Abstract
Native arbuscular mycorrhizal fungi (AMF) generally provide more effective assistance for phytoremediation to remove heavy metal (HM) from polluted soils than non-native AMF. Nevertheless, it is a time-consuming work to isolate, identify, and propagate AMF inoculum for practical application. This study aims to explore an alternative method to improve the phytoremediation efficiency of Bidens parviflora using domesticated AMF under HM stress condition for a certain period of time. Our results showed that Funneliformis mosseae inoculation alleviated oxidative damage to plant membranes by enhancing activities of superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase. Furthermore, mycorrhizal plants had higher chlorophyll concentration, photosynthesis efficiency, and root Pb content to protect the aerial parts from damage. These protective mechanisms were found to be more efficient in domesticated AMF inoculation compared with non-domesticated AMF inoculation. Overall, this study suggests that F. mosseae domesticated for 12 months could greatly enhance plant root Pb accumulation and plant growth mainly through strengthening antioxidant defenses as well as the photosynthesis efficiency under Pb stress conditions. Plants inoculated with pre-domesticated AMF provided a promising new strategy to enhance phytoremediation of Pb-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to rapid industrialization and urbanization, heavy metal (HM) contamination is increasingly becoming a serious environmental problem around the world because of their toxic effects on living beings, food security, and the entire ecological function (Briffa et al. 2020). Although HMs are naturally occurring elements in soil, anthropogenic activities (mining, smelting, industrial emissions, agricultural fertilization, etc.) are still responsible for excessive quantities of HM input to the environment. Among the HMs, Pb was found to be the most toxic heavy element in the environment due to its abundance, toxic, and non-biodegradable behavior (Ab Latif Wani and Usmani 2015). China has the second largest Pb reserves in the world and produced 1.9 million metric tons of Pb in 2020, accounting for 43% of the world’s total mine production (Global No. 1 Business Data Platform 2021). Soil Pb contamination has become a serious problem in China due to its detrimental effects on human health and the potential threat to the construction of the ecological civilization proposed by Chinese government in 2017 (Hansen et al. 2018). Therefore, HM contamination of soils has become a worldwide problem which requires urgent remediation actions.
Various strategies are currently used for the remediation of HM-polluted soils, mainly including physical, chemical, and biological methods (Lajayer et al. 2019). Despite high efficiency, physical and chemical techniques suffer from serious limitations such as high cost, intensive labor, soil property destruction, and soil microorganism disturbance (Sharma et al. 2016). Phytoremediation is a promising technology that uses metal-accumulating plants to remove toxic metals or render them harmless from environment (Raskin et al. 1997). It is accepted as one of the safer, cost-effective, and environment-friendly techniques, which has attracted more and more attentions over the recent decades (Marques et al. 2009). However, there are several practical limitations associated to phytoremediation, mainly related to the slow growth rate, small biomass yields, and low HM accumulation of plants. The limitations of phytoremediation can be partly overcome using plants having large biomass, fast growth rate, high HM accumulation, and ability to adapt with wide range of environmental stress.
On the other hand, the application of plant-associated microbe can be considered as an alternatively promising strategy to enhance phytoremediation efficiency (Rajkumar et al. 2012). Arbuscular mycorrhizal fungi (AMF) are a group of soil microorganisms that form symbiotic association with most terrestrial plants (Smith and Read 2008). The benefits of AMF in phytoremediation of HM-contaminated soils has been widely accepted, as their ability to improve plant biomass, growth rate, stress resistance, and HM accumulation (Ezawa et al. 2002; Smith et al. 2011; Yang et al. 2015a; Salazar et al. 2018; Bhantana et al. 2021). The beneficial effects of AMF on phytoremediation efficiency are related to the source of AMF and their adaptability to HM-contaminated soils (Yang et al. 2016). Generally, the inoculation of native AMF species is more effective in promoting plant growth and HM accumulation compared with the non-native fungi (Klironomos 2003; Orłowska et al. 2005; Pellegrino et al. 2011). However, it is a time-consuming process to isolate native AMF species from HM-contaminated soils, select and identify the most effective AMF strain, and propagate it to obtain enough inoculum for practical application. Alternatively, a simple and feasible method known as stress-driven adaptive evolution experiments has been proposed to improve the adaptability and growth of microbial species to particular stress conditions (Sun et al. 2018). This is because microorganisms can adapt rapidly to changing environments through regulation of their gene expression in order to survive and reproduce (López-Maury et al. 2008). Recently, this strategy has been successfully used in a number of microbe (especially microalgal species) to obtain domesticated strain with high growth and stress resistance advantages in stress environments. However, whether the domestication treatment can enhance growth and stress tolerance of AMF and their beneficial effects on phytoremediation efficiency is still unclear. We have a hypothesis here: AMF can gain the capacity to cope with HM stress and enhance positive impact on phytoremediation through consuming nutrients efficiently, regulating gene expression, and influencing HM accumulation.
Bidens is an annual herbaceous plant belonging to compositae family and distributes widely all over the world (Sun et al. 2009). It has been suggested that Bidens parviflora Willd. has the ability to accumulate large amount of HM and can be considered as a potential hyperaccumulator for restoration of Cd- and Pb-contaminated soil (Deng et al. 2019). In our field study, the roots of B. parviflora were found highly colonized by AMF in HM-polluted soil. The exact role of domesticated AMF with different domestication durations in phytoremediation efficiency remains unclear. Therefore, the objectives of this study were to (1) determine the ability of B. parviflora to accumulate and translocate Pb from soil to plant organs, (2) evaluate the impacts of AMF on growth and HM accumulation of B. parviflora, and (3) explore whether the domesticated AMF under HM stress had more beneficial effects on phytoremediation efficiency.
Materials and methods
Plant, AMF inoculum, and substrate
B. parviflora was selected as a host plant in this study because it is a potential used hyperaccumulator in northeast China. The seeds of B. parviflora were collected from Longwan National Natural Reserve, Jilin Province, China (42°20′56″N, 126°22′51″E) in October. The seeds were surface-sterilized with 0.5% sodium hypochlorite and 75% ethanol (v/v) for 10 min and 5 min, respectively. Subsequently, the seeds were washed carefully with sterile distilled water five times and then were sown on moistened filter paper arranged in 9 cm diameter Petri dishes with 7 mL distilled water. The Petri dishes were incubated in the dark at 27 °C for 2 days before the germinated seeds were transplanted into the sterilized pots.
The AM fungus Funneliformis mosseae (T.H. Nicolson & Gerd.) C. Walker & A. Schüßler (BGC GZ01A, formerly Glomus mosseae) was purchased from Beijing Academy of Agriculture & Forestry Sciences (BAAFS). The fungus is a widely distributed AMF species all over the world and can form symbionts with most plants (e.g., B. parviflora) to enhance their resistance to stress conditions (Huang et al. 2019). The AMF isolate (F. mosseae) was chosen based on its widely distribution and beneficial effects on plant growth as well as its predominance in HM-contaminated soils (Yang et al. 2015b). The AMF strain was propagated in sterilized sand (diameter < 1 mm) pots using Zea mays L. and Trifolium repens L. as host plants for 6, 12, and 24 months. The plants were uniformly irrigated with distilled water and Hoagland’s nutrient solution every 2 days and 1 week, respectively. Pots were divided into two groups. In the first group, pots were irrigated with pure Hoagland’s nutrient solution (no Pb addition), and the fungus was expressed as non-domesticated F. mosseae (ND6Fm, ND12Fm, and ND24Fm). In the second group, pots were irrigated with Hoagland’s nutrient solution contained 1000 mg kg−1 Pb, and the arbuscular mycorrhizal fungus was expressed as domesticated F. mosseae (D6Fm, D12Fm, and D24Fm). Finally, the spore density in sand substrate was determined using the wet-sieving and decanting methods under light dissecting microscope (Gerdemann and Nicolson 1963). The average spore densities in soils were shown in Table 1, and only dried substrate was mixed homogeneously to use as the AMF inoculum to ensure that all treatments could receive the same number of spores.
Soil was collected from 0 to 20 cm soil depth at the Experimental Station of Jilin Agricultural University, Changchun, China (43˚49′07″N, 125˚23′56″E). The area is located in the temperate continental monsoon climate, with mean annual temperature of 6.7 °C and mean annual precipitation of 600–700 mm. The soil type was classified as black soil (Phaeozems) and has a silty loam texture. The collected soil samples were passed through a 2-mm mesh sieve to remove large coarse sand and gravel particles as well as to improve the homogeneity. The sieved soil was air-dried at room temperature for 20 days, added into a clean cloth bag, and then sterilized with two cycles of the autoclave at 121 °C, 0.11 Mpa for 2 h to eliminate all microorganisms. The basic properties of soil used for the substrate in the current study were listed in Table S1. The soil was mixed with equal volume of deionized water or Pb(NO3)2 solution at final concentrations of 0, 500, and 2000 mg kg−1 Pb. Subsequently, the soils were equilibrated for 2 weeks, undergoing five cycles of saturation with deionized water and air-drying.
Experimental design
The pot experiment was carried out in a solar greenhouse of Northeast Normal University, Changchun city (Jilin province, China), and lasted 90 days. We carried out the study in a randomized complete block design with five replications: AMF inoculation (three levels, without AMF inoculation, non-domesticated, and domesticated F. mosseae inoculation); domestication duration (three levels, 6, 12, and 24 months), and Pb addition (three levels, 0, 500, and 2000 mg kg−1 soil). The plastic pots (15 cm diameter top, 12 cm diameter base, and 15 cm depth) filled with 2.5 kg sterilized soil with different concentrations of Pb. A total of 40 g of mycorrhizal inoculum (containing 1000 spores) was placed in each plastic pot at a depth of 2 cm below the seeds and covered with soil. Subsequently, eight germinated seeds of B. parviflora were planted into each plastic pot and then covered with a thin layer of the substrate. Plant positions were repositioned at random, and plants were watered every 2 days based on the pot weight to maintain the soil water field capacity (FC) at 75%.
Plant and soil analysis
After 90 days of treatments, the net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (Gs), and transpiration rate (Tr) were assessed with a LI-COR LI-6400 portable gas exchange system on the fifth leaf of each plant from 8:30 to 11:00 am (Yang et al. 2014).
The fifth leaf was then harvested to measure the chlorophyll concentration (Chl a, Chl b, Chl a + b, and Chl a/b). Chlorophyll pigments were extracted by chilled acetone (80%, v/v) using a mortar and pestle at room temperature in the dark, and the optical density (OD) of the extract was read at 663 and 646 nm using a UV-5500PC spectrophotometer (Shanghai Metash Instruments Co., Ltd., China). The concentration of chlorophyll pigments was calculated according to the formulas of Lichtenthaler and Buschmann (2001).
The activities of antioxidant enzymes (catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR)) in leaves were determined according to the method used in our previous study (Yang et al. 2020). Briefly, fresh leaf samples were harvested separately, grinded in liquid nitrogen, and homogenized with 25 mM potassium phosphate buffer solution (pH 7.8) containing 0.5 mM EDTA-Na2 and 1 mM ascorbate. The homogenate was then centrifuged at 10,000 rpm for 20 min, and the supernatant was stored in a refrigerator (4 °C) for the measurement of antioxidative enzyme activities. The CAT activity was assessed by monitoring the decrease in the absorbance at 240 nm as a consequence hydrogen peroxide (H2O2) decomposition (Aebi 1984). The SOD activity was assayed according to Aebi (1984) by measuring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm. The APX activity was calculated by detecting the decrease of ascorbate oxidation at 290 nm (Nakano and Asada 1981). The GR activity was assayed according to Foyer and Halliwell (1976) based on the rate of glutathione at 420 nm.
The hydrogen peroxide (H2O2) content in leaves was estimated as described by Patterson et al. (1984) by detecting the absorbance of the titanium–peroxide complex at 410 nm. The malondialdehyde (MDA) content in leaves was performed using the thiobarbituric acid method as described by Heath and Packer (1968).
Plant roots were washed with tap water, cleared in 10% KOH, bleached in H2O2, acidified with 1% lactic acid, and then stained with 0.05% trypan blue (Phillips and Hayman 1970). The mycorrhizal colonization was determined according to Biermann and Linderman (1981) by monitoring the proportion of root length colonized by AMF. AMF spores were extracted from 20 g soil using wet-sieving and decanting method (Gerdemann and Nicolson 1963), and the number was counted under a dissecting microscope. The extracted hyphae of AMF in soil were stained with a 0.05% (w/v) trypan blue solution, and the hyphal length density (HLD) was determined by grid-line intersection method (Jakobsen et al. 1992).
Plants were harvested and separated into roots, stems, and leaves. The samples were rinsed with distilled water three times, blotted with filter paper, and oven-dried at 80 °C until constant mass to recorded plant biomass. Plant organs (leaves, stems, and roots) were firstly digested in Teflon vessels with concentrated HF and HNO3 acid solutions, and then the Pb concentrations in different plant organs were determined using flame atomic absorption spectrometry (FAAS, AA-7003A, Beijing, China). The bioconcentration factor (BCF) and translocation factor (TF) were estimated to depict the ability of the plants to accumulate HM from soil, which is calculated as follows:
where \({C}_{soil}\), \({C}_{root}\), \({C}_{stem}\), and \({C}_{leaf}\) are the concentrations of Pb in the soil, plant root, stem, and leaf, respectively.
Statistical analysis
Prior to data analysis, the Kolmogorov–Smirnov test and Levene test were used to evaluate the normality and homogeneity of data using SPSS 21 (SPSS Inc., Chicago, IL, USA) for Windows 10, respectively. The significant differences among the mean values of different treatments were compared using one-way ANOVA followed by the Duncan’s multiple range tests using SPSS 21. p values less than 0.05 were considered statistically significant. The coefficient of variation of each parameter determined in this study could be seen in Table S2. The correlations among all observed variables were computed by applying the Pearson correlation method at a significance level of 0.05. Mantel test was performed to assess the correlations between AMF growth index and plant physiochemical parameters, and the results were visualized using the “ggplot2” package in R (version 4.0.4). A bubble plot was constructed using the R packages “ggplot2” and “reshape2.”
Results
AMF growth parameters
The good symbiosis between F. mosseae and B. parviflora could be detected using microscopic assessment, whereas no mycorrhizal structures were found in the roots of non-mycorrhizal plants. Pb2000 treatment (2000 mg kg−1) considerably decreased mycorrhizal colonization (MC) of plants colonized by non-domesticated F. mosseae (NDFm), while plants inoculated with domesticated F. mosseae (DFm) had significantly higher MC and HLD than NDFm plants (Fig. 1). D12Fm and D24Fm plants had higher MC compared with D6Fm plants, while no significant difference was detected between D12Fm and D24Fm plants under Pb2000 treatment. By contrast, no significant difference in spore density (SPD) was found among all treatments (p > 0.05).
Mycorrhizal colonization (MC, a), spore density (SPD, b), and hyphal length density (HLD, c) of B. parviflora inoculated with F. mosseae (Fm) under different Pb stress levels. Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; NM, non-mycorrhizal inoculation; NDFm, non-domesticated F. mosseae inoculation; DFm, domesticated F. mosseae inoculation. The subscript numbers present the duration of domestication (month). Data represent mean ± SD for biological replicates (n = 5). The same letter indicates no significant difference at each Pb stress level (Duncan’s test, p < 0.05)
Plant growth parameters
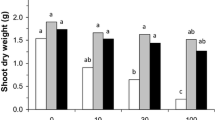
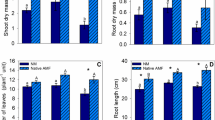
Compared with non-mycorrhizal plants, AMF inoculation increased root, stem, and leaf dry weights under Pb stress conditions (Fig. 2). The magnitude of the growth response to domesticated AMF was more effective than non-domesticated AMF in terms of plant root, stem, and leaf dry weights under Pb500 and Pb2000 treatments. However, no significant difference in stem dry weight, leaf dry weight, and R/S ratio was detected between non- and mycorrhizal plants under Pb0 treatment. The R/S ratio of non-mycorrhizal plants was lower compared with the mycorrhizal plants under Pb2000 treatment, although no significant difference could be detected under Pb500 treatment (p > 0.05). DFm plants had significantly higher leaf and stem dry weights than non-mycorrhizal and NDFm plants, while no difference was found between D12Fm and D24Fm plants under Pb2000 treatments (p > 0.05).
Plant root dry weight, stem dry weight, leaf dry weight, and root:shoot (R/S) ratio of B. parviflora inoculated without or with F. mosseae (Fm) under different Pb stress levels. Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; NM, non-mycorrhizal inoculation; NDFm, non-domesticated F. mosseae inoculation; DFm, domesticated F. mosseae inoculation. The subscript numbers present the duration of domestication (month). Data represent mean ± SD for biological replicates (n = 5). The same letter indicates no significant difference at each Pb stress level (Duncan’s test, p < 0.05)
Leaf gas exchange parameters
The leaf gas exchange parameters of B. parviflora among different treatments are showed in Fig. 3. The Pn, Gs, and Tr were negatively correlated with the increasing Pb concentration (R2 = − 0.582, − 0.612, and − 0.630, respectively; all p < 0.001). Ci was positively correlated with Pb concentration (R2 = 0.586, p < 0.001). The Pn, Gs, and Tr of non-mycorrhizal plants under Pb2000 versus Pb0 treatments decreased by 41.5, 46.4, and 42.8%, respectively. Plants inoculated with domesticated F. mosseae (D6Fm, D12Fm, and D24Fm) had higher Pn, Gs, and Tr but lower Ci compared with the plants colonized by non-domesticated F. mosseae (ND6Fm, ND12Fm, and ND24Fm) under Pb2000 treatment (p < 0.05).
Leaf net photosynthetic rate (Pn, a), stomatal conductance (Gs, b), intercellular CO2 concentration (Ci, c), and transpiration rate (Tr, d) of B. parviflora inoculated without or with F. mosseae (Fm) under different Pb stress levels. Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; NM, non-mycorrhizal inoculation; NDFm, non-domesticated F. mosseae inoculation; DFm, domesticated F. mosseae inoculation. The subscript numbers present the duration of domestication (month). Data represent mean ± SD for biological replicates (n = 5). The same letter indicates no significant difference at each Pb stress level (Duncan’s test, p < 0.05)
Leaf chlorophyll concentration
With the increase of Pb stress level, the chlorophyll a, b, and a + b concentrations of DFm plants firstly increased and then decreased, reaching a maximum under Pb500 treatment (Fig. 4). There were no significant differences in the chlorophyll a, b, and a + b concentrations or chlorophyll a/b ratio between non- and mycorrhizal plants under Pb0 treatment. Non-mycorrhizal plants had significantly lower chlorophyll a, b, and a + b concentrations compared with mycorrhizal plants, while the chlorophyll a/b ratio was considerably higher than mycorrhizal plants. The chlorophyll b concentration showed a similar change trend as the chlorophyll a concentration, and DFm plants had higher chlorophyll b concentration and chlorophyll a/b ratio than NDFm plants under Pb stress conditions (p < 0.05).
Chlorophyll concentration and chlorophyll a/b ratio in leaf of B. parviflora inoculated without or with F. mosseae (Fm) under different Pb stress levels. Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; NM, non-mycorrhizal inoculation; NDFm, non-domesticated F. mosseae inoculation; DFm, domesticated F. mosseae inoculation. The subscript numbers present the duration of domestication (month). Data represent mean ± SD for biological replicates (n = 5). The same letter indicates no significant difference at each Pb stress level (Duncan’s test, p < 0.05)
Antioxidant enzyme activities
The activities of SOD and CAT in leaves of D12Fm and D12Fm plants were higher than other plants under Pb500 treatment (Fig. 5). There was no significant difference in SOD, CAT, APX, and GR activities in leaves of non- or mycorrhizal plants under Pb0 treatment, but D12Fm and D24Fm plants had considerably higher SOD, CAT, and APX activities compared with the control and other mycorrhizal plants under Pb2000 treatment (p < 0.05). Furthermore, the SOD, CAT, and GR activities in leaves of mycorrhizal plants were significantly greater than non-mycorrhizal plants under Pb2000 treatment (p < 0.05).
The activities of SOD (a), CAT (b), APX (c), and GR (d) in leaf of B. parviflora inoculated without or with F. mosseae (Fm) under different Pb stress levels. Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; NM, non-mycorrhizal inoculation; NDFm, non-domesticated F. mosseae inoculation; DFm, domesticated F. mosseae inoculation. The subscript numbers present the duration of domestication (month). Data represent mean ± SD for biological replicates (n = 5). The same letter indicates no significant difference at each Pb stress level (Duncan’s test, p < 0.05)
Leaf hydrogen peroxide and malondialdehyde contents
Pb stress significantly increased the hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents in plant leaves (Fig. 6), while there was no significant difference in the H2O2 and MDA contents between non- and mycorrhizal plants under the control treatment (0 mg kg−1 Pb). Non-mycorrhizal plants had higher H2O2 content compared with mycorrhizal plants under Pb addition treatments. Interestingly, DFm plants had lower H2O2 and MDA contents compared with NDFm plants, while no significant difference in H2O2 and MDA contents among D6Fm, D12Fm, and D24Fm was found under Pb stress conditions.
The hydrogen peroxide (H2O2, a) and malondialdehyde (MDA, b) contents in leaf of B. parviflora inoculated without or with F. mosseae (Fm) under different Pb stress levels. Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; NM, non-mycorrhizal inoculation; NDFm, non-domesticated F. mosseae inoculation; DFm, domesticated F. mosseae inoculation. The subscript numbers present the duration of domestication (month). Data represent mean ± SD for biological replicates (n = 5). The same letter indicates no significant difference at each Pb stress level (Duncan’s test, p < 0.05)
Pb accumulation in plant
The Pb concentrations in the roots, stems, and leaves of B. parviflora seedlings performed an increasing trend following an increase in Pb concentration in soil (Fig. 7). Plant roots had the highest Pb concentration followed by the leaves, while the stems showed the lowest amounts of Pb. Mycorrhizal plants accumulated significantly higher Pb in the roots compared with non-mycorrhizal plants at all Pb stress levels, but no difference in stem and leaf Pb concentrations was detected between non- and mycorrhizal plants under Pb0 treatment (p > 0.05). Pb concentrations in the leaves and stems of D12Fm and D24Fm plants were lower compared with the NDFm plants under Pb stress conditions (p < 0.05). However, there was no difference in leaf, stem, or root Pb concentrations between D12Fm and D24Fm plants under all treatments (p > 0.05).
Pb concentration in leaf (a), stem (b), and root (c) of B. parviflora inoculated without or with F. mosseae (Fm) under different Pb stress levels. Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; NM, non-mycorrhizal inoculation; NDFm, non-domesticated F. mosseae inoculation; DFm, domesticated F. mosseae inoculation. The subscript numbers present the duration of domestication (month). Data represent mean ± SD for biological replicates (n = 5). The same letter indicates no significant difference at each Pb stress level (Duncan’s test, p < 0.05)
Discussion
Lead (Pb) contamination in soils has been reported in many countries throughout the world, with the most severe problems found in Asia. Using plants that can hyperaccumulate specific metals has been considered as a promising, environmentally friendly, and low-cost technology for remediation of Pb-contaminated soils (Salt et al. 1995). In northeast China with cold temperature, B. parviflora is a more suitable phytoremediator for Pb than the other hyperaccumulator because of its high tolerance to cold stress. The native AMF isolates have beneficial effects on phytoremediation efficiency, but it is a time-consuming and laborious process to isolate and identify the most effective AMF strain from HM-contaminated soils (Vivas et al. 2003). Our study provided an alternative strategy to enhance positive impact of AMF strain on phytoremediation efficiency by domesticating it under HM-contaminated soil for 12 months.
The Pb hyperaccumulator is defined as being able to accumulate more than 1,000 mg kg−1 Pb in plant aerial organs on a dry-weight basis (Baker and Brooks 1989). Furthermore, it is suggested that only species with both BCF and TF greater than one had the potential to be used for phytoextraction (Baker 1981), while the BCF is greater than one, and TF less than one in the plants had the potential for phytostabilization (Yoon et al. 2006). The current study showed that B. parviflora grown in the Pb-contaminated soils contained higher concentration of Pb than that of the uncontaminated soils (Fig. 7). Although plants had the ability to accumulate Pb, the Pb concentrations in plant roots and leaves among all treatments were below but similar to the above-mentioned criterion. Based on the average BCFs of different plant organs (Table S2), the Pb was most efficiently accumulated in plant leaves (BCF = 0.67) and followed by plant roots (BCF = 0.65) under Pb500 treatment. Based on the average TFs (Table S2), the Pb was most efficiently translocated from roots to leaves under Pb2000 treatment (TF = 1.10) and followed by Pb500 treatment (TF = 1.08). The results indicated that B. parviflora could be identified as a potential Pb hyperaccumulator (Fig. S2).
Several studies have indicated that AMF inoculation can promote plant growth, nutrient uptake, and tolerance to HM stress (Yang et al. 2015a; Adeyemi et al. 2021). Zhan et al. (2018) reported that the root and shoot biomass of maize inoculated with F. mosseae were significantly higher than that of non-mycorrhizal plants in HM-contaminated soils. Our findings were consistent with these previous studies and indicated that the beneficial effects of AMF on plant growth could be attributed to MC and HLD based on the mantel test (Fig. 8). Additionally, the current study further revealed that the domesticated AMF was more effective than the non-domesticated fungi in promoting plant growth under Pb stress treatments (Figs. 2, S1). This indicated that an ecophysiological adaptation of F. mosseae to the Pb-contaminated soil may have occurred, and the non-domesticated AMF may not function well even when colonizing plants to high levels in sterile soils. However, our study found that the domesticated F. mosseae showed negative effect on plant biomass under the control treatment (0 mg kg−1 Pb) (Fig. 2). It is quite possible that the domesticated F. mosseae had adapted well to the Pb-contaminated soil and could not form a functional symbiosis with B. parviflora in uncontaminated soils. This speculation could be supported by the lower MC and HLD in the roots of DFm plants compared with the NDFm plants under the control treatment (Fig. 1).
Pairwise comparisons of correlations between AMF growth index (MC, SPD, and HLD) and plant physiochemical parameters (dry weight, chlorophyll concentration, leaf gas exchange parameters, H2O2, and MDA concentrations). Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; MC, mycorrhizal colonization; SPD, spore density; HLD, hyphal length density; DW, dry weight; Pn, net photosynthetic rate; Ci, intercellular CO2 concentration; Gs, stomatal conductance; Tr, transpiration rate; Chla, chlorophyll a concentration; Chlb, chlorophyll b concentration; Chla/b, chlorophyll a/b ratio; H2O2, hydrogen peroxide content; MDA, malondialdehyde. Pearson’s correlation is shown in a color gradient. AMF growth index based on Bray–Curtis distance were correlated to plant physiochemical parameters by Mantel test, with edge representing Mantel’s r for correlations, and the color corresponding to the significance
The more beneficial effect of domesticated F. mosseae on plant growth and stress tolerance compared with non-domesticated fungi was mainly attributed to the maintenance of the photosynthesis and improvement of antioxidant enzyme activities under Pb stress conditions. In the present study, the chlorophyll concentration, Pn, Gs, and Tr of plants decreased significantly under Pb2000 treatment, and the decrease of chlorophyll concentration, Pn, Gs, and Tr of NDFm plants was higher than that of DFm plants (Figs. 3, 4). This indicated that NDFm plants were more sensitive to Pb stress and showed a greater reduction in photosynthetic rate, and DFm plants were able to maintain photosynthetic functionality for longer in response to Pb-contaminated soil. The inoculation of domesticated F. mosseae probably resulted in upregulated chloroplast gene expression, thereby contributing to higher PSII efficiency and enhancing photosynthetic capacity under Pb stress conditions (Chandrasekaran et al. 2019). Furthermore, chlorophyll is able to absorb energy from sunlight and then transform water and carbon dioxide to carbohydrates and oxygen. Therefore, chlorophyll is essential for plant photosynthesis, and high concentration of chlorophyll in DFm plantscan leads to high photosynthetic activity (Yang et al. 2014; Mahama et al. 2016).
Exposure of plants to stress environment is known to induce formation of reactive oxygen species (ROS), which are involved in damage mechanisms and then results in the inhibition of plant growth and development (Smimoff 1995; Das and Roychoudhury 2014). In our study, domesticated F. mosseae inoculation increased the SOD, CAT, APX, and GR activities significantly in the leaves of B. parviflora compared with the non-domesticated F. mosseae inoculation in Pb-contaminated soils, resulting in low H2O2 and MDA contents (Figs. 5, 6). SOD dismutates superoxide radicals to H2O2, which is then converted into H2O and O2 by CAT and APX, while GR sustains the reduced status of GSH via ascorbate–glutathione pathway and plays an essential role in maintenance of sulfhydryl (–SH) group (Yousuf et al. 2012). Therefore, we can infer that the domesticated F. mosseae inoculation was more effective than the commercial F. mosseae inoculation in promoting plant growth and resistance to oxidative damage caused by reducing excessive accumulation of ROS in Pb stress conditions. This might be attributed to the strong ability of domesticated AMF in activating the expression of genes encoding antioxidant enzymes to maintain the cellular oxidative equilibrium and reduce the free radical damage (Riaz et al. 2020).
The beneficial effects of the domesticated F. mosseae on phytoremediation efficiency were attributed to not only large plant biomass but also high Pb accumulation in plant organs (Fig. S2). In the present study, the higher Pb concentration in the roots of DFm plants was observed compared with that of NDFm or non-mycorrhizal plants under Pb stress treatments (Fig. 7). Several studies indicated that mycorrhizal plants could confer greater Pb concentration in the roots through mechanisms such as immobilization and chelation of metals in hyphae and compartmentalization within fungal cells (Zhang et al. 2010). Additionally, AMF-produced glomalin, tightly bound in AMF hyphae and spore walls, is a kind of glycoprotein, which has the ability to efficiently sequestrate Pb in soils (Malekzadeh et al. 2016).
In this study, B. parviflora presented to be a good candidate for the Pb phytoaccumulator (Fig. 9), due to the high metal accumulation in plant roots and leaves (Deng et al. 2004). Stoltz and Greger (2002) reported that mycorrhizal inoculation increased exclusion strategy of plants on the basis of translocation factor. It is supported by our previous study where it was found that AMF inoculation significantly increased Pb concentration in the roots but decreased Pb concentration in the stems and leaves of Robinia pseudoacacia seedlings (Yang et al. 2015a). Our study further confirmed that the inoculation of domesticated F. mosseae could greatly enhance Pb accumulation in plant roots compared with the non-domesticated fungus (Fig. 9). This phenomenon might be partly explained by the high MC and HLD of domesticated F. mosseae under Pb stress treatments (Fig. 1). However, DFm plants had a significantly lower stem and leaf Pb concentrations than NDFm plants grown in Pb-contaminated soils (Fig. 7). The result was consistent with Vogel-Mikuš et al. (2006) who suggested that a large amount of HMs could be concentrated in mycorrhizal structures, such as fungal mycelia and vesicle, thereby minimizing metal translocation from the root to the aerial parts of the plant. The domesticated F. mosseae was more effective in providing a barrier against Pb transfer to plant leaf and protecting the photosystems from Pb-induced damage.
The bioconcentration factor (BCF) and translocation factor (TF) of Pb in different organs of B. parviflora inoculated without or with F. mosseae (Fm) under different Pb stress levels. Pb0, 0 mg kg−1 Pb stress level; Pb500, 500 mg kg−1 Pb stress level; Pb2000, 2000 mg kg−1 Pb stress level; NM, non-mycorrhizal inoculation; NDFm, non-domesticated F. mosseae inoculation; DFm, domesticated F. mosseae inoculation. The subscript numbers present the duration of domestication (month). The same letter indicates no significant difference at each Pb stress level (Duncan’s test, p < 0.05)
Conclusions
Phytoremediation is receiving more and more attention in recent years. The potential importance of AMF in phytoremediation of Pb-contaminated soils has been demonstrated due to their beneficial effects on plant growth and Pb accumulation. The present study demonstrated that the domesticated AMF were more potentially useful for the phytostabilization of Pb-contaminated soils than non-domesticated AMF, for possible control of Pb translocation in food chains, and subsequently for reducing environmental risks. For phytostabilization purposes, further studies were required to identify domesticated AMF species having more positive responsiveness to host plant. There is also a need to conduct systematic studies to screen for host-AMF compatibility and to understand the mechanisms of how domesticated AMF contribute to the enhancement of phytostabilization.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adeyemi NO, Atayese MO, Sakariyawo OS, Azeez JO, Ridwan M (2021) Arbuscular mycorrhizal fungi species differentially regulate plant growth, phosphorus uptake and stress tolerance of soybean in lead contaminated soil. J Plant Nutr 44:1633–1648
Aebi H (1984) Catalase in Vitro. Method Enzymol 105:121–126
Baker AJ (1981) Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Baker AJ, Brooks R (1989) Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1(2):81–126
Bhantana P, Rana MS, Sun XC, Moussa MG, Saleem MH, Syaifudin M, Shah A, Poudel A, Pun AB, Bhat MA, Mandal DL (2021) Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 84:19–37
Biermann B, Linderman RG (1981) Quantifying vesicular-arbuscular mycorrhizae: a proposed method towards standardization. New Phytol 87:63–67
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Chandrasekaran M, Chanratana M, Kim K, Seshadri S, Sa T (2019) Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress-a meta-analysis. Front Plant Sci 10:457
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Env Sci-Switz 2:53
Deng H, Ye ZH, Wong MH (2004) Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ Pollut 132:29–40
Deng Q, Deng Q, Wang Y, Li L, Long X, Ren S, Fan Y, Lin L, Xia H, Liang D, Wang J (2019) Effects of intercropping with Bidens species plants on the growth and cadmium accumulation of Ziziphus acidojujuba seedlings. Environ Monit Assess 191:1–8
Ezawa T, Smith SE, Smith FA (2002) P metabolism and transport in AM fungi. Plant Soil 244:221–230
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244
Global No. 1 Business Data Platform. Available online: https://www.statista.com/statistics/273652/global-lead-reserves-by-selected-countries (Accessed on 24 November 2021)
Hansen MH, Li H, Svarverud R (2018) Ecological civilization: interpreting the Chinese past, projecting the global future. Global Environ Chang 53:195–203
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Huang L, Chen D, Zhang H, Song Y, Chen H, Tang M (2019) Funneliformis mosseae enhances root development and Pb phytostabilization in Robinia pseudoacacia in Pb-contaminated soil. Front Microbiol 10:2591
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. New Phytol 124:61–68
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Lajayer BA, Moghadam NK, Maghsoodi MR, Ghorbanpour M, Kariman K (2019) Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environ Sci Pollut Res 26:8468–8484
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids-measurement and characterisation by UV-VIS. Current Protocols in Food Analytical Chemistry (CPFA), (Supplement 1), F4.3.1-F4.3.8. John Wiley, New York
López-Maury L, Marguerat S, Bähler J (2008) Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet 9(8):583–593
Mahama GY, Prasad PV, Roozeboom KL, Nippert JB, Rice CW (2016) Response of maize to cover crops, fertilizer nitrogen rates, and economic return. AGRON J 108:17–31
Malekzadeh E, Aliasgharzad N, Majidi J, Abdolalizadeh J, Aghebati-Maleki L (2016) Contribution of glomalin to Pb sequestration by arbuscular mycorrhizal fungus in a sand culture system with clover plant. Eur J Soil Biol 74:45–51
Marques AP, Rangel AO, Castro PM (2009) Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Crit Rev Environ Sci Technol 39:622–654
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Orłowska E, Ryszka P, Jurkiewicz A, Turnau K (2005) Effectiveness of arbuscular mycorrhizal fungal (AMF) strains in colonisation of plants involved in phytostabilisation of zinc wastes. Geoderma 129(1–2):92–98
Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492
Pellegrino E, Bedini S, Avio L, Bonari E, Giovannetti M (2011) Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol Biochem 43:367–376
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. T Brit Mycol Soc 55:158–161
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574
Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol 8:221–226
Riaz M, Kamran M, Fang Y, Wang Q, Cao H, Yang G, Deng Lu, Wang Y, Zhou Y, Anastopoulos I, Wang X (2020) Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: a critical review. J Hazard Mater 402:123919
Salazar MJ, Menoyo E, Faggioli V, Geml J, Cabello M, Rodriguez JH, Marro N, Pardo A, Pignata ML, Becerra AG (2018) Pb accumulation in spores of arbuscular mycorrhizal fungi. Sci Total Environ 643:238–246
Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13:468–474
Sharma S, Rana S, Thakkar A, Baldi A, Murthy RSR, Sharma RK (2016) Physical, chemical and phytoremediation technique for removal of heavy metals. J Heavy Metal Toxicol Dis 1:1–15
Smimoff N (1995) Antioxidant systems and plant response to the environment; in Environment and Plant Metabolism. Bios Scientific Publishers, Oxford
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, New York, NY
Stoltz E, Greger M (2002) Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environ Exp Bot 47:271–280
Sun XM, Ren LJ, Zhao QY, Ji XJ, Huang H (2018) Microalgae for the production of lipid and carotenoids: a review with focus on stress regulation and adaptation. Biotechnol Biofuels 11(1):1–16
Sun Y, Zhou Q, Wang L, Liu W (2009) Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J Hazard Mater 161:808–814
Vivas A, Vörös A, Biró B, Barea JM, Ruiz-Lozano JM, Azcón R (2003) Beneficial effects of indigenous Cd-tolerant and Cd-sensitive Glomus mosseae associated with a Cd-adapted strain of Brevibacillus sp. in improving plant tolerance to Cd contamination. Appl Soil Ecol 24:177–186
Vogel-Mikuš K, Pongrac P, Kump P, Nečemer M, Regvar M (2006) Colonisation of a Zn, Cd and Pb hyperaccumulator Thlaspi praecox Wulfen with indigenous arbuscular mycorrhizal fungal mixture induces changes in heavy metal and nutrient uptake. Environ Pollut 139(2):362–371
Yang Y, Cao Y, Li Z, Zhukova A, Yang S, Wang J, Tang Z, Cao Y, Zhang Y, Wang D (2020) Interactive effects of exogenous melatonin and Rhizophagus intraradices on saline-alkaline stress tolerance in Leymus chinensis. Mycorrhiza 30:357–371
Yang Y, Han X, Liang Y, Ghosh A, Chen J, Tang M (2015) The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS One 10:e0145726
Yang Y, Liang Y, Han X, Chiu TY, Ghosh A, Chen H, Tang M (2016) The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. SCI REP-UK 6:1–14
Yang Y, Song Y, Scheller HV, Ghosh A, Ban Y, Chen H, Tang M (2015b) Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils. Soil Biol Biochem 86:146–158
Yang Y, Tang M, Sulpice R, Chen H, Tian S, Ban Y (2014) Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J Plant Growth Regul 33:612–625
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2–3):456–464
Yousuf PY, Hakeem KUR, Chandna R, Ahmad P (2012) Role of glutathione reductase in plant abiotic stress. In Abiotic stress responses in plants (pp. 149-158). Springer, New York, NY
Zhan F, Li B, Jiang M, Yue X, He Y, Xia Y, Wang Y (2018) Arbuscular mycorrhizal fungi enhance antioxidant defense in the leaves and the retention of heavy metals in the roots of maize. Environ Sci Pollut Res 25:24338–24347
Zhang HH, Tang M, Chen H, Zheng CL, Niu ZC (2010) Effect of inoculation with AM fungi on lead uptake, translocation and stress alleviation of Zea mays L. seedlings planting in soil with increasing lead concentrations. Eur J Soil Biol 46:306–311
Funding
This research was supported by the National Natural Science Foundation of China (41807052) and the Program of Introducing Talents of Discipline to Universities (B16011).
Author information
Authors and Affiliations
Contributions
YY and XW designed and conceived the experiment. YY, BH, and JX carried out the experiments and collected the empirical data. ZT and ZL performed the data analysis. YY and XW wrote the paper with contributions from ZT and ZL.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Huang, B., Xu, J. et al. Heavy metal domestication enhances beneficial effects of arbuscular mycorrhizal fungi on lead (Pb) phytoremediation efficiency of Bidens parviflora through improving plant growth and root Pb accumulation. Environ Sci Pollut Res 29, 32988–33001 (2022). https://doi.org/10.1007/s11356-022-18588-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18588-2