Abstract
The coastal ocean receives nutrient pollutants from various sources, such as aerosols, municipal sewage, industrial effluents and groundwater discharge, with variable concentrations and stoichiometric ratios. The objective of this study is to examine the response of phytoplankton to these pollutants in the coastal water under silicate-rich and silicate-poor coastal waters. In order to achieve this, a microcosm experiment was conducted by adding the pollutants from various sources to the coastal waters during November and January, when the water column physicochemical characteristics are different. Low salinity and high silicate concentration were observed during November due to the influence of river discharge contrasting to that observed during January. Among the various sources of pollutants used, aerosols and industrial effluents did not contribute silicate whereas groundwater and municipal sewage contained high concentrations of silicate along with nitrate and phosphate during both the study periods. During November, an increase in phytoplankton biomass was noticed in all pollutant-added samples, except municipal sewage, due to the limitation of growth by nitrate. On the other hand, an increase in biomass and abundance of phytoplankton was observed in all pollutant-added samples, except for aerosol, during January. Increase in phytoplankton abundance associated with decrease in biomass was observed in aerosol-added sample due to co-limitation of silicate and phosphate during January. A significant response of Thalassiothrix sp. was observed for industrial effluent–added sample during November, whereas Chaetoceros sp. and Skeletonema sp. increased significantly during January. Higher increase in phytoplankton biomass was observed during November associated with higher availability of silicate in the coastal waters in January. Interestingly, an increase in the contribution of dinoflagellates was observed during January associated with low silicate in the coastal waters, suggesting that the concentration of silicate in the coastal waters determines the response of the phytoplankton group to pollutant inputs. This study suggested that silicate concentration in the coastal waters must be considered, in addition to the coastal currents, while computing dilution factors for the release of pollutants to the coastal ocean to avoid occurrence of unwanted phytoplankton blooms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The continuously escalating occurrence and distribution of algal blooms have become a genuine global concern over the past many decades (Hallegraeff 1993; Anderson 2009). Two major challenges faced due to this phenomenon include intensifying oxygen-minimum zones (OMZs; Naqvi et al. 2000; Bristow et al. 2017; Breitburg et al. 2018) and the spread of harmful algal blooms (HABs; Anderson 2009; Karlson et al. 2021). Several studies suggested that the rapid mass production of phytoplankton is stimulated by prolonged supplementation of nutrient loading, which is generally limited in the coastal systems (Vollenweider 1968; Smayda 1990; de Jonge et al 2002). The ongoing pattern of emerging eutrophication zones nearshore has been thoroughly documented by several researchers and is primarily attributed to the generation of nutrient and organic load released either naturally by rivers (Chen et al. 2003; Wang et al. 2007) and deposition of atmospheric dust (Yadav et al. 2016; Kumari et al. 2022) or through anthropogenically induced actions including industrial effluents, port activities, agricultural run-off and domestic sewage discharge (Khan and Mohammad 2014; Withers et al. 2014; Hasler 1947). These changes have resulted in the accelerated development of hypoxia over several coastal oceans in the world. Blooms are complex phenomena that can be influenced by a variety of factors, including the biogeochemical cycling of nutrients and physicochemical and biological processes (Turkoglu and Koray 2004). These factors can vary with reference to time, season and location, making it challenging to predict and manage blooms. Understanding these factors is crucial for mitigating the impacts of blooms on ecosystems and human health.
The Bay of Bengal (BoB), the north-eastern part of the Indian Ocean, receives a huge amount of discharge from major rivers including the Ganges, Brahmaputra, Mahanadi and Godavari with a peak influx during summer (June to September) compared to other seasons (UNESCO 1979; Schott and McCreary 2001; Shankar et al. 2002; Papa et al. 2010). The East India Coastal Current (EICC) displays seasonal reversing trends along the coast that spread riverine water along the coast (Murty et al. 1993; Shetye et al. 1996). The equatorward flow of EICC during October–December distributes low saline water, discharged by rivers, along the east coast of India (Rao et al. 1994; Naqvi et al. 1994; Kumar et al. 1996; Sarma et al. 2013). The poleward EICC flow (Shetye et al. 1993; Sanilkumar et al. 1997; Babu et al. 2003; Gangopadhyay et al. 2013) from January to June (Shetye et al. 1993) carries high saline waters. Therefore, the coastal stratification is mainly controlled by the seasonal reversal in EICC. Sarma et al. (2013) observed that nutrient concentrations in the coastal waters are significantly modulated by the flow of EICC. High concentrations of silicate (> 2 µM) and low nitrate and phosphate were reported from October to December when nitrate is a limiting nutrient associated with low salinity. In contrast, silicate limits the phytoplankton biomass from January to June associated with high salinity. The discharge of pollutants from different sources mostly brings nitrogen and phosphorus nutrients to the coastal waters, but not silicate; therefore, the response of phytoplankton may vary with season. Several studies were conducted to examine the response of phytoplankton composition and dominance due to alterations of nutrient ratios and concentrations in the coastal waters (e.g. Jickells 1998; Officer and Ryther 1980, Piehler et al. 2004). For instance, Raman and Ganapati (1986) reported occurrence of Skeletonema costatum blooms with increase in Chl-a biomass from 2 to 49.4 mg/m3 in the coastal Bay of Bengal, off the Visakhapatnam harbour, which is attributed to the input of untreated sewage and fertilizer factory effluents. Karthikeyan et al. (2010) noticed that industrial effluent input to the coastal Bay of Bengal increased the growth of marine diatoms, Chaetoceros simplex, and the response of diatom was increased with increase in the pollutant concentrations. Enhanced concentration of phytoplankton biomass, especially potential harmful species such as Trichodesmium, Microcystis and Pseudo-nitzschia, was observed due to discharge of sewage along the coastal Dar es Salaam, Tanzania (Hamisi and Mamboya 2014). However, there were no studies to our knowledge that examined how coastal water concentrations of nutrients would determine the response of phytoplankton to pollutants from different sources. In addition to this, most of the studies either focused on municipal sewage or industrial effluents, and no studies were conducted simultaneously to examine the impact of variable sources of pollutants on coastal water phytoplankton.
The present study focuses on the coastal water of the Visakhapatnam region, where industrial and sewage discharge spots are widespread across the coast. The intensely colonized coastal areas engage in numerous urban developments, industrial activities and over-utilization of marine resources. Commercial and residential conglomerates around the coast, thus, raise the concern of intensifying anthropogenic pollution and aggravating environmental degradation (Häder et al. 2020). The coastal city, Visakhapatnam, is an important hub for industrial activities along the east coast of India, harbouring 1132 registered commercial and industrial enterprises during 2019–2020 (State report 2023). Visakhapatnam ranks among the most swiftly growing industrial city in Asia. Semi- or untreated effluents are generated and discharged by circa 1,960,000 inhabitants into the intertidal region. With an immense discharge rate of ~ 305 MLD of industrial and ~ 50 MLD of domestic sewage, the city is listed among the 19 hotspots across the country with significant levels of pollution requiring year-round monitoring of discharge (CPCB 2008; Madeswaran et al. 2018). The study region also receives a high amount of atmospheric pollutants throughout the year (Kumari et al. 2022) and contributes to excessive coastal water nutrients and ocean acidification (Kumari et al. 2021). The groundwater along Indian coastal waters is heavily polluted with reference to nitrogen nutrients (Kumar et al. 2021) mainly driven by agricultural pollutants. The objective of this study is to examine the response of coastal phytoplankton to the discharge of pollutants from different sources during November (silicate-rich coastal waters) and January (silicate-poor coastal waters) using microcosm experiments.
Material and methods
Sample collection
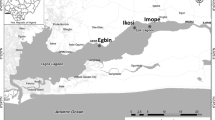
Coastal water samples and pollutants, including aerosol, municipal sewage and industrial effluent, and groundwater samples were collected from different locations in the city of Visakhapatnam (Fig. 1). The details of the sampling locations and methods are given below.
The study regions where the incubation experiments were conducted. The locations of pharmaceutical industries, from where industrial effluents were collected, the discharge locations of municipal sewage, the location of groundwater discharge, aerosol sampling regions and the region of coastal water sampling are shown. AR aerosols, IE industrial effluent, GW groundwater and MS municipal sewage
Coastal waters
Coastal water sample was collected using a polyvinyl bucket from Ramakrishna Beach (17.71° N; 83.33° E), Visakhapatnam. Samples were then transferred to 20-L pre-cleaned transparent polycarbonate Nalgene bottles by filtering through zooplankton mesh (200 microns) to remove mesozooplankton. However, the smaller zooplankton, passed through the mesh, may remain in the sample, and they may influence the response of phytoplankton. Therefore, the actual response of phytoplankton may be slightly impacted by grazing of smaller zooplankton in our experiments. The experiment was done twice, first in the month of November 2021 and a second time in January 2022. Coastal water sampling during both experiments was done from the same place following the same procedure.
Aerosol
Aerosol was collected from close to the coast (17.72° N; 83.33° E) for 24 h on a pre-combusted and pre-weighted quartz filter with a high volume sampler (Envirotech 430) at a flow rate of 1.2 m3 min−1 during November 2021 and January 2022. For aerosol extraction, the filter was shredded into small fragments and sonicated with 130 mL ion-free water in a Bransonic water bath sonicator for 20 min at 2000 rpm. The extracted sample was filtered through a 0.22-µm pore size filter, and 100 mL of the filtered extract was used for subsequent incubation experiments and 30 mL was used for measuring initial nutrient concentrations.
Effluents from municipal sewage and industrial discharge
A municipal sewage sample was collected from open sewer drainage situated 250 m away from the coast at Jalari Peta, Visakhapatnam (17.73° N; 83.34° E), using a pre-cleaned 10-L container on the day of experiment (Fig. 1). Industrial effluent discharge sample, after treatment, from the guard pond was collected in a pre-cleaned 20-L polyvinyl container from a pharmaceutical company located in Annavaram, Visakhapatnam, at 17.91° N; 83.47° E. Both the samples were filtered through a 0.2-μm pore size Millipore filter (47-mm diameter) to remove turbidity and bacteria to arrest biological modification of inoculum. Both municipal sewage and industrial effluent were collected during November and January. These effluents may undergo environmental degradation before they leach into the water column such as photodegradation, oxidation and chemical degradation. Since the municipal sewage was collected about 250 m away from the coastal waters, the environmental modifications may be minimal before they discharge to the coastal waters. However, the sewage composition varies with reference to space and time; caution is warranted as the experimental results may give an idea of the typical impact on phytoplankton composition. In contrast, the industrial effluents were collected from the guard pond and thus were not subjected to complete natural degradation. Both sewage and industrial effluents also increase turbidity at the location of mixing before dispersion. Since we filtered the pollutants, before mixing with coastal waters, the impact of pollutants on coastal phytoplankton may be considered on the higher side.
Groundwater
A mechanical hand pump located near Lawson’s Bay area, Visakhapatnam (17.73° N; 83.33° E), was used for a groundwater sample (Fig. 1). Before utilization for the incubation experiment, this sample was also filtered through a 0.2-μm pore size Millipore filter. The groundwater samples were collected separately during the two experiments.
Microcosm incubation experiment
Seawater samples were collected in 10 Nalgene bottles of 20-L capacity, and the experiment was conducted in duplicate. In two bottles each, 50 mL of the filtered aerosol extract and 1 L each of the municipal sewage, industrial effluent and groundwater were added representing 5% of pollutant in each bottle. The addition of 5% of pollutant source to the experimental tank was determined from the measurements made in the pollutants’ released location (Dr. M.S. Krishna personal communications). At marine discharge locations, nutrient concentrations were collected from the point of discharge, 0.5 km, 1.0 km and 5 km away from the discharge, and also some data were collected from the non-discharge locations (> 20 km away from the discharge). Based on the enrichment of nutrients in the discharge location from that of non-discharge location, volume of pollutant to the waters was computed that varied between 1 and 5% in different locations and the higher during calm seasons when currents are weaker. We considered 5% here to represent the higher side of the impact.
In two bottles, no pollutant was added and called control where natural growth was recorded (Fig. 2). The bottles were thoroughly mixed and capped. The incubation bottles were kept in the plastic crates filled with circulating water to maintain the temperature to avoid evaporation and overheating. The bottles were frequently mixed at 1-h interval during daytime to avoid settling of the plankton. The bottles were covered with black plastic sheet in nighttime to avoid light penetration due to local electrification. Both pollutants added and control were treated under similar conditions. The bottles were gently shaken before subsampling for different parameters for analysis to avoid settling of particulate matter including phytoplankton.
2.3 Sampling of aliquots from the experimental bottles
Subsamples were collected in the evening to track the gradual alterations of chemical and biological parameters. About 100 mL of water sample was collected from all bottles for nutrients (dissolved nitrate, phosphate and silicate) and 1 L each for phytoplankton biomass and enumeration were collected into the plastic bottles before addition of pollutant inoculum. Subsampling was carried out on day 1 and day 3 after addition of pollutants to the incubation bottles. The day 0 data represents initial and day 3 represents response. In the case of January, subsampling was done on day 4 also. Nutrient samples were poisoned with mercuric chloride whereas phytoplankton samples with 0.5% Lugol’s iodine solution (Throndsen 1978).
2.4 Analysis of nutrients
Nutrients, including nitrate, nitrite, phosphate and silicate, were analysed following colorimetric procedure of Grasshoff et al. (2009) using EcoLab autoanalyser. The precisions of nitrate, phosphate and silicate were ± 0.02, 0.01 and 0.02 µM, respectively.
Analysis of biochemical parameters
Phytoplankton biomass (chlorophyll-a) was estimated by filtering 1 L of water sample through a 47-mm-diameter GF/F filter (0.7-μm pore size) using a low-vacuum suction pump (Tarsons, India) at reduced light conditions to avoid photodegradation. The filters were soaked in 90% acetone for extraction of chlorophyll-a on the filter for 12 h. Fluorescence of the extract was measured following the standard protocol (Suzuki and Ishimaru 1990) using a Trilogy fluorometer (Turner Designs, USA). The analytical precision was ± 4%.
Sea water sample taken for phytoplankton abundance is concentrated before analysis to reduce the volume up to 25 mL by siphoning through a 10-μm mesh. Phytoplankton enumeration and identification were carried out using the Sedgwick-Rafter cell counting chamber observed under IX-71 Olympus microscope (Olympus, Japan) by following procedures given elsewhere (Santra 1993; Hasle et al. 1996; Baker 2012; Bharathi et al. 2018. For some organisms, the identification was not possible, so they were grouped as others.
Various ecological parameters including species richness, diversity, evenness and abundance were calculated. Species richness (S) was calculated as the count of total species present in the sample. Abundance was measured as the total number of individuals present in the sample. The phytoplankton diversity expressed as Shannon-Weiner diversity index (H′) (Shannon and Weaver 1998) was calculated as:
where pi is the proportion of the number of individuals of ith species in the sample population.
The evenness of community (J') was expressed by Pielou’s evenness index (Pielou 1966) as:
where H′ is the number derived from the Shannon diversity index and H′max is the maximum value of H′ derived as H′max = ln (S).
The active chlorophyll measurement was done using the fast repetition rate fluorometer with an FRRF3 sensor (FastOcean, Chelsea Technologies Group, Ltd. UK). The samples were taken every day in a 10-L black can with an open-wide mouth and allowed to adapt in the dark for 5 min before analysis. Single turn-over protocol was followed with 100 flashlights each of 2-µs pitch. The excitation wavelength was provided by LEDs of royal blue (450 nm), green (530 nm) and red (624 nm) light. The maximum quantum efficiency of the phytoplankton in the dark-adapted state in each sample was measured as fv/fm with reference to Kitajima and Butler (1975).
Results
Initial conditions of coastal water during November and January
The coastal waters were relatively low saline during November (29.54) compared to January (33.12) with higher temperatures during the former (31.23 °C) than the latter (27.54 °C) (Table 1). The concentration of nitrate in the coastal seawater was 2.14 μM and 6.07 μM, respectively during November and January, whereas 0.46 and 0.32 μM, respectively, for phosphate. The N:P ratio was higher (19) during January than in November (5) (Table 1). The concentration of dissolved silicate was high during November (5.53 μM) compared to January (0.85 μM). The N:Si ratio was 0.4 and 7.1 during November and January, respectively. The phytoplankton biomass, in terms of Chl-a, was higher during January (15 mg m−3) than in November (10.1 mg m−3) (Table 1).
The phytoplankton abundance was more than double during January (45,687 cells L−1) than in November (15,792 cells L−1; Table 1). Diatoms dominated the total phytoplankton abundance with Chaetoceros sp. and Skeletonema sp., respectively, in November and January. Among the phytoplankton, 58% were contributed by Chaetoceros and 11% by Skeletonema sp. during November (Fig. 3). Following diatoms, the contribution of dinoflagellates was observed only in January, when silicate concentration was low, and they contributed < 5% of total phytoplankton abundance (Table 1). The species richness was high in January (30) compared to November (13), and similarly, Shannon–Wiener diversity was higher in January (2.37) than in November (1.52) (Table 1).
Concentrations and stoichiometric ratios of nutrients in the inoculum or pollutants
The nutrient concentrations of different inocula (pollutants), such as municipal sewage, industrial effluent, groundwater and aerosols, are given in Table 2. Groundwater and industrial effluents contained a high concentration of nitrate (7798 and 5764 μM, respectively), while low nitrate was found in municipal sewage (76 μM) and aerosol (34 μM) in November. Simultaneously, a lower phosphate concentration was observed in the aerosols (0.05 μM) than in other sources of pollutants (16–64 μM; Table 2). The highest concentration of silicate was observed in groundwater (883 μM) followed by municipal sewage (167 μM) and the least in industrial effluent (25 μM) and aerosol (non-detectable) during November (Table 2). The N:P ratio was the highest in aerosol (680), due to a lack of phosphate supply, followed by groundwater (170) compared to industrial effluent (90) and municipal sewage (5), whereas a higher N:Si ratio was noticed in industrial effluent (231) compared to groundwater (9) and municipal sewage (0.46) during November. The concentration of nitrate was more by an order of magnitude in all sources of pollution during January with the highest being the same, i.e., groundwater and industrial effluents (3520 and 3286 μM, respectively), and the least in aerosol (168 μM). Phosphate concentrations were lower during January than in November and varied between 0.2 and 60 μM. Silicate concentrations were high in the groundwater (728 µM) and municipal sewage (124 μM) in January, but they were lower than in November. Similarly, the high N:P ratio was observed in aerosol (840) followed by groundwater (320) and industrial effluent (55), whereas the high N:Si ratio was observed in industrial effluent (219) followed by groundwater (5) and municipal sewage (3) during January (Table 2).
Nutrient concentration in the incubation tanks after the addition of inoculum
After the addition of inoculum from various sources of pollution to the coastal water, the concentrations of nutrients were significantly increased from that of seawater concentrations (Table 3). The initial normalized nutrient concentrations with different pollutant treatments are shown in Fig. 4. The highest increase in nitrate concentrations was observed in the industrial effluent- and groundwater-added samples during November and January (Fig. 4). A significant increase in phosphate was observed due to the addition of industrial effluent, groundwater and municipal sewage, but the contribution is too low for aerosols. A significantly higher increase in silicate was observed only in the groundwater and municipal sewage–added tanks than in other sources. In addition to concentrations, the stoichiometric ratios were also changed. For instance, the N:P ratio in the seawater was 5 during November, which has been changed to 0.8 to 137 with higher ratios in the industrial effluent– and groundwater-added samples, whereas the N:Si ratio changed from 0.4 in the initial value to 0.06–22 in the inoculum-added samples (Table 3).
Chemical and biological responses after incubation
After incubating seawater with different inoculums for 4 days, variable chemical and biological responses were observed. In the control sample, both nitrate and phosphate were reduced during November by ~ 16 to 35% during incubation of 3 days (Table 4) associated with an increase in phytoplankton biomass by 20% and in abundance by 3%. Similarly, a decrease in nutrient concentration was observed in the incubation samples with different inoculums. For instance, nitrate, phosphate and silicate decreased in all pollutant-added samples by 50–77%, 22–40%, and 41–62%, respectively. These changes were associated with increase in phytoplankton abundance in industrial effluent (by 69%)– and groundwater (by 134%)-added samples, while a slight decrease by 12% was observed in the aerosol-added sample, and insignificant changes in municipal sewage–added sample (0.7%) were observed. An increase in phytoplankton biomass was observed (168–350%) in all treated samples except municipal sewage which decreased by 50% (Table 4). The response of the total phytoplankton abundance, biomass and diatoms and dinoflagellate abundance was normalized to the initial value and is shown in Fig. 5. It is clearly shown that during November, an increase in phytoplankton abundance was noticed in both industrial effluent– and groundwater-added samples, whereas aerosol- and municipal sewage–added samples were close to that of the initial. In the case of biomass, all pollutant-added samples, except municipal sewage, responded positively. The significant response of diatom abundance was noticed in industrial effluent– and groundwater-added samples with weak/no response of dinoflagellates (Fig. 5). Despite Chaetoceros sp. being dominant in the seawater during November, Pseudo-nitzschia sp. and Thalassiothrix sp. responded significantly in aerosol-, industrial effluent– and municipal sewage–added samples, whereas Chaetoceros sp. along with Pseudo-nitzschia sp., Skeletonema sp. and Thalassiothrix sp. responded in the groundwater-added sample (Fig. 6). The increase in species richness and diversity was noticed in the groundwater (+ 23%, + 20%)- and industrial effluent (+ 38%, + 31%)–added samples, while an insignificant change in the municipal sewage–added sample in comparison to the day 0 control and a slight decrease in the aerosol-added sample were noticed (Table 4). The contribution of different groups of phytoplankton was variable after the addition of pollutants. For instance, Chaetoceros sp. contributed ~ 58% in the control which has decreased to 33% and 41% in industrial effluent– and groundwater-added samples, respectively, whereas almost a similar contribution was noticed in aerosol- and municipal sewage–added samples (Fig. 3). An increase in Thalassiothrix sp. was observed in groundwater-, industrial effluent– and municipal sewage–added samples.
The response of phytoplankton abundance (cells/L) and biomass (mg m−3) to the addition of pollutants from various sources in the microcosm experiments after 3 days of incubation during November and January. All the data was normalized to the initial value of the seawater. C control, AR aerosols, IE industrial effluent, GW groundwater and MS municipal sewage
After fertilization in January, both nitrate and phosphate were reduced by ~ 14 to 67% in all pollutant-incubated samples associated with an increase in phytoplankton abundance by 362 to 606% in all treatments. An increase in biomass by 107–239% was also observed in the industrial effluent–, groundwater- and municipal sewage–added samples, but a decrease of 38% was noticed in the aerosol-added sample (Table 4). Among the major contributors (≥ 10%), Skeletonema sp., Chaetoceros sp., Nitzschia sp. and Thalassiothrix sp. were dominant in the control sample (Fig. 3). In all pollutant-added samples, Chaetoceros sp. was increased considerably; in addition, the responses of Thalassiothrix sp. and Nitzschia sp. were found most in municipal sewage– and groundwater-added samples and Skeletonema sp. in industrial effluent– and aerosol-added samples (Fig. 6b). Interestingly, dinoflagellates increased in all samples except in the aerosol-added sample (Fig. 5b). A significant increase in dinoflagellate abundance from day 0 control (849 Nos/L) was observed in municipal sewage (1328 Nos/L, 56%)–, industrial effluent (1855 Nos/L, 118%)– and groundwater (2011 Nos./L, 137%)-added samples (Table 4). Chaetoceros sp. contributed 10% in the control and the same increased to 52–59% in aerosol- and municipal sewage–added samples, whereas it increased to 24–32% in groundwater- and industrial effluent–added samples (Fig. 3). Similarly, the contribution of Nitzschia sp. increased from 11% in the control to 26% in the groundwater-added sample, whereas a significant increase in Skeletonema sp. from 29 to 46% was observed in the industrial effluent–added sample. Though response in phytoplankton abundance was significantly higher in all samples, the species richness, diversity and evenness were decreased compared to initial seawater in all treatments during January (Table 4).
Response of phytoplankton physiology
The fv/fm ratio of the control was ~ 0.38 during both November and January experiments, and the same was increased to a maximum of 0.55 in the industrial effluent-added sample and ~ 0.5 in the groundwater-added sample. A decrease in fv/fm ratio was observed in the municipal sewage–added sample during November, but an increase was noticed in January. A decrease in fv/fm was noticed in both experiments in the aerosol-added sample (Fig. 7).
Discussion
The phytoplankton composition in the coastal waters of the Bay of Bengal was dominated by diatoms (Shaik et al. 2015; Viswanadham et al. 2016; Bandyopadhyay et al. 2017). The present study also noticed the dominance of diatoms in the coastal Bay of Bengal during November and January. Coastal phytoplankton responds to nutrient enrichment due to the addition of pollutants from various sources. The response is not uniform for each source of inoculum (pollutant) added and depends on the magnitude and ratio of nutrients in the pollutants added and also in the seawater nutrient concentrations, especially silicate.
Response of phytoplankton to the addition of aerosols
Aerosols are a significant source of nitrogen but a poor source of phosphate and no source of silicate (Kumari et al. 2022). The nitrate concentration of inoculum during November was low (34 μM) compared to January (168 μM) with the N:P ratio of 680 and 840, respectively. The difference in concentrations is caused by the wind trajectories. The HYSPLIT wind trajectory suggests that wind comes from the western region during November, whereas it was the northeast region during January that brought anthropogenic aerosols from the Indo-Gangetic Plain (IGP; Kumari et al. 2022). Several investigators reported high levels of both total and anthropogenic aerosol optical depth (AOD) in north-eastern India (Sudheer and Sarin 2008) with a higher rate of increase over the past two decades (Yadav et al. 2021). Since aerosol is not a significant source of phosphate and silicate, its deposition enhances the N:P and N:Si ratios of the coastal waters (Kumari et al. 2021). After the addition of aerosol to coastal seawater, the concentration of nitrate increased by 12 and 9% during November and January, respectively, whereas phosphate increased by 0–2% only, and no significant increase in silicate was observed. Though aerosol did not enrich silicate in the incubated seawater, however, phytoplankton (mainly diatoms) growth did not limit by silicate due to high concentrations (5.53 μM) in the coastal waters during November. In contrast, low concentrations of silicate (0.85 μM) in the coastal waters during January limited the phytoplankton growth in the aerosol-added sample (Table 1). Higher silicate during November than in January is caused by a seasonal reversal in East India Coastal Current (EICC; Shetye et al. 1993). The EICC flows equatorward from October to December that brings silicate-rich riverine water along the coast, whereas it turns poleward during January that brings high saline water from the south with poor nutrients, including silicate (Sarma et al. 2013; Table 1). Sarma et al. (2021) found that coastal water chemical characteristics are controlled by the direction of EICC. Therefore, the availability of silicate due to changes in coastal currents influences the biological response in the coastal Bay of Bengal. The increase in nutrient concentration due to the addition of aerosols resulted in phytoplankton biomass increase by 168% during November, whereas it decreased by 38% during January due to availability of more silicate in the former than the latter period. In addition to this, the phosphate availability was also low in the coastal waters during January (0.36 μM) with a high N:P ratio (19) compared to November (5) (Table 1); therefore, both phosphate and silicate severely limited the phytoplankton growth during January. In contrast, a decrease in phytoplankton abundance was observed during November by 12%, whereas an increase (431%) was noticed during January (Table 4). Since enough silicate is available during November, microplankton responded dominantly, resulting in a decrease in abundance but increase in phytoplankton biomass. In contrast, low silicate concentrations did not allow microplankton growth, resulting in an increase in smaller diatoms and other groups of phytoplankton during January. It was further noticed that an insignificant abundance of dinoflagellates and cyanobacteria was found during November, while their contribution was more during January (Fig. 5). Kumari et al. (2022) conducted experiments on the dissolution of monthly atmospheric aerosols in coastal waters and found that dominant fucoxanthin (marker pigment for diatoms) increased from October to December associated with high silicate and contribution of peridinin (marker pigment for dinoflagellates) during January to March in the coastal Bay of Bengal.
Despite Chaetoceros sp. being a dominant contributor during November, it decreased by 15%, whereas Pseudo-nitzschia sp. increased by 89% with reference to their abundance (Fig. 6). Various diatom species exhibit different rates of silicon uptake and deposition. While all diatoms maintain internal silicon concentrations within a certain range, however, the sizes of silicon pools vary among species, potentially influenced by the timing of silicic acid uptake and its incorporation into the frustule (Chisholm et al. 1978; Martin-Jézéquel et al. 2000). Thus, the demand for dissolved silicate by diatoms appears to differ with species (Mochizuki et al. 2002), and inter-specific differences in silica requirements may be one of the reasons for the observed difference in abundance in different groups of diatoms. Chaetoceros sp. uptake dissolved silicate more efficiently at a much faster rate relative to other large diatoms (Tréuer et al. 1991) which may be attributed to the near-simultaneous nature of silicate uptake and deposition processes in Chaetoceros sp. (Chisholm et al. 1978). This could be the reason for the low Chaetoceros sp. response during November due to the low concentration of silicate. The increase in Pseudo-nitzschia sp. was observed as they have the ability to respond to a wide range of silicate (Pan 1994, 1996). On the other hand, all dominant phytoplankton (Chaetoceros sp., Nitzschia sp., Skeletonema sp., Thalassionema sp., Thalassiothrix sp.) increased by 5 to 2595% from that of the control during January with a higher response by Chaetoceros sp. and Skeletonema sp. The variable response of phytoplankton groups may be caused by the availability of nutrients (N, P, and Si). As a result, phytoplankton species richness decreased from 13 in the control to 11 in the aerosol added sample during November and from 30 to 26 during January. The Shannon–Wiener diversity decreased insignificantly from 1.52 to 1.50 and from 2.37 to 1.55 during November and January, respectively. The evenness changed from 0.59 to 0.63 and from 0.70 to 0.48 during November and January, respectively, due to the addition of aerosols (Table 4).
In order to examine the phytoplankton physiological status, the maximum quantum efficiency of photosynthesis (Fv/Fm) is measured that is used as an indicator of nutrient stress (Sakshaug et al. 1997; Kolber et al. 1990; Falkowski and Raven 2013), and physiological changes in phytoplankton (Suggett et al. 2009; Alderkamp et al. 2012; Garrido et al. 2013; Erga et al. 2014) and also the impact of various substances on the photosynthetic apparatus of algae (Matorin et al. 2009). The Fv/Fm values can be between 0.65 and 0.70 for phytoplankton under optimum growth conditions (Juneau et al. 2005; Suggett et al. 2009) that the ratio denotes absorbed energy maximum photochemical efficiency of PSII and indicates the fraction of the absorbed energy channelled to photosynthesis by PSII reaction centres. The Fv/Fm ratio was ~ 0.38 in the coastal waters during November and January and decreased by 0.06 due to the addition of aerosols, suggesting that phytoplankton’s physiological status did not differ significantly during the experimental period (Fig. 7).
The deposition of aerosols significantly modified the nutrient (mainly nitrate) concentration, resulting in an alteration of the stoichiometry of the nutrients and a change in their diversity, abundance, and biomass. Since aerosols did not add significant concentrations of phosphate and no silicate, the response of phytoplankton depended on the concentration of these two nutrients in the coastal waters.
Response of phytoplankton to the addition of industrial effluent
The industrial effluent is highly enriched with nitrate and phosphate, but poor in silicate; hence, the response of phytoplankton may depend on the availability of silicate in the coastal waters, where it is released. Coastal ocean receives silicate from the rivers where weathering of the silicate minerals releases dissolved silicate (Dupre et al. 2003; Tipper et al. 2021 and references therein). Nitrate concentration after the addition of inoculum during November and January was 107 and 22 times higher than the day 0 control, whereas a 7–8 times increase in phosphate concentration was observed. The marginal increase in silicate was observed due to the addition of industrial effluent to coastal waters (Fig. 4). As a result, an abnormal increase in N:P ratios was observed (49–66%) due to enriched inorganic nitrogen in the industrial effluent (Table 3). As a response to the addition of industrial effluent, a significant increase in phytoplankton abundance and biomass was observed in both the experiments with a higher response in biomass during November (350%) than in January (143%); in contrast, a higher increase in abundance was observed during January (362%) than in November (69%). The increase in biomass during November is associated with high concentrations of nitrate and phosphate in the inoculum along with high concentration of silicate in the coastal waters. The abundance of Thalassiothrix sp., Pseudo-nitzschia sp. and Skeletonema sp. responded positively, whereas Chaetoceros sp. was declined due to the addition of industrial effluent during November. In contrast, an increase in Chaetoceros sp., Nitzschia sp., Skeletonema sp., Thalassionema sp. and Thalassiothrix sp. was observed during January (Fig. 6). Despite higher nitrate and phosphate concentration during January than in November, the concentration of silicate was low leading to a lower biomass increase in January (143%) than in November (350%). The silicate concentration at the end of the experiment was at the limiting levels (< 0.9 µM) in January, further confirming that silicate is a limiting nutrient during this period (Table 4). This is consistent with Tréuer et al. (1991) who suggested that the response of diatoms is dependent on the availability of the silicate. The coastal waters contained a high abundance of diatoms during November (15,288 no./L) and January (42,605 no./L), resulting in their response to the nutrient enrichment by industrial effluent mixing. It was noticed that Chaetoceros sp. reduced by 4%, while Thalassiothrix sp. increased to 438% compared to the control in November (Fig. 6). On the other hand, Chaetoceros sp. and Skeletonema sp. responded the highest compared to other phytoplankton in January (Fig. 6). In addition to diatoms, an increase in the response of dinoflagellate was observed in January (Fig. 5) associated with higher nitrate and phosphate and lower silicate, suggesting that lack of silicate in the coastal waters might have triggered dinoflagellates abundance. Officer and Ryther (1980) noticed the proliferation of dinoflagellate growth over diatoms in the regions where silicate concentrations were low. A significant increase in species richness and diversity was noticed during November (Table 4). This study suggested that industrial effluent contributes to high concentrations of nitrate and phosphate to the coastal waters leading to a significant increase in phytoplankton abundance and biomass and initiation of a shift in phytoplankton from diatoms to dinoflagellates noticed during the period when coastal waters contain low silicate levels associated with the poleward flow of EICC. The Fv/Fm ratio was ~ 0.38 in the coastal waters during November and January and increased to 0.52 due to the addition of industrial effluent, suggesting that phytoplankton’s physiological status improved from that of the control (Fig. 7).
Response of phytoplankton to the addition of municipal sewage
Unlike aerosols, municipal sewage contains a high concentration of nitrate, phosphate and silicate compared to seawater concentrations (Table 2). A relatively lower concentration of nitrate (76 μM) and phosphate (16 μM) was observed in the municipal sewage during November compared to January (347 μM and 124 μM, respectively) (Table 2; Fig. 4). Since municipal sewage–added sample during November contained a low concentration of nitrate (2.7 μM; Table 3), it has been consumed during the incubation period (< 0.5 μM; Table 4), resulting in severe limitation of phytoplankton growth. As a result, a decrease in phytoplankton biomass by 50% was observed with insignificant modifications in the abundance (Fig. 5), suggesting a possible shift in phytoplankton abundance from siliceous to non-siliceous. Since we conducted the experiment for 3 days only, we could not identify such a shift. In contrast, a significant increase in phytoplankton biomass and abundance was noticed during January associated with high concentrations of nitrate, phosphate and silicate (Table 4). The increase in abundance (584%) and biomass (239%) was observed considerably during January (Table 4; Fig. 5). A slight increase in Thalassiothrix sp. was noticed, whereas a slight decrease in Chaetoceros sp. and Pseudo-nitzschia sp. was observed during November. In contrast, significant increase in Chaetoceros (3848%) and a moderate increase in Nitzschia sp., Skeletonema sp., Thalassionema sp. and Thalassiothrix sp. (145–297%) were noticed during January (Fig. 6b). Several studies on the addition of municipal sewage to the coastal water suggested that the response is dependent on the amount of nitrate and phosphate added. At lower addition of nitrate (< 12 \(\mu\)M), the general enrichment of the existing species was observed without change in species structure. In contrast, at higher concentrations of nitrate (> 36 \(\mu\)M), an eightfold increase in phytoplankton growth with an altered species structure, was noticed (Johnson et al., 1973; Topping et al., 1976). A slight increase in species richness and diversity was noticed during November, whereas it was significantly high during January due to the high concentration of nitrate and phosphate input compared to November (Table 4). The Fv/Fm ratio did not differ significantly from that of the control (~ 0.38) due to the addition of municipal sewage during November, whereas it increased to 0.50 during January, suggesting that phytoplankton physiological status improved during the latter period (Fig. 7).
Response of phytoplankton to the addition of groundwater
The groundwater contained high concentrations of nitrate, phosphate and silicate with high N:P and N: Si ratios compared to seawater (Table 2). Kumar et al. (2021) reported that most of the groundwater along the Indian coast was contaminated with agricultural inputs. Groundwater discharge into the coastal ocean is considered to be a significant source of nutrients and may contribute to 10% of the riverine supply (Taniguchi et al. 2002; Slomp and Van Cappellen 2004; Liu et al. 2014; Windom et al. 2006; Kim et al. 2005). A range of impacts due to groundwater discharge was reported such as eutrophication (Knee and Paytan 2011; Rabalais et al. 2009), deoxygenation (Breitburg et al. 2018) and global nutrient budget (Galloway et al. 2008; Slomp and Van Cappellen 2004). Due to the removal of phosphate in association with particles, the N:P ratios in the groundwater were high during November (170) and January (320) (Table 2). The concentration of nitrate was the highest in groundwater compared to other pollutants including industrial effluent, aerosol and municipal sewage during both the experimental periods. The addition of groundwater to the coastal seawater enhanced both concentrations and stoichiometric ratios from that of seawater values (Table 2). After 3 days of incubation, nitrate, phosphate and silicate concentrations were above the limiting levels for phytoplankton growth. A significant increase in phytoplankton abundance (134%) and biomass (278%) was observed during November, whereas an increase in phytoplankton abundance (606%) and slight increase in biomass (107%) was observed in January compared to November (Table 4; Fig. 5). The concentration of phosphate was low (0.6 μM) after 3 days of incubation in January, suggesting severe limitation might have restricted the increase in phytoplankton biomass. Though coastal waters contain low concentrations of silicate during January, however, silicate will not limit the phytoplankton growth as groundwater is a good source of silicate to the coastal ocean. Chaetoceros sp. was dominated in the control, and the same was increased by 65% due to the addition of groundwater. In addition, the increase in Pseudo-nitzschia sp., Thalassiothrix sp. and Skeletonema sp. also increased significantly due to the addition of groundwater during November. An increase in the abundance of Chaetoceros sp., Nitzschia sp., Thalassionema sp. and Thalassiothrix sp. was observed in January (Fig. 6). An increase in the abundance of dinoflagellates was also noticed, and a similar increase was noticed in the municipal sewage- and industrial effluent–added samples (Table 4). An increase in species richness and diversity was noticed compared to the control during both experiments. The Fv/Fm ratio increased from ~ 0.38 in the control to 0.50 in the groundwater-added sample during both November and January, suggesting that phytoplankton physiological status improved from that of the control (Fig. 7).
Multivariant analysis
In order to examine the significance of the phytoplankton response to different pollutant additions, multivariant analysis (principal component analysis) was conducted (Fig. 8a, b). This analysis suggests that the biological response was insignificant in the control, aerosol and municipal sewage addition, whereas significant in the groundwater and industrial effluent additions during November (Fig. 8a). In the case of January, phytoplankton response by addition of municipal sewage, groundwater and industrial effluents was significant, but aerosols were not significant (Fig. 8b). Even though increased phytoplankton abundance and biomass were observed in the aerosol-added samples, however, the nitrate addition is smaller through aerosols compared to other pollutants (Table 2). The concentration of nitrate in the municipal sewage was five times lower during November compared to January (Table 2), resulting in less increase in its concentration due to its addition (Table 3). As a result, only marginal increase in abundance was observed with decrease in biomass of phytoplankton due to addition of municipal sewage during November, whereas significant increase was found during January due to high concentrations of nutrients.
Principal component analysis of the experimental data during a November and b January. The dendrogram of the response of phytoplankton to different pollutants input is drawn for c November and d January experiments. C control, AR aerosols, IE industrial effluent, GW groundwater and MS municipal sewage. The incubation days of each treatment are also given
The dendrograms suggested that the phytoplankton response due to addition of industrial effluent and groundwater were grouped together at 30% of similarity, whereas aerosols, municipal sewage and control formed a separate group (Fig. 8c) during November. In contrast, municipal sewage–, industrial effluent– and groundwater-added samples grouped together at > 35% similarly during January. The nutrient addition through aerosols was higher during January than November (Table 2); hence, the phytoplankton responded to aerosol addition on days 1 and 2 along with groundwater, industrial effluent and municipal sewage during January (Fig. 8d), whereas they were grouped along with control and municipal sewage during November (Fig. 8c). Therefore, this study suggested that discharge of industrial effluents and groundwater have significant impact on the coastal water phytoplankton, whereas influence of municipal sewage may show large intra-annual variability and marginal or insignificant influence was observed due to addition of aerosols.
Summary and Conclusion
The response of phytoplankton abundance and biomass to addition of pollutants from various sources was different during silicate-rich coastal waters during November and silicate-poor waters during January as summarized in Fig. 9. All pollutants contained nitrate and phosphate, whereas significant amount of silicate was brought by groundwater and municipal sewage. A severe limitation of phosphate was noticed in the aerosol-added sample, whereas nitrate limited phytoplankton abundance in municipal sewage–added samples during November. On the other hand, the limitation of silicate was noticed in the aerosol and industrial effluent–added sample during January. Increase in dinoflagellate abundance and biomass was noticed during January associated to low concentrations of silicate in the coastal waters, suggesting that the release of nitrogen and phosphorus pollutants without silicate, either in the pollutant or coastal waters, may possibly trigger harmful algal blooms that may destroy the ecosystem. The multivariant analysis suggested that additions of industrial effluent and groundwater significantly influenced coastal phytoplankton abundance and biomass, whereas influence of municipal sewage may have large variability with time depending on the composition of sewage, but the influence of aerosol deposition varies between insignificant and marginal influences.
The governing agencies generally limit the volume of discharge of industrial effluents to coastal waters and design the discharge rate such that 100 to 300 times dilution occurs. While designing, the current speed, diffusion rates and mixing parameters are taken into consideration. This experiment suggests that the seasonal variations in the concentration of silicate in the coastal waters also should be considered. It is recommended to use high dilution factors during the period of silicate-poor coastal waters and vice versa during silicate-rich coastal waters to avoid possible formation of harmful algal blooms.
There are some limitations in our experiment due to removal of only main grazers, i.e. mesozooplankton sizing above 200 µm. However, smaller grazers and bacteria would remain in the experimental tank, and their grazing may influence the phytoplankton response. Since it is very difficult to remove all grazers from the natural samples, the response of phytoplankton observed in our experiment may be underestimated due to grazing of smaller zooplankton.
As an emerging economy, India is still struggling with environmental pollution as an inescapable outcome of the ongoing industrial development. With multinational organizations investing to set up manufacturing units in India, Chandrika et al. (2022) suggested the country is likely to become a pollution haven. Data from Yale University’s Environmental Performance Index, 2022, showed that India was the worst performer with an EPI score of 18.90, ranking last among 180 countries (Wolf et al. 2022). Although to ensure effective monitoring and management of the release of wastewater, the Indian government has a streamlined regulatory framework. There has been a continuous effort towards the expansion of wastewater treatment facilities throughout the country. The total number of sewage treatment plants (STPs) has increased from 746 in 2014 to 1496 in 2020. The daily wastewater treatment capacity almost doubled since 2014 with 31,841 million litres per day (MLD) in 2020 (CPCB 2022). Regardless of the accelerated growth of treatment premises and adequate legislation, the demands are not yet fully met. This leads to high nutrient input through industrial and municipal discharges. The coastal area near the industrial set-ups is susceptible to severe environmental catastrophes leading to biodiversity loss and prolonged influence on human health. Therefore, the current situation of human-induced pollution influx needs to be acted upon more expeditiously.
Data availability
The data is derived through laboratory experiments, and all the data is presented in the manuscript. The data are available upon request to the corresponding author by email (sarmav@nio.org).
References
Alderkamp AC, Kulk G, Buma AG, Visser RJ, Van Dijken GL, Mills MM, Arrigo KR (2012) The effect of iron limitation on the photophysiology of Phaeocystis antarctica (Prymnesiophyceae) and Fragilariopsis cylindrus (Bacillariophyceae) under dynamic irradiance 1. J Phycol 48(1):45–59. https://doi.org/10.1111/j.1529-8817.2011.01098.x
Anderson DM (2009) Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast Manage 52:7. https://doi.org/10.1016/j.ocecoaman.2009.04.006
Babu MT, Sarma YVB, Murty VSN, Vethamony P (2003) On the circulation in the Bay of Bengal during Northern spring inter-monsoon (March-April 1987). Deep-Sea Res Part II: Topical Stud Oceanogr 50:5. https://doi.org/10.1016/S0967-0645(02)00609-4
Baker AL, et al (2012) Phycokey—an image based key to algae (PS Protista), Cyanobacteria, and other aquatic objects. University of New Hampshire Center for Freshwater Biology. http://cfb.unh.edu/phycokey/phycokey.htm
Bandyopadhyay D, Biswas H, Sarma VVSS (2017) Impacts of SW monsoon on phytoplankton community structure along the western coastal BOB: an HPLC approach. Estuaries Coasts 40:1066–1081. https://doi.org/10.1007/s12237-016-0198-6
Bharathi MD, Sarma VVSS, Ramaneswari K (2018) Intra-annual variations in phytoplankton biomass and its composition in the tropical estuary: Influence of river discharge. Mar Pollut Bull 129(1):14–25. https://doi.org/10.1016/j.marpolbul.2018.02.007
Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, Garçon V, Gilbert D, Gutiérrez D, Isensee K, Jacinto GS (2018) Declining oxygen in the global ocean and coastal waters. Science 359(6371):eaam7240. https://doi.org/10.1126/science.aam7240
Bristow LA, Callbeck CM, Larsen M, Altabet MA, Dekaezemacker J, Forth M, Gauns M, Glud RN, Kuypers MM, Lavik G, Milucka J (2017) N2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone. Nat Geosci 10(1):24–29. https://doi.org/10.1038/ngeo2847
Chandrika R, Mahesh R, Tripathy N (2022) Is India a pollution haven? Evidence from cross-border mergers and acquisitions. J Clean Prod 376:134355. https://doi.org/10.1016/j.jclepro.2022.134355
Chen C, Zhu J, Beardsley RC, Franks P (2003) Physical-biological sources for dense algal blooms near the Changjiang River. Geophys Res Lett 30:10. https://doi.org/10.1029/2002gl016391
Chisholm SW, Azam F, Eppley RW (1978) Silicic acid incorporation in marine diatoms on light:dark cycles: use as an assay for phased cell division 1. Limnol Oceanogr 23(3):518–529. https://doi.org/10.4319/lo.1978.23.3.0518
CPCB (2008). Status of groundwater quality in India- part II, Groundwater quality series: GWQS/10/2007–2008. https://cpcb.nic.in/publication-details.php?pid=MTM
CPCB (2022). Annual report 2020–21. https://cpcb.nic.in/annual-report.php
Dupre B, Dessert C, Oliva P, Goddéris Y, Viers J, François L, Millot R, Gaillardet J (2003) Rivers, chemical weathering and Earth’s climate. CR Geosci 335(16):1141–1160. https://doi.org/10.1016/j.crte.2003.09.015
Erga SR, Ssebiyonga N, Hamre B, Frette Ø, Hovland E, Hancke K, Drinkwater K, Rey F (2014) Environmental control of phytoplankton distribution and photosynthetic performance at the Jan Mayen Front in the Norwegian Sea. J Marine Syst 130:193–205. https://doi.org/10.1016/j.jmarsys.2012.01.006
Falkowski PG, Raven JA (2013) Aquatic photosynthesis. Princeton University Press
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320(5878):889–892. https://doi.org/10.1126/science.1136674
Gangopadhyay A, Bharat Raj GN, Chaudhuri AH, Babu MT, Sengupta D (2013) On the nature of meandering of the springtime western boundary current in the Bay of Bengal. Geophys Res Lett 40:10. https://doi.org/10.1002/grl.50412
Garrido, M., Cecchi, P., Vaquer, A., & Pasqualini, V. (2013). Effects of sample conservation on assessments of the photosynthetic efficiency of phytoplankton using PAM fluorometry. Deep-Sea Res Part I: Oceanogr Res Papers, 71. https://doi.org/10.1016/j.dsr.2012.09.004
Grasshoff K, Kremling K, Ehrhardt M (Eds.). (2009) Methods of seawater analysis. John Wiley & Sons
Häder DP, Banaszak AT, Villafañe VE, Narvarte MA, González RA, Helbling EW (2020) Anthropogenic pollution of aquatic ecosystems: emerging problems with global implications. Sci Total Environ 713:136586. https://doi.org/10.1016/j.scitotenv.2020.136586
Hallegraeff GM (1993) A review of harmful algal blooms and their apparent global increase. Phycologia 32:2. https://doi.org/10.2216/i0031-8884-32-2-79.1
Hamisi, M.I. and Mamboya, F.A., 2014. Nutrient and phytoplankton dynamics along the ocean road sewage discharge channel, Dar es Salaam, Tanzania. J Ecosyst, 2014. https://doi.org/10.1155/2014/271456
Hasle GR, Syvertsen EE, Steidinger KA, Tangen K, Tomas CR (1996) Chapter 2: Marine diatoms. In: Identifying marine diatoms and dinoflagellates, Elsevier, pp 5–385. https://doi.org/10.1016/B978-012693015-3/50005-X
Hasler AD (1947) Eutrophication of lakes by domestic drainage. Ecology 28:4. https://doi.org/10.2307/1931228
Jickells TD (1998) Nutrient biogeochemistry of the coastal zone. Science 281(5374):217–222. https://doi.org/10.1126/science.281.5374.217
de Jonge VN, Elliott M, Orive E (2002) Causes, historical development, effects and future challenges of a common environmental problem: eutrophication. Hydrobiologia 475–476. https://doi.org/10.1023/A:1020366418295
Juneau P, Green BR, Harrison PJ (2005) Simulation of pulse-amplitude-modulated (PAM) fluorescence: limitations of some PAM-parameters in studying environmental stress effects. Photosynthetica 43:1. https://doi.org/10.1007/s11099-005-5083-7
Karlson B, Andersen P, Arneborg L, Cembella A, Eikrem W, John U, West JJ, Klemm K, Kobos J, Lehtinen S, Lundholm N (2021) Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 102:101989. https://doi.org/10.1016/j.hal.2021.101989
Karthikeyan P, Jayasudha S, Sampathkumar P, Manimaran K, Santhoshkumar C, Ashokkumar S, Ashokprabu V (2010) Effect of industrial effluent on the growth of marine diatom, Chaetoceros simplex (Ostenfeld, 1901). J Appl Sci Environ Manage 14:4. https://doi.org/10.4314/jasem.v14i4.63253
Khan MN, Mohammad F (2014) Eutrophication: challenges and solutions. Eutrophication: Causes. Consequences Control: 2:1–15. https://doi.org/10.1007/978-94-007-7814-6_1
Kim G, Ryu JW, Yang HS, Yun ST (2005) Submarine groundwater discharge (SGD) into the Yellow Sea revealed by 228Ra and 226Ra isotopes: implications for global silicate fluxes. Earth Planet Sci Lett 237(1–2):156–166. https://doi.org/10.1016/j.epsl.2005.06.011
Kitajima MBWL, Butler WL (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochimica et Biophysica Acta (BBA)-Bioenerg 376(1):105–115. https://doi.org/10.1016/0005-2728(75)90209-1
Knee K, Paytan A (2011) 4.08 submarine groundwater discharge: a source of nutrients, metals, and pollutants to the Coastal Ocean. Treatise Estuar Coast Sci 4:205–234
Kolber Z, Wyman KV, Falkowski PG (1990) Natural variability in photosynthetic energy conversion efficiency: a field study in the Gulf of Maine. Limnol Oceanogr 35(1):72–79. https://doi.org/10.4319/lo.1990.35.1.0072
Kumar MD, Naqvi SWA, George MD, Jayakumar DA (1996) A sink for atmospheric carbon dioxide in the northeast Indian Ocean. J Geophys Res: Oceans 101(C8):18121–18125. https://doi.org/10.1029/96JC01452
Kumar BSK, Viswanadham R, Kumari VR, Rao DB, Prasad MHK, Srinivas N, Sarma VVSS (2021) Spatial variations in dissolved inorganic nutrients in the groundwaters along the Indian coast and their export to adjacent coastal waters. Environ Sci Pollut Res 28:9173–9191. https://doi.org/10.1007/s11356-020-11387-7
Kumari VR, Yadav K, Sarma VVSS, Dileep Kumar M (2021) Acidification of the coastal Bay of Bengal by aerosols deposition. J Earth Syst Sci 130:1–13. https://doi.org/10.1007/s12040-021-01723-x
Kumari VR, Neeraja B, Rao DN, Ghosh VRD, Rajula GR, Sarma VVSS (2022) Impact of atmospheric dry deposition of nutrients on phytoplankton pigment composition and primary production in the coastal Bay of Bengal. Environ Sci Pollut Res 29:82218–82231. https://doi.org/10.1007/s11356-022-21477-3
Liu Q, Charette MA, Henderson PB, McCorkle DC, Martin W, Dai M (2014) Effect of submarine groundwater discharge on the coastal ocean inorganic carbon cycle. Limnol Oceanogr 59(5):1529–1554. https://doi.org/10.4319/lo.2014.59.5.1529
Madeswaran P, Murthy MVR, Rajeevan M, Ramanathan V, Patra S, Sivasdas SK, Boopathi T, Bharathi MD, Anuradha S, Muthukumar C (2018) Status report: seawater quality at selected locations along Indian coast. Nat. Centre Coastal Res, 2018, pp-177, available at NCCR’s Web site at http://www.nccr.gov.in
Martin-Jézéquel V, Hildebrand M, Brzezinski MA (2000) Silicon metabolism in diatoms: implications for growth. J Phycol 36(5):821–840. https://doi.org/10.1046/j.1529-8817.2000.00019.x
Matorin DN, Osipov VA, Seifullina NK, Venediktov PS, Rubin AB (2009) Increased toxic effect of methylmercury on Chlorella vulgaris under high light and cold stress conditions. Microbiology 78:321–327. https://doi.org/10.1134/S0026261709030102
Mochizuki M, Shiga N, Saito M, Imai K, Nojiri Y (2002) Seasonal changes in nutrients, chlorophyll a and the phytoplankton assemblage of the western subarctic gyre in the Pacific Ocean. Deep Sea Res Part II 49(24–25):5421–5439. https://doi.org/10.1016/S0967-0645(02)00209-6
Murty VSN, Suryanarayana A, Rao DP (1993) Current structure and volume transport across 12 N in the Bay of Bengal. http://nopr.niscpr.res.in/handle/123456789/37746
Naqvi SWA, Jayakumar DA, Nair M, Kumar MD, George MD (1994) Nitrous oxide in the western Bay of Bengal. Mar Chem 47(3–4):269–278. https://doi.org/10.1016/0304-4203(94)90025-6
Naqvi SWA, Jayakumar DA, Narvekar PV, Naik H, Sarma VVSS, D’souza W, Joseph S, George MD (2000) Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature 408(6810):346–349. https://doi.org/10.1038/35042551
Officer CB, Ryther JH (1980) The possible importance of silicon in marine eutrophication. Mar Ecol Prog Ser 3(1):83–91
Pan Y, Subba Rao DV, Mann KH, Brown RG, Pocklington R (1996) Effects of silicate limitation on production of domoic acid, a neurotoxin, by the diatom Pseudo-nitzschia multiseries. I. Batch culture studies. Marine Ecol Prog Ser 131:225–233. https://doi.org/10.3354/meps131225
Pan Y (1994) Production of domoic acid, a neurotoxin, by the diatom Pseudonitzchia pungens f. multiseries Hasle under phosphate and silicate limitation
Papa F, Durand F, Rossow WB, Rahman A, Bala SK (2010) Seasonal and interannual variations of the Ganges-Brahmaputra River Discharge, 1993–2008 from satellite altimeters. J Geophys Res 115:C12013. https://doi.org/10.1029/2009JC006075
Piehler MF, Twomey LJ, Hall NS, Paerl HW (2004) Impacts of inorganic nutrient enrichment on phytoplankton community structure and function in Pamlico Sound, NC, USA. Estuar Coast Shelf Sci 61(2):197–209. https://doi.org/10.1016/j.ecss.2004.05.001
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144. https://doi.org/10.1016/0022-5193(66)90013-0
Rabalais NN, Turner RE, Díaz RJ, Justić D (2009) Global change and eutrophication of coastal waters. ICES J Mar Sci 66(7):1528–1537. https://doi.org/10.1093/icesjms/fsp047
Rao CK, Naqvi SWA, Kumar MD, Varaprasad SJD, Jayakumar DA, George MD, Singbal SYS (1994) Hydrochemistry of the Bay of Bengal: possible reasons for a different water-column cycling of carbon and nitrogen from the Arabian Sea. Mar Chem 47(3–4):279–290. https://doi.org/10.1016/0304-4203(94)90026-4
Sakshaug E, Bricaud A, Dandonneau Y, Falkowski PG, Kiefer DA, Legendre L, Morel A, Parslow J, Takahashi M (1997) Parameters of photosynthesis: definitions, theory and interpretation of results. J Plankton Res 19(11):1637–1670. https://doi.org/10.1093/plankt/19.11.1637
Sanilkumar KV, Kuruvilla TV, Jogendranath D, Rao RR (1997) Observations of the western boundary current of the Bay of Bengal from a hydrographic survey during March 1993. Deep Sea Res Part I 44(1):135–145. https://doi.org/10.1016/S0967-0637(96)00036-2
Santra SC (1993) Biology of Rice-fields blue-green algae. Daya Books
Sarma VVSS, Sridevi B, Maneesha K, Sridevi T, Naidu SA, Prasad VR, Venkataramana V, Acharya T, Bharati MD, Subbaiah CV, Kiran BS (2013) Impact of atmospheric and physical forcings on biogeochemical cycling of dissolved oxygen and nutrients in the coastal Bay of Bengal. J Oceanogr 69:229–243. https://doi.org/10.1007/s10872-012-0168-y
Sarma VVSS, Krishna MS, Srinivas TNR, Kumari VR, Yadav K, Kumar MD (2021) Elevated acidification rates due to deposition of atmospheric pollutants in the coastal Bay of Bengal. Geophys Res Lett 48(16):e2021GL095159. https://doi.org/10.1029/2021GL095159
Schott FA, McCreary JP Jr (2001) The monsoon circulation of the Indian Ocean. Prog Oceanogr 51(1):1–123. https://doi.org/10.1016/S0079-6611(01)00083-0
Shaik AR, Biswas H, Reddy NPC, Srinivasa Rao V, Bharathi MD, Subbaiah CV (2015) Time series monitoring of water quality and microalgal diversity in a tropical bay under intense anthropogenic interference (SW coast of Bay of Bengal, India). Environ Impact Assess Rev 55:169–181. https://doi.org/10.1016/j.eiar.2015.08.005
Shankar D, Vinayachandran PN, Unnikrishnan AS (2002) The monsoon currents in the north Indian Ocean. Prog Oceanogr 52(1):63–120. https://doi.org/10.1016/S0079-6611(02)00024-1
Shannon CE, Weaver W (1998) The mathematical theory of communication, 1949 rep. Urbana, University of Illinois Press, Foreword by Richard E. Blahut and Bruce Hajek
Shetye SR, Gouveia AD, Shenoi SSC, Sundar D, Michael GS, Nampoothiri G (1993) The western boundary current of the seasonal subtropical gyre in the Bay of Bengal. J Geophys Res: Oceans 98(C1):945–954. https://doi.org/10.1029/92JC02070
Shetye SR, Gouveia AD, Shankar D, Shenoi SSC, Vinayachandran PN, Sundar D, Michael GS, Nampoothiri G (1996) Hydrography and circulation in the western Bay of Bengal during the northeast monsoon. J Geophys Res: Oceans 101(C6):14011–14025. https://doi.org/10.1029/95JC03307
Slomp CP, Van Cappellen P (2004) Nutrient inputs to the coastal ocean through submarine groundwater discharge: controls and potential impact. J Hydrol 295(1–4):64–86. https://doi.org/10.1016/j.jhydrol.2004.02.018
Smayda TJ (1990) Novel and nuisance phytoplankton blooms in the sea: evidence for a global epidemic. Toxic marine phytoplankton, pp 29–40
State report (2023). District Visakhapatnam: Economy, Government of Andhra Pradesh. https://visakhapatnam.ap.gov.in/economy/
Sudheer AK, Sarin MM (2008) Carbonaceous aerosols in MABL of Bay of Bengal: influence of continental outflow. Atmos Environ 42(18):4089–4100. https://doi.org/10.1016/j.atmosenv.2008.01.033
Suggett DJ, Moore CM, Hickman AE, Geider RJ (2009) Interpretation of fast repetition rate (FRR) fluorescence: signatures of phytoplankton community structure versus physiological state. Mar Ecol Prog Ser 376:1–19. https://doi.org/10.3354/meps07830
Suzuki R, Ishimaru T (1990) An improved method for the determination of phytoplankton chlorophyll using N, N-dimethylformamide. J Oceanogr Soc Jpn 46:190–194. https://doi.org/10.1007/BF02125580
Taniguchi M, Burnett WC, Cable JE, Turner JV (2002) Investigation of submarine groundwater discharge. Hydrol Process 16(11):2115–2129. https://doi.org/10.1002/hyp.1145
Throndsen J (1978) Preservation and storage. In: Sournia A (ed) Phytoplankton manual. UNESCO, Pans, pp 69–74
Tipper ET, Stevenson EI, Alcock V, Knight AC, Baronas JJ, Hilton RG, Bickle MJ, Larkin CS, Feng L, Relph KE, Hughes G (2021) Global silicate weathering flux overestimated because of sediment–water cation exchange. Proc Natl Acad Sci 118(1):e2016430118. https://doi.org/10.1073/pnas.2016430118
Tréuer P, Lindner L, van Bennekom AJ, Leynaert A, Panouse M, Jacques G (1991) Production of biogenic silica in the Weddell-Scotia Seas measured with 32Si. Limnol Oceanogr 36(6):1217–1227. https://doi.org/10.4319/lo.1991.36.6.1217
Turkoglu M, Koray T (2004) Algal blooms in surface waters of the Sinop Bay in the Black Sea. Turk Pak J Biol Sci 7(9):1577–1585. https://doi.org/10.3923/pjbs.2004.1577.1585
UNESCO (1979) Discharge of Selected Rivers of the World, Paris
Viswanadham R, Bharathi MD, Sarma VVSS (2016) Variations in concentrations and fluxes of dimethylsulfide (DMS) from the Indian estuaries. Estuaries Coasts 39:695–706. https://doi.org/10.1007/s12237-015-0039-z
Vollenweider RA (1968) Scientific fundamentals of the eutrophication of lakes and flowing waters, with particular reference to nitrogen and phosphorus as factors in eutrophication. Paris (France) 192:14
Wang XL, Lu YL, Han JY, He GZ, Wang TY (2007) Identification of anthropogenic influences on water quality of rivers in Taihu watershed. J Environ Sci 19(4):475–481. https://doi.org/10.1016/S1001-0742(07)60080-1
Windom HL, Moore WS, Niencheski LFH, Jahnke RA (2006) Submarine groundwater discharge: a large, previously unrecognized source of dissolved iron to the South Atlantic Ocean. Mar Chem 102(3–4):252–266. https://doi.org/10.1016/j.marchem.2006.06.016
Withers PJ, Neal C, Jarvie HP, Doody DG (2014) Agriculture and eutrophication: where do we go from here? Sustainability 6(9):5853–5875. https://doi.org/10.3390/su6095853
Wolf MJ, Emerson JW, Esty DC, de Sherbinin A, Wendling ZA (2022) 2022 Environmental Performance Index. New Haven, CT: Yale Center for Environmental Law & Policy. https://epi.yale.edu
Yadav K, Sarma VVSS, Rao DB, Kumar MD (2016) Influence of atmospheric dry deposition of inorganic nutrients on phytoplankton biomass in the coastal Bay of Bengal. Mar Chem 187:25–34. https://doi.org/10.1016/j.marchem.2016.10.004
Yadav K, Rao VD, Sridevi B, Sarma VVSS (2021) Decadal variations in natural and anthropogenic aerosol optical depth over the Bay of Bengal: the influence of pollutants from Indo-GangeticPlain. Environ Sci Pollut Res 28(39):55202–55219. https://doi.org/10.1007/s11356-021-14703-x
Acknowledgements
We would like to thank Director INCOIS for funding the project entitled “Visakhapatnam Coastal time-series Observatory to monitor biogeochemical processes”. We would like to thank Mr Dipin and Satyanarayana for their help in nutrient analysis. We would like to thank two anonymous reviewers for their constructive comments to improve the presentation of our results. This has NIO contribution number is 7226.
Author information
Authors and Affiliations
Contributions
AS—collection of samples and conducting the experiment and analysing the samples.
VVSS—concept of the work, guidance and finalizing the manuscript.
Both authors contributed to writing, building concepts and finalizing the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A., Sarma, V.V.S.S. Response of coastal phytoplankton to pollution from various sources in the coastal Bay of Bengal. Environ Sci Pollut Res 31, 31787–31805 (2024). https://doi.org/10.1007/s11356-024-33354-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33354-2