Abstract
The growth and sustainability of freshwater aquaculture are highly dependent on economic feed which is the major running cost. Fish feed industries depend on the high-priced fish meal (FM) as protein source in feed formulations. In this context, a nutrient-rich, and palatable insect meal–based fish feed was developed incorporating the black soldier fly (BSF) (Hermetia illucens) prepupae meal (BSFPM) reared on organic waste imparting additional benefit of waste bioremediation to make cost-effective feed. Feeding trial was conducted to evaluate growth performance on monosex Nile tilapia (Oreochromis niloticus). The different treatments were (1) dried BSF prepupae, (2) BSF prepupae and BSFPM-based feed in 1:1 proportion, (3) BSFPM feed, and (4) control feed with FM. The survival, growth, feed efficiency, and haematological parameters were not significantly different between BSFPM and control feed. Fish fed with control feed and BSFPM feed showed significantly higher (P ≤ 0.05) weight gain, specific growth rate, and percentage weight gain. Lowest food conversion ratio (P ≤ 0.05) was recorded for fish fed control feed with a significantly higher feed efficiency ratio (0.65d ± 0.034) and protein efficiency ratio (2.11a ± 0.063). The mean corpuscular volume of blood in fish fed BSF prepupae (128.5a ± 3.2) is significantly higher. The good growth of fish fed BSFPM feed may be attributed to the essential amino acids which are not limiting in feed. Absence of microbes and safe level heavy metals in BSFPM feed ensures safety of the ingredient. Hence, it can be used as a suitable protein source in feed formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of the aquaculture sector is highly dependent on the nutritionally balanced fish feed which is a major recurring cost. The major protein component used in fish feed formulations is the fish meal and the aquaculture profitability is affected by the high cost of fish meal. Overexploitation of fish stocks for fish meal affects the sustainability of these resources in the wild. Hence, an alternative ingredient to replace fish meal in aquaculture feed which is economically and environmentally viable is of primary interest worldwide. Insects can be a good alternative as a feed ingredient as they can be reared on organic wastes by sustainable and cost-effective farming methods (Meneguz et al. 2018). The possibility and potential of the exploitation of insects as an animal feed ingredient are being exploited worldwide with promising results (Borgogno et al. 2017; Dumas et al. 2018; Vargas et al. 2018). During the last few years, there have been investigations on the efficacy of insect meal in the formulation of fish feed for several cultivable freshwater fish species. Among several potential insect species which can be utilised as animal feed, particularly the black soldier fly (BSF) (Hermetia illucens) Linnaeus 1758 (Diptera: Stratiomyidae) gained most attention on a commercial scale (Lock et al. 2015) for being a potential source of protein in feed formulations (Cammack and Tomberlin 2017).
The BSF can be cultivated on organic wastes such as agricultural by-products, animal manure (Diener et al. 2011), waste from fin fishes, and shell fishes (Sealey et al. 2011; Villazana and Alyokhin 2019). They convert these wastes to quality protein (Newton et al. 1977; Wang and Shelomi 2017), which consequently act as a means of bioremediation. The BSF can also be reared on home-based food wastes which are usually generated in large quantities in urban areas and are potential environmental pollutants causing human health hazards (Li et al. 2011). Thus, the rearing of BSF larvae is an eco-friendly waste management option (Warburton and Hallman 2002). BSF is reported to breed prolifically in organic wastes, producing protein rich biomass utilising 50% of manure waste (Sheppard et al. 1994). Bio-waste degradation using biological means is an efficient way of waste disposal (Diener et al. 2009) and BSF control the growth of pathogenic bacteria like Salmonella sp. and Escherichia coli during organic waste decay (Lalander et al. 2013).
Considering the nutritional quality of BSFL, it is rich in protein (Cummins et al. 2017), lipids (Li et al. 2016), minerals (Spranghers et al. 2017), and contains chitin (Caligiani et al. 2018). In addition, BSFL possesses an essential amino acid profile, which is comparable to the amino acid profile of fish meal, rendering it most suitable as a fish feed. But they remain rich in saturated fatty acids compared to polyunsaturated fatty acids unlike fish (Barragan-Fonseca et al. 2017). They also have high ash as well as crude protein content than many insects that are used as feed ingredients (Barroso et al. 2014). Hence, to characterise the potentiality of BSFL as a feed and feed ingredient, feeding trials were conducted to evaluate the growth of monosex Nile tilapia on different feed forms of dried BSF pre-pupae with a control excluding BSFL. The potential utilisation of BSFL meal in fish feeds could reduce feed manufacturers’ and fish farmers’ dependency on fishmeal as an alternate protein source which could eventually lead fish farming into an economically and environmentally viable venture. Therefore, the present study evaluated the effect of replacement of fishmeal with BSFL meal on feed efficiency, growth rates, serum biochemistry, and haematology in juvenile Nile tilapia. Haematological and serum biochemical parameters of the fish are indicators to monitor the nutritional effects and health status of the fish. These parameters were evaluated to understand the stress related to nutrition if any. The tilapia fish in particular was selected for evaluating different experimental feeds containing BSF prepupae as it is a widely cultured fish and has an omnivorous feeding habit that accepts a wide selection of food.
Materials and methods
The experiment was conducted following the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 2011). The feeding trial was carried out for a period of 90 days to evaluate the effect of BSFL on the growth performance of tilapia. The experimental fish were procured from a government hatchery in Kerala, India. The experimental setup consisted of 12 glass tanks of 172-L water capacity. During the trial, the temperature averaged 25 ± 2 °C, dissolved oxygen was maintained above 5 mg L−1, and nitrogenous compounds were below 0.02 mgL−1.
The experiment was based on a completely randomised design comprising four treatments with three replicates. The different treatments are detailed in Table 1. Prior to the start of the experiment, the fish were acclimatised for 2 weeks. Fish of average weight (3.98 ± 0.07 g) and length (6.3 ± 0.26 cm) were stocked at the rate of 150 no.m−3. The feeding rate was maintained at 5% of body weight per day. The daily ration was divided into two equal doses a day offered at 09:00 and 16:00 h. Water exchange was carried out once in 2 weeks to ensure the water quality parameters to be in the optimum range for fish growth. Faecal matter accumulated was collected daily before the morning feed. At the termination of the experiment, fish were weighed individually taking representative samples from each tank following 1 day of feed deprivation. The length and weight of the fish were measured every 2 weeks and the amount of feed provided to the fish was adjusted accordingly.
BSF rearing and prepupae production
BSF was reared at ICAR–National Bureau of Agricultural Insect Resources (NBAIR), Bangalore (77°34ʹ E; 13°54ʹ N) using organic wastes. For the rearing, equal proportions (500 g each) of vegetable and fruit wastes collected from the local markets were stored in a rearing box (20 × 20 cm) in the laboratory (60 ± 10% RH, 27 ± 2 °C temperature, 60–70% moisture). About a hundred numbers of 1-day-old BSF larvae were inoculated over the rearing substrate in plastic rearing boxes covered with a black coloured muslin cloth. The substrate was monitored on daily basis for feeding of the larvae till pupation. After completion of the larval stage, the prepupae were manually harvested from the substrate. The harvested prepupae after thorough washing was killed by keeping in refrigerator for 1 day. The dead prepupae was then sun dried for 7 days and then used for feed preparation (Panikkar et al. 2018a). The dried BSF prepupae with a crude protein content of 32.53% and crude lipid of 22.1% was ground to make powder before incorporating in feed. The prepupae development and feeding are graphically represented in Fig. 1.
Analysis of chitin content in dried BSF prepupae
The chitin content in BSF prepupae was analyzed according to Caligiani et al. (2018). The BSF prepupae dried to a constant weight was ground to powder using a mixer. One-molar HCl was added to the powder for demineralisation at an hour interval for 6 h. The demineralised powder was then washed with distilled water, followed by de-proteinisation using 1 M NaOH keeping at 80 °C for 24 h. The de-proteinised extract was then filtered and de-coloured using 1% KMnO4. The de-coloured filtrate was washed with distilled water and dried to constant weight before weighing.
Analysis of amino acid profile of dried BSF prepupae and BSFL diet
The amino acid composition was determined by using high-performance liquid chromatography (HPLC) (1525, Waters) equipped with a C18 reverse-phase column (WAT052885) and a fluorescence detector (2475, Waters) following Ishida et al. (1981) and Sastry and Tammuru (1985).
Analysis of the fatty acid profile of dried BSF prepupae
Fatty acid compositions of the sample were determined by gas chromatography–mass spectrometry (GC–MS). The identification and quantification were done using a GC (Trace GC Ultra, Thermo Scientific) equipped with a capillary column (TR-FAME, 30 m × 0.25 mm, 0.25 μm film thickness) and an MS (ITQ 900, Thermo Scientific) attached to it following the procedure described by Folch et al. (1957), Metcalfe et al. (1966), and Mohanty et al. (2013)
Microbial analysis of BSFL
The microbial analysis of BSF prepupae was carried out. The dried BSF prepupae was ground to powder and 25 g of this powder was added to phosphate buffer to make a 250-mL mixture by blending in a stomacher for homogenous mixing. Aliquots of different dilutions (10−1, 10−2, 10−3, 10−4, 10−5, and 10−6) were prepared using phosphate buffer for enumeration. The presence of Salmonella and Escherichia coli was analysed based on the standard biochemical procedures (Alfrad 2007; Marjan et al. 2014).
Analysis of heavy metals in BSFPM-based feed
The possibility of occurrence of heavy metal in BSFL-incorporated feed was analysed as the BSFL was reared on vegetable waste. The analysis was carried out to estimate the presence of arsenic, cadmium, chromium, lead, nickel, and mercury in the formulated feed. The feed samples were digested (Al-Weher 2008) and analysed for heavy metals using atomic absorption spectrophotometer, and the recovery for each metal was calculated (Uba et al. 2008).
Biochemical analysis of BSFL-incorporated feed and experimental fish
Biochemical analysis of feed and fish was done to estimate the proximate composition following standard methods of AOAC (1995). Moisture, crude protein, ether extract, ash, and total carbohydrate composition of BSFPM feed and fish after the 90 days of feeding trial were estimated. The fish were individually weighed and dried for proximate composition estimation at the termination of the experiment.
Growth parameters of O. niloticus
On the termination of the feeding trial, the fish were counted and weighed to estimate various parameters such as percentage weight gain, specific growth rate (SGR), feed efficiency ratio (FER), protein efficiency ratio (PER), and food conversion ratio (FCR). Feeding was suspended for a day prior to taking the body weight measurement. The growth performance indices were calculated using the following formulae:
Effect of BSFL-based diet on haematology of O. niloticus
The effect of BSF prepupae-based diet on the haematology of O. niloticus was evaluated based on the parameters such as haemoglobin (Hb) content, haematocrit (HCT) value, red blood cell (RBC) count, white blood cell (WBC) count, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC). Blood samples were collected from the caudal vein of the fish, using a 2-mL sterile disposable plastic syringe (23 G) coated with anticoagulant (10% EDTA). The blood was then transferred into vials containing EDTA to avoid clotting and was analysed immediately after collection. The total RBC and WBC counts were estimated using an improved Neubauertype haemocytometer (Pradhan et al. 2014; Guijarro et al. 2003). The number of RBCs/WBCs was expressed as cells per cubic millimetre and was calculated as follows:
The haemoglobin (Hb) content was estimated by acid–haematin method using Sahli’s haemocytometer. The amount of haemoglobin was directly read in g/dL. The haematocrit (Hct) was determined by using microhaematocrit reader and the values were expressed in % (Wintrobe 1974). The erythrocyte indices such as mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC) were calculated using the respective formula (Dacie and Lewis 1994):
Serum biochemistry of O. niloticus
Serum total protein was estimated using the total protein kit (Biuret method) and albumin was estimated using the albumin kit (BCG dye-binding method) of Merck Specialities Pvt Ltd., Mumbai. Globulin was calculated by subtracting the albumin values from total protein. Globulin (g%) = total protein (g%) − albumin (g%).
The albumin globulin (A/G) ratio was calculated by dividing albumin values by globulin values.
Statistical analysis
Data on growth parameters are represented as mean with standard error of the mean. One-way analysis of variance (ANOVA) test using Duncan’s multiple range test (Duncan 1955) was done to compare the means at 95% confidence level (P ≤ 0.05) for growth parameters and proximate body composition of the fish. Statistical package SPSS 16 version was used and a P-value ≤ 0.05 was treated as statistically significant to reject the null hypothesis.
Results
Proximate analysis of the feeds
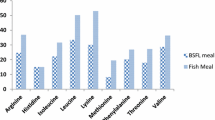
The crude protein content of the BSFPM feed was 29.97% with crude lipid content of 12.9%, moisture 4.47%, ash content 6.54%, and total carbohydrate as 50%. The chitin content was found to be 11.87% in BSF prepupae. The amino acid profile of the BSFPM-based feed (Table 2) showed that the 10 essential amino acids, methionine, arginine, threonine, tryptophan, histidine, isoleucine, lysine, leucine, valine, and phenyalanine are not limiting in the BSFPM-incorporated feed. The fatty acid profile of dried black soldier fly prepupae is depicted in Table 3. The total saturated fatty acid content in black soldier fly prepupae meal was 52.53%, wherein margaric acid and myristic acid dominated the fraction. The monounsaturated fatty acid dominated by oleic acid was 41.31%. The polyunsaturated fatty acids comprised only of linoleic acid which contributed to 6.16% of the total fatty acids.
Microbial and heavy metal analysis in feed
Microbial analysis of BSF prepupae showed the absence of E. coli and Salmonella. In BSFPM feed the heavy metals such as arsenic, cadmium, chromium, lead, nickel, and mercury are well within the safe limits as represented in Table 4.
Feed acceptability, survival, growth rate, and feed efficiency
The fish readily accepted all the feeds and hence no palatability issues were recorded during the study. The survival rate, feed intake, feed efficiency, and growth performances were found to be satisfactory in all the treatments (Table 5). Fish fed with control feed and BSFPM feed showed significantly higher (P ≤ 0.05) final weight and mean weight gain when compared to other treatments. Percentage weight gain recorded significantly highest value (P ≤ 0.05) in fish fed with control feed (725c.51 ± 3.698) and BSFPM feed (706c.69 ± 8.814) compared with other treatments. SGR also showed a similar pattern as that of PWG, with the highest values (P ≤ 0.05) in fish fed with control feed (2.42c ± 0.007) and BSFPM feed (2.39c ± 0.009). The FCR of all the treatments differed significantly (P ≤ 0.05) with the lowest FCR (1.54a ± 0.081) recorded for fish fed with control feed. Likewise FER and PER also differed significantly for treatments with significantly higher FER (0.65d ± 0.034) and PER (2.11a ± 0.063) in fish fed with control feed. The survival rate of fish ranged from 74% (T1) to 86% (T2).
There was no significant difference (P ≥ 0.05) in crude lipid, ash, and total carbohydrate content in fish of different treatments after the feeding trial. But the crude protein content (57.86 ± 0.023) of fish fed with dried BSF prepupae was significantly lower (P ≤ 0.05) than other treatments (Table 6).
The haematological and some of the serum biochemical parameters of T. niloticus are presented in Table 7, which shows that there is no significant difference in the Hb content, HCT value, RBC count, MCH, MCHC, and total WBC count. Hb range from 4.67 ± 0.26 to 4.97 ± 0.78 gm/dL, HCT from 23.03 ± 1.00 to 29.8 ± 1.04%, RBC count from 2.01 ± 0.05 to 2.44 ± 0.19 million/mL, MCH from 20.77 ± 0.64 to 24.87 ± 1.88 pg, and MCHC from 19.47 ± 0.44 to 20.83 ± 0.23 g/100 mL. The total WBC count range from 25,167 ± 593 to 26,367 ± 982 cells/mL. The MCV of T1 (128.5 ± 3.2) is significantly higher than the T3 (111.8 ± 6.9). The effect of different feeds on serum biochemical parameters such as serum total protein, albumin, and A/G ratio was non-significant. While the serum globulin of T1 and T3 were significantly higher than T2 and T4.
Discussion
BSF larva is reported to be a rich source of protein with a well-balanced profile particularly the essential amino acids (Makkar et al. 2014; Spranghers et al. 2017; Wang and Shelomi 2017). However, the proximate composition of BSF larvae varies widely based on the substratum on which they are reared. In our study, the protein content of BSF larvae was found to be 32.53% on dry matter basis which differed from those of earlier reports, ranging from 38.5% when reared on fruits and vegetables to 62.7% when reared on the liver as the culture medium (Nguyen et al. 2015). Caligiani et al. (2018) recorded 32% protein, 37% lipid, and 19% minerals in BSF larvae which are in conjunction with our study. The essential amino acids (EAA) are not lacking in the BSFPM and the formulated feed. Henry et al. (2015) and Liland et al. (2017) have reported that the BSF larvae have an amino acid (AA) profile equivalent to the FM except for methionine and lysine. However, in our study methionine was not lacking and the EAA composition of the pellet feed appears to be adequate with no limiting AA for Nile tilapia as observed in other freshwater fish as reported by Peres and Oliva-Teles (2007) and Magalhaes (2017).
The crude lipid content of 22.1% is well within the range of 6.63% when reared BSFL on restaurant waste (Zheng et al. 2012) and 39.2% on fruit and vegetable wastes (Nguyen et al. 2015). The published reports on proximate composition show large differences within species which is attributed to the substrates on which the insect larvae are reared (Sanchez-Muros et al. 2015).
The BSFL exoskeleton encompasses chitin which is a cell wall component in insects. This can affect the digestibility of the feed and thereby affect the growth of the fish. The chitin content of 11% in the present study was found to be within the acceptable levels in fish feed formulations as reported by Fontes et al. (2019). Tilapia fed insect meal–based feed containing 12% chitin exhibited satisfactory growth (Fontes et al. 2019). The chitin content in BSF prepupae varied from 6 to 7% (Spranghers et al. 2017) to around 9% (Caligiani et al. 2018) on dry matter basis.
Since the BSF is grown on organic wastes, there are chances of microbial contaminations. Hence it is a prerequisite to conduct the microbial analysis of the insect meal to be used as ingredients in feed formulations. The study conducted by Wynants et al. (2019) reported the presence of Salmonella and Bacillus cereus in BSF larvae pointing to the importance of decontamination of the larvae before use in feed formulations. In the present investigation, the microbial analysis of dried BSF prepupae as well as BSFPM-based feed showed the absence of E. coli and Salmonella, making it safe as a feed ingredient. This could be attributed to the resistance of BSFL to ecological parameters and the ability to lessen or destroy microbes (Choi et al. 2012; Jeon et al. 2011) which makes them suitable as a feed ingredient.
Insects are susceptible to toxin accumulation or heavy metal accretion through the feed or water they consume (Marone 2016). Purschke et al. (2017) reported that BSF larvae accumulated heavy metals from the contaminated feed at lower concentrations than the initial substrate concentration, other than for cadmium and lead. This report was later backed by the findings of Shumo et al. (2019) who reported low concentrations of cadmium and lead in BSF larvae and recommended the examination of BSF larvae-incorporated feeds for possible contamination to ensure food safety. The heavy metal analysis of BSFPM-incorporated feed in the present study showed that arsenic, cadmium, chromium, lead, nickel, and mercury are well within the safe limits approved by the EU for heavy metals in fish feed.
The growth performance of the fish was satisfactory with BSFPM feed and there was no disease outbreak during the feeding trials. The experimental and control feed were readily accepted by the fish. The comparative growth analysis showed that the tilapia fed solely on BSF prepupae exhibited poor growth compared to other treatments, and this may be due to the poor digestibility of the chitin content in BSF prepupae. The study conducted by Shakil-Rana et al. (2015) has reported a similar observation in BSFL-fed tilapia fry.
The lowest FCR recorded in fish fed with fish meal feed can be due to the increased digestibility and nutrient profile of the feed especially the fatty acid profile, the polyunsaturated fatty acid which is lacking in BSFP.
In our earlier study on Amur common carp (Cyprinus carpio), it was evident that the fish meal can be substituted up to 75% in formulated feed without interrupting the normal growth of the fish (Panikkar et al. 2018a). Further, Belghit et al. (2019) reported that complete substitution of fish meal with BSF larvae meal in salmon feed did not hamper the feed utilisation and growth performance of sea-water Atlantic salmon. Other studies also showed promising results when tried the BSFL incorporation in feeds for the culture of Psetta maxima (Kroeckel et al. 2012), Salmo salar (Lock et al. 2015), Dicentrarchus labrax (Magalhaes et al. 2017), Danio rerio (Vargas et al. 2018), and Sparus aurata (Karapanagiotidis et al. 2014).
The proximate composition analysis revealed that the crude protein content of fish fed only dried BSF prepupae was lower than other treatments. The body protein content in African catfish was not affected when replaced the fish meal with feather meal, maggot meal, and chicken offal meal but with significantly higher lipid (Adewolu et al. 2010). Lipid composition in BSFL is characterised by the high level of saturated fatty acids as reported by Liland et al. (2017) and St-Hilaire et al. (2007). However, there was no difference in crude lipid content of the fish on the completion of the feeding trials of the present study. Belghit et al. (2019) also reported similar findings in Salmo salar with no effect on whole body protein, lipid, as well as amino acid composition when fed with BSFL-incorporated feed replacing fish meal. The ash and carbohydrate composition also had no significant difference when compared with other treatments. Goda et al. (2007) reported no significant differences in whole-body proximate composition (gross energy and ash content) of African catfish on replacement of the fish meal in feed with other protein sources, which shows that the protein source of feed has not much influence on the protein deposition in the animal. The level of protein and lipid in BSFL can vary depending on the rearing medium as reported by Tschimer and Simon (2015), while the AA profile is not too dependent on the substrate (Spranghers et al. 2017).
Haematology and blood biochemistry are used as indicators of the health of an organism (Panikkar et al. 2018b; Manna et al. 2021). Among other extrinsic factors, diet can also affect the haematological indices as reported by Rao et al. (2015). In the present study, there is no significant difference in Hb content, HCT value, MCH, MCHC, RBC, and total WBC counts of the experimental fish which shows that the different diets have not caused any nutrition-related stress. As reported by Javed et al. (2016), a high MCV value in Channa punctatus is attributable to the macrocytic condition due to heavy metal exposure. In our study, the MCV values of the fish fed the BSFPM diet are non-significant with the control diet indicating the absence of any macrocytic condition. Further, the Hb, HCT, RBC count, MCH, MCHC values are similar to the values reported by Bittencourt et al. (2003) in O. niloticus grown under semi-intensive culture systems. These values are also in consistent with the TEC, Hb, and HCT values in Barbodes carnaticus as reported by Panikkar et al. (2018b) from a river system in India. The increase in WBC of fish indicates an alteration in defence mechanism as a protective response against stress (Das 1998). However, the WBC counts of BSFPM diet and control diet were comparable indicating the absence of stress related to malnutrition or other chance variables. The higher serum globulin of fish fed exclusively on BSF prepupae and the mixed diet of BSF prepupae and BSFPM feed may be associated with a stronger innate immune response in fish (Wiegertjes et al. 1996).
Conclusions
Observations from the present investigation augment well for the utilisation of BSF prepupae meal as a cost-effective protein source in formulation of freshwater fish feeds. At present, utilisation of BSFL as fish feed is being tried globally on experimental scale. Research needs to be focussed on effective utilisation of this detritivorous insect which can replace the feed ingredients in limited supply. This would enable environment friendly and sustainable way of waste reduction as well as reducing the feed cost in aquaculture industry. Inclusion of cost-effective, eco-friendly, and locally available feed ingredients in freshwater fish feed formulations could support the sustainable intensification of aquaculture practices contributing to the nutritional security.
Data availability
Data can be made available to researchers on genuine request to improve science.
References
Adewolu MA, Ikenweiwe NB (1822) Mulero SM (2010) Evaluation of an animal protein mixture as a replacement for fishmeal in practical diets for fingerlings of Clarias gariepinus (Burchell. Isr J Aquacult Bamid 62(4):237–244
Alfrad EB (2007) Bensons microbiological applications. McGraw-Hill Book Company, New York
Al-Weher SM (2008) Levels of heavy metal Cd, Cu and Zn in three fish species collected from the Northern Jordan Valley, Jordan. Jordan J Biol Sci 1:41–46
AOAC (1995) Official methods of analysis of AOAC International, Vol. 1, 16thedn. (ed. Cunniff, P. A.). AOAC International, Arlington, USA
Barragan-Fonseca KB, Dicke M, van Loon JJA (2017) Nutritional value of the black soldier fly (Hermetia illucens L) and its suitability as animal feed–a review. J Insects Food Feed 3(2):105–120. https://doi.org/10.3920/JIFF2016.0055
Barroso FG, Haro C, Sanchez-Muros MJ, Venegas E, Martinez-Sanchez A, Perez-Banon C (2014) The potential of various insect species for use as food for fish. Aquaculture 422:193–201. https://doi.org/10.1016/j.aquaculture.2013.12.024
Belghit I, Liland NS, Gjesdal P, Biancarosa I, Menchetti E, Li Y, Waagbo R, Krogdahl A, Lock E (2019) Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 503(30):609–619. https://doi.org/10.1016/j.aquaculture.2018.12.032
Bittencourt NDR, Molinari LM, de Oliveira DS, Pedroso RB, Nakamura CV, Ueda-Nakamura T, de Abreu FAB, Filho BPD (2003) Haematological and biochemical values for Nile tilapia Oreochromis niloticus cultured in semi-intensive system. Acta Scientiarum Biol Sci Maringa 25(2):385–389
Borgogno M, Dinnella C, Iaconisi V, Fusi R, Scarpaleggia C, Schiavone A, Monteleone A, Gasco L, Parisi G (2017) Inclusion of Hermetia illucens larvae meal on rainbow trout (Oncorhynchus mykiss ) feed: effect on sensory profile according to static and dynamic evaluations. J Sci 97(10):3402–3411. https://doi.org/10.1002/jsfa.8191
CaligianiA MA, Leni G, Baldassarre S, Maistrello L, Dossena A, Sforza S (2018) Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res Int 105:812–820. https://doi.org/10.1016/j.foodres.2017.12.012
Cammack JA, Tomberlin JK (2017) The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L) (Diptera: Stratiomyidae). Insects 8:56. https://doi.org/10.3390/insects8020056
Choi W, Yun J, Chu J, Chu K (2012) Antibacterial effect of extracts of Hermetia illucens (Diptera: Stratiomyidae) larvae against Gram-negative bacteria. Entomol Res 42:219–226. https://doi.org/10.1111/j.1748-5967.2012.00465.x
Cummins VC, Rawles SD, Thompson KR, Velasquez A, Kobayashi Y, Hager J, Webster CD (2017) Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for pacific white shrimp (Litopenaeus vannamei). Aquaculture 473:337–344. https://doi.org/10.1016/j.aquaculture.2017.02.022
Dacie JV, Lewis SM (1994) Practical Haematology, 8th ed. pp. 49–82. Longman Group Ltd, Hong Kong
Das BK (1998) Studies on the effects of some pesticides and commonly used chemicals on Indian major carp and their ecosystem. Dissertation, Orissa University of Agriculture and Technology
Diener S, Zurbrugg C, Tockner K (2009) Conversion of organic material by black soldier fly larvae: establishing optimal feeding rates. Waste Manag Res 27(6):603–610. https://doi.org/10.1177/0734242X09103838
Diener S, Solano NMS, Gutierrez FR, Zurbrugg C, Tockner K (2011) Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valoriz 2:357–363. https://doi.org/10.1007/s12649-011-9079-1
Dumas A, Raggi T, Barkhouse J, Lewis E, Weltzien E (2018) The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 492:24–34. https://doi.org/10.1016/j.aquaculture.2018.03.038
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemist 226:497–509
Fontes TV, de Oliveira KRB, Almeida ILG, Orlando TM, Rodrigues PB, Costa DV, Rosa PV (2019) Digestibility of insect meals for Nile tilapia fingerlings. Animals 9:181. https://doi.org/10.3390/ani9040181
Goda AM, El-Haroun ER, Kabir-Chowdhury MA (2007) Effect of totally or partially replacing fish meal by alternative protein sources on growth of African catfish Clarias gariepinus (Burchell, 1822) reared in concrete tanks. Aquac Res 38(3):279–287. https://doi.org/10.1111/j.1365-2109.2007.01663.x
Guijarro AI, Lopez-Patino MA, Pinillos ML, Isorna E, Alonso-Gomez AL, Alonso-Bedate M, Delgado MJ (2003) Seasonal changes in haematology and metabolic resources in the tench. J Fish Biol 62:803–815. https://doi.org/10.1046/j.1095-8649.2003.00066.x
Henry M, Gasco L, Piccolo G, Fountoulaki E (2015) Review on the use of insects in the diet of farmed fish: past and future. Anim Feed Sci Technol 203:1–22. https://doi.org/10.1016/j.anifeedsci.2015.03.001
Ishida Y, Fujita T, Arai K (1981) New detection and separation methods for amino acid by high performance liquid chromatography. J Chromatogr 204:143–148. https://doi.org/10.1016/s0021-9673(00)81650-7
Javed M, Ahmad I, Ahmad A, Usmani N, Ahmad M (2016) Studies on the alterations in haematological indices, micronuclei induction and pathological marker enzyme activities in Channa punctatus (spotted snakehead) perciformes, channidae exposed to thermal power plant effluent. Springer plus 5(1):761. https://doi.org/10.1186/s40064-016-2478-9
Jeon H, Park S, Choi J, Jeong G, Lee S, Choi Y, Lee SJ (2011) The intestinal bacterial community in the food waste-reducing larvae of Hermetia illucens. Curr Microbiol 62:1390–1399. https://doi.org/10.1007/s00284-011-9874-8
Karapanagiotidis IT, Daskalopoulou E, Vogiatzis I, Rumbos C, Mente E, Athanassiou CG (2014) Substitution of fishmeal by fly Hermetia illucens prepupae meal in the diet of gilthead seabream (Sparus aurata). Hydromedit, Volos, Greece 2014:110–114
Kroeckel S, Harjes AGE, Roth I, Katz H, Wuertz S, Susenbeth A, Schulz C (2012) When a turbot catches a fly: evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute - Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 364:345–352. https://doi.org/10.1016/j.aquaculture.2012.08.041
Lalander C, Diener S, Magri ME, Zurbrugg C, Lindstrom A, Vinneras B (2013) Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—from a hygiene aspect. Sci Total Environ 458:312–318. https://doi.org/10.1016/j.scitotenv.2013.04.033
Li Q, Zheng LY, Qiu N, Cai H, Tomberlin JK, Yu ZN (2011) Bioconversion of dairy manure by black soldier fly (diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag 31:1316–1320. https://doi.org/10.1016/j.wasman.2011.01.005
Li S, Ji H, Zhang B, Tian J, Zhou J, Yu H (2016) Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 465:43–52. https://doi.org/10.1016/j.aquaculture.2016.08.020
Liland NS, Biancarosa I, Araujo P, Biemans D, Bruckner CG, Waagbo R, Torstensen BE, Lock E (2017) Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enrichedmedia. PLOS ONE 12(8):e0183188. https://doi.org/10.1371/journal.pone.0183188
Lock E, Arsiwalla T, Waagbo R (2015) Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) post smolt. Aquac Nutr 22:1202–1213. https://doi.org/10.1111/anu.12343
MagalhaesR S-L, Leal RS, Martinez-Llorens S, Oliva-Teles A, Peres H (2017) Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 476:79–85. https://doi.org/10.1016/j.aquaculture.2017.04.021
Makkar HPS, Tran G, Heuze V, Ankers P (2014) State-of-the-art on use of insects as animal feed. Anim Feed Sci Technol 197:1–33. https://doi.org/10.1016/j.anifeedsci.2014.07.008
Manna SK, Das N, Bera AK, Baitha R, Maity S, Debnath D, Panikkar P, Nag SK, Sarkar SD, Das BK, Patil PK (2021) Reference haematology and blood biochemistry profiles of striped catfish (Pangasianodon hypophthalmus) in summer and winter seasons. Aquac Rep 21:100836. https://doi.org/10.1016/j.aqrep.2021.100836
Marjan S, Das KK, Munshi SK, Noor R (2014) Drug-resistant bacterial pathogens in milk and some milk products. Nutrition and Food Science 44(3):241–248. https://doi.org/10.1108/NFS-05-2013-0061
Marone PA (2016) Food safety and regulatory concerns. In: Dossey AT, Morales-Ramos JA, Rojas MG (ed) Insects as sustainable food ingredients, Academic Press, pp 203–221. https://doi.org/10.1016/B978-0-12-802856-8.00007-7
Meneguz M, Achille S, Francesco G, Andrea D, Carola L, Manuela R, Gasco, L (2018) Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J Sci 98. https://doi.org/10.1002/jsfa.9127
Metcalfe LD, Schmitz AA, Petha JR (1966) Rapid preparation of fatty acid esters from lipids for gas chromatography analysis. Anal Chem 38:514–517. https://doi.org/10.1021/ac60235a044
Mohanty BP, Bhattacharjee S, Paria P, Mahanty A, Sharma AP (2013) Lipid biomarkers of lens aging. App Biochem and Biotech 169:192–200. https://doi.org/10.1007/s12010-012-9963-6
Newton GL, Booram CV, Barker RW, Hale OM (1977) Dried Hermetia illucens larvae meal as a supplement for swine. J Anim Sci 44(3):395–400. https://doi.org/10.2527/jas1977.443395x
Nguyen TT, Tomberlin JK, Vanlaerhoven S (2015) Ability of black soldier fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ Entomol 44:406–410. https://doi.org/10.1093/ee/nvv002
Panikkar P, Amala U, Jesna PK, Khan FM, Das BK, Ballal CR, Hassan MA (2018) Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diet for amur carp (Cyprinus carpio). J Inland Fish Soc India 50(2):33–38
Panikkar P, Jagadeesh TD, Sarkar UK (2018) Haematological changes In Barbodes Carnaticus (Jerdon, 1849) In Relation to seasonal variation in River Cauvery Basin. J Exp Zool India 21(1):393–398
Peres H, Oliva-Teles A (2007) Effect of the dietary essential amino acid pattern on growth, feed utilization and nitrogen metabolism of European sea bass (Dicentrarchus labrax). Aquaculture 267:119–128. https://doi.org/10.1016/j.aquaculture.2007.01.010
Pradhan SC, Patra AK, Pal A (2014) Haematological and plasma chemistry of Indian major carp, Labeo rohita (Hamilton, 1822). J Appl Ichthyol 30:48–54. https://doi.org/10.1111/jai.12297
Purschke B, Scheibelberger R, Axmann S, Adler A, Jager H (2017) Impact of substrate contamination with mycotoxins, heavy metals and pesticides on growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit Contam Part A. https://doi.org/10.1080/19440049.2017.1299946
Raes K, De Smet S, Demeyer D (2001) Effect of double-muscling in Belgian Blue young bulls on the intramuscular fatty acid composition with emphasis on conjugated linoleic acid and polyunsaturated fatty acids. Anim Sci 73:253–260. https://doi.org/10.1017/S1357729800058227
Rao K, Podeti BG (2015) Haematological changes in fresh water fish, Channa striatus diagnosed with the epizootic ulcerative syndrome (EUS). Int J Adv Biotech Res 6(2):238–244
Sanchez-Muros MJ, De Haro C, Sans A, Villareces S, Barroso FG (2015) Nutritional evaluation of Teneribo molitor meal as fish meal substitute for Tilapia (Oreochromis niloticus) diet. Aquac Nutr 22(5):943–955. https://doi.org/10.1111/anu.12313
Sastry CSP, Tammuru MK (1985) Spectrophotometric determination of tryptophan in protein. J Food Sci Tech 22:146–147
Sealey WM, Gaylord TG, Barrows FT, Tomberlin JK, McGuire MA, Ross C, St-Hilaire S (2011) Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J World Aquacult Soc 42:34–45. https://doi.org/10.1111/j.1749-7345.2010.00441.x
Shakil-Rana KM, Salam MA, Hashem S, Islam MDA (2015) Development of black soldier fly larvae production technique as an alternate fish feed. Int J Res Fish Aquacult 5(1):41–47
Sheppard DC, Newton LG, Thompson SA, Savage S (1994) A value added manure management system using the black soldier fly. Bioresour Technol 50:275–279. https://doi.org/10.1016/0960-8524(94)90102-3
Shumo M, Osuga IM, Khamis FM, Tanga CM, Fiaboe KKM, Subramanian S, Ekesi S, van-Huis A, Borgemeister C (2019) The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci Rep 9:10110. https://doi.org/10.1038/s41598-019-46603-z
Spranghers T, Ottoboni M, Klootwijk C, Ovyn A, Deboosere S, De-Meulenaer B, Michiels J, Eeckhout M, De-Clercq P, De-Smet S (2017) Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J Sci 97:2594–2600. https://doi.org/10.1002/jsfa.8081
St-Hilaire S, Sheppard C, Tomberlin JK, Irving S, Newton L, McGuire MA, Mosley EE, Hardy RW, Sealey W (2007) Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J World Aquacult Soc 38:59–67. https://doi.org/10.1111/j.1749-7345.2006.00073.x
Tschirner M, Simon A (2015) Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J Insect Food Feed 1(3):1–12. https://doi.org/10.3920/JIFF2014.0008
Uba SA, Uzairu GFS, Harrison M, Balarabe L, Okunola OJ (2008) Assessment of heavy metals bioavailability in dumpsites of Zaria Metropolis. Nigeria Afric J Biotechnol 7(2):122–130
Vargas A, Randazzo B, Riolo P et al (2018) Rearing zebrafish on black soldier fly (Hermetia illucens): biometric, histological, spectroscopic, biochemical and molecular implications. Zebrafish 15(4):404–419. https://doi.org/10.1089/zeb.2017.1559
Villazana J, Alyokhin A (2019) Development of black soldier fly larvae (Diptera: Stratiomyidae) on seafood wastes. J Insects as Food Feed 5(4):313–319. https://doi.org/10.3920/JIFF2019.0008
Wang Y-S, Shelomi M (2017) Review of black soldier fly (Hermetia illucens) as Animal Feed and Human Food. Foods 6(10):91. https://doi.org/10.3390/foods6100091
Warburton K, Hallman V (2002) Processing of organic materials by the soldier fly, Hermetia illucens. In: Warburton K, Pillai-Mc Garry U, Ramage D (eds) Integrated biosystems for sustainable development. Proceedings of the InFoRM 2000 National Workshop on Integrated Food Production and Resource Management. Rural Industries Research and Development Corporation Publication no. 01/174: 115–126. https://rirdc.infoservices.com.au/items/01–174
Wiegertjes GF, Stet RM, Parmentier HK, van Muiswinkel WB (1996) Immunogenetics of disease resistance in fish: a comparative approach. Dev Comp Immunol 20(6):365–381. https://doi.org/10.1016/s0145-305x(96)00032-8
Wintrobe MM (1974) Clinical haematology. Lea and Febiger, Philadelphia
Wynants E, Frooninckx L, Crauwels S, Verreth C, De Smet J, Sandrock C, Wohlfahrt J, Van Schelt J, Depraetere S, Lievens B, Van Miert S, Claes J, Van Campenhout L (2019) Assessing the microbiota of black soldier fly larvae (Hermetia illucens) reared on organic waste streams on four different locations at laboratory and large scale. Microb Ecol 77:913–930. https://doi.org/10.1007/s00248-018-1286-x
Zheng L, Hou Y, Li W, Yang S, Li Q, Yu Z (2012) Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 47:225–229. https://doi.org/10.1016/j.energy.2012.09.006
Acknowledgements
The authors are grateful to Dr. B. P. Mohanty, Assistant Director General of Fisheries (Inland), Indian Council of Agricultural Research (ICAR) and Dr. M. A. Hassan, Principal Scientist, ICAR-CIFRI, Barrackpore for the support during the research work. This work was supported by the institute project fund of ICAR-Central Inland Fisheries Research Institute, Barrackpore and ICAR–National Bureau of Agricultural Insect Resources, Bangalore, India.
Funding
This work was supported by the institute project fund of ICAR-Central Inland Fisheries Research Institute, Barrackpore and ICAR-National Bureau of Agricultural Insect Resources, Bangalore, India.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis: Preetha Panikkar, Jesna Parakkandi, and Amala Udayakumar. Writing—original draft preparation: Preetha Panikkar and Jesna Parakkandi. Formal analysis and investigation: Feroz Khan, Mahesh Yandigeri, and Vijaykumar Muttanahalli Eregowda. Writing—review and editing and supervision: Basanta Kumar Das. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The work was approved by the Institute ethical committee of ICAR-Central Inland Fisheries Research Institute, Barrackpore, India.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panikkar, P., Parakkandi, J., Khan, F. et al. Use of black soldier fly (Hermetia illucens) prepupae reared on organic waste as feed or as an ingredient in a pellet-feed formulation for Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res 29, 72968–72978 (2022). https://doi.org/10.1007/s11356-022-20926-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20926-3