Abstract
Melatonin, a ubiquitous molecule found in almost all organisms, is considered an important regulator in plant growth. However, little is known about the interactive effect of melatonin and arbuscular mycorrhizal (AM) fungi on plant resistance against soil salinity and alkalinity. To fill in such a gap in knowledge, we conducted three experiments to explore (1) whether exogenous melatonin and an AM fungus had interactive effects on plant response to saline-alkaline stress, (2) whether the influence of melatonin on mycorrhizal plant stress tolerance was attributable to effect on the AM fungus, and (3) whether the effect of melatonin application was due to changes in soil salinity and alkalinity. We found interactive effects between melatonin and the AM fungus on alleviating ROS burst, decreasing malondialdehyde content and protecting Leymus chinensis photosynthetic activity through activation of antioxidant enzyme and gene expression (superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase) in plant shoots and roots. Our results showed that exogenous melatonin promoted spore germination and hyphal length of the AM fungus under Petri-dish conditions. However, exogenous melatonin application did not exhibit significant effects on soil salinity and alkalinity. This study provides an insight into the beneficial effects of exogenous melatonin on saline-alkaline stress tolerance in mycorrhizal L. chinensis through regulating antioxidant systems, protecting photosynthetic activity, and promoting associated AM fungal growth without changing soil salinity and alkalinity. It also reveals potential applications of exogenous melatonin and AM fungi for the restoration of saline-alkaline degraded grassland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saline-alkaline stress has been considered one of the major abiotic stresses that cause a significant reduction in agricultural crop production, terrestrial biodiversity, and ecological health, particularly in arid and semiarid climates. The area of saline and alkaline soil continues to expand due to increases in irrigated areas (Shrivastava and Kumar 2015). Plants grown in saline-alkaline soils are adversely affected by osmotic stress, ion injury, and high-pH toxicity (Yang et al. 2009). A high level of soil salinity and alkalinity generally exerts an adverse influence on photochemical reactions due to stomatal closure, intercellular CO2 concentration restriction, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity reduction, and reactive oxygen species (ROS) accumulation (Maroco et al. 2002; Pancha et al. 2015). The ROS such as superoxide (O2−), singlet oxygen (1O2), hydroxyl radicals (OH−), and hydrogen peroxide (H2O2) can seriously disrupt regular metabolism via oxidative injury to carbohydrates, lipids, proteins, and nucleic acids. Fortunately, plants possess various enzymatic and non-enzymatic antioxidants that protect cells from the adverse effects of excessive ROS by scavenging them in different steps (Nafees et al. 2019). Superoxide dismutase (SOD) is the first defense against oxygen-derived free radicals, catalyzes the dismutation of the superoxide anion O2− into hydrogen peroxide H2O2, which is subsequently detoxified into H2O and O2 by catalase (CAT) and ascorbate peroxidase (APX) (Papalia et al. 2018).
Plants also can cope with abiotic stress through establishing symbiotic associations with arbuscular mycorrhizal (AM) fungi, which form mutualistic relationships with more than 80% of terrestrial plants and exist widely in saline and alkaline soils (Aliasgharzadeh et al. 2001). Although salinity and alkalinity can decrease colonization by AM fungi, numerous studies have indicated that AM fungi play an important role in enhancing saline-alkaline stress tolerance of host plants through promoting nutrient uptake ability, improving root functions, maintaining leaf photosynthetic efficiency, and reducing damage by free radicals (Ruiz-Lozano et al. 2012; Kumar et al. 2015; Bhattacharjya et al. 2018). It also has been demonstrated that AM fungi have multiple beneficial effects on membrane stability, photosynthetic pigment enhancement, defense compound stimulation, and osmotic and ion balance protection of plant cells (Augé et al. 2014; Lin et al. 2017).
Nevertheless, the effects of AM fungi on plant performance under stress highly depend on conditions such as soil properties, salinity and alkalinity levels, plant physiological status, and developmental stage. Therefore, a promising technique for the restoration of degraded ecosystems is to select biological or non-biological agents that improve mycorrhizal symbiotic efficiency. One agent, melatonin (N-acetyl-5-methoxytryptamine), a low molecular-weight molecule with an indole ring in its structure, was first reported in animals in 1958 (Lerner et al. 1960). Melatonin has several physiological functions in animals such as affecting sleep physiology, circadian rhythms, and the immune system. Subsequent studies discovered that melatonin also exists widely in plants and microorganisms (Tan et al. 2011; Arnao and Hernández-Ruiz 2015). In plants, melatonin has been considered to be synthesized from tryptophan through four sequential enzymatic steps involving tryptophan decarboxylase (TDC), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), and N-acetylserotonin methyltransferase (ASMT) with the activation of TDC, T5H, SNAT, and ASMT genes in the biosynthetic pathway (Back et al. 2016). A previous study found that a low accumulation of melatonin improved IAA biosynthesis resulting in the promotion of root growth in Brassica juncea, although the association between melatonin and IAA remains unclear (Chen et al. 2009). Furthermore, pre-treatment with melatonin significantly alleviates plant growth inhibition under salinity conditions, thus enabling plants to maintain a robust root system and increase photosynthetic capacity (Li et al. 2012).
Salinity interferes with plant growth through physiological drought and ion toxicity, which cause enhanced generation of ROS, but melatonin can act to limit the generation of ROS by promoting the expression of stress related genes, increasing the enzymatic antioxidant system, and thereby reducing malondialdehyde (MDA) and H2O2 levels (Reiter et al. 2015). Sarropoulou et al. (2012) indicated that exogenous melatonin not only prevented ROS but also protected the proteins related to chlorophyll and photosynthesis. Although the role of melatonin in alleviating abiotic stress has been recognized for many species, including Arabidopsis thaliana (Zhang et al. 2019), maize (Okant and Kaya 2019), and tomato (Ahammed et al. 2019), little is known about how melatonin affects grass saline-alkaline stress tolerance.
Most investigators of melatonin effects on plants have conducted experiments by using a leaf spaying method, but Huang et al. (2019) suggested that root-irrigation is most effective and that root-irrigated seedlings with 100 μM melatonin exhibited healthy growth with significantly increased plant height and biomass. However, we are not aware of any reports regarding whether the beneficial effects of melatonin on plant saline-alkaline stress tolerance should be attributed to its direct influence on soil properties versus changes in plant physiological status.
Although the benefits of melatonin in mitigating the damage caused by abiotic stresses are known (Bajwa et al. 2014), there is little information in the literature regarding the interactive effects between AM fungi and exogenous melatonin on saline-alkaline stress tolerance in the important grass, Leymus chinensis (Trin.) Tzvel. L. chinensis, a dominant or co-dominant perennial rhizomatous clonal C3 species, is widely distributed across the eastern region of the Eurasian Steppe, from the western part of the Northeast Plain to the eastern part of the Mongolian Plateau in China (Wang and Ba 2008). This grass offers various important economic and ecological values because it not only contains relatively large amounts of trace minerals, vitamins, high-quality protein, and carbohydrates but also grows rapidly with high biomass (Liu et al. 2012; Li et al. 2019). An increasing number of studies have indicated that L. chinensis inoculated with appropriate arbuscular mycorrhizal (AM) fungus could be an effective tool for restoration of meadow steppe in north China (Zhang et al. 2011). Therefore, the objectives of this study were (1) to reveal any interactive effects between an AM fungus and exogenous melatonin on saline-alkaline stress tolerance of L. chinensis in a pot experiment; (2) to assess the effects of exogenous melatonin on that AM fungus in a Petri-dish experiment; and (3) to determine the effects of exogenous melatonin on soil salinity and alkalinity in an incubation experiment. We also will discuss the potential application of exogenous melatonin and AM fungi for degraded grassland restoration.
Materials and methods
Experiment 1: effects of an AM fungus and exogenous melatonin on saline-alkaline stress tolerance in L. chinensis
Seeds of L. chinensis were obtained from the Grassland Ecological Research Station of Northeast Normal University, Jilin Province, China (123o44′E, 44o44′N), in July. The seeds were surface-sterilized with sodium hypochlorite (0.5%, v/v) for 5 min, washed three times with sterile water, treated with 70% ethanol for 5 min, and then washed three times again with sterile water. Surface sterilized seeds were pre-germinated on moist filter paper in Petri dishes (9 cm in diameter) in the dark for 2 days at 28 °C before they were transferred into plastic pots.
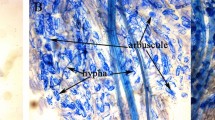
The AM fungus, Rhizophagus intraradices (N.C. Schenck & G.S. Sm.) C. Walker & A. Schüßler (BGC BJ09) was obtained from Beijing Academy of Agriculture and Forestry Sciences, Beijing, China. The fungus was propagated on maize (Zea mays L.) and white clover (Trifolium repens L.) grown in pot cultures for 4 months. The AM colonization (91.8%) and spore density (501 per 10 g of air-dried soil) were determined after harvest. The AM fungal inoculum consisted of a mixture of spores, mycelium, colonized root fragments of maize and white clover, and air-dried substrate.
The growth substrate consisted of soil and sand (1:1, v/v). Soil samples were collected from the top layer (0–15 cm) in Songnen Grassland Ecological Field Station, Northeast Normal University, Jilin Province, China (123o44′E, 44o44′N). The district has a temperate continental arid climate and a mixed salt-alkali meadow soil, with an annual rainfall of 300–450 mm and a mean annual temperature of 4.6–6.4 °C. The obtained soil samples were air-dried for 20 days, then passed through a 2-mm sieve to remove root-stone residue and ensure homogeneity. The sieved soil used for the growth substrate has the following properties: pH 8.1 (1: 2.5, soil: water, m/v); 21.3 mg g−1 soil organic matter; 0.41 mg g−1 total phosphorus; 5.13 mg kg−1 available phosphorus (Olsen’s P); 1.18 mg g−1 total nitrogen; 72.5 mg kg−1 available nitrogen; 131.6 mg kg−1 available potassium; and 219.7 μS cm−1 electrical conductivity. The mixture of sieved soil and washed sand was placed into a clean cloth bag and autoclaved at 121 °C for 1 h twice to eliminate all possible mycorrhizal propagules and other soil microorganisms.
Experiment design
The experiment was a 2 × 2 × 2 fully crossed design with mycorrhizal inoculation (2 levels; AM fungus inoculation or not), melatonin application (2 levels; 0 μM or 100 μM), and salinity-alkalinity addition (2 levels; 0 mM or 150 mM) to test the interaction effects of melatonin and AM fungi on plant response to saline-alkaline stress. Each treatment had ten replicates for a total of 80 plastic pots.
The pre-germinated seeds of L. chinensis were transplanted into each plastic pot (15 cm upper diameter, 12-cm lower diameter, and 15-cm depth) filled with 2.5 kg sterilized growth substrate. In each pot, 20 g of AM fungal inoculum was placed 2 cm below the seeds and covered with the substrate. Non-mycorrhizal control plants received 15 mL of the filtered leachate (20 μm) from the AM fungal inoculum to correct possible differences in soil microbial and non-AM fungi communities. After emergence (10 days after transplantation), seedlings were thinned to a final density of 10 plants per pot and thereafter were treated with saline-alkaline stress (10 days after thinning). Four salts NaCl, Na2SO4, NaHCO3, and Na2CO3 were mixed in a 9:1:1:9 M ratios to simulate a range of mixed saline-alkaline stress conditions according to the ion composition of salt-alkali soil in Northeast China. To avoid osmotic shock, the saline-alkaline solution (150 mM, 300 mL) was introduced gradually by successively adding 100 mL every 2 days after thinning. An equivalent volume of distilled water was added to the control pots. A saucer was placed under each pot to ensure that no excess leaching from the pot occurred. To obtain melatonin solution, melatonin (Sigma-Aldrich, St. Louis, USA) was first dissolved in 100% ethanol and then diluted to 100 μM using distilled water. The melatonin solution (100 μM, 150 mL) was added to the substrate three times on the same day as the addition of neutral and alkaline salts. Moreover, irrigation with melatonin was conducted during the dark stage because melatonin is a photosensitive molecule (De Luca et al. 2013). Soil moisture probes (Field Scout TDR 100, USA) were used to measure the relative water content (RWC) daily and the amount of lost water was supplied to each pot to keep the RWC (70%) according to our previous study (Yang et al. 2014). Beginning 2 weeks after treatment, each pot was irrigated with 50 mL half-strength Hoagland’s nutrient solution (Hoagland and Arnon 1950) every 2 weeks throughout the growth period of seedlings.
After 65 days of treatment (the plants were about to reach the reproductive stage), five pots (n = 5) from ten replicative pots in each treatment were randomly selected, and then the parameters of plant height, leaf gas exchange, and chlorophyll fluorescence were determined. Subsequently, substrate and plant shoots and roots in these five pots were harvested and mixed homogeneously for measurements of AM colonization (MC), spore density (SPD), hyphal length density (HLD), enzyme activities, hydrogen peroxide concentration, lipid peroxidation, and gene expression level. The plants in the other five pots (n = 5) within each treatment were harvested and then dried at 80 °C to measure plant shoot dry weight, root dry weight, and total dry weight.
AM colonization, spore density, and hyphal length density
The plant roots in pots of each treatment were harvested to stain with trypan blue (0.05%) following Phillips and Hayman (1970), and MC was assessed using the Biermann and Linderman method (Biermann and Linderman 1981). At least 100 root fragments per treatment were used to measure MC using a Nikon Eclipse Ni light microscope (Tokyo, Japan) under bright-field illumination (200× magnification). The percentage of mycorrhizal structures in each 1-cm root fragment was assessed as 0, 10, 20...100%. The MC was calculated according to Eq. (1):
where N0, N10, N20, … N100 are the number of root fragments with the certain estimated percentage of AM fungal vesicles, arbuscules, or hyphae.
AM fungal spores were isolated from the rhizosphere soil of L. chinensis using the wet sieving and decanting method (Gerdemann and Nicolson 1963), and the number of AM fungal spores was counted under a dissecting microscope using a hand tally counter according to our previous study (Yang et al. 2015a).
Soil subsamples (5 g) were blended for 20 s with distilled water. The suspension was then poured through a 500-μm and a 32-μm sieve to separate hyphae. The soil sample was suspended again in 250 mL distilled water, transferred to a beaker, shaken for 30 s, and then left to settle for 5 min at room temperature. The supernatant (containing hyphae) was filtered 4 times on a filtration apparatus under vacuum. The extracted hyphae were stained with a 0.05% (w/v) trypan blue solution and then were measured by the gridline intercept method at 200× magnification. The hyphal length density (HLD) of AM fungus was presented in units of m g−1 dry soil (Jakobsen et al. 1992).
Plant growth, photosynthetic pigments, gas exchange, and chlorophyll fluorescence
All seedlings in each pot of the same treatment were collected, separated into above- and belowground parts, dried at 80 °C to constant weight, and finally weighed.
Fresh leaf samples (0.25 g) per pot were harvested, mixed homogeneously, cleaned with deionized water to remove any surface contamination, and then thoroughly homogenized in chilled acetone (80%) with a mortar and pestle in the dark at 4 °C. The homogenates were centrifuged at 10,000×g for 20 min, and the supernatants were collected to determine the absorbance of the acetone extracts using a UV/Vis spectrophotometer (UV-5500PC, Shanghai Metash Instruments Co., Ltd., China) at 663 and 646 nm. The concentrations of chlorophyll (Chl) a, Chl b, and Chl a/b ratio were calculated according to the method described by Wellburn (1994).
The net photosynthetic rate by unit of leaf area (Pn), transpiration rate (Tr), intercellular CO2 concentration (Ci), and stomatal conductance (Gs) were measured with a portable gas exchange system (Li-6400, Li-COR, USA) between 9:00 A.M. and 11:00 A.M. before harvest according to our previous study (Yang et al. 2014). During the period of measurements, a 6400-02B LED source provided a photosynthetic photon flux density of 1300 μmol m−2 s−1, and the CO2 concentration in the growth chamber was maintained at 400 g m−3.
Chlorophyll fluorescence parameters were recorded using a portable gas exchange system (LI-6400, Li-COR, USA) coupled with an integrated fluorescence chamber head (LI-6400-40 leaf chamber fluorometer) according to a previous study (Huang et al. 2004). Photochemical quenching coefficient (qP), non-photochemical quenching coefficient (qN), and effective photochemical efficiency of PSII (ΦPSII) were measured by the following equations: qP = (Fm′ – Fs)/(Fm′ – Fo′); qN = (Fm – Fm′)/(Fm – F0); ΦPSII = (Fm′ – Fs)/Fm′ (Genty et al. 1989).
Enzyme extraction and assays
Fresh leaf and root samples per pot of the same treatment were harvested separately and then thoroughly homogenized at 4 °C to prepare for enzyme extractions according to our previously reported procedures (Yang et al. 2015b). Protein concentration was assayed by the Lowry spectrophotometric method, using bovine serum albumin as a standard.
SOD activity was assayed by measuring the inhibition in the photoreduction of nitroblue tetrazolium (NBT) at 560 nm according to a method previously described (Giannopolitis and Ries 1977). One unit (U) of SOD activity was taken as the amount of enzyme causing 50% inhibition of photochemical reduction of NBT. SOD activity was expressed in enzyme units per gram fresh weight per hour (U g−1 FW h−1).
CAT activity was determined spectrophotometrically at 240 nm by monitoring the decrease in absorbance of the reaction mixture resulting from the decomposition of hydrogen peroxide (H2O2) (Aebi 1984). One unit (U) of CAT activity was defined as the amount of enzyme that caused 1 μL H2O2 decomposition in 1 min. CAT activity was expressed as enzyme units per gram fresh weight per minute (U g−1 FW min−1).
APX activity was measured according to the method described by Nakano and Asada (1981). The reaction mixture contained 0.1 mM EDTA, 0.5 mM ascorbate, 1.0 mM H2O2, 100 μL enzyme extract, and 50 mM phosphate buffer (pH 7.0) in a final volume of 1.0 mL. The decrease in absorbance at 290 nm was recorded, and the amount of ascorbate oxidized was calculated using an extinction coefficient (2.8 mM−1 cm−1) for 1 min. One unit of APX was defined as the amount of the enzyme required to oxidize 1 μmoL ascorbate per mg of soluble protein per min. The enzyme activity was expressed as U g−1 FW min−1.
Glutathione reductase (GR) activity was assayed according to Foyer and Halliwell (1976). One unit of GR is defined as the amount of enzyme that catalyzes the oxidation of 1 μmol of NADPH per minute. GR activity was expressed as U g−1 protein min−1.
Hydrogen peroxide concentration
Hydrogen peroxide (H2O2) concentration was determined according to the method described by Loreto and Velikova (2001). Approximately 0.5 g of fresh leaves or roots were cut into pieces, ground in liquid nitrogen with a mortar and pestle, and homogenized in an ice bath with 5 mL of 1% trichloroacetic acid (TCA). Subsequently, the homogenate was then centrifuged at 12,000×g for 10 min at 4 °C and 0.5 mL of supernatant was added to 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide. The absorbance of the supernatant was measured at 390 nm with a spectrophotometer (Shanghai Metash Instruments Co., Ltd., China) and the concentration of H2O2 was expressed as μmol g−1 FW.
Lipid peroxidation
The level of lipid peroxidation was determined from the measurement of malondialdehyde (MDA) concentration resulting from the thiobarbituric acid (TBA) reaction (Heath and Packer 1968). Fresh leaf or root tissue (0.5 g) was ground in liquid nitrogen using a pre-chilled mortar and pestle in 5 mL extraction buffer of 1% TCA. The homogenate was centrifuged at 12,000×g for 10 min. A total of 1 mL of the supernatant was added to 2 mL of a reaction solution containing 20% (v/v) TCA and 0.5% (v/v) TBA. The homogenate was heated at 95 °C for 30 min and then cooled in an ice bath to stop the reaction. After the solution was centrifuged at 10,000×g for 10 min, the absorbance of the supernatant was read at 532 and 600 nm. Nonspecific absorbance at 600 nm was subtracted from that at 532 nm, and MDA content was calculated using this adjusted absorbance and the extinction coefficient of 155 mM−1 cm−1. The concentration of MDA was expressed as nmol g−1 FW.
Total RNA isolation and cDNA synthesis
Total RNA was extracted from 100 mg of the frozen leaf using the Plant RNA isolation kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s instructions. To eliminate genomic DNA, the extracted RNA was treated with the RNase-free DNase I supplement provided with the kit. UV absorption and gel electrophoresis were employed to evaluate the RNA quantity and quality. First-strand cDNA was synthesized from 2 μg of total RNA using 50 μM oligo (dT) primers and reverse transcriptase M-MLV (RNase H) according to the manufacturer’s instructions (Takara, Otsu, Japan).
Quantitative real time-polymerase chain reaction (qRT-PCR) analysis
Four plant genes of interest, i.e., Cu/Zn-SOD, CAT, APX, and GR genes were previously searched from the L. chinensis Transcriptome Database to perform qRT-PCR (Chen et al. 2014). The corresponding specific primers are detailed in Table S1, and the actin gene was used as the reference according to Zhou et al. (2014). The primer specificity and formation of primer-dimers were determined by dissociation curve analysis and agarose gel electrophoresis on a 3% agarose gel. The qRT-PCR was performed using a CFX96™ real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and an SYBR Premix ExTaq II (2×) Kit (Takara) under cycling conditions of 95 °C for 5 min, 40 cycles of 95 °C for 20 s, and 60 °C for 60 s. These qRT-PCR experiments were performed in three replicates, based on three separate RNA extracts from three leaf samples. The relative gene expressions were analyzed using the 2-ΔΔCT method according to Livak and Schmittgen (2001).
Experiment 2: effects of exogenous melatonin on AM fungal growth under Petri-dish conditions
Spores of R. intraradices were isolated from the rhizosphere of L. chinensis using the wet-sieving and decanting method (Gerdemann and Nicolson 1963). The collected spores were surface sterilized three times for 10 min with a 2% solution of Chloramine-T containing a drop of Tween 80, thoroughly rinsed with several changes of sterile tap water and soaked in a solution of antibiotics (streptomycin, 200 mg L−1; gentamycin, 100 mg L−1; and chloromycetin, 50 mg L−1) (Karandashov et al. 2000). The sterilized spores were immersed in a sterilized melatonin solution (100 μM; 500 μL sterilized by filtration through a 0.22 μm Micropore filter and transferred to a Spin Filter [MP Biomedicals, FastDNA Spin Kit for Soil]) or sterilized solution without melatonin for 30 min at room temperature, centrifuged at 10,000×g for 2 min, and transferred to a 1% water agar plate using fine forceps. Four salts (NaCl, Na2SO4, NaHCO3, and Na2CO3) were mixed in a 9:1:1:9 M ratios to simulate natural saline-alkaline stress conditions according to the ion composition of the salt-alkali soil in Northeast China. Agar powder was mixed with the salt solution (150 mM) before sterilization to yield agar gel (1%, w/v). Each Petri plate contained 25 spores, and 5 plates were utilized for each treatment with either 0 mM or 150 mM saline-alkaline stress. The Petri dishes were incubated at 25 °C in the dark for 2 weeks (14 days). Spore germination was detected under a dissecting microscope and was considered as successful germination if the germ tubes were longer than the spore diameter (> 200 μm). The hyphal elongation of the germinated spores was determined by the 2-mm grid method according to a previous study (Bécard et al. 1992).
Experiment 3: effects of exogenous melatonin application on soil salinity and alkalinity
A soil incubation experiment was conducted to evaluate whether the effect of exogenous melatonin on mycorrhizal plant stress tolerance was attributed to changes of soil salinity and alkalinity. Air dried substrate (500 g) was weighed and distributed to sterile plant tissue culture vessels (10 cm diameter × 13 cm height). A saline-alkaline stress treatment (150 mM) was introduced by adding 60 mL of the solution of neutral and alkaline salts in distilled water, while a melatonin solution (100 μM, 20 mL) was added into the substrate as in the description of the pot experiment. A full factorial combination of the two factors and two levels for each factor produced four experimental treatments, and each treatment had five replicates. As a result, we had 20 incubation soil samples in total with 4 treatments (2 saline-alkaline stress levels × 2 melatonin application levels) in a climatic incubator (BSC-400, Shanghai Boxun Industry & Commerce Co., Ltd. Medical Equipment Factory, Shanghai, China) at 28 °C. The vessels were covered by screw caps with small holes to reduce water loss via evaporation while allowing gas exchange. The moisture of the soil was kept at field capacity condition (34.33%) with distilled water. After 20 days of incubation, soil samples in the vessels were collected and air-dried at room temperature. The pH, electrical conductivity (EC), soluble anions, and soluble cations were measured using saturated soil extracts (Richards 1968). Soil pH was determined using the glass electrode method, and the EC was measured using a 1 cm conductivity cell, dip-type probe. Exchangeable cations were determined using ammonium acetate extraction. Soil Na+ and K+ were measured using flame emission spectroscopy (Model 410 Flame photometer, Sherwood Scientific, Ltd., New York, USA), while Ca2+ and Mg2+ were determined by atomic absorption spectrophotometry (Super 990F, Beijing Purkinje General Instrument Co. Ltd. Beijing, China). Soil CO32− and HCO3− were evaluated by the neutralization method (Tahira et al. 2011), while Cl−, and SO42− were assessed using anion chromatography (DX-300 ion chromatographic system, Dionex Company, Sunnyvale, USA). The exchangeable sodium percentage (ESP) and sodium adsorption ratio (SAR) were calculated as Eqs. (2) and (3), respectively.
where Na+ is the measured exchangeable Na (cmol kg−1) and CEC is the cation exchange capacity (cmol kg−1).
where Na+, Ca2+, and Mg2+ are the measured exchangeable Na, Ca, and Mg (cmol kg−1).
Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences, for Windows version 16.0 (SPSS 16; Chicago, IL, USA). The assumptions of normal distribution and homogeneity of variance were checked with the Kolmogorov-Smirnov test (P > 0.05) and Levene’s test (P > 0.05), respectively. When necessary, the data were log-transformed to meet assumptions of normality and homogeneity. For Experiment 1, a three-way analysis of variance (ANOVA) followed by Duncan’s test at P < 0.05 was applied to reveal the influences of saline stress (SS), mycorrhizal inoculation (MF), and melatonin addition (MT) on morpho-physiological characteristics of L. chinensis. Two-way ANOVA was used to examine the effects of SS and MT on mycorrhizal fungus parameters. Two-way ANOVAs followed by Duncan’s test also was used to examine the results of Experiments 2 and 3.
Results
Experiment 1: effects of an AM fungus and exogenous melatonin on saline-alkaline tolerance in L. chinensis
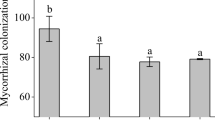
Microscopic assessment confirmed that symbiosis had been established between L. chinensis seedlings and R. intraradices at harvest, while no MC was detected in the roots of non-mycorrhizal plants. For the AM fungus inoculation treatment (+MF), saline-alkaline stress had a strong inhibitory effect on AM fungal growth, leading to significant decreases in MC and hyphal length density (HLD) by 20.0% and 24.3%, respectively (Fig. 1; Table S2). However, no significant difference was found in spore density (SPD) between non-stress (NS) and saline-stress (SS) treatments. Melatonin application (+MT) significantly increased MC, SPD, and HLD by 52.0%, 39.3%, and 38.1% compared with non-melatonin application (−MT) treatments, respectively. Saline-alkaline stress did not exhibit adverse effects on MC in mycorrhizal L. chinensis when the plants were pretreated with exogenous melatonin.
Effects of saline-alkaline stress, AM fungus inoculation, and exogenous melatonin application on AM colonization (MC), spore density (SPD), and hyphal length density (HLD) in roots and rhizosphere soils of L. chinensis. Bars (means ± SD, n = 5) topped by the same letter do not differ significantly at P < 0.05 according to Duncan’s multiple range test. NS, non-stress; SS, saline-alkaline stress; −MT, non-melatonin application; +MT, melatonin application
Plant growth
The −MT plants exposed to saline-alkaline stress had significantly lower shoot dry weight (DW), root DW, and total DW compared with the plants under the NS treatment (Table 1). The plants colonized by the AM fungus and pretreated with exogenous melatonin under the NS treatment had the highest root, shoot, and total DWs at 1.27, 0.43, and 1.71 g plant−1, respectively, while the control plants under the SS treatment had the lowest DWs at 0.94, 0.26, and 1.20 g, respectively. A significant difference in shoot/root ratio of control and −MT+MF plants between the NS and SS treatments was detected, while no difference was found among +MT plants.
Photosynthetic pigments
Chlorophyll (Chl) b concentration was higher for plants grown in the NS treatment than for those grown in the SS treatment (Fig. 2). In contrast, no significant difference in Chl a concentration of mycorrhizal plants was found between −MT and +MT treatments. Mycorrhizal plants had significantly higher Chl a and Chl b concentrations compared with non-mycorrhizal plants in −MT treatment under saline-alkaline stress. AM fungus inoculation led to a decrease in Chl a/b ratio by 14.77% and 5.47% under NS and 21.31% and 12.98% under the SS condition in −MT and +MT treatments, respectively. MT × MF interaction exhibited a significant influence on Chl b concentration and Chl a/b ratio, while no effect was found on Chl a concentration (Table S3).
Effects of saline-alkaline stress, AM fungus inoculation, and exogenous melatonin application on chlorophyll (Chl) a concentration, Chl b concentration, and Chl a/b ratio in leaves of L. chinensis. Bars or points (means ± SD, n = 5) associated with the same letter for the same parameter do not differ significantly at P < 0.05 according to Duncan’s multiple range test. NS, non-stress; SS, saline-alkaline stress; −MT, non-melatonin application; +MT, melatonin application; −MF, non-AM fungus inoculation; +MF, AM fungus inoculation
Gas exchange
The plants exposed to saline-alkaline stress showed lower net photosynthetic rate (Pn), stomatal conductance (Gs), and transpiration rate (Tr) in all treatments than the NS control, while the intercellular CO2 concentration (Ci) of mycorrhizal plants in the SS treatment was significantly higher compared with the plants in the NS treatment (Table 2). AM fungus inoculation and/or exogenous melatonin pretreatment significantly increased Pn, Gs, and Tr in plant leaves under both the NS and SS conditions but decreased Ci under the SS condition compared with the control. Both SS × MF and MT × MF interactions had significant effects on Pn and Ci, while no notable effects were found on Gs and Tr (Table 2).
Chlorophyll fluorescence
The mixed saline-alkaline stress caused a decline in the maximal quantum yield of PSII (Fv/Fm), effective photochemical efficiency of PSII (ΦPSII) and photochemical quenching coefficient (qP), but an increase in non-photochemical quenching coefficient (qN) in leaves of L. chinensis in all treatments (Table 3). The mycorrhizal plants had significantly higher ΦPSII and qP compared with non-mycorrhizal plants no matter whether they were under stress condition. The highest value of Fv/Fm (8.55) could be detected in +MT+MF plants, although no significant difference was observed between +MT+MF plants and −MT+MF plants under the NS condition. The greast ΦPSII (0.46) and qP (0.56) was found in +MT+MF plants grown under NS condition, while the largest qN (7.83) was detected in −MT−MF plants when they were exposed to saline-alkaline stress. MT × MF interaction had a significant effect on PSII (ΦPSII), qN, and qP.
Antioxidant enzymes
AM fungus inoculation significantly increased the activities of ascorbate peroxidase (APX) and glutathione reductase (GR) in leaves of L. chinensis in all treatments, while no notable difference was found in the activities of root APX and GR between non-mycorrhizal and mycorrhizal plants in the NS treatment (Fig. 3). Mycorrhizal plants had higher activities of leaf superoxide dismutase (SOD) and catalase (CAT) compared with non-mycorrhizal plants in the SS treatment, but no difference was observed in +MT plants under the NS condition. Melatonin application significantly increased the activity of CAT in leaves and roots of mycorrhizal plants under the NS and SS conditions but did not affect that in leaves of non-mycorrhizal plants. MT × MF interaction exhibited a significant impact on activities of APX and GR in plant roots, whereas SS × MT × MF interaction did not influence the activities of SOD, CAT, APX, and GR.
Effects of saline-alkaline stress, AM fungus inoculation, and exogenous melatonin application on activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) in leaves and roots of L. chinensis. Bars (means ± SD, n = 5) topped by the same letter for the same parameter do not differ significantly at P < 0.05 according to Duncan’s multiple range test. NS, non-stress; SS, saline-alkaline stress; −MT, non-melatonin application; +MT, melatonin application; −MF, non-AM fungus inoculation; +MF, AM fungus inoculation
Expression of genes encoding antioxidant enzymes
Saline-alkaline stress significantly enhanced the expression levels of Cu/Zn-SOD and CAT in both leaves and roots of L. chinensis in +MT and −MT+MF treatments, while it did not influence the expression level of leaf Cu/Zn-SOD in −MT−MF plants under the NS condition (Fig. 4). Mycorrhizal plants had higher expression levels of Cu/Zn-SOD, CAT, and APX in leaves and roots compared with non-mycorrhizal plants in the SS treatment, whereas no difference was found when plants were exposed to melatonin under the NS condition. Melatonin application significantly increased expression levels of Cu/Zn-SOD, CAT, and APX in leaves and roots under saline-alkaline stress, while no difference was detected in the expression level of root APX in mycorrhizal plants between −MT and +MT treatments. MT × MF interaction had a significant impact on the expression level of leaf Cu/Zn-SOD, whereas SS × MT × MF interaction did not affect the expression levels of these antioxidant enzymes in either plant leaves or roots.
Effects of saline-alkaline stress, AM fungus inoculation, and exogenous melatonin application on expresion levels of genes encoding superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) in leaves and roots of L. chinensis. Bars (means ± SD, n = 5) topped by the same letter for the same parameter do not differ significantly at P < 0.05 according to Duncan’s multiple range test. NS, non-stress; SS, saline-alkaline stress; −MT, non-melatonin application; +MT, melatonin application; −MF, non-AM fungus inoculation; +MF, AM fungus inoculation
Hydrogen peroxide and malondialdehyde concentrations
Saline-alkaline stress significantly increased hydrogen peroxide (H2O2) and malondialdehyde (MDA) concentrations in both leaves and roots of L. chinensis in all treatments. In contrast, the plants inoculated by the AM fungus or pretreated with melatonin had higher H2O2 and MDA concentrations compared with the controls in the SS treatment, while no difference was observed in the NS treatment (Fig. 5). For +MT plants, the AM fungus inoculation significantly decreased MDA concentration in both leaves and roots under the SS condition but did not impact it under the NS condition. The H2O2 and MDA concentrations in leaves of mycorrhizal plants were significantly lower in +MT treatment than in −MT treatment, but no difference was found for the values in roots of mycorrhizal plants.
Effects of saline-alkaline stress, AM fungus inoculation, and exogenous melatonin application on hydrogen peroxide (H2O2) and malondialdehyde (MDA) contents in leaves and roots of L. chinensis. Bars (means ± SD, n = 5) topped by the same letter for the same parameter do not differ significantly at P < 0.05 according to Duncan’s multiple range test. NS, non-stress; SS, saline-alkaline stress; −MT, non-melatonin application; +MT, melatonin application; −MF, non-AM fungus inoculation; +MF, AM fungus inoculation
Experiment 2: effects of exogenous melatonin on AM fungal growth under Petri-dish conditions
There were significant differences in AM fungus spore germination and hyphal growth among different treatments at 14 days (Table 4). Saline-alkaline stress had significantly negative effects on both spore germination and hyphal growth, and the minimum values (25.0% for spore germination and 30.4 mm spore−1 for hyphal length) occurred when spores were not pretreated with melatonin under saline-alkaline stress. In contrast, the spores pretreated with melatonin had the highest spore germination (82.2%) and hyphal length (114.0 mm spore−1) under the NS condition. Hyphal length in the control was much higher than that recorded under saline-alkaline stress but was slightly lower than that of +MT treatment. The interaction between SS and MT did not have a significant influence on hyphal length.
Experiment 3: effects of exogenous melatonin application on soil salinity and alkalinity
The concentrations of exchangeable Na2+, CO32−, HCO3−, Cl−, and SO42− increased noticeably in the SS treatment compared with the NS treatment (Fig. 6), with mean values of 4.71, 0.25, 1.32, 0.39, and 0.03 cmol kg−1, respectively. No significant difference in concentrations of Ca2+, Mg2+, and K+ were found between NS and SS treatments, although the Mg2+ concentration was a bit low in +MT treatment compared with −MT treatment. In addition, soil pH, EC, CEC, ESP, and SAR were greater in the SS treatment than in the NS treatment. Notably, the addition of exogenous melatonin did not significantly influence soil properties related to the salinity and alkalinity in either the NS treatment or SS treatment.
Effects of exogenous melatonin application on soil salinity and alkalinity in an incubation experiment (Experiment 3). Bars (means ± SD, n = 5) topped by the same letter do not differ significantly at P < 0.05 according to Duncan’s multiple range test. NS, non-stress, SS, saline-alkaline stress, −MT, non-melatonin application; +MT, melatonin application; EC, electrical conductivity; Na+, sodium; K+, potassium; Ca2+, calcium; Mg2+, magnesium; SO42−, sulphate; CO32−, carbonate; HCO3−, bicarbonate; Cl−, chloride; CEC, cation exchange capacity; ESP, exchangeable sodium percentage; SAR, sodium adsorption ratio
Discussion
Soil salinity and alkalinity are major threats to crop yield worldwide and are among the most common land degradation processes. The Songnen Plain in Northeast China is one of the three largest soda saline-alkaline regions in the world. The symbiotic association between AM fungi and L. chinensis has been considered as one of the most promising strategies for degraded grassland restoration in the region. However, the practical effect of this technology mainly depends upon AM fungi species, soil conditions, and stress levels. This study found that exogenous melatonin could promote the growth of one AM fungus and saline-alkaline resistance of mycorrhizal L. chinensis. Such findings support the application of melatonin in degraded grassland restoration.
Soil salinization and alkalization limit plant growth and development through inhibiting a series of physiological and biochemical mechanisms (Shi and Sheng 2005). Plant biomass accumulation and allocation are functional parameters used to evaluate the plant strategies for resource acquisition and environment adaptation (Duru et al. 2009). Our results showed that the induction of saline-alkaline stress was associated with significant decreases in shoot DW, root DW, total DW, and shoot/root ratio of plants (Table 1). In accordance with previous studies (Sheng et al. 2008), our data also suggested that inoculation with an AM fungus significantly alleviated this growth inhibition. Melatonin, as a type of new plant growth promoter, has been implicated as involved in plant tolerance to both abiotic and biotic stresses (Antoniou et al. 2017). This study agreed with previous findings that exogenous melatonin treatment led to a significant improvement in plant growth and biomass production. Moreover, it is interesting to note that significantly higher shoot, root, and total DW of plants was found in the +MF/+MT treatments compared with single MF or MT treatments (Table 1). To help explain this phenomenon, we found that melatonin pretreatment significantly increased spore germination and hyphal length of the AM fungus in Petri dishes and also increased MC, SPD, and HLD in pots under both NS and SS conditions. However, the mechanisms involved in melatonin-mediated tolerance to saline-alkaline stress in mycorrhizal L. chinensis remain unknown. Therefore, we evaluated the influences of exogenous melatonin on photosynthesis, chlorophyll fluorescence, antioxidant defense systems, and ROS accumulation in mycorrhizal plants under saline-alkaline stress. The current study suggested that the application of exogenous melatonin plays a beneficial role in AM fungal growth and mycorrhizal plant physiology against saline-alkaline stress.
Photosynthesis is an important physiological process to promote plant growth, maintain plant survival, and alleviate tolerance to environmental stress (Walters 2005). Chlorophyll fluorescence has been widely used for decades to elucidate the organization, function, and acclimation of the photosynthetic apparatus at the subcellular and leaf levels (Porcar-Castell et al. 2014). Net photosynthesis is reduced due to regulatory mechanisms that include a decrease in stomatal conductance and chlorophyll concentrations when plants are exposed to saline-alkaline stress, as demonstrated in this study (Table 2). However, we found that melatonin increased the photosynthetic rate and maintained higher Fv/Fm values during saline-alkaline stress (Table 3), consistent with previous findings (Liang et al. 2018). The higher stomatal apertures and chlorophyll concentrations in leaves from melatonin-treated mycorrhizal plants might explain why plants in the +MF/+MT treatment could maintain higher photosynthetic values under saline-alkaline stress. These results suggest that exogenous melatonin can cause stomata to open, contributing to an efficient Photosystem II and alleviating the inhibition of photosynthesis caused by saline-alkaline stress in mycorrhizal plants.
Excessive accumulation of ROS has been found to cause lipid peroxidation, enzyme inactivation, and membrane destruction, although a low level is indispensable to plants (Mittler 2002). As one of the most abundant ROS, H2O2 participates in a series of processes for plant growth, development, and stress responses. In this study, the H2O2 content was significantly induced by saline-alkaline stress, but AM fungus inoculation and/or exogenous melatonin application alleviated stress-triggered ROS accumulation (Fig. 5). Membrane damage is an indicator of the level of lipid destruction under stress conditions, while MDA concentration is generally considered as a reliable indicator for cell membrane stability (Chen et al. 2018). To validate the effects of the AM fungus and melatonin on plant membrane damage, we measured MDA in L. chinensis leaves. We observed a lower concentration of MDA in AM fungus inoculation and melatonin-treated seedlings under saline-alkaline stress (Fig. 5). Moreover, the H2O2 and MDA concentrations in leaves of mycorrhizal L. chinensis were significantly reduced by melatonin pretreatment under the SS treatment. These results indicate that melatonin can decrease membrane damage caused by over-accumulation of ROS in mycorrhizal plants.
It has been suggested that melatonin functions as a natural antioxidant, providing additional protection against oxidative damage by scavenging most ROS (Arnao and Hernández-Ruiz 2014). Inconsistently, melatonin itself acts as not only as a strong antioxidant but also as a trigger of enzymatic antioxidants to minimize the rate of ROS accumulation. The activities of the antioxidant enzymes SOD, CAT, APX, and GR were higher in roots and leaves of L. chinensis under saline-alkaline stress under AM fungus inoculation and/or melatonin pretreatment (Fig. 3). To further substantiate this, we determined the effects of exogenous melatonin on the expression of antioxidant enzyme biosynthesis genes (Cu/Zn-SOD, CAT, and APX). Application of exogenous melatonin significantly increased the transcription levels of Cu/Zn-SOD, CAT, and APX in both roots and leaves of mycorrhizal and non-mycorrhizal plants (Fig. 4). These results indicate that exogenous melatonin plays an important role in promoting antioxidant enzyme activities in roots and leaves of mycorrhizal L. chinensis to maintain cellular ROS at a relatively low level. However, further study is required to examine whether the stimulatory effect results from the direct action of melatonin on enzymes or through signal transduction mechanisms that control related gene expression and change the production of these enzymes (Ke et al. 2018).
Conclusions
In summary, the data provided here indicated that AM fungus inoculation and/or exogenous melatonin application can greatly improve seedling growth and mitigate the effects of stress-related damage (Fig. S1). The study provides the first evidence for the protective effects of exogenous melatonin in the symbiotic association between R. intraradices and L. chinensis in response to direct oxidative stress. The underlying mechanisms may relate to the improvement of AM fungal growth, protection of photosynthetic pigments, maintenance of the photosynthetic system, activation of antioxidant enzymes, and reduction of MDA and H2O2 concentrations. Our findings provide a basis for developing future practices to produce sheepgrass tolerant of abiotic stress.
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Ahammed GJ, Xu W, Liu A, Chen S (2019) Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ Exp Bot 161:303–311
Aliasgharzadeh N, Rastin SN, Towfighi H, Alizadeh A (2001) Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 11:119–122
Antoniou C, Chatzimichail G, Xenofontos R, Pavlou JJ, Panagiotou E, Christou A, Fotopoulos V (2017) Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J Pineal Res 62(4):e12401
Arnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19(12):789–797
Arnao MB, Hernández-Ruiz J (2015) Functions of melatonin in plants: a review. J Pineal Res 59:133–150
Augé RM, Toler HD, Saxton AM (2014) Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: a meta-analysis. Front Plant Sci 5:562
Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res 61(4):426–437
Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK (2014) Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J Pineal Res 56:238–245
Bécard G, Douds DD, Pfeffer PE (1992) Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Appl Environl Microb 58:821–825
Bhattacharjya S, Bhaduri D, Sahu A (2018) Arbuscular mycorrhizal fungi: a potential tool for enhancing crop productivity in salt affected soil. Int J Agric Biol 11:871–880
Biermann B, Linderman RG (1981) Quantifying vesicular-arbuscular mycorrhizae: a proposed method towards standardization. New Phytol 87:63–67
Chen Q, Qi WB, Reiter RJ, Wei W, Wang BM (2009) Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J Plant Physiol 166(3):324–328
Chen S, Cai Y, Zhang L, Yan X, Cheng L, Qi D, Zhou Q, Li X, Liu G (2014) Transcriptome analysis reveals common and distinct mechanisms for sheepgrass (Leymus chinensis) responses to defoliation compared to mechanical wounding. PLoS One 9:e89495
Chen YE, Mao JJ, Sun LQ, Huang B, Ding CB, Gu Y, Liao J, Hu C, Zhang Z, Yuan S, Yuan M (2018) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plantarum 164:349–363
De Luca M, Tauler R, Ioele G, Ragno G (2013) Study of photodegradation kinetics of melatonin by multivariate curve resolution (MCR) with estimation of feasible band boundaries. Drug Test Anal 5(2):96–102
Duru M, Khaled RAH, Ducourtieux C, Theau JP, Quadros FLFD, Cruz P (2009) Do plant functional types based on leaf dry matter content allow characterizing native grass species and grasslands for herbage growth pattern? Plant Ecol 201:421–433
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agricultural Experiment Station. Circular-347
Huang B, Chen Y, Zhao Y, Ding C, Liao J, Hu C, Zhou L, Zhang Z, Yuan S, Yuan M (2019) Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front Plant Sci 10:677
Huang ZA, Jiang DA, Yang Y, Sun JW, Jin SH (2004) Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 42:357–364
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. New Phytol 124:61–68
Karandashov V, Kuzovkina I, Hawkins HJ, George E (2000) Growth and sporulation of the arbuscular mycorrhizal fungus Glomus caledonium in dual culture with transformed carrot roots. Mycorrhiza 10:23–28
Ke Q, Ye J, Wang B, Ren J, Yin L, Deng X, Wang S (2018) Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front Plant Sci 9:914
Kumar A, Dames JF, Gupta A, Sharma S, Gilbert JA, Ahmad P (2015) Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: a biotechnological perspective. Crit Rev Biotechnol 35(4):461–474
Lerner AB, Case JD, Takahashi Y (1960) Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem 235:1992–1997
Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, Jia D, Fu M, Ma F (2012) The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res 53(3):298–306
Li J, Meng B, Chai H, Yang X, Song W, Li S, Lu A, Zhang T, Sun W (2019) Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front Plant Sci 10:499
Liang B, Ma C, Zhang Z, Wei Z, Gao T, Zhao Q, Ma F, Li C (2018) Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ Exp Bot 155:650–661
Lin J, Wang Y, Sun S, Mu C, Yan X (2017) Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci Total Environ 576:234–241
Liu J, Wang L, Wang D, Bonser SP, Sun F, Zhou Y, Gao Y, Teng X (2012) Plants can benefit from herbivory: stimulatory effects of sheep saliva on growth of Leymus chinensis. PLoS One 7:e29259
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Maroco JP, Rodrigues ML, Lopes C, Chaves MM (2002) Limitations to leaf photosynthesis in field-grown grapevine under drought-metabolic and modelling approaches. Funct Plant Biol 29:451–459
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nafees M, Fahad S, Shah AN, Bukhari MA, Ahmed I, Ahmad S, Hussain S (2019) Reactive oxygen species signaling in plants. In: Plant Abiotic Stress Tolerance. Springer, Cham
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Okant M, Kaya C (2019) The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ Sci Pollut Res 26(12):11864–11874
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348
Papalia T, Panuccio MR, Sidari M, Muscolo A (2018) Reactive oxygen species and antioxidant enzymatic systems in plants: role and methods. In: Advances in Plant Ecophysiology Techniques. Springer, Cham
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Porcar-Castell A, Tyystjärvi E, Atherton J, Van der Tol C, Flexas J, Pfündel EE, Moreno J, Frankenberg C, Berry JA (2014) Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: mechanisms and challenges. J Exp Bot 65:4065–4095
Reiter RJ, Tan DX, Zhou Z, Cruz MHC, Fuentes-Broto L, Galano A (2015) Phytomelatonin: assisting plants to survive and thrive. Molecules 20(4):7396–7437
Richards LA (1968) Diagnosis and improvement of saline soils U.S. Salinity Laboratory Staff, agriculture handbook no. 60. Oxford and IBH publishing co, New Delhi
Ruiz-Lozano JM, Porcel R, Azcón C, Aroca R (2012) Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot 63:4033–4044
Sarropoulou VN, Therios IN, Dimassi-Theriou KN (2012) Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb). J Pineal Res 52(1):38–46
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18(6–7):287–296
Shi D, Sheng Y (2005) Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ Exp Bot 54(1):8–21
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Tahira JJ, Khan SN, Suliman R, Anwar W (2011) Evaluation of soil quality on the basis of chemical and microbial health for potential use in agriculture. Afr J Agr Res 6(16):3713–3717
Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (2011) Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot 63:577–597
Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56(411):435–447
Wang D, Ba L (2008) Ecology of meadow steppe in Northeast China. Rangel J 30:247–254
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Yang CW, Xu HH, Wang LL, Liu J, Shi DC, Wang DL (2009) Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants. Photosynthetica 47:79–86
Yang Y, Tang M, Sulpice R, Chen H, Tian S, Ban Y (2014) Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J Plant Growth Regul 33:612–625
Yang Y, Liang Y, Ghosh A, Song Y, Chen H, Tang M (2015a) Assessment of arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead-zinc mine area: potential applications for phytoremediation. Environ Sci Pollut R 22:13179–13193
Yang Y, Han X, Liang Y, Ghosh A, Chen J, Tang M (2015b) The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS One 10:e0145726
Zhang YF, Wang P, Yang YF, Bi Q, Tian SY, Shi XW (2011) Arbuscular mycorrhizal fungi improve reestablishment of Leymus chinensis in bare saline-alkaline soil: implication on vegetation restoration of extremely degraded land. J Arid Environ 75:773–778
Zhang J, Li D, Wei J, Ma W, Kong X, Rengel Z, Chen Q (2019) Melatonin alleviates aluminum-induced root growth inhibition by interfering with nitric oxide production in Arabidopsis. Environ Exp Bot 161:157–165
Zhou Q, Jia J, Huang X, Yan X, Cheng L, Chen S, Liu G (2014) The large-scale investigation of gene expression in Leymus chinensis stigmas provides a valuable resource for understanding the mechanisms of poaceae self-incompatibility. BMC Genomics 15(1):399
Acknowledgments
We gratefully acknowledge Dr. Yanhong Xiao and Dr. Gang Zhang from the School of Environment of Northeast Normal University for their help with parameter measurement and statistical analyses. Also, we thank Prof. Lee Liu of the University of Central Missouri for his help with manuscript revision.
Funding
This study was supported by the National Key Research and Development Program of China (2016YFC0500602), the National Natural Science Foundation of China (31770520, 41807052), the Program for Introducing Talents to Universities (B16011), the State Major Basic Research Programme of China (2016YFC0501202), and the 13th Five-year Project for Science and Technology of Jilin Province (JJKH20180026KJ).
Author information
Authors and Affiliations
Contributions
YY, ZL, and DW participated in the design and coordination of the study. YY, YC, AZ, SY, JW, ZT, and YZ carried out the experiment. YY, ZT, YC, and DW performed the statistical analysis. YY and DW prepared the draft for the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 346 kb)
Rights and permissions
About this article
Cite this article
Yang, Y., Cao, Y., Li, Z. et al. Interactive effects of exogenous melatonin and Rhizophagus intraradices on saline-alkaline stress tolerance in Leymus chinensis. Mycorrhiza 30, 357–371 (2020). https://doi.org/10.1007/s00572-020-00942-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-020-00942-2