addition of aerosols
The concentration of TSP over the study region displayed significant variability with higher concentration from June to February, whereas the concentration of nutrients was higher from August to November than in other months (Fig. 2b, c). The variability in the concentration of TSP and nutrients in aerosols is related to the source and size of the particles modulated by atmospheric processes (Church and Jickells 2004). The aerosols were received from northeast (Southwest Asia) between October and December and northeast (Southeast Asia) during January and February to the study region when moderately higher concentrations of TSP and nitrate concentrations were observed compared to March to May (Fig. 1) when marine winds were observed. Winds turned from western (terrestrial; Indian peninsular) between June and September, when both TSP and nitrate concentrations were significantly increased from that of previous months (Tstat = − 2.62; p < 0.001) due to the transport of anthropogenic aerosols from the Indian subcontinent (Fig. 1). The measurable levels of phosphate were observed only from December to February when winds were blowing from the northwest to the study region suggesting it is sourced from Indo-Gangetic Plain (Kumar et al. 2008; Srinivas et al. 2011).
The concentration of nutrients (nitrate and phosphate) in the coastal BoB is low due to strong stratification driven by the river discharge (Sarma et al. 2013a, 2013b). Krishna et al. (2015) reported that most of the river-borne nutrients are utilized within the estuary itself and a small fraction (< 5%) are fluxed to the coastal waters. Sarma et al. (2013) found that nitrate is the major limiting nutrient in the coastal BoB. The concentration of nitrate is always present in the atmospheric aerosols over the coastal BoB, but displayed significant monthly variability (Fig. 2b) and its deposition may increase not only its concentration but also N:P ratios. The mean N:P ratio of the coastal BoB was < 5, and the same increased by 0.4 to 9.5 from that of control due to the addition of atmospheric nitrogen and phosphorus during different months (Table 1). The concentration of silicate was > 3 μM in the coastal waters, and they are much above the limiting levels (< 1 μM) throughout the year (Sarma et al. 2013) suggesting it is not a limiting nutrient.
The time-series measurement of nutrients for 4 days, after the addition of aerosols, found that nitrate was consumed within 2 to 3 days and come to the under-detection levels during all months suggesting that nitrate is a strong limiting nutrient in the coastal BoB. The fertilization experiment conducted using a mixing of nutrient-rich deep water with surface water also suggested that nitrate is a limiting nutrient in the coastal BoB (Sarma et al. 2013). The concentration of phosphate was at a measurable level, even after 4 days of incubation, whereas silicate concentration was significantly high (> 3 μM) throughout the study (Figure is not shown) indicating silicate is not a limiting nutrient.
The coastal waters were dominated by Bacillariophyceae (fucoxanthin), Dinophyceae (peridinin), blue-green algae (Chl-b), Prochlorococcus (divinyl Chl-a) and Cyanophyceae (zeaxanthin) with relatively higher biomass of Bacillariophyceae (45 ng/l) and the least of Dinophyceae (32 ng/l). The ratio of Bacillariophyceae to Cyanophyceae (bac/cya) was < 2 and Bacillariophyceae to Dinophyceae (bac/din) was ~ 2 in the coastal BoB. After the addition of aerosols to the coastal waters, the major pigments, peridinin, fucoxanthin, Chl-b and Div-Chl-a, increased significantly (Table 2). A significant increase in the concentration of peridinin (Dinophyceae) was observed from July to November associated with high N:P ratios (> 7; Table 1) suggesting that enhanced inorganic nitrogen input enhanced the biomass of dinoflagellates. A significant increase in Bacillariophyceae (fucoxanthin) was observed (6.9 to 194.0 ng/l) associated with an increase in nitrate concentration and N:P ratios. Make a note here that the less increase in both dinoflagellates (peridinin) and diatoms (fucoxanthin) was noticed during March when the least increase in nitrate and lower N:P ratio was noticed (Table 1). The increase in Prochlorococcus (marker pigment of Div-Chl-a) displayed a significant increase throughout the year with a higher increase during winter (December to February) followed by summer (June to October). In contrast, decrease in zeaxanthin (cyanobacteria) was observed by almost 50% from that of initial conditions (Fig. 3d). The ratio of bac/cya increased from < 2 in the coastal water to > 7 after the addition of aerosols from January to February, July to August and November suggesting a significant response of Bacillariophyceae to nutrient increase. On the other hand, the bac/din ratio decreased from ~ 2 in the coastal waters to 0.8 from January to April but slightly increased to 1.2 from June to December suggesting that significant increase in Dinophyceae in response to an increase in nutrients. This study suggested that the dominant response of Bacillariophyceae from December to June and Dinophyceae from July to November in the coastal BoB is due to the addition of aerosols.
In response to the increase in concentration of nutrients, the concentration of phytoplankton biomass (as Chl-a) increase from 0.4 to 6.4 μg/l and displayed significant linear relationship with concentration of nitrate increased after addition of aerosols (r2 = 0.66; p < 0.01), concentration of nitrate in the TSP (r2 = 0.53; p < 0.05) and N:P ratios (r2 = 0.88; p < 0.001). Similarly, the concentration of nitrate increased in the coastal waters due to aerosol addition exhibited linear relation with a concentration of peridinin, fucoxanthin, Chl-b, alloxanthin and divinyl Chl-a (Table 3), whereas it was not significant in the case of zeaxanthin, 19′HF and 19′BF. The N:P ratios, after the addition of aerosols to coastal waters, displayed linear relation with peridinin, fucoxanthin, zeaxanthin, 19′BF and divinyl Chl-a suggesting that both increase in nitrate and N:P ratios due to aerosol addition significantly modified phytoplankton composition in the coastal waters (Table 3).
The increase in the contribution of microplankton relative to control was observed due to the addition of aerosols. The increase in microplankton contribution was higher from June to November than in other months (Fig. 4a). In contrast, a decrease in the contribution of picoplankton was observed in the experiment compared to the control (Fig. 4c), whereas insignificant modifications were noticed in the case of nanoplankton (Fig. 4b). This experiment suggested that aerosol addition promoted microphytoplankton growth that may lead to a possible increase in export production to deep and efficient carbon sequestration.
Response of primary production to aerosol nutrients addition in the coastal BoB
Despite strong stratification and weak nutrient inputs through vertical mixing to the surface waters, the mean primary production in the photic zone during the dry period is higher in the BoB (340 ± 139 mgC m−2 d−1; Gauns et al. 2005; Kumar and Ramesh 2005; Prasanna Kumar et al. 2007; Jyothibabu et al. 2008; Ramaiah et al. 2010) than the Arabian Sea (200 ± 10 mgC m−2 d−1). Yadav et al. (2016) reported that 3–33% of the coastal primary production is contributed by atmospheric deposition. These estimations were carried out based on the computation of atmospheric deposition using the dry depositional velocity of 0.1 and 1 cm/s for PM2.5 and PM10, respectively (Duce et al., 1991), and assuming that all the deposited nutrients were consumed by the phytoplankton. The dry depositional flux includes the uncertainty of a factor of 3, which is caused due to several factors that include wind speed, terrain roughness, particle composition, etc. (Srinivas et al 2011).
The experiments conducted in this study suggested that aerosol dissolution in the coastal waters increased primary production by 3 to 19% (0.6 to 7.6 mgC m−3 d−1) due to the input of nutrients from aerosols. The increase was higher from June to November with maxima in September than in other months. The variations in an increase in primary production displayed a significant linear relationship with an increase in nitrate (r2 = 0.85; p < 0.001) and phosphate (r2 = 0.46; p < 0.05) in the coastal waters after the addition of aerosols suggesting that increase in nutrients enhanced coastal primary production (Fig. 5). Similarly, the concentration of nitrate in the TSP also displayed a significant linear relationship with an increase in primary production (r2 = 0.65; p < 0.001). Based on this relationship, it is estimated that 1 μg m−3 of nitrate in the aerosols deposited on the surface waters enhances primary production by ~ 1.7 ± 0.5 mgC m−3 d−1 in the coastal waters.
Influence of acidification on phytoplankton composition and primary production
Sarma et al. (2015) reported a decrease in pH of surface water due to the deposition of atmospheric pollutants due to the formation of nitric and sulphuric acid from NO3 and SO4 in the aerosols. Biswas et al. (2015) noticed that a significant increase in phytoplankton biomass and primary production was observed upon a decrease in pH in the microcosm experiments. The increase in primary production in the present study displayed a significant linear relationship with sulphates and nitrate (Fig. 5) in the aerosols suggesting that an increase in primary production may be contributed by both decreases in pH and also increase in nutrients. To account contribution of pH and nutrients, additional experiments were conducted.
The addition of 1 μg m−3 of atmospheric aerosols in the 20 L of surface seawater decreased pH by 0.01 to 0.07 units during different months. The decrease in pH due to aerosol dissolution displayed a linear relationship with an increase in primary production (Fig. 6b) suggesting that acidification of waters enhanced phytoplankton production in the surface waters. Since the addition of atmospheric aerosols decreases pH and increases nutrients together, we had conducted another experiment to understand the impact of pH and nutrients individually by decreasing pH by 0.03 and 0.06 by addition of acid (HCl) in two sets of surface water bottles and addition of 0.2 and 0.8 μM of potassium nitrate in another two sets of surface water bottles. It was noticed that a decrease in pH by 0.03 and 0.06 resulted in an increase in primary production by 22 ± 1% and did not show a significant difference between the two sets (Table 4). On the other hand, an increase in nitrate by 0.2 and 0.8 μM of nitrate enhanced primary production by 10 and 50%, respectively, from that of control, respectively, suggesting that higher influence of nitrate than acidification (Table 4). Based on these experiments, the mean increase in primary production due to a decrease in pH accounted for 22% of the total production increase, whereas the remaining increase was contributed by the increase in nutrients.
Both decrease in pH and increase in nitrate significantly modified the phytoplankton pigment composition. In the control, the concentration of Chl-b and fucoxanthin was relatively higher (~ 30 ng/l) compared to other pigments detected (< 10 ng/l). After a decrease in pH and an increase in nitrate concentrations, both fucoxanthin and Chl-b were increased significantly (Table 4) by 22–38 and 15–109%, respectively, with the higher response of Chl-b associated with higher addition of nitrate. The response of peridinin was noticed (10%) only at the high increase in nitrate (0.8 μM) than others (Table 4). The rate of increase in Chl-b was higher (100%) followed by Chl-a (37%) due to a high decrease in pH and a high increase in nitrate in the coastal waters. This experiment suggested that both fucoxanthin and Chl-b responded positively to the increase in nutrients and acidification, whereas zeaxanthin decreased.
In addition to this, the addition of acid decreased the contribution of micro and nanoplankton to the total phytoplankton biomass, whereas increased picoplankton contribution by ~ 20%. In contrast, an increase in nitrate and both nitrate and acid enhanced contribution of microplankton biomass to total biomass but decreased picophytoplankton (Fig. 7). Therefore, atmospheric deposition of pollutants enhanced acidification and nutrients that lead to modification in the size structure of the phytoplankton towards enhancing bigger phytoplankton that allows them to sinks faster to a deeper depth and efficiently sequester atmospheric carbon dioxide.
Summary and conclusions
Aerosols carry several inorganic elements, and their deposition over the surface waters may acidify and fertilize coastal waters that may have a significant impact on the phytoplankton composition and primary production. To test this hypothesis, aerosol samples were collected at a monthly interval over the central east coast of India. Aerosols over the study region were mixed with the coastal waters, and the biological response was observed through microcosm experiments. The higher concentration of nitrate and sulphate were observed in the TSP during summer followed by winter and the least during intermonsoon periods due to dominant terrestrial during the former two seasons and marine sources in the latter season. The mixing of monthly aerosols to the surface waters increases fucoxanthin (marker pigment for Bacillariophyceae), and peridinin (dinophyceae) and Chl-b (Chlorophyceae) were observed, whereas a decrease in zeaxanthin (Cyanophyceae) was noticed compared to control. A significant linear relationship was observed between phytoplankton biomass response and the amount of increase in nitrate and phosphate concentrations and their ratios. The addition of aerosols enhanced the primary production of the coastal waters by 0.6 to 7.6 mgC m−3 d−1 (3 to 19%) from that of control. Similar to phytoplankton biomass, an increase in primary production exhibited significant linear relation with a concentration of sulphate and nitrate in the atmospheric aerosols suggesting both acidification and enhanced nutrients concentrations due to aerosols’ increased primary production. The contribution of an increase in acidification to primary production was estimated to be ~ 22% of the increase in production, and the remaining fraction was contributed by the increase in nutrients concentrations. The decrease in pH led to a decline in the contribution of micro and nanoplankton by 10% whereas increase in picophytoplankton. In contrast, an increase in nitrate enhanced the contribution of microplankton by 20%. This study suggests that deposition of aerosols enhances coastal acidification and fertilisation resulting in a modification in the phytoplankton composition, size structure, and an increase in primary production. Recent studies (Yadav et al. 2021) suggested that anthropogenic aerosol levels, through aerosol optical depth, are increasing at the highest rate over the BoB than elsewhere in the world between 2001 and 2020. Therefore, the impact of atmospheric aerosols on surface ocean biogeochemistry in the BoB may be highly significant. Since the northern Indian Ocean is surrounded by landmasses on three sides, the impact of aerosols is significant, and more efforts are required to include these processes in the regional model to evaluate the impact of the climate change on the ocean biogeochemistry in the northern Indian Ocean.
Data availability
The data is derived through laboratory experiments, and all the data is presented in the manuscript. The data are available upon request to the corresponding author by email (sarmav@nio.org).
References
Banerjee P, Prasanna Kumar S (2014) Dust-induced episodic phytoplankton blooms in the Arabian Sea during winter monsoon. J Geophys Res Oceans 119:7123–7138. https://doi.org/10.1002/2014JC010304
Biswas H, Gadi SD, Ramana VV, Bharathi MD, Priyan RK, Manjari DT, Kumar MD (2012) Enhanced abundance of tintinnids under elevated CO2 level from coastal Bay of Bengal. Biodivers Conserv 21(5):1309–1326
Biswas H, Jie J, Li Y, Zhang G, Zhu ZY, Wu Y, Zhang GL, Li YW, Liu SM, Zhang J (2015) Response of a natural phytoplankton community from the Qingdao coast (Yellow Sea, China) to variable CO2 levels over a short-term incubation experiment. Cur Sci 108(10):1901–1909
Biswas H, Rahman SAU, Bandyopadhyay D, Chowdhury N (2017) CO2 induced growth response in a diatom dominated phytoplankton community from SW Bay of Bengal coastal water. Estuar Coastal Shelf Sci 198:29–42
Boyd PW, Mackie DS, Hunter KA (2010) Aerosol iron deposition to the surface ocean- modes of iron supply and biological responses. Mar Chem 120:130–145
Church TM, Jickells TD (2004) Atmospheric chemistry in the coastal ocean: a synopsis of processing, scavenging and inputs. Indian Jl Mar Sci 33(1):71–76
Doney SC, Mahowald N, Lima I, Feely RA, Mackenzie FT et al (2007) Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proc Nat Acad of Sci USA 104:14580–14585. https://doi.org/10.1073/pnas.0702218104
Draxler RR (2002) Forecasting dust storms using HYSPLIT, paper presented at Sino-Us Workshop on dust storms and their effects on human health. Raleigh, NC
Duce RA, Liss PS, Merrill JT, Atlas EL, Buat-Meanrd P et al (1991) The atmospheric input of trace species to the world ocean. Global Biogeochem Cycles 5(3):193–259
Duce RA, LaRoche J, Altieri K, Baker AR, Cadone DG, Cornell S et al (2008) Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 320(5878):893–897. https://doi.org/10.1126/science.1150369
Gauns M, Madhupratap M, Ramaiah N, Jyothibabu R, Fernandes V, Paul JT, Prasanna Kumar S (2005) A comparative estimate of carbon flux in the Arabian Sea and the Bay of Bengal. Deep-Sea Res II 52:2003–2017p
Grasshoff K, Ehrhardt M, Kremling K (1992) Methods of seawater analysis. Verlag Chemie, Weinheim
Guieu C, Al Azhar M, Aumont O, Mahowald N, Levy M, Ethe C, Lachkar Z (2019) Major impact of dust deposition on the productivity of the Arabian Sea. Geophys Res Lettrs 46:6736–6744
Hagens M, Keith AK, Liss PK, Middelburg JJ (2013) Biogeochemical context impacts seawater pH changes resulting from atmospheric sulfur and nitrogen deposition. Am Geophys Union. https://doi.org/10.1002/2013GL058796
Hama T, Miyazaki T, Ogawa Y, Iwakuma T, Takahashi M, Otsuki A, Ichimura S (1983) Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope. Mar Biol 73:31–36
Jickells TD (1998) Nutrient biogeochemistry of the coastal zone. Science 10:217–222
Jyothibabu R, Madhu NV, Maheswaran PA, Jayalakshmy KV, Nair KKC, Achuthankutty CT (2008) Seasonal variation of microzooplankton (20–200 [mu]m) and its possible implications on the vertical carbon flux in the western Bay of Bengal. Cont Shelf Res 28(6):737–755
Krishna MS, Prasad VR, Sarma VVSS, Reddy NPC, Hemalatha KPJ, Rao YV (2015) Fluxes of dissolved organic carbon and nitrogen to the Northern Indian Ocean from the Indian monsoonal rivers. J Geophys Res (G: Biogeosciences) 120(10):2067–2080
Kumar S, Ramesh R (2005) Productivity measurements in the Bay of Bengal using the 15N tracer: Implications to the global carbon cycle. Indian J Mar Sci 34(2):153–162
Kumar A, Sudheer AK, Sarin MM (2008) Chemical characteristics of aerosols in MABL of Bay of Bengal and Arabian Sea during spring inter-monsoon: a comparative study. Jl of Earth Sys Sci 117:325–332
Kumar BSK, Viswanadham R, Kumari VR, Rao DB, Prasad MHK, Srinivas N, Sarma VVSS (2021) Spatial variations in dissolved inorganic nutrients in the groundwaters along the Indian coast and their export to adjacent coastal waters. Environ Sci Pollu Res 28:9173–9191
Kumari VR, Yadav K, Sarma VVSS, Kumar MD (2021) Acidification of the coastal Bay of Bengal by aerosol deposition. J Earth Sys Sci 130:223
Lachkar Z, Mehari M, Azhar MA, Lévy M, Smith S (2021) Fast local warming is the main driver of recent deoxygenation in the northern Arabian Sea. Biogeosciences 18:5831–5849
Mackey KRM, Chia-Te-Chien Post AF, Saito MA, Paytan A (2015) Rapid and gradual modes of trace metal dissolution in seawater. Front Microbiol 5. https://doi.org/10.3389/fmicb.2014.00794
Mahowald N, Lindsay K, Rothenberg D, Doney SC, Moore JK, Thronton P, Randerson JT, Jones CD (2011) Desert dust and anthropogenic aerosol interactions in the community climate system model coupled-carbon-climate model. Biogeosciences 8:387–414. https://doi.org/10.5194/bg-8-387-2011
Patra PK, Kumar MD, Mahowald N, Sarma VVSS (2007) Atmospheric deposition and surface stratification as controls of contrasting chlorophyll abundance in the North Indian Ocean. J Geophys Res 112:C05029. https://doi.org/10.1029/2006JC003885
Prasanna Kumar S et al (2007) Eddy-mediated biological productivity in the Bay of Bengal during fall and spring intermonsoons. Deep Sea Res Part I 54(9):1619–1640
Prospero JM, Barrett K, Church T, Dentener F, Duce RA, Galloway JN, Levy H, Moody J, Quinn P (1996) Atmospheric deposition of nutrients to the North Atlantic. Biogeochemistry 35:27–73
Ramaiah N, Fernandes V, Paul J T, Jyothibabu R, Mangesh G, and Jayraj EA (2010) Seasonal variability in biological carbon mass standing stock and production in the surface layers of Bay of Bengal. Indian J Mar Sci 39(3)
Sarma VVSS, Lenton A, Law RM, Metzl N, Patra PK, Doney S, Lima ID, Dlugokencky E, Ramonet M Valsala V (2013a) Sea–air CO2 fluxes in the Indian Ocean between 1990 and 2009. Biogeosciences 10:7035–7052. https://doi.org/10.5194/bg-10-7035-2013
Sarma VVSS, Sridevi B, Maneesha K, Sridevi T, Naidu SA, Prasad VR, Venkataramana V, Acharya T, Bharati MD, Subbaiah ChV, Kiran BS, Reddy NPC, Sarma VV, Sadhuram Y, Murty TVR (2013b) Impact of atmospheric and physical forcings on biogeochemical cycling of dissolved oxygen and nutrients in the coastal Bay of Bengal. J Oceanogr. https://doi.org/10.1007/s10872-012-0168-y
Sarma VVSS, Krishna MS, Paul YS, Murthy VSN (2015) Observed changes in ocean acidity and carbon dioxide exchange in the coastal Bay of Bengal - a link to air pollution. Tellus B 67:24638
Sarma VVSS, Krishna MS, Srinivas TNR, Kumari VR, Yadav K, Kumar MD (2021) Elevated acidification rates due to deposition of atmospheric pollutants in the coastal Bay of Bengal. Geophys Res Lett 48: e2021GL095159
Scherner F, Pereira CM, Duarte G, Horta PA, Castro CB, Barufi JB, Pereira SMB (2016). Effects of ocean acidification and temperature increases on the photosynthesis of tropical reef calcified macroalgae. https://doi.org/10.1371/journal.pone.0154844
Schott FA, McCreary JP Jr (2001) The monsoon circulation of the Indian Ocean. Prog Oceanogr 51:1–123
Sridevi B, Sarma VVSS (2021) Role of river discharge and warming on ocean acidification and pCO2 levels in the Bay of Bengal. Tellus B 73:1–20. https://doi.org/10.1080/16000889.2021.1971924
Srinivas B, Sarin MM, Sarma VVSS (2011) Atmospheric dry deposition of inorganic and organic nitrogen to the Bay of Bengal: impact of continental outflow. Mar Chem 127:170–179
Uitz J, Claustre H, Morel A, Hooker SB (2006) Vertical distribution of phytoplankton communities in open ocean: an assessment based on surface chlorophyll. J Geophys Res Oceans 111. https://doi.org/10.1029/2005JC003207
Van Heukelem L, Thomas CS (2001) Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J Chromatogr A 910(1):31–49
Yadav K, Sarma VVSS, Rao DB, Kumar MD (2016) Influence of atmospheric dry deposition of inorganic nutrients on phytoplankton biomass in the coastal Bay of Bengal. Mar Chem 187:25–34
Yadav K, Rao VD, Sridevi B, Sarma VVSS (2021) Decadal variations in natural and anthropogenic aerosols optical depth over the Bay of Bengal: influence of pollutants from Indo-Gangetic Plain. Environ Sci Pollu Res. https://doi.org/10.1007/s11356-021-14703-x
Zhang J, Reid JS (2010) A decadal regional and global trend analysis of the aerosol optical depth using a data-assimilation grade over-water MODIS and Level 2 MISR aerosol products. Atmos Chem Phy 10:10949–10963
Acknowledgements
We would like to thank Director and Scientist-In-Charge for their encouragement and support. The support of Mr D. Vasu during coastal sampling is highly appreciated. Several project staff supported the collection of monthly sampling of aerosols, and their efforts are appreciated. We would like to thank three anonymous reviewers for their comments and suggestions to improve the presentation of our manuscript. This publication has the NIO contribution number.
We would like to thank the Director, Centre for Marine Living Resources and Ecology (CMLRE), Ministry of Earth Science for funding through a project entitled “Comprehensive assessment of the rate of ocean acidification and their drivers along the east coast of India”. We would like to thank three anonymous reviewers for their comments and suggestions to improve the presentation of our manuscript. This publication has the NIO contribution number of 6935
Author information
Authors and Affiliations
Contributions
VRK: A collection of monthly aerosol samples and analysis of the atmospheric composition.
BN: Conducting in vitro experiments along with DNR and VRDG and analysis of samples.
DNR: Measurement of primary production, conducting experiments for primary production.
VRDG: Measurement of nutrients, conducting an experiment.
VVSS: The concept of the work, guidance and finalizing of the manuscript.
All authors contribute in writing, building concepts and finalizing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, V.R., Neeraja, B., Rao, D.N. et al. Impact of atmospheric dry deposition of nutrients on phytoplankton pigment composition and primary production in the coastal Bay of Bengal. Environ Sci Pollut Res 29, 82218–82231 (2022). https://doi.org/10.1007/s11356-022-21477-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21477-3