Abstract
This study aimed to explore the relationship between chronic diarrhea and urinary phthalate metabolite concentrations in US adults from the 2005–2010 NHANES study. After adjusting for potential confounding factors, logistic regression was used to explore the relationship between phthalates (PAEs) concentrations and chronic diarrhea, Bayesian kernel machine regression (BKMR), and quantile g calculation (quantile-based g calculation, qgcomp) which was used to study the combined and independent effects of PAEs on gastrointestinal infections. In the current study, 4260 adult participants over the age of 20 from the NHANES study were included, of whom 542 (12.72%) were assessed as having chronic diarrhea. In multivariate logistic regression analysis, after adjusting for all relevant covariates, the results showed that urinary phthalate metabolite concentrations were significantly associated with the risk of chronic diarrhea (P<0.001). Various PAEs were risk factors for chronic diarrhea, among which MiBP (OR=1.419, 95% CI: 1.416–1.423) and MCPP (OR=1.237, 95% CI: 1.235–1.239) were more significant. The BKME results showed a significant increase in the risk of chronic diarrhea with increasing total levels of the PAEs mixture. Mixed exposure to PAEs can promote the occurrence of chronic diarrhea, and the effect was more pronounced in obese people. Notably, most PAEs showed some degree of protection in overweight people. The risk effect of PAEs was more significant in the middle-aged and older population than in the younger population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the increasing harmfulness of phthalates and their metabolites, owing to their low cost, appealing properties, and absence of suitable alternatives, phthalates are utilized in plastics as plasticizers and in countless consumers. Large-scale use as an additive in products continues (Wang et al. 2021). Globally, few epidemiological or clinical human researches have revealed that phthalates (PAEs) pose a prominent ecological risk due to their multiple toxicity potential and ubiquitous detection in the environment (Hamid et al. 2020). Human ingests more than a dozen phthalates and their metabolites by passive ingestion from breathed air, general environment, food, beverages, and everyday household products that can result in a variety of functional impairments, comprising health hazards to adolescents and children, epigenetic regulation, male and female reproductive toxicity, overweight and obesity, type II diabetes and insulin resistance, skeletal deformities, etc. (Benjamin et al. 2017). PAEs are a typical class of endocrine disruptors that can accumulate in organisms and interfere with their secretion systems (Zhu et al. 2022). An increasing number of studies have investigated how endocrine-disrupting chemicals (EDCs) affect the gut microbiota of a range of animals, and resident bacteria in the gut and other parts of the body can greatly influence the host response (Rosenfeld 2021).
The intestine is an important digestive organ and the largest immune organ in the human body (Guo et al. 2021). Intestinal homeostasis is the dynamic balance of interactions between the host’s intestinal mucosa, immune barrier, gut microbiome, nutrients, and metabolites. Once homeostasis is out of balance, it increases the risk of intestinal disease (Shen et al. 2022). Chronic diarrhea is a prevalently presenting symptom in both primary care and specialist gastroenterology departments. Over 5% of the global population is believed to suffer from chronic diarrhea. (Alonso-Cotoner et al. 2021). Diarrhea is the main pathological feature of inflammatory bowel disease, and the expression and function of major intestinal ion transporters are significantly reduced (Jayawardena et al. 2020). Recently, some research evidence has shown that exposure to DEHP contamination significantly increases intestinal permeability and enhances intestinal inflammation (Deng et al. 2020).
Inflammatory dysregulation is a common thread connecting the most important chronic diseases and conditions in all physiological systems and associated comorbid conditions (Dietert 2012). As a common chronic disease, chronic diarrhea is closely related to inflammation, and some studies have shown that exposure to EDCs can increase inflammation in certain populations (Zota et al. 2018). The relationship between EDCs and inflammatory bowel diseases such as chronic diarrhea has also attracted more and more attention, but as typical EDCs, there are few studies on the relationship between EDCs and intestinal diseases. Hence, this study’s objective was to evaluate if variations in phthalate exposure, as estimated by urine concentrations, were related to variations in chronic diarrheal disease in US adults samples.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a biennial cross-sectional survey that is utilized for evaluating the health and nutritional status of children and adults in the USA (Zand et al. 2015). NHANES, a critical program of the National Center for Health Statistics (NCHS), guarantees that participants are selected from diverse geographic and racial/ethnic groups, thus providing a representative sample total population of the USA. For complete information on the data provided by NHANES and precise details of data collection, please refer to its specific website (http://www.cdc.gov/nchs/nhanes.htm). Data pooled in the study from participants during the 2005–2006, 2007–2008, and 2009–2010 NHANES surveys, excluding individuals with (1) incomplete gut health questionnaire assessment data, (2) colon cancer and/or rectal cancer, (3) lack of baseline status, and (4) population under 20 years of age. The final analysis of chronic diarrhea comprised 4260 patients aged 20 years and older (2117 males and 2143 females) (Fig. 1). Additionally, weighted estimates were established and extrapolated to sample data to get nationwide estimates.
Exposure assessment and outcome assessment
For analysis, urine specimens were stored, processed, and transported to the Department of Laboratory Sciences, the National Center for Environmental Health, and the Centers for Disease Control and Prevention. The vials were kept under proper freezing (20°C) until they were transported to the National Center for Environmental Health for testing. The following phthalate metabolites were detected in urine utilizing high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS). NHANES was replaced for every chemical concentration below the limit of detection (LOD) by dividing the LOD value by the square root of 2. Environmental pollution exposures include mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono(carboxynonyl) phthalate (MCNP), mono-benzyl phthalate (MBzP), mono-(3-carboxypropyl) phthalate (MCPP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(carboxyoctyl) phthalate (MCOP), mono-n-butyl phthalate (DCHP), mono-ethyl phthalate (MEP), mono-(2-ethyl)-hexyl phthalate (MEHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono-isobutyl phthalate(MiBP).
The BSFS and frequency criteria were utilized for the evaluation of chronic constipation, both from the Gut Health Questionnaire, a gender-related standardized question panel. The BSFS utilized a colored picture card with textual descriptions and pictures of seven stool types and provided them to recipients. The following written question was asked: “Look at this card and tell me which stool type resembles your normal or commonest stool type.” Diarrhea was identified as type 6 (fluffy, flaky, mushy stools, and rough edges) or type 7 (water sample, no solid flakes). Bowel function is also measured by the bowel movements frequency per week and answers the question: “How often do you normally experience bowel movements per week?” According to previous data from NHANES, it is less than 3 times (constipation), 3–21 times (normal), and exceed 21 times (diarrhea).
Covariates
According to previous studies, multiple potential variables were included in multivariate models confounding the relation between urine phthalate metabolite concentrations and chronic diarrhea, including sex (male/female), age (20–35, 36–45, 46–55, 56, and older), race/ethnicity(Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, other/multi-racial), body mass index (BMI) category, poverty income ratio (PIR, continuous), creatinine, marital status (married, divorced/widowed/separated, never married, living with partner), and education (less than 9th grade, 9th–11th grade, high school grad/GED or equivalent, some college or AA degree, college grad, or above). In the current study, we defined BMI as three levels: <25.00 (underweight/normal weight), 25.00–30.00 (overweight), and >30.00 (obese). The PIR is estimated by dividing the household income (relied on the poverty criteria specific to household size) by the year and state for indicating the participant’s socioeconomic status within the family.
Statistical analysis
All PAEs and associated covariates distributions were evaluated. PAEs and other continuous covariates were reported as interquartile and medians ranges. Other categorical covariates are number and percentage. For additional model analysis, all concentrations of PAEs were natural log (ln) transformed. The chi-square test (categorical variables) or Wilcoxon rank-sum test (skewed distribution) were utilized for assessing variations in PAEs and covariates between the diarrhea and non-diarrhea groups. For investigating pairwise correlations between raw PFAS values, Spearman rank correlation analysis was utilized. Prior to regression analysis, missing values for covariates were imputed utilizing multiple imputations of the fully conditional normative (FCS-MI) method. This method enables the specification of multivariate imputation models on a variable-by-variable basis and gives a principled but malleable approach for addressing missing data. Ten datasets were established and modeled individually with missing data imputation. The results in single contaminant and exposure analyses of mixtures of PAEs were aggregated for obtaining final impact parameters. Logistic regression models were utilized for evaluating the relation between PAEs and chronic diarrhea. PAEs homologues associated with chronic diarrhea were analyzed one at a time. Variance inflation factor (VIF) values and tolerance are calculated for evaluating multicollinearity among covariables, with tolerances <0.1 and VIF > 10 indicating multicollinearity. For education, sex, marital status, BMI, age, poverty income ratio, race, and creatinine concentration, all models were adjusted. In our regression model, MINP, MnOP, MMP, and MCHP were not incorporated owing to their low detection rate.
The individual and overall impacts of PAEs on the chronic diarrhea incidence were estimated utilizing Bayesian kernel machine regression (BKMR). BKMR is a statistical method that can flexibly model the combined effects of pollutant mixtures and can be used to estimate the effects of individual mixture components, the effects of overall mixture components, and the interactions between mixture components. Fitting exposure-response relationships for binary outcomes using a probabilistic link function. The core of the BKMR model is kernel machine regression (KMR), which iteratively regresses the exposure-response function using a Gaussian kernel function. PAEs were ln-transformed, centered at 0, and scaled using standard deviation 1 for all BKMR analyses to account for skewness and minimize the extreme values effects and various value scales within the variable. As previously stated, for the corresponding covariates, each BKMR model was adjusted. The overall mixed PAEs effect was assessed by subtracting the mean of the results at the 25th percentile for all PAEs at the 75th percentile by subtracting the mean of the results at the 75th percentile for all PAEs. Likewise, individual effects of every PAEs mixture component were calculated by subtracting the mean outcome value when the provided PAEs were at their 25th percentile minus provided PAEs at their 75th percentile while keeping other PAEs homologues fixed at their median unchanged. Plots were utilized for analyzing the overall association of a PAEs mixture with several measures of glucose homeostasis and for demonstrating the exposure-response relation for every PAE isoform, whereas maintaining other exposures at their 50% percentile and covariates constant.
Though BKMR benefits from exposure-response function modeling’s flexibility and permits for nonlinear and non-additive associations between several PAEs and chronic diarrhea, it lacks concise parametric reasoning (e.g., single coefficient of change in results). Additionally, depending on parametric inference, we utilize a newly developed quantile-based g calculation approach for exploring joint effects. Quantile g calculation is a parameter-based, generalized linear model-based implementation of g-computation used to estimate the change in quantile results while increasing all exposures in a particular mixture. This method comprises weighted quantile regression (WQS) inference simplicity with g-computation flexibility, whereas accounting for BKMR’s nonlinearity and non-additivity resulting in lower bias and more robustness. SAS V9.4 (SAS Institute Inc., Cary, NC, USA) and R (version 3.6.1, R Development Core Team 2019) were utilized for conducting all statistical analyses. A α level of 5% (two-sided) was deemed statistically significant.
Results
Population characteristics
Table 1 illustrates the demographic characteristics of the overall population enrolled in the study. In the current study, 4260 adult participants over the age of 20 from the NHANES study were included, of whom 542 (12.72%) were assessed as having chronic diarrhea. The ages of the participants were mainly distributed between 20 and 35 years old and 56 years old and above. Moreover, the obesity group accounted for a higher proportion (43.7%) in the chronic diarrhea population. Most subjects were non-Hispanic white. Between the chronic diarrhea group and the normal group, there were significant variations in age, BMI, PIR, and education level (P<0.05).
Urinary phthalate metabolites
Ten of the eleven phthalate metabolites investigated in this study were observed in over 92% of the study population, while one was observed in over 69% of the population (Table 2). The detection frequencies of most phthalate metabolites were roughly similar except for mono-ethyl phthalate (MEP) and mono-isobutyl phthalate (MiBP). The difference between the normal and diarrhea groups was statistically significant (P<0.05). Pairwise correlation analysis showed that some of the PAEs metabolites had significant correlations. For different PAEs, correlation coefficients varied from 0 to 0.97, with strong correlations among several PAEs metabolites (MEHP, MEHHP, MEOHP, and MECPP) (Fig. 2).
The relationship urinary phthalate metabolite concentrations and chronic diarrhea
The relation between different PAEs types and the risk of chronic diarrhea is illustrated in Table 3. In multivariate logistic regression analysis, after adjusting for all relevant covariates, the results showed that urinary phthalate metabolite concentrations were significantly associated with the risk of chronic diarrhea (P<0.001). The risk of chronic diarrhea increases with increasing concentrations of PAEs. Various PAEs were risk factors for chronic diarrhea, among which MiBP (OR=1.419, 95% CI: 1.416–1.423) and MCPP (OR=1.237, 95% CI: 1.235–1.239) were more significant, whereas MCNP, MEHP, and MEOHP are protective factors for chronic diarrhea (Supplementary Fig. 1). For further exploring the specific correlation between the various phthalate metabolites concentrations and chronic diarrhea, we performed a subgroup analysis based on BMI and age, and the results showed (Supplementary Table 1) that in the obese population, various PAEs were risk factors for chronic diarrhea (P<0.001). In overweight people, most PAEs showed some degree of protection (Supplementary Table 2). The risk effect of PAEs was more significant in the middle-aged and elderly population (>46) compared with the younger population (<46). The risk effect of MCPP on chronic diarrhea in the 46–55 age group (OR=2.308, 95% CI: 2.299–2.318) was more than double that of the 20–35 age group (OR=1.189, 95% CI: 1.185–1.194).
PAEs joint and individual exposure with chronic diarrhea based on BKMR
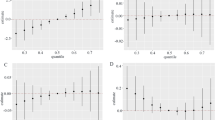
Figure 3 A illustrates the overall association between PAEs mixture and chronic diarrhea. The risk of chronic diarrhea elevated significantly as the total mixture of PAE levels elevated. Additionally, we evaluated the individual exposure-response function of PAEs and chronic diarrhea (Fig. 3C). Changes from 25 to 75% in MECPP, MEHHP, and DCHP were associated with an increased risk of chronic diarrhea (Fig. 3B). MCOP, MiBP, and MCPP exposure were positively correlated with an increased risk of chronic diarrhea. There was no interaction between different PAEs (Fig. 3D).
The association between phthalate metabolites and chronic diarrhea in adults was studied utilizing the BKMR model. The model is based on age and gender, race/ethnicity, education, poverty income ratio (PIR ), marital status, body mass index, and creatinine. A Cumulative effects of phthalate metabolites (estimates and 95% confidence intervals). B Univariate response functions for every phthalate metabolite have 95% confidence bands, and other metabolites are fixed at the median. C Single phthalate metabolite effect (estimated value and 95% confidence interval). D Bivariate exposure-response function for every of the two phthalate metabolites among patients with chronic diarrhea.
Sensitivity analysis
In the content of sensitivity analysis, the results of the analysis based on quantile g calculations were used to assess the robustness and stability of the BKMR and multivariate logistic regression modeling results. Combined effects analysis showed that mixed exposure to PAEs contributed to the increased risk of chronic diarrhea. MECPP had the largest positive weight, indicating a greater association with chronic diarrhea. At the same time, by comparing the results of the regression data before and after weighting, it was found that the data results before and after weighting are consistent (Supplementary Table 3).
Discussion
According to some studies, adults have a prevalence of chronic diarrhea ranging from 1 to 5%. (Burgers et al. 2020). Common causes include inflammation, tumors, malabsorption, infection, vascular and irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). Other causes include food intolerances, medications side effects, or post-operative conditions. Additionally, diarrhea can be a symptom of systemic disorders, such as hyperthyroidism or diabetes (Hammer 2021).
One of the major causes of global disease burden is thought to be ambient air pollution. The association of air pollution with respiratory and cardiovascular disease was first recognized, and then accumulated data suggested that the gut may also be compromised (Vignal et al. 2021). PAEs (phthalates) are commonly utilized in industrial production to soften hard plastics (plasticizers), and their related products are widely distributed in our everyday lives as emerging pollutants. As PAEs are not chemically bonded to the product, they are easily transported to the surrounding environment (Xiong and Pei 2021). However, the research between PAEs and intestinal health is very scarce. The BKMR results of this study indicated that the overall effect of mixed exposure to PAEs promoted the occurrence and development of chronic diarrhea to a certain extent. Studies have shown that the accumulation of PAEs in the liver and intestines was significantly increased in mice exposed to PAEs pollution (Deng et al. 2021). Endocrine disrupting chemicals/mixtures (EDCs) are chemicals that disrupt hormone homeostasis in the body. They are critical in the function of the endocrine system, including the hypothalamic-pituitary-gonadal axis (HPG axis). Some common and widely studied EDCs have negative effects on the endocrine system and a wide range of activities including PAEs (Czarnywojtek et al. 2021). Exposure to EDCs was strongly associated with dysregulated inflammatory responses, and exposure to PAEs was positively associated with both pro- and anti-inflammatory markers. These findings underscore the impact of EDCs on the regulation of inflammation, which may be closely related to a range of chronic diseases (Liu et al. 2022), while there is evidence that PAE exposure is associated with oxidative stress, adiponectin, and inflammatory cytokines in diabetic patients (Duan et al. 2017). In neonates, phthalate-mediated PPAR-γ inhibition by neutrophils is more sensitive, which may be related to a decrease in anti-inflammatory signaling (Vetrano et al. 2010). Results from animal studies indicate that certain phthalates can affect immune and inflammatory function in rodents when administered at appropriate doses and by appropriate routes, but no consistent pattern has emerged. Results range from enhanced immune or inflammatory responses to no effect, suppressive, or immunosuppressive activity (Kimber and Dearman 2010). Interestingly, an important role for phthalates in promoting inflammation was not supported in the findings of one study, although some statistically significant results were observed (Trim et al. 2021). As a common chronic disease, the relationship between chronic diarrhea and PAEs exposure still needs more research and analysis in the future, as well as its underlying mechanism.
Chronic diarrhea is a common problem in all age groups but poses special challenges in the elderly as a special patient population (Schiller 2019). An increase in body mass index (BMI) is correlated with an elevation in all-cause mortality and diseases associated with higher mortality, including diabetes, cardiovascular disease, and cancer, comprising diseases of the gastrointestinal system. Gastrointestinal symptoms such as vomiting and diarrhea are more common in obese patients than in normal-weight patients (Moayyedi 2008). Obesity increases the risk of various gastrointestinal and liver diseases. Studies have provided strong evidence that in a nationally representative US adult population, obesity is positively associated with chronic diarrhea, and the risk of diarrhea increases with the severity of obesity (Ballou et al. 2019). However, the mechanism of the relationship between obesity and diarrhea still needs to be further explored. Current research points to changes including bile acid absorption and intestinal physiology (increased colonic motility and intestinal permeability). And the link between gut microbiota and this obesity and diarrhea is very likely (Sayuk and Elwing 2019). Bile acid diarrhea is the commonest unexplained cause of chronic diarrhea. Various pathophysiological causes can lead to chronic diarrhea. Even after excluding more common causes, 5% of the population is affected by unexplained chronic diarrhea (Storr et al. 2021). Although the majority of studies have demonstrated decreased diversity and richness of the gut microbiota in obese people, there is still much debate on the actual microbial signature of healthy or obese gut microbiota (Vallianou et al. 2019).
The current study has numerous limitations. Firstly, though the NHANES database comprises sampling weights for facilitating the NHANES sample extrapolation to the general population of the USA, but weighting methods based on selected demographics may still fail to take into account the complex interactions between factors such as race, income level, and BMI. Therefore, the diarrhea patients addressed in this article may not be representative of all people in the USA who suffer from chronic diarrhea. Secondly, NHANES is cross-sectional in nature and hence unable to detect the temporal and causal relation between the onset of chronic diarrhea and exposure to PAEs. Since the NHANES data are based on self-reports, there may be some recall bias when asking about common or most common stool types.
At the same time, the present study has some advantages. First, constipation, as a common disease state, is not easy to assess at a small population level. The study used data from a representative large population survey (NHANES) that included study data from three periods from 2005 to 2010, enabling a relatively accurate assessment of constipation. Secondly, due to the comprehensive nature of the NHANES cohort, we were able to adjust for many of the established risk factors for constipation. To our knowledge, this study is the first in-depth investigation of the relationship between PAEs and chronic diarrhea. Finally, BKMR can flexibly model exposure to address interactions and nonlinear relationships. In this study, the BKMR model was able to assess the overall relationship between PAEs and chronic diarrhea risk while identifying a single potential interaction.
Conclusion
Mixed exposure to PAEs can promote the occurrence of chronic diarrhea, and the effect was more pronounced in obese people. Notably, most PAEs showed some degree of protection in overweight people. The risk effect of PAEs was more significant in the middle-aged and older population than in the younger population. Future studies should further explore the complex pathophysiological interactions between PAEs exposure, obesity, and intestinal symptoms and their underlying mechanisms.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request
References
Alonso-Cotoner C, Abril-Gil M, Albert-Bayo M, Mall JG, Expósito E, González-Castro AM, Lobo B, Santos J (2021) The role of purported mucoprotectants in dealing with irritable bowel syndrome, functional diarrhea, and other chronic diarrheal disorders in adults. Adv Ther 38:2054–2076
Ballou S, Singh P, Rangan V, Iturrino J, Nee J, Lembo A (2019) Obesity is associated with significantly increased risk for diarrhoea after controlling for demographic, dietary and medical factors: a cross-sectional analysis of the 2009-2010 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 50:1019–1024
Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA (2017) Phthalates impact human health: epidemiological evidences and plausible mechanism of action. J Hazard Mater 340:360–383
Burgers K, Lindberg B, Bevis ZJ (2020) Chronic diarrhea in adults: evaluation and differential diagnosis. Am Fam Physician 101:472–480
Czarnywojtek A, Jaz K, Ochmańska A, Zgorzalewicz-Stachowiak M, Czarnocka B, Sawicka-Gutaj N, Ziółkowska P, Krela-Kaźmierczak I, Gut P, Florek E, Ruchała M (2021) The effect of endocrine disruptors on the reproductive system - current knowledge. Eur Rev Med Pharmacol Sci 25:4930–4940
Deng Y, Yan Z, Shen R, Wang M, Huang Y, Ren H, Zhang Y, Lemos B (2020) Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ Int 143:105916
Deng Y, Yan Z, Shen R, Huang Y, Ren H, Zhang Y (2021) Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus). J Hazard Mater 406:124644
Dietert RR (2012) Misregulated inflammation as an outcome of early-life exposure to endocrine-disrupting chemicals. Rev Environ Health 27:117–131
Duan Y, Wang L, Han L, Wang B, Sun H, Chen L, Zhu L, Luo Y (2017) Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environ Int 109:53–63
Guo Y, Wang B, Wang T, Gao L, Yang ZJ, Wang FF, Shang HW, Hua R, Xu JD (2021) Biological characteristics of IL-6 and related intestinal diseases. Int J Biol Sci 17:204–219
Hamid N, Junaid M, Manzoor R, Jia PP, Pei DS (2020) Prioritizing phthalate esters (PAEs) using experimental in vitro/vivo toxicity assays and computational in silico approaches. J Hazard Mater 398:122851
Hammer HF (2021) Management of chronic diarrhea in primary care: the gastroenterologists’ advice. Dig Dis (Basel, Switzerland) 39:615–621
Jayawardena D, Tyagi S, Nazmi A, Olivares-Villagómez D, Dudeja PK (2020) Ion transport basis of diarrhea in a mouse model of adoptive T cell transfer colitis. Dig Dis Sci 65:1700–1709
Kimber I, Dearman RJ (2010) An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology 271:73–82
Liu Z, Lu Y, Zhong K, Wang C, Xu X (2022) The associations between endocrine disrupting chemicals and markers of inflammation and immune responses: a systematic review and meta-analysis. Ecotoxicol Environ Saf 234:113382
Moayyedi P (2008) The epidemiology of obesity and gastrointestinal and other diseases: an overview. Dig Dis Sci 53:2293–2299
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rosenfeld CS (2021) Xenoestrogen effects on the gut microbiome. Curr Opin Endoc Metab Res 19:41–45
Sayuk GS, Elwing JE (2019) Editorial: obesity and chronic diarrhoea-a hill of evidence for causation? Aliment Pharmacol Ther 50:1137–1138
Schiller LR (2019) Chronic diarrhea evaluation in the elderly: IBS or something else? Curr Gastroenterol Rep 21:45
Shen H, Zhao Z, Zhao Z, Chen Y, Zhang L (2022) Native and engineered probiotics: promising agents against related systemic and intestinal diseases. Int J Mol Sci 23:594
Storr M, Gross M, Madisch A, von Arnim U, Mönnikes H, Walters J, Krammer H, Keller J (2021) Bile acid diarrhea, stepchild of chronic diarrhea - prevalence, diagnosis and treatment. Update 2021. Z Gastroenterol 59:580–591
Trim A, Hankinson SE, Liu S, Shadyab AH, Meliker J, Bao W, Luo J, Liu B, Manson JE, Tinker L, Bigelow C, Reeves KW (2021) Biomarkers of phthalates and inflammation: findings from a subgroup of women’s health Initiative participants. Int J Hyg Environ Health 234:113743
Vallianou N, Stratigou T, Christodoulatos GS, Dalamaga M (2019) Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: current evidence and perspectives. Curr Obes Rep 8:317–332
Vetrano AM, Laskin DL, Archer F, Syed K, Gray JP, Laskin JD, Nwebube N, Weinberger B (2010) Inflammatory effects of phthalates in neonatal neutrophils. Pediatr Res 68:134–139
Vignal C, Guilloteau E, Gower-Rousseau C, Body-Malapel M (2021) Review article: epidemiological and animal evidence for the role of air pollution in intestinal diseases. Sci Total Environ 757:143718
Wang J, Shi J, Zhao Y, Xue L, Li G, Wang B, Huang J, Wu S, Guo X (2021) Cardiorespiratory responses in healthy young adults with exposure to indoor airborne PAEs: a randomized, crossover trial of air purification. Environ Int 156:106761
Xiong YH, Pei DS (2021) A review on efficient removal of phthalic acid esters via biochars and transition metals-activated persulfate systems. Chemosphere 277:130256
Zand A, van Deen WK, Inserra EK, Hall L, Kane E, Centeno A, Choi JM, Ha CY, Esrailian E, DHaens GR, Hommes DW (2015) Presenteeism in inflammatory bowel diseases: a hidden problem with significant economic impact. Inflamm Bowel Dis 21:1623–1630
Zhu X, Jiang L, Tu Y, Tian Y, Xu G, Wu D, Li A, Xie X (2022) In situ monitoring of phthalate esters (PAEs) pollution and environmental risk assessment in Poyang Lake Basin by DGT Technology using cyclodextrin polymer as binding phase. Sci Total Environ 808:151892
Zota AR, Geller RJ, Romano LE, Coleman-Phox K, Adler NE, Parry E, Wang M, Park JS, Elmi AF, Laraia BA, Epel ES (2018) Association between persistent endocrine-disrupting chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and biomarkers of inflammation and cellular aging during pregnancy and postpartum. Environ Int 115:9–20
Funding
Comparative study on multiple prevention and treatment schemes for esophageal stricture after ESD surgery, Hebei Provincial Health Commission (Medical Science Research Project Planning Guiding Project).
Author information
Authors and Affiliations
Contributions
Weirui Ren and Junmin Wang conceived and designed the study. All authors wrote the article. Xiaoya Wang downloaded and screened the data from NHANES database. Weirui Ren and Chuang zhang participated in analyzing the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval from the ethical board for this study was not required because of the public nature of all the data.
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, W., Zhang, C., Wang, X. et al. Investigating associations between urinary phthalate metabolite concentrations and chronic diarrhea: findings from the National Health and Nutrition Examination Survey, 2005–2010. Environ Sci Pollut Res 29, 77625–77634 (2022). https://doi.org/10.1007/s11356-022-21123-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21123-y