Abstract
Purpose of Review
Chronic diarrhea is a common problem in all age groups but is a particularly challenging diagnostic problem in the elderly, since many different conditions need to be considered. The purpose of this review is to discuss the evaluation of chronic diarrhea in older individuals. It highlights those conditions that seem to occur with increased frequency in the elderly, discusses the diagnostic tests that are of greatest value in sorting out these problems, and presents an approach to evaluation that is both practical and affordable.
Recent Findings
There appears to be little value in distinguishing irritable bowel syndrome with diarrhea (IBS-D) from functional diarrhea in most patients, including older individuals. Both conditions need a thoughtful analysis of potential causes that may lead to more focused treatment. Older individuals may be more at risk of having certain structural disorders, and these need to be considered when constructing a differential diagnosis. In addition, elderly patients may have atypical presentations of specific disorders that require an increased index of suspicion. Diagnostic tests generally seem to perform well in older patients but have not been validated in this cohort of patients. Although the pretest probabilities of certain diseases are different in the elderly, the conventional algorithm for assessment of chronic diarrhea should lead to a diagnosis in most cases.
Summary
Better studies are needed to adequately quantitate the likelihood of different diagnoses and the operating characteristics of diagnostic tests in older patients with chronic diarrhea. Lacking that information, physicians can still do a good job of making a diagnosis in these patients by adopting a stepwise approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic diarrhea—the habitual passage of unformed stools for > 4 weeks—is a common symptom in people of all ages, occurring in 6.6% of the population [1]. It has a broad differential diagnosis, encompassing many different structural problems, absorptive and biochemical anomalies, infections, and functional problems. Sorting out the different causes of chronic diarrhea can be difficult. The standard evaluation of patients with chronic diarrhea begins with a detailed history, complemented by a careful physical examination and basic diagnostic tests [2•, 3••]. Initially, thought needs to be given to several possibilities: (a) fecal incontinence masquerading as diarrhea, (b) iatrogenic diarrhea due to drugs, surgery, or therapeutic radiation, (c) chronic infections, and (d) irritable bowel syndrome with diarrhea (IBS-D) for those patients with abdominal pain related to changes in stool form or frequency who meet the published criteria. Most patients with IBS-D have underlying food intolerances, bile acid malabsorption, or small intestinal bacterial overgrowth which can be evaluated with therapeutic trials or further diagnostic testing. If a diagnosis has not been reached after this initial evaluation, most patients should then undergo an evaluation for structural problems with abdominal imaging (CT or MRI scan with enterography) and colonoscopy with mucosal biopsies. If diarrhea still evades diagnosis, comprehensive stool analysis can help to categorize the type of diarrhea and direct further evaluation [4].
The main question of this review is to what extent this diagnostic approach needs to be altered for older patients. The likelihood of having specific problems varies as patients go through life, but there is no sharp age cut-off for disease prevalence. Likewise, there is no precipitous change in gastrointestinal physiology as we age that makes one arbitrary age more relevant to this discussion than another. For purposes of this discussion though, I will define “older persons” as age 65 and above, roughly the last quarter of the average lifespan in economically advantaged countries.
Epidemiology of Chronic Diarrhea in the Elderly
Relatively few population-based epidemiologic studies of chronic diarrhea have been published from US data. Two patient-reported symptom surveys in the general population conducted 25 years apart suggest that diarrhea is common but is reported less often by older respondents than younger individuals [5, 6]. A survey in Olmsted County, MN, focused on elderly community residents aged 65–93 yielded an age- and sex-adjusted prevalence of chronic diarrhea of 14.2% (95% CI, 10.1–18.2%) [7]. A more recent national survey showed prevalences of 9.7% and 9.6% in respondents aged 60–69 and 70+ (95% CI, 6.2–13.2% and 6.3–12.9%, respectively) [1]. These studies suggest that chronic diarrhea may be somewhat more common in the elderly than in the general population. No information is available from these studies about the causes of diarrhea. Despite the widespread use of electronic health records in the USA, there is no good picture of the frequency of specific diagnoses in either younger or older patients with chronic diarrhea. Most information about disease frequency in the USA comes from tertiary centers and is subject to referral bias. Epidemiologic studies from Europe are more inclusive of data from broader populations but are still subject to variations in diagnosis coding.
Incidence and prevalence data also are complicated by the fact that older individuals tend to have more coexisting illnesses and take more medications than younger individuals. For example, the prevalence of diabetes increases in the elderly. Diabetes has been associated with an increased prevalence of diarrhea (11.2% vs. 6.0% in non-diabetics, p < 0.0001) [8]. In any given individual, it may be difficult to attribute diarrhea to diabetes or one of its complications, such as small intestinal bacterial overgrowth, pancreatic exocrine insufficiency, celiac disease, and autonomic neuropathy. Just by being older, elderly individuals will have had more time to develop chronic diseases and may be taking medications which may be associated with diarrhea. In addition, different diagnoses may become more common as patients age, making the prior probabilities of specific diagnoses different than in younger individuals.
All of this makes it difficult to assign accurate pretest probabilities for various problems that might be causing diarrhea in older patients. Currently, clinicians rely on their “gut feelings” about the likelihood of specific problems and use this to inform their selection of diagnostic tests in individual patients. In the future, computer-assisted algorithms will depend on more accurate assessments of prior probabilities and the operating characteristics of diagnostic tests. This information will have to come from population-based sources, such as interoperable electronic health records.
Conditions which Seem to Be More Likely Causes of Diarrhea in Older Patients
Table 1 lists the different categories of diarrhea by stool characteristics and highlights those conditions under each category which seem to be more common in older individuals. For the reasons stated above, precise prevalence data are unavailable for most of these. This list also does not take into account coexisting conditions which may make diarrhea more or less likely to occur.
Osmotic Diarrhea
Patients with watery diarrhea due to ingestion of poorly absorbed substances are said to have osmotic diarrhea [4]. While ingestion of these materials in sufficient amounts will cause diarrhea at any age, some poorly absorbed substances are more likely to be ingested by older individuals. For example, many older adults are advised to take calcium and magnesium supplements to try to prevent osteopenia. There may be sufficient magnesium in the dose ingested to cause diarrhea. Similarly, many older individuals take liquid medications to avoid swallowing a pill. Many of these liquid medications include sorbitol as a sweetener; this too can induce diarrhea if taken in large enough quantities.
Dietary components also may contribute to osmotic diarrhea. The best example of this is lactose intolerance in patients with lactase deficiency. Juvenile mammals depend on mucosal lactase activity to digest and absorb lactose in milk. Most mammals decrease lactase activity after weaning when it is no longer needed. Humans tend to have down-regulation of lactase activity toward the end of adolescence except for those who have inherited a mutation for lactase persistence [9, 10]. These individuals can continue to consume lactose into adult life. Even in this group, however, lactase activity may decrease in time, and previously tolerated amounts of lactose may now produce excess flatus and osmotic diarrhea. Older individuals are more likely than younger individuals to have this age-related reduction in lactase activity. Enteral feeding is another way in which poorly absorbed substances may enter the alimentary tract; it to may be associated with diarrhea in some patients [11].
Secretory Diarrhea
Secretory diarrhea has a broad differential diagnosis, but relatively few of these conditions are particularly likely to happen in older individuals. Medications are a frequent cause of secretory diarrhea, and it is important to assess each patient’s exposure to potential drug-induced diarrhea by reviewing the medication list [12, 13]. Because of the increasing likelihood of concurrent diseases as patients age, the number of medications consumed may go up with time making this a more likely possibility.
Despite its name (which might suggest an inflammatory type of diarrhea), microscopic colitis usually presents with a watery, secretory diarrhea. Microscopic colitis has been attributed to various medications and immune phenomena but frequently occurs without a remediable cause [14•, 15]. Epidemiologic studies from Sweden and Olmsted County show a marked increase in the incidence and prevalence of microscopic colitis in the seventh and eighth decades of life [16, 17]. Anecdotal experience in referral centers suggests that microscopic colitis is among the most common causes of secretory diarrhea in the elderly. The frequency with which it is found in older individuals makes it important to obtain colonoscopic biopsies in elderly patients being evaluated for chronic diarrhea.
Fatty Diarrhea
Several conditions causing malabsorption may occur more frequently in the elderly [18, 19]. The most common presentation of Whipple’s disease, infection of the gut with Tropheryma whipplei, is in older patients with weight loss, fatty stools, and systemic manifestations (e.g., arthritis, heart disease, neuropathy) [20]. Two other rare syndromes producing malabsorption in older patients are autoimmune enteropathy and drug-induced enteropathy (typically due to ingestion of angiotensin II receptor blockers, such as olmesartan) [21, 22]. Celiac disease does not appear to be more common in older individuals but can present for the first time at an advanced age and needs to be considered [23]. Another condition typically affecting older patients and sometimes producing fatty diarrhea is chronic mesenteric ischemia, although the weight loss in this condition may be more due to food avoidance than to malabsorption [24]. These conditions typically produce histologic changes in the small bowel mucosa and point out the need for obtaining small intestinal biopsies in older patients presenting with fatty diarrhea.
Not all conditions producing steatorrhea are associated with mucosal changes, however. In the absence of underlying diseases, aging does not produce enough reduction in exocrine pancreatic secretion to produce steatorrhea [25]. It is conceivable that aging-associated problems like diabetes may produce exocrine pancreatic insufficiency and thus the prevalence of pancreatic insufficiency may be higher in older patients than in younger patients. The course of chronic pancreatitis may differ from that in younger patients with more pseudocysts, more pancreatic exocrine insufficiency, and less abdominal pain [26]. Pancreatic insufficiency should be part of the differential diagnosis of steatorrhea, and imaging of the pancreas, functional testing, or a therapeutic trial of pancreatic enzyme replacement therapy may be part of the diagnostic evaluation.
One condition causing steatorrhea that probably is more common in older individuals is small intestinal bacterial overgrowth (SIBO) [27, 28•]. This may occur as a result of drugs inhibiting gastric acid secretion (e.g., proton pump inhibitors), structural abnormalities (e.g., jejunal diverticulosis or previous gastric surgery), or motility disorders of the upper gastrointestinal tract which may inhibit clearance of luminal bacteria (e.g., scleroderma). Since many of these underlying causes are more likely to occur in the elderly, SIBO should be a consideration in older patients presenting with steatorrhea. Frail elders may be at special risk [29]. Quantitative culture of small bowel contents or breath testing (typically with glucose) can be used to confirm or lend credence to this diagnosis [28•].
Inflammatory Diarrhea
Although there may be a second peak in incidence of inflammatory bowel disease (IBD) in older patients, most patients with ulcerative colitis or Crohn’s disease present at a younger age. Unfortunately, when IBD does occur in older individuals, it often has a severe course. Anyone with chronic diarrhea who has blood and/or pus in stool should be evaluated for IBD with colonoscopy regardless of age [30,31,32]. In addition to IBD, colonoscopy in patients with inflammatory diarrhea may reveal other problems that occur in older patients with increased prevalence, such as malignancy or radiation enteritis.
Invasive infections can cause inflammatory diarrhea, but most of these present as acute illnesses in young and old alike [33, 34]. One exception is recurrent Clostridioides difficile infection, which seems to occur predominantly in older individuals who may have senescent immune systems [35, 36]. This infection occurs both in institutional and free-living settings and is an important consideration in elderly patients in whom the risk of mortality is higher than in younger patients. Initial diagnosis of C. difficile typically involves a screening test, such as a glutamate dehydrogenase assay (GDH) or nucleic acid amplification test (NAAT), with positive results evaluated with a second stage test (sensitive enzyme immunoassay for C. difficile toxin A/B or toxigenic culture). Other potential causes for loose stools, such as medications or laxative use, should be excluded by history before ordering testing for C. difficile, and only liquid stools should be sent for assay. Diagnosis of recurrence is tricky; organisms and spores may be excreted for weeks after the infection has subsided. Very sensitive tests, such as multiplex PCR testing, may remain positive for weeks. Confirmation of recurrence should involve toxin testing or toxigenic culture. Several recent guidelines address the management of recurrent C. difficile infection [37••, 38].
IBS-D and Functional Diarrhea in the Elderly
Older patients with chronic diarrhea may meet Rome IV criteria for IBS-D or functional diarrhea [39••]. Epidemiologic studies suggest that the incidence (new cases) of these diagnoses is lower in older individuals than in younger patients [40]. Moreover, plausible alternative diagnoses may be more common than in younger individuals. In my opinion, this alters the equipoise of the standard advice to make a diagnosis of IBS-D based on history alone and proceed with treatment with minimal or no evaluation. To my knowledge, this has not been studied scientifically as yet in older subjects; it should be. Clinicians should keep an open mind toward the further evaluation of chronic diarrhea in older patients and not settle on IBS-D as a default diagnosis.
Scheme for Evaluating Chronic Diarrhea in the Elderly
Although the pretest probabilities of specific diagnoses may be different in the elderly than in younger adults, I think that the same diagnostic approach previously advocated for adults with chronic diarrhea will lead to an expeditious diagnosis and specific treatment for most elderly patients [3••].
It starts with a comprehensive history, thoughtful physical examination including digital rectal examination, and simple laboratory evaluation (complete blood count, comprehensive metabolic profile, C-reactive protein level, fecal occult blood test, fecal lactoferrin (or calprotectin or smear for white blood cells), and microbiology studies). The clinician then should consider one of five different possibilities: (a) fecal incontinence, (b) iatrogenic diarrhea, (c) chronic infections, (d) IBS-D, or (e) everyone else with chronic diarrhea, including those in the other categories who have not improved with empiric therapy.
Fecal incontinence is a prevalent problem in older individuals. It comes up in this context because most patients consider incontinence to be a manifestation of severe diarrhea and use the term “diarrhea” when describing fecal incontinence. In point of fact, incontinence usually is related to malfunctions of the nerves and muscles that mediate continence and is present in approximately 5% of elderly subjects [41]. Being clear about what the patient means when they complain of diarrhea, asking specifically about the accidental passage of stool, and assessing continence dynamics with a digital rectal examination are the most straightforward ways to assess this possibility. One important problem to recognize in institutionalized elders is overflow incontinence related to fecal impaction; digital rectal examination can detect impaction if present [42]. If fecal incontinence seems likely, assessment of anorectal dynamics with anorectal manometry may be helpful, and biofeedback training may mitigate the problem.
Iatrogenic diarrhea due to drug therapy, previous surgery, and radiation therapy also may be more common in the elderly, but its exact prevalence is not known. Older people accrete more chronic illnesses over time that may be treated with drugs. Obviously, a careful history including a review of the medication list can be an important clue. A surprisingly large number of drugs have been associated with diarrhea as a side effect [12, 13]. The only way to confirm drug therapy as a cause for diarrhea is to discontinue the medication and see if diarrhea goes away. Rechallenge with the potentially offending agent can strengthen the association. If the drug is essential and cannot be stopped, reducing the dosage or adding a nonspecific antidiarrheal drug may improve symptoms.
Gastrointestinal surgeries, including bariatric surgery, bowel resection, and cholecystectomy, have been associated with diarrhea in some patients [43]. Again, older individuals are more likely than younger patients to have had surgeries because of their longer lifespans. Several different mechanisms may cause diarrhea postoperatively, including reduced fluid and electrolyte absorptive capacity, bile acid malabsorption, SIBO, and altered motility. Defining the mechanism for postsurgical diarrhea may help with treatment. Therapy with opioid antidiarrheal agents may optimize absorption by allowing more time for absorption to take place by slowing motility [44]. Bile acid binders may help some patients with relatively small ileal resections by reducing intracolonic bile acid concentrations below the cathartic threshold, and antibiotics may suppress SIBO [28•, 45]. The extent to which diagnostic tests for these mechanisms are needed versus therapeutic trials depends on the utility of the diagnostic specific tests available to the clinician. Radiation therapy may produce ideal dysfunction or frank radiation enterocolitis which often is treated empirically with bile acid binders or opioid antidiarrheal drugs [46].
Infections usually cause acute diarrhea which subsides within a few days or weeks. Some agents can produce chronic diarrhea and should be considered in any patient presenting with diarrhea. Traditional culture and microscopy techniques can be applied, but newly developed multiplex PCR testing can identify most enteric pathogens within a few hours with a single test. As mentioned before, results of PCR testing need to be interpreted with care, however, because the tests are so sensitive [47]. PCR evidence of pathogens in the stool may reflect only transient carriage and may persist for weeks after clinical evidence of infection has subsided. When the infection is the cause of chronic diarrhea, antibiotic therapy usually is needed.
The fourth possibility to consider after the initial assessment is IBS-D or functional diarrhea. It is important that patients meet the published criteria for IBS-D before assigning this diagnosis [39••]. New onset IBS-D may be less common in the elderly than in younger patients, but it does occur. The diagnostic thought process should not stop with a diagnosis of IBS-D. Most individuals with IBS-D have one of three underlying diagnoses: food intolerances, bile acid malabsorption, or SIBO (usually without steatorrhea) [3••]. The relative frequencies of these disorders may be different in older patients with IBS-D, but this has not been studied. A food and symptom diary can help identify potential food intolerances and direct elimination diets; alternatively, a therapeutic trial of a low FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet may be tried [48•]. Diagnostic testing for bile acid malabsorption with a SeHCAT retention test (where available) or serum C4 level may be helpful in identifying bile acid malabsorption as a mechanism for IBS-D, but a therapeutic trial of a bile acid binder may be a more direct strategy in most settings [45, 49•]. SIBO can be assessed with the quantitative culture of small bowel contents or breath hydrogen testing with a variety of substrates [28•]. Alternatively, patients can receive a therapeutic trial of antibiotics targeted at SIBO. Antibiotic therapy can mitigate diarrhea due to SIBO but does nothing to address the underlying cause of SIBO, and so recurrence is likely.
Patients not falling into any of the four categories and those that do not respond to treatment for the suspected cause need further evaluation. This stage begins with additional laboratory tests (if not previously done), including testing for celiac disease (IgA anti-tissue transglutaminase and total IgA level) and a qualitative or quantitative fecal fat test. The next step is to examine the structure of the gastrointestinal tract with CT or MRI imaging with enterography and colonoscopy with biopsies. Upper gastrointestinal tract endoscopy with small bowel biopsy also should be done, if steatorrhea is present. These studies will identify many potential causes of diarrhea, including inflammatory bowel disease, microscopic colitis, malignancies, fistulas, mucosal diseases, and pancreatic disease.
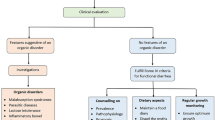
If no structural problems are identified, more clues need to be assembled. This can be done by quantitative chemical analysis of stool (stool electrolytes, pH, osmolality, fat excretion) [4]. Along with testing for fecal leukocytes and red blood cells, these tests can be used to categorize diarrhea into one of three types: watery (with subtypes of osmotic and secretory diarrhea), inflammatory, and fatty. Each category has a more restricted differential diagnosis than the whole and a suite of tests that can lead to a diagnosis (Fig. 1).
Chronic diarrhea workup by type. Classification by stool characteristics limits differential diagnosis and directs further diagnostic testing. In most cases, this allows the establishment of a likely diagnosis and initiation of effective therapy (from: Schiller LR. Chronic diarrhea in the older adult. In: Geriatric Gastroenterology, 2nd ed., C. S. Pitchumoni and T. S. Dharmarajan, eds. Springer-Verlag, New York, in press)
Conclusions
Chronic diarrhea in the elderly presents a challenging differential diagnosis which may differ some from that in younger individuals, particularly in the pretest probabilities of specific diagnoses. Epidemiological studies should be conducted to better define the prevalence and pretest probabilities of conditions causing chronic diarrhea in older patients. The evaluation of older patients with chronic diarrhea can follow the same staged diagnostic approach advocated for other adults with a good likelihood that a diagnosis can be reached. IBS-D may be less common in older patients than in younger individuals. The canny clinician should look beyond a diagnosis of IBS-D in older patients with chronic diarrhea; often the diagnosis will prove to be something else.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Singh P, Mitsuhashi S, Ballou S, Rangan V, Sommers T, Cheng V, et al. Demographic and dietary associations of chronic diarrhea in a representative sample of adults in the United States. Am J Gastroenterol. 2018;113(4):593–600.
• Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: diagnosis and management. Clin Gastroenterol Hepatol. 2017;15(2):182–93. A series of issues that frame the evaluation and management of chronic diarrhea.
•• Schiller LR. Evaluation of chronic diarrhea and irritable bowel syndrome with diarrhea in adults in the era of precision medicine. Am J Gastroenterol. 2018;113(5):660–9. Basic approach to evaluation of chronic diarrhea in adults.
Steffer KJ, Santa Ana CA, Cole JA, Fordtran JS. The practical value of comprehensive stool analysis in detecting the cause of idiopathic chronic diarrhea. Gastroenterol Clin N Am. 2012;41(3):539–60.
Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Grant Thompson W, et al. U. S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38(9):1569–80.
Almario CV, Ballal ML, Chey WD, Nordstrom C, Khanna D, Spiegel BMR. Burden of gastrointestinal symptoms in the United States: results of a nationally representative survey of over 71,000 Americans. Am J Gastroenterol. 2018;113(11):1701–10.
Talley NJ, O’Keefe EA, Zinsmeister AR, Melton LJ 3rd. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102(3):895–901.
Sommers T, Mitsuhashi S, Singh P, Hirsch W, Katon J, Ballou S, et al. Prevalence of chronic constipation and chronic diarrhea in diabetic individuals in the United States. Am J Gastroenterol. 2019;114(1):135–42.
Bayless TM, Brown E, Paige DM. Lactase non-persistence and lactose intolerance. Curr Gastroenterol Rep. 2017;19(5):23. https://doi.org/10.1007/s11894-017-0558-9.
Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):738–46.
Buchman AL. Intestinal failure and rehabilitation. Gastroenterol Clin N Am. 2018;47(2):327–40.
Philip NA, Ahmed N, Pitchumoni CS. Spectrum of drug-induced chronic diarrhea. J Clin Gastroenterol. 2017;51(2):111–7.
Abraham BP, Sellin JH. Drug-induced, factitious, & idiopathic diarrhoea. Best Pract Res Clin Gastroenterol. 2012;26(5):633–48.
• Pardi DS. Diagnosis and management of microscopic colitis. Am J Gastroenterol. 2017;112(1):78–85. Review of a common cause of chronic diarrhea in older patients.
Gentile N, Yen EF. Prevalence, pathogenesis, diagnosis, and management of microscopic colitis. Gut Liver. 2018;12(3):227–35.
Bergman D, Clements MS, Khalili H, Agréus L, Hultcrantz R, Ludvigsson JF. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther. 2019;49(11):1395–400.
Gentile NM, Khanna S, Loftus EV Jr, et al. The epidemiology of microscopic colitis in Olmsted County from 2002–2010: a population-based study. Clin Gastroenterol Hepatol. 2014;12(5):838–42.
Holt PR. Intestinal malabsorption in the elderly. Dig Dis. 2007;25:144–50.
Schiller LR. Diarrhea and malabsorption in the elderly. Gastroenterol Clin N Am. 2009;38(3):481–502.
Marth T. Tropheryma whipplei, immunosuppression and Whipple’s disease: from a low-pathogenic, environmental infectious organism to a rare, multifaceted inflammatory complex. Dig Dis. 2015;33(2):190–9.
Ahmed Z, Imdad A, Connelly JA, Acra S. Autoimmune enteropathy: an updated review with special focus on stem cell transplant therapy. Dig Dis Sci. 2018;64:643–54. https://doi.org/10.1007/s10620-018-5364-1.
Ebrahim VS, Martin J, Murthy S, Odstrcil E, Huang H, Polter D. Olmesartan-associated enteropathy. Proc (Baylor Univ Med Cent). 2017;30(3):348–50.
Collin P, Vilppula A, Luostarinen L, Holmes GKT, Kaukinen K. Review article: coeliac disease in later life must not be missed. Aliment Pharmacol Ther. 2018;47(5):563–72.
Sherid M, Samo S, Sulaiman S, et al. Comparison of ischemic colitis in the young and the elderly. WMJ. 2016;115(4):196–202.
Löhr JM, Panic N, Vujasinovic M, Verbeke CS. The ageing pancreas: a systematic review of the evidence and analysis of the consequences. J Intern Med. 2018;283(5):446–60.
Hirth M, Härtel N, Weiss C, Hardt P, Gubergrits N, Ebert MP, et al. Clinical course of chronic pancreatitis in elderly patients. Digestion. 2019;10:1–8. https://doi.org/10.1159/000494349.
Quigley EMM. The spectrum of small intestinal bacterial overgrowth (SIBO). Curr Gastroenterol Rep. 2019;21(1):3. https://doi.org/10.1007/s11894-019-0671-z.69.
• Rezaie A, Pimentel M, Rao SS. How to test and treat small intestinal bacterial overgrowth: an evidence-based approach. Curr Gastroenterol Rep. 2016;18(2):8. https://doi.org/10.1007/s11894-015-0482-9. Review of the evaluation and management of SIBO in adults.
Mitsui T, Shimaoka K, Goto Y, Kagami H, Kinomoto H, Ito A, et al. Small bowel bacterial overgrowth is not seen in healthy adults but is in disabled older adults. Hepatogastroenterology. 2006;53(67):82–5.
van Walree IC, van Tuyl SA, Hamaker ME. Late-onset inflammatory bowel disease in the very elderly. Neth J Med. 2015;73(1):4–9.
Butter M, Weiler S, Biedermann L, Scharl M, Rogler G, Bischoff-Ferrari HA, et al. Clinical manifestations, pathophysiology, treatment and outcome of inflammatory bowel diseases in older people. Maturitas. 2018;110:71–8.
John ES, Katz K, Saxena M, Chokhavatia S, Katz S. Management of inflammatory bowel disease in the elderly. Curr Treat Options Gastroenterol. 2016;14(3):285–304.
White AE, Clampa N, Chen Y, et al. Characteristics of Campylobacter, Salmonella infections and acute gastroenteritis in older adults in Australia, Canada, and the United States. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciy1142.
Jump RLP, Crnich CJ, Mody L, Bradley SF, Nicolle LE, Yoshikawa TT. Infectious diseases in older adults of long-term care facilities: update on approach to diagnosis and management. J Am Geriatr Soc. 2018;66(4):789–803.
Asempa TE, Nicolau DP. Clostridium difficile infection in the elderly: an update on management. Clin Interv Aging. 2017;12:1799–809.
Donskey CJ. Clostridium difficile in older adults. Infect Dis Clin N Am. 2017;31(4):743–56.
•• McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987–94. https://doi.org/10.1093/cid/ciy149. Current consensus viewpoint on the management of C. difficile.
Ooijevaar RE, van Beurden YH, Terveer EM, Goorhuis A, Bauer MP, Keller JJ, et al. Update of treatment algorithms for Clostridium difficile infection. Clin Microbiol Infect. 2018;24(5):452–62.
•• Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150:1393–407. Rome IV definitions of functional bowel disorders, including IBS-D.
Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80.
Yu SW, Rao SS. Anorectal physiology and pathophysiology in the elderly. Clin Geriatr Med. 2014;30(1):95–106.
Serrano Falcón B, Barceló López M, Mateos Muñoz B, Álvarez Sánchez A, Rey E. Fecal impaction: a systematic review of its medical complications. BMC Geriatr. 2016;16:4. https://doi.org/10.1186/s12877-015-0162-5.
Borbély YM, Osterwalder A, Kröll D, et al. Diarrhea after bariatric procedures: diagnosis and therapy. World J Gastroenterol. 2017;23(26):4689–700.
Schiller LR. Antidiarrheal drug therapy. Curr Gastroenterol Rep. 2017;19(5):18. https://doi.org/10.1007/s11894-017-0557-x.
Camilleri M. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015;9(3):332–9.
Teo MTW, Sebag-Montefiore D, Donnellan CF. Prevention and management of radiation-induced late gastrointestinal toxicity. Clin Oncol. 2015;27:656–67.
Zboromyrska Y, Vila J. Advanced PCR-based molecular diagnosis of gastrointestinal infections: challenges and opportunities. Expert Rev Mol Diagn. 2016;16(6):631–40.
• Schumann D, Klose P, Lauche R, Dobos G, Langhorst J, Cramer H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24–31. Systematic review of the impact of the low FODMAPs diet in IBS.
• Vijayvargiya P, Camilleri M. Update on bile acid malabsorption: finally ready for prime time? Curr Gastroenterol Rep. 2018;20(3):10. https://doi.org/10.1007/s11894-018-0615-z. This review focuses on bile acid malabsorption as a common mechanism of chronic diarrhea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lawrence Schiller reports personal fees from Allergan, Ardelyx, Ironwood, Salix/Bausch, Shire, Commonwealth Laboratories, Prometheus Laboratories, Abbvie and Romark, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gastroenterology in Geriatric Patients
Rights and permissions
About this article
Cite this article
Schiller, L.R. Chronic Diarrhea Evaluation in the Elderly: IBS or Something Else?. Curr Gastroenterol Rep 21, 45 (2019). https://doi.org/10.1007/s11894-019-0714-5
Published:
DOI: https://doi.org/10.1007/s11894-019-0714-5