Abstract
Rationale
Cadmium (Cd) is an environmental and occupational toxin that represents a serious health hazard to humans and other animals. One of the negative consequences of cadmium exposure is testicular injury.
Objective
This study aimed to investigate the therapeutic effect of etanercept against cadmium chloride-induced testicular damage and the probable underlying mechanisms of its action.
Methods
A total of sixty rats were divided into six groups: control, cadmium chloride (CdCl2) (7 mg/ kg i.p.), and CdCl2 treated with etanercept (5,10 and 15 mg/kg s.c.) and etanercept only (15 mg/kg s.c.). CdCl2 was administrated as a single dose, while etanercept was administered every 3 days for 3 weeks.
Results
CdCl2 reduced serum testosterone, testicular glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD). However, it elevated the levels of malondialdehyde (MDA) and microtubule-associated protein light chain 3B (LC3B) in the testes. Cadmium caused pathogenic alterations as well as increased levels of inflammatory biomarkers such as tumor necrosis factor-alpha (TNF-α) and nuclear factor-kappa B (NF-κB). Besides, the gene expressions of caspase-3 and inducible nitric oxide synthase (i-NOS) and Beclin-1 protein increased with CdCl2 exposure. Interestingly, etanercept relieved the previous toxic effects induced by CdCl2 in a dose-dependent manner as evidenced by inhibition of oxidative stress, inflammatory markers, Beclin-1, LC3B, and caspase-3 accompanied by improvement in histopathological changes.
Conclusion
Etanercept provides a potential therapeutic approach to treat testicular tissue against the damaging effects of Cd by reducing oxidative stress, inflammation, apoptosis, and autophagy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Testicular damage is primarily induced by chemical exposure leading to infertility (Ilieva et al. 2020). Heavy metals, such as Cd, lead, and mercury, are environmental toxic contaminants (Fu and Xi, 2020). Cd causes harmful effects at low doses once absorbed by the body (Ciarrocca et al. 2013). Cdcl2 is an extremely poisonous heavy metal that causes cancer in humans and animals (Odewumi et al. 2016). The potential toxicity of Cd to male reproductive organs may cause testicular steroidogenesis inhibition, sperm-cell apoptosis, testes necrosis, and prostate cancer, which finally result in male infertility (Habib et al. 2019a). Moreover, Cd can pass through the blood–testes barrier, causing changes in the hypothalamic–pituitary–testicular axis and DNA damage (Yang et al. 2021).
However, there is a limited and inconsistent research on the mechanism of Cd-induced testicular injury. The key variables involved in cadmium-induced tissue damage include oxidative stress and inflammation (Arafa et al. 2014). Oxidative stress is triggered by reactive oxygen species (ROS) and an imbalance between ROS and antioxidant enzymes activities (Antar et al. 2021). As a result, oxidative stress activates the NF-κB signaling pathway, which controls several genes implicated in inflammatory responses such as TNF-α and iNOS (Abdelrazek et al. 2016; Fouad et al. 2013).
The mechanism of interaction between apoptosis and autophagy in testicular damage induced by Cd exposure, still remains unknown. Apoptosis and autophagy are two types of programmed cell death that are important for the development and regulation of male reproductive functions (Bustamante-Marín et al. 2012). Oxidative stress induces Ca2 + channel dysfunction and activation of the intrinsic apoptotic pathway (Knight et al. 2019). In addition, TNF-α acts as a death ligand and activates extrinsic apoptosis (Mukhopadhyay et al. 2014).
Autophagy is activated in response to cellular stress, such as oxidative stress (Singh et al. 2018). Increased generation of ROS can activate mitogen-activated protein kinases (MAPKs) (Son et al. 2011), which induce autophagy by phosphorylating Bcl-2. Phosphorylated Bcl-2 cannot form a complex with Beclin-1 (Filomeni et al. 2015; Kroemer et al. 2010). Hence, Beclin-1 expression may represent the autophagic state of a cell. LC3B is also a marker of autophagic activity that is required for phagophore elongation (Ni et al. 2011; Wang et al. 2015).
Etanercept is a recombinant dimeric fusion protein that binds TNF-α and is composed of the extracellular ligand-binding region of the 75-kDa human TNF receptor coupled to the Fc component of human immunoglobulin G1 (Chadwick et al. 2018). Several inflammatory illnesses, such as rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis, are treated with etanercept (Aghdashi et al. 2020). In addition to reducing inflammatory disorders, anti-TNF drugs may improve sperm parameters and hormone levels (Ramonda et al. 2014b). When spermatozoa are exposed to high levels of TNF-α, their genomic and functional integrity may be damaged. Thus, TNF-α may play a role in the pathophysiology of testicular damage (Morsy et al. 2020). Etanercept has shown an effect on testicular function and semen in spondyloarthritis patients (Ramonda et al. 2014b). Moreover, intratesticular expression of mRNAs of both interferon γ and TNF-α is significantly increased in experimental orchitis in mice, indicating that etanercept could be a valuable therapeutic agent in testicular damage (Terayama et al. 2011). Administration of etanercept has been found to promote anti-inflammatory and antioxidant responses in a model of testicular injury (Pascarelli et al. 2017); however, the mechanisms mediating this effect have not been well established and require further research. The above-mentioned possible roles of TNF-antagonists have motivated the desire to explore their potential experimental effects in this regard. Therefore, the current study was conducted to assess the potential therapeutic role of etanercept against chemically induced testicular damage. Several aspects of the activity of etanercept, including its antioxidants, anti-apoptotic, anti-autophagy, and anti-inflammatory potential, have been investigated.
Materials and methods
Drugs and chemicals
Etanercept (Enbrel 50 mg/ 1 ml) prefilled syringe was purchased from Pfizer, Egypt and was diluted with saline. Cadmium chloride was a generous gift from the Analytical Department, Faculty of Pharmacy, Mansoura University, with 96% purity and was dissolved in distilled water.
Animals and experimental design
A total of sixty Wistar male albino rats weighing 190 to 220 g at the start of the experiment were used. The Modern Veterinary Office for Laboratory Animals (Cairo, Egypt) provided for all rats. The rats were allowed to acclimatize under laboratory conditions for two weeks before the experiment. Rats were housed under controlled temperature (25 ºC ± 1) in a 12-h light/dark cycle. Food and water were allowed ad libitum during the study period. The study protocol was approved by the research ethics committee, Faculty of Pharmacy, Suez Canal University (Ismailia, Egypt), according to the Canadian Council on Animal Care Guidelines (License number 201911MA3).
The rats were randomly divided into six experimental groups as follows: in control, rats received saline once daily; in the CdCl2 group, rats received CdCl2 (7 mg/kg; i.p) single dose (Akunna et al. 2017); the third, fourth, and fifth groups received single dose of CdCl2 (7 mg/kg; i.p) and treated with etanercept (5 mg/kg, 10 mg/kg, and 15 mg/kg s.c), respectively, every 3 days for 3 weeks (Totoson et al. 2016); and the sixth group received etanercept only (15 mg/kg/3 days; i.p). Treatment began 8 weeks post-CdCl2 administration.
Killing and biological sample collection
At the end of the experiment, the rats were anesthetized with thiopental sodium (50 mg/kg) and then killed. A dry Eppendorf tube was used to collect blood from a cardiac puncture. The blood samples were allowed to settle for 30 min, followed by centrifugation at 2000 × g for 15 min. The separated serum was stored at -20ºC for further biochemical analyses (Antar et al. 2021). Testes were isolated and collected. One testis was fixed in bouin solution, 5% acetic acid, 9% formaldehyde, and 1.5% picric acid in aqueous solution (Ellenburg et al. 2020) and used in histopathological investigations. Another group of testes was homogenized by phosphate buffer saline and then centrifuged at 4000 rpm for 15 min; then, the supernatant was separated, collected in clean tubes, and stored at (-20ºC) for ELISA analysis. The remaining tissues were frozen at -80 °C for RT-PCR analysis and Western blot analysis.
Assessment of serum cadmium and testicular cadmium
The serum cadmium level and testicular cadmium content were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Cat. No. MBS3809624, MyBioSource®, USA) according to the manufacture's instructions. Standard 50 µl was added to the standard well followed by testing sample 10 µl. Then, 100 µl of HRP-conjugate reagent was added to each well and incubated for 60 min at 37 °C. Each well was washed. Chromogen solution A (50 µl) and chromogen solution B (50 µl) were added to each well. They were gently mixed and incubated for 15 min at 37 °C protected from light. The color in the wells changed from blue to yellow. The optical density (O.D.) at 450 nm was read using a microtiter plate reader within 15 min.
Estimation of serum testosterone, testicular TNF-α, and LC3B
The serum testosterone level was determined using an ELISA kit (Cat. No. MBS262661, MyBioSource®, USA) according to the manufacturer's protocol. One hundred microliters of samples or different concentrations of rat testosterone standard samples were added to corresponding wells. The biotinylated rat testosterone antibody liquid was added. Then, 100 µl of color reagent liquid was added to individual well. When color for the high concentration of standard curve became darker and color gradient appeared, 100 µl color reagent C was added. Optical density at (450 nm) was read within 10 min.
The TNF-α content in the testes was determined using a rat TNF-ELISA kit (Cat. No. MBS355371, MyBioSource®, USA) according to the manufacturer’s instructions. The testicular content of LC3B was determined using an ELISA kit (Cat. No. MBS938189, MyBioSource®, USA) following the manufacturer's protocol.
Assessment of testicular oxidative stress biomarkers
The MDA levels in the tissues were evaluated using an ELISA kit (MyBioSource®, USA, Cat. No. MBS355371). The levels of SOD in tissues were measured using a rat ELISA kit (Cat. No. MBS036924, MyBioSource®, USA). GSH levels in tissues were measured using an ELISA kit (Cat. No. E02G0367, Shang Hai Blue Gene Biotech CO). Another ELISA kit (Cat. No. MBS) was used to determine the amount of CAT in the tissues. The microtiter plate provided in these kits was pre-coated with an antibody specific to MDA, SOD, GSH, or CAT. Standards or samples were then added to the appropriate wells with a biotin-conjugated polyclonal antibody preparation specific for MDA, SOD, GSH, or CAT, and avidin conjugated to horseradish peroxidase (HRP) was added to each microplate well and incubated. Then, a TMB substrate solution was added to each well. Only those wells that contained MDA, SOD, GSH, or CAT, biotin-conjugated antibody, and enzyme-conjugated avidin exhibited a color change. The enzyme–substrate reaction was terminated by the addition of a sulfuric acid solution, and the color change was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of MDA, SOD, GSH, or CAT in the samples was then determined by comparing the O.D. of the samples to the standard curve.
Assessment of iNOS and caspase-3
iNOS and caspase-3 gene expression was evaluated using reverse transcription-polymerase chain reaction (RT-PCR). In brief, pure RNA was extracted using a total RNA Purification Kit according to the manufacturer’s protocol (Thermo Scientific, Fermentas, #K0731). A high-capacity cDNA reverse transcription kit was utilized to convert the total RNA (0.5 to 2 µg) to cDNA. The cDNA samples were then stored at − 20 ◦C. The isolated cDNA was amplified using 2X Maxima SYBR Green/ROX qPCR Master Mix following the manufacturer’s protocol (Thermo Scientific, Waltham, MA, USA). The qRT-PCR assay with the following gene-specific primer sets was optimized with the annealing temperature (iNOS; forward primer: /5 GACCAGAAACTGTCTCACCTG/3, reverse primer: /5 CGAACATCGAACGTCTCACA/3, Caspase3; forward primer:: /5 GGTATTGAGACAGACAGTGG/3, reverse primer: /5 CATGGGATCTGTTTCTTTGC/3, β-actin; forward primer:: /5 AAGTCCCTCACCCTCCCAAAAG/3, reverse primer:: /5 AAGCAATGCTGTCACCTTCCC/3 Real-time PCR amplification and analysis were performed to measure the expression of mRNAs of target genes in the tissue relative to β-actin mRNA expression as an internal reference. The applied biosystems software version 3.1 (StepOneTM, USA) was used to evaluate the expression of mRNAs of target genes in the tissue, with β-actin as an internal reference.
Assessment of NF-κB and Beclin-1 by western blot
Proteins were extracted from testicular tissue using an extraction buffer. Lysates were allowed to remain on ice for 30 min and then centrifuged at 15,000 rpm for 30 min at 4 °C. The soluble lysates were mixed at a 1:4 ratio with 5-ml Laemmle buffer and heated for 4 min at 94 °C. Next, 20 µg of protein was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA), and then blocked with 5% defatted milk in TBS-Tween buffer for 3 h at 4 °C (Daiichikagaku, Tokyo, Japan). The membranes were incubated with specific primary antibodies including anti-Beclin-1 (sc-4834, Santa Cruz, USA) and NF-κB (ab 16,502, Abcam Company). On the next day, β-actin monoclonal antibody (sc- 47,778, Santa Cruz, USA) was added and incubated for 1 h on a roller shaker at 4 °C. To remove unbound primary antibody, the membranes were washed 5 times for 5 min each in TBS-Tween. The membrane incubated with the appropriate secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG) diluted at 1:5000 in blocking solution for 1 h at room temperature and then washed three times with 1 × TBS-T. The densitometry analysis of protein bands was performed using Image J. software. The density of each band was normalized with β-actin.
Histopathological examination
The testicular samples collected from all groups were fixed in the bouin solution and then dried using repeated dilutions of alcohol after fixation. Tissues were prepared, embedded in paraffin, sectioned at 5-mm intervals, and stained with hematoxylin and eosin (H&E). For histological investigation under a light electric microscope, 4-µm-thick slices were cut using a microtome and stained with H&E.. The investigation was conducted by a skilled pathologist without previous information about the treatments.
Statistical analysis
All data are presented as a as mean ± SD. One-way analysis of variance (ANOVA) was used to determine statistical significance, followed by Tukey’s multiple comparison analysis. The level of significance was fixed at a p-value of < 0.05.
Results
Effect of etanercept on testes/body weight index
In the CdCl2 control group, CdCl2 significantly decreased testes/body weight index by approximately 0.61-fold compared with that in the control group. In a dose-dependent way, treatment with etanercept (5 mg/kg and 10 mg/kg) significantly increased the testes/body weight index by 21.85% and 36.02%, respectively, compared with the CdCl2 group. Treatment with 15 mg/kg etanercept induced a further significant increase in testes/body weight index by 46.83% in comparison with the CdCl2 group (Table 1).
Table 1 Effect of etanercept (5,10, 15 mg/kg) on the testes/body weight index in experimental groups.
Results are expressed as mean ± SD. All data were analyzed using one-way ANOVA followed by Tukey's multiple comparison test at P<0.05. * Significantly different from control, & significantly different from CdCl2 group, # significantly different from 5 mg/kg etanercept-treated group, $ significantly different from 10 mg/kg etanercept-treated group.
Effect of etanercept on testosterone level
The testosterone level in the CdCl2 control group was significantly decreased by threefold compared to the control group. In a dose-dependent way, treatment with etanercept (5, 10, and 15 mg/kg) significantly increased the serum testosterone by 24. 94%, 47.71%, and 90.86%, respectively, compared with the CdCl2 group. In comparison with the CdCl2 group, treatment with etanercept alone (15 mg/kg) resulted in a significant increase in serum testosterone level by 152.21%. These findings indicate favorable effect of etanercept on the testicular steroidogenic changes following Cd exposure (Table 2).
Results are expressed as mean ± SD. All data were analyzed using one-way ANOVA followed by Tukey's multiple comparison test at P<0.05. * Significantly different from control & significantly different from CdCl2 group, # significantly different from 5 mg/kg etanercept-treated group, $ significantly different from 10 mg/kg etanercept-treated group.
Effect of etanercept on serum cadmium and testicular cadmium levels
In comparison with the control group, the serum cadmium level was significantly increased by threefold in the CdCl2 group. Treatment with etanercept (5, 10, and 15 mg/kg) significantly reduced the serum cadmium level by 36.27%, 39.72%, and 58.87%, respectively, compared with the CdCl2 group.
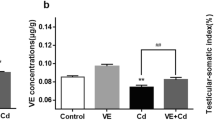
The testicular cadmium content was also markedly increased by 9.19-fold in the CdCl2 group compared with the control group. Treatment with etanercept (5 mg/kg) reduced testicular cadmium content by 70.64% compared with the CdCl2 group, and treatment with etanercept (10 mg/kg) significantly reduced the testicular cadmium content by 75.56%, compared with the CdCl2 group. Optimum enhancement was observed with etanercept (15 mg/kg) treatment with a significant decrease in testicular cadmium content by 87.48% compared with the CdCl2 group (Fig. 1).
Effect of etanercept (5 mg/kg, 10 mg/kg, 15 mg/kg) on: (a) serum cadmium level, (b) content of testicular cadmium in experimental groups. Results are expressed as mean ± SD. All data were analyzed using one-way ANOVA followed by Tukey's multiple comparison test at P < 0.05. *Significantly different from control, &significantly different from CdCl2 group, #Significantly different from 5 mg/kg etanercept-treated group, $Significantly different from 10 mg/kg etanercept-treated group (+ : present, -: absent)
Effect of etanercept on CdCl 2 -induced changes in testes oxidative and anti-oxidative stress markers
Exposure to cadmium chloride raised the testicular MDA content by fourfold and reduced the testicular SOD, CAT, and GSH contents by approximately (7), (7), and (5.6) folds, respectively, in the CdCl2 group compared with the control group. Treatment with etanercept (5, 10, and 15 mg/kg) significantly ameliorated CdCl2-induced damage to different ranges. Etanercept at (5 mg/kg) decreased the testicular MDA content and increased the SOD, GSH, and CAT contents by 32.21%, 53.58%, 37.75%, and 56.25%, respectively, compared with those in the CdCl2 group, and etanercept at (10 mg/kg) significantly decreased testicular MDA content and restored SOD, GSH, and CAT contents by 102.61%, 72.02, 68.53%, and 71.27%, respectively, compared with those in the CdCl2 group. The dose of (15 mg/kg) etanercept provided the best results, with a considerable decrease in the testicular MDA content and an increase in the SOD, GSH, and CAT levels by 188.16%, 80.37%, 76.98%, and 78.77%, respectively, compared with those in the CdCl2 group as shown in Fig. 2
Effect of etanercept (5 mg/kg, 10 mg/kg, 15 mg/kg) on oxidative stress biomarkers in experimental groups. (a) Testicular content of malondialdehyde. (b) Testicular content of superoxide dismutase enzyme. (c) Testicular content of glutathione. (d) Testicular content of catalase enzyme. Results are expressed as mean ± SD. All data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test at P < 0.05. *Significantly different from control, &significantly different from CdCl2 group, #Significantly different from 5 mg/kg etanercept-treated group, $Significantly different from 10 mg/kg etanercept-treated group(+ : present, -: absent).
Effect of etanercept on testicular inflammatory markers (TNF-α, iNOS, and NF-κB)
The content of TNF-α was markedly elevated by 6.45-fold. Likewise, iNOS gene expression was significantly increased by approximately 5.9-fold along with a significant rise in NF-κB protein expression by approximately 2.43-fold, in the CdCl2 group compared with those in the control group.
Treatment with etanercept (5, 10, and 15 mg/kg) lowered testicular TNF-α content by 31.08%, 50.09%, and 64.13%, respectively, compared with the CdCl2 group. The testicular iNOS expressions were also reduced by 35.97%, 46.64%, and 62.65%, respectively, compared with that in the CdCl2 control group. Similarly, treatment with etanercept (5, 10, and 15 mg/kg) reduced the testicular NF-κB protein expression by 33.88%, 37.77%, and 56.80%, respectively, compared with the CdCl2 control group (Fig. 3).
Effect of etanercept (5 mg/kg, 10 mg/kg, 15 mg/kg) on inflammatory biomarkers in experimental groups. (a) Testicular content of TNF-α. (b) Testicular gene expression of i-NOS. (c) Western blot analysis showing protein expression of NF-κB and β-actin. (d) Testicular protein expression of NF-κB. Results are expressed as mean ± SD. All data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test at P < 0.05. *Significantly different from control, &significantly different from CdCl2 group, #Significantly different from 5 mg/kg etanercept-treated group, $Significantly different from 10 mg/kg etanercept-treated group(+ : present, -: absent).
Effect of etanercept on caspase-3
Exposure to cadmium chloride increased the testicular caspase-3 gene expression by threefold compared with that in the control group. Treatment with etanercept (5 and 10 mg/kg) reduced the testicular caspase-3 gene expression by 29.48% and 41.34%, sequentially compared with the CdCl2 group. Treatment with (15 mg/kg) etanercept provided the best results, with a significant reduction in the testicular caspase-3 gene expression by 53.52% (Fig. 4).
Effect of etanercept (5 mg/kg, 10 mg/kg, 15 mg/kg) on testicular caspase-3 gene expression in experimental groups. Results are expressed as mean ± SD. All data were analyzed using one-way ANOVA followed by Tukey's multiple comparison test at P < 0.05. *Significantly different from control, &significantly different from CdCl2 group, #Significantly different from 5 mg/kg etanercept-treated group, $Significantly different from 10 mg/kg etanercept-treated group(+ : present, -: absent)
Effect of etanercept on autophagy biomarkers
Beclin-1 protein expression was significantly elevated by approximately 3.45-fold. Similarly, the LC3B content was significantly increased by approximately 5.62-fold in the CdCl2 group compared with the control group. Treatment with etanercept (5, 10 and 15 mg/kg) reduced the testicular Beclin-1 protein expression by 17.98%, 34.52%, and 52.05%, respectively, compared with the CdCl2 group. Similarly, the testicular LC3B content was, respectively, reduced by 43.08%, 47.38%, and 71.07% compared with that in the CdCl2 group (Fig. 5).
Effect of etanercept (5 mg/kg, 10 mg/kg, 15 mg/kg) on autophagy biomarkers in experimental groups (a) Western blot analysis showing protein expression of Beclin-1 and β-actin. (b) Testicular protein expression of Beclin-1. (c) Testicular content of LC3-B. Results are expressed as mean ± SD. All data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test at P < 0.05. *Significantly different from control, &significantly different from CdCl2 group, #Significantly different from 5 mg/kg etanercept-treated group, $Significantly different from 10 mg/kg etanercept-treated group(+ : present, -: absent).
Histopathological examination
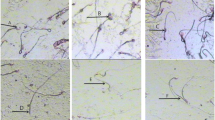
The H&E-stained testicular sections of the control group exhibited normal seminiferous tubules lined with spermatogenic cell layers (arrows) with the presence of free sperms within their lumen (arrowheads). The CdCl2 group revealed severe testicular degeneration within the spermatogenic cell layers (arrows), hyperplasia of Leydig cells within edematous (arrowheads) and atypia within some spermatogenic cells (tailed-arrow). CdCl2 with etanercept (5 mg/kg) treatment led to a decrease in testicular degeneration within the spermatogenic cell layers (arrows) with a core of desquamative spermatogenic cells (arrowheads). CdCl2 with etanercept (10 mg/kg) treatment resulted in a marked decrease in testicular degeneration within the spermatogenic cell layers (arrows) with the appearance of free sperms within the lumen (arrowheads). Finally, CdCl2 with etanercept (15 mg/kg) treatment induced a remarkable decrease in the spermatogenic cell layer damage (arrows) and an increase in spermatogenesis (arrowheads indicate free sperms). Furthermore, testes sections of the group treated with etanercept (15 mg/kg)-treated group only revealed a normal seminiferous tubule lined with spermatogenic cell layers (arrows) with the presence of free sperms within their lumen (arrowheads) as shown in Fig. 6.
Discussion
Human exposure to environmental pollutants that negatively affect the male reproductive function has become the most pressing public health issue (Ma et al. 2019). In the present study, it was observed that CdCl2 exposure led to a remarkable reduction in the testes/body weight index. These results were in agreement with the other studies, which have shown that CdCl2 reduced the weight of sex organs (Bashandy et al. 2016; Erboga et al. 2016; Nna et al. 2017). CdCl2 was found to cause necrotic degeneration of testicular tissues as a result of oxidative stress and inflammation. Interestingly, the testicular weight primarily depends on the mass of undifferentiated spermatogenic cells. Hence, CdCl2 may have a detrimental impact on the number of germ cells and elongated spermatids, resulting in a reduction of testicular weight (Ola-Mudathir et al. 2008). Moreover, CdCl2 has the potential to degrade the functional and structural integrity of testicular tissues (CHEN et al. 2013).
There are different therapeutic agents used in management of cd-induced testicular damage such as natural products and monoclonal antibodies. Previous studies showed that kolaviron and quercetin have protective effects on cadmium-induced testicular damage and endocrine pathology in rats by their antioxidant activity (Farombi et al. 2012). Moreover, fenugreek seed powder mitigates cadmium-induced testicular damage and hepatotoxicity in male rats by its antioxidant and anti-inflammatory activities (Arafa et al. 2014). On the other hand, infliximab, anti-TNF-α, abrogates cadmium-induced testicular damage and spermiotoxicity via enhancement of steroidogenesis and suppression of inflammation and apoptosis mediators (Habib et al. 2019b). In the current study, etanercept, anti-TNF-α, showed therapeutic effect against cadmium-induced testicular damage by suppressing oxidative stress, inflammation, apoptosis, and autophagy. Indeed, anti-TNF-α drugs can improve testicular function (Ramonda et al. 2014a).
The most common side effects linked to etanercept use in clinical trials are non-upper respiratory tract infections followed by upper respiratory infections (Scheinfeld, 2004). Etanercept is associated with serious bacterial infections (Elwood et al. 2003). It can also promote the reactivation of tuberculous, though to a lesser extent of infliximab. Infliximab has been related to viral and bacterial infection. As foreign substances, TNF-α blockers are immunogenic and can result in the development of neutralizing antibodies. Treatment with infliximab can be associated with the development of neutralizing antibodies to infliximab in approximately 10% of patients. On the other hand, 5% of patients treated with etanercept for rheumatoid and psoriatic arthritis develop antibodies to etanercept. However, these antibodies are not related to the side effects or efficacy of etanercept (Scheinfeld, 2004).
Treatment with etanercept restored the testes weight in a dose-dependent manner. Consistent with this result, treatment with infliximab (anti-TNF-α) was found to significantly alleviate Cd-induced weight loss in the reproductive organs. High levels of TNF-α "the most important inflammatory mediator" played an essential role in reducing the quantity of germ cells and elongated spermatids (Bashandy et al. 2016). Therefore, TNF-α inhibition by etanercept could be responsible for its effect on the testicular weight increase in the present study.
Treatment with etanercept decreased Cd concentration. Administration of Cd to rats causes acute tubular necrosis, which results in decreasing its elimination from the body and increasing its serum levels (Aoyagi et al. 2003). Previous studies showed that etanercept is a nephroprotective drug by its antioxidant properties, decreasing serum creatinine, and proteinuria (Kim et al. 2013). It has been reported that simultaneous administration of CdCl2 and quercetin decreased Cd accumulation in serum, testis, and epididymis (Nna et al. 2017). Quercetin improved renal function in cadmium-exposed rats by lowering oxidative stress in the kidneys, with increase in creatinine, urea, and cadmium clearance (Morales et al. 2006; Renugadevi et al. 2010). By the same mechanism of decreasing oxidative stress, etanercept could improve renal function, decrease proteinuria, and increase Cd clearance from the body. In addition, it has been reported that Cd triggers disturbances in the lipid composition so that the macrophages will release TNF-α and increase oxidative stress in organs. In addition, there is a linear relationship between Cd concentration and TNF-α protein levels (Rumahlatu et al. 2019). Thus, it may be considered that the inhibition of TNF-α by etanercept is responsible for the decreased serum cadmium concentration.
Oxidative stress is considered as the main mechanism by which CdCl2 mediates testicular injury. It was observed that CdCl2 increased the MDA content and decreased the GSH, SOD, and CAT contents. These results came in line with previous research, which revealed that Cd can initiate oxidative stress and cause an indirect increase in ROS by binding to the sulfhydryl (-SH) groups of the ROS scavenging proteins and non-protein molecules like GSH (Djuric et al. 2015; Erboga et al. 2016; Nna et al. 2017). Lipid peroxidation is also evidence of oxidative stress induced by Cd exposure (Al Olayan et al. 2020). In addition, CdCl2 interacts with the structure of enzyme protein, inhibiting catalytic activity, and replaces the other divalent cation required for antioxidant enzyme activity (Almeer et al. 2018). Treatment with etanercept reduced the testicular oxidative stress, as evidenced by lower MDA levels and increased GSH, SOD, and CAT activities. A previous study showed that etanercept exerts a protective effect against myocardial ischemia/reperfusion injury in rats, which could be due to its ability to reduce lipid peroxidation and increase antioxidant enzyme activity (Yang et al. 2014).
Inflammation is a crucial process in Cd-induced testicular damage. CdCl2 increased the testicular TNF-α content and induced the expression of NF-κB and iNOS leading to excessive production of nitric oxide (NO). These results matched with previous experimental studies, which showed that CdCl2 induced the NF-κB signaling pathway by dissociation from its inhibitory IκBα (inhibitory kappa B) as a result of oxidative stress. This signaling promotes the production of proinflammatory cytokines such as TNF-α (Ansari et al. 2017; Habib et al. 2019b; Jiaxin et al. 2020). CdCl2 also triggers cytotoxicity by increasing the expression of iNOS. The activation of macrophages and other leukocytes by a high amount of NO intensifies inflammation, thus contributing to testicular disease (Elmallah et al. 2017). However, treatment with etanercept dose-dependently decreased the testicular TNF-α content together with inhibiting the expression of NF-κB and iNOS. This result can be explained by the following sequence: etanercept is a competitive inhibitor of TNF-α, which regulates other inflammatory mediators such as NF-κB and iNOS (Goffe and Cather 2003; Yang et al. 2014). So, the inhibition of TNF-α by etanercept is followed by inhibition of NF-κB and iNOS.
Testosterone is required for normal spermatogenesis and the maintenance of normal seminiferous tubule structure (Sadik 2008). Moreover, male sex organ mass loss is considered as the main sign of androgenic status alterations. CdCl2 administration decreased the levels of serum testosterone. It has been demonstrated that the effects of CdCl2 on Leydig cells and the hypothalamic–pituitary–testicular axis probably cause endocrine disruption (Siu et al. 2009). CdCl2 accumulates in the hypothalamus and pituitary gland, stimulating oxidative stress, and adversely affects the hormonal secretions of these organs (Nna et al. 2017). Moreover, inflammatory stimuli, in particular TNF-α, enhance the iNOS expression, which catalyzes the synthesis of a large quantity of NO. Then, NO interacts with superoxide anion to form peroxynitrite radicals, which cause cell damage and steroidogenesis suppression in Leydig cells and the adrenal cortex (Cameron and Hinson, 1993; Sokanovic et al. 2013).
In the current study, etanercept treatment increased the serum testosterone levels in a dose-dependent manner. This effect could be due to its antioxidant properties, which protect Leydig cells against CdCl2-induced oxidative stress and improve testicular steroidogenesis (Arafa et al. 2014). These findings are in parallel with studies showing that etanercept may have a potential impact on the management of TNF-α-induced infertility (Pascarelli et al. 2017). TNF-α has been shown to impair gonadal activities, specifically gene expressions of steroidogenic enzymes and steroidogenesis in Leydig cells (Hong et al. 2004; Sadasivam et al. 2015). In addition, etanercept inhibits the effect of the aromatase enzyme “the enzyme that converts testosterone to estrogen” because proinflammatory cytokines, such as TNF-α, activate the aromatase enzyme (Atzeni et al. 2008; Cutolo et al. 2006).
Cadmium exposure induces DNA damage of testicular cells. Apoptosis is a process in which cells with substantial DNA damage die (Han et al. 2020). Excessive apoptosis of testicular germ cells is caused by direct alterations of hormonal support from Leydig cells (Fouad et al. 2009). Low intratesticular testosterone levels in response to Cd-induced toxicity may cause germ cell detachment from the seminiferous epithelium and apoptosis of germ cells because, in seminiferous tubules, testosterone is required for the attachment of distinct generations of germ cells (Sadik 2008). This coincides with the result obtained in the present study showing the upregulation of caspase-3 expression by CdCl2.
Oxidative stress stimulates the mitochondrial pathway of apoptosis by Cd exposure. ROS generation and accumulation cause Ca+2 channel dysregulation, which alters mitochondrial membrane permeability and cytochrome c release into the cytoplasm. In the presence of adenosine triphosphate (ATP), cytochrome c can activate apoptotic protease activating factor-1 (Apaf-1) that results in the activation of caspase-9. Activated caspase-9 cleaves caspase-3 zymogen that produces activated caspase-3, which causes DNA fragmentation (Kassab et al. 2020; Knight et al. 2019; Ye et al. 2007). Inflammation also triggers apoptosis by stimulating death receptor signaling pathways. TNF-α binds to TNFR1, the death receptor (DR), on the plasma membrane. Activation of DR can recruit and activate caspase-8. Caspase-8 recruitment stimulates caspase-3. CdCl2 was found to cause an upregulation of DR expression (Jiaxin et al. 2020). Furthermore, NF-κB activation induces the expression of apoptotic markers (Behl et al. 2008; Ye et al. 2019).
Etanercept administration downregulated the expression of caspase-3 in the testicular tissue. TNF-α was found to change the expression of vascular adhesion molecules, allowing lymphocytes and macrophages to reach the target site, activate the inflammation, and trigger apoptosis by the release of cytotoxic substances. A study demonstrated that etanercept could preserve the retina of diabetic rats by reducing the leakage of retina and apoptosis of retinal cells (Ye et al. 2019). The anti-apoptotic effect of etanercept may be due to its antioxidant and anti-inflammatory properties (Yildirim et al. 2016).
Simultaneously, strong autophagy induces cell death and apoptosis, whereas weak autophagy prevents apoptosis and maintains cells alive (Gump and Thorburn 2011). The link between autophagy and apoptosis is still unclear because the 2 processes occur independently and may either induce or oppose one other (Mi et al. 2016). Autophagy is involved in a variety of physiological and pathological processes, including cell survival, cell death, cell metabolism, and immunity (Chuang et al. 2014; Circu and Aw 2012). Under stress conditions, autophagy plays a vital role in preventing cell death by removing harmful particles and protein aggregates. However, the protective effects of autophagy on the body are limited. When the dosage and exposure period of cadmium surpass the safety threshold, it can induce irreversible cell damage by causing autophagic death (Wang et al. 2017b).
In the present study, CdCl2 exposure increased the Beclin-1 protein expression and testicular content of LC3B. These results were supported by previous experimental research (Wang et al. 2017a). Cadmium prevents the synthesis of functional metallothionein, which primarily reduces inflammatory responses. Therefore, increasing levels of inflammatory cytokines, such as TNF-α, caused by cadmium absorption lead to an increase in the number of cells undergoing autophagy (Inoue, 2013; Lee et al. 2015). Moreover, cadmium stimulates the endoplasmic reticulum, causing calcium to leak out and cause autophagic cell death (Kalogeris et al. 2012). Cd also harms cells by prohibiting cell communication via gap junctions. This impact can be amplified by autophagy, resulting in significant harm (Zou et al. 2015). Etanercept treatment, especially at a high dose, reduced the expression of Beclin-1 and LC3B. These results may be attributed to the effect of TNF-α in autophagy induction (Yuan et al. 2018). TNF-α promotes hepatocyte apoptosis and autophagy in humans, according to an earlier study (Ezquerro et al. 2019). Therefore, etanercept could inhibit the overactivity of autophagy induced by cadmium exposure.
Histopathological examination showed that Cdcl2 led to severe testicular degeneration within the spermatogenic cell layers, hyperplasia of Leydig cells within edematous as well as atypia in some spermatogenic cells. The presence of atypical germ cells is the characteristic finding of testicular damage (Michalova et al. 2020). In contrast, etanercept treatment dose-dependently led to a remarkable decrease in the degenerative changes within the spermatogenic cell layers and interstitial cell proliferation and an increase in spermatogenesis. This result supports that etanercept may have a potential impact on the management of TNF-α induced infertility (Pascarelli et al. 2017).
Conclusion
Current data demonstrated a new mechanistic pathway by which etanercept alleviated Cd-induced testicular damage. The observed improvement in testicular function and physiology was achieved through a variety of mechanisms including reducing oxidative stress, improving the antioxidant activity, inhibition of pro-inflammatory cytokines (TNF-α and i NOS), suppression of autophagy markers (Beclin-1 and LC3B) and testicular damage-induced apoptosis. It is worth noting that anti-inflammatory, antioxidant, and anti-apoptotic properties of etanercept are dose-dependent. More investigations are needed for explaining the molecular mechanisms of etanercept protective effect.
References
Abdelrazek HM Helmy SA Elsayed DH Ebaid HM Mohamed RMJRb 2016 Ameliorating effects of green tea extract on cadmium induced reproductive injury in male Wistar rats with respect to androgen receptors and caspase3 16 300 308
Aghdashi MA, Khadir M, Dinparasti-Saleh R (2020) Antinuclear antibodies and lupus-like manifestations in rheumatoid arthritis and ankylosing spondylitis patients at 4 months’ follow-up after treatment with infliximab and etanercept. Curr Rheumatol Rev 16:61–66
Akunna G, Obikili E, Anyawu G, Esom EJJoP, Toxicology (2017): Evidences for spermatozoa toxicity and oxidative damage of cadmium exposure in rats. 12, 50-6
Al Olayan EM, Aloufi AS, AlAmri OD, Ola H, Moneim AEAJSotTE (2020): Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis. 723, 137969
Almeer RS, Soliman D, Kassab RB, AlBasher GI, Alarifi S, Alkahtani S, Ali D, Metwally D, Abdel Moneim AEJIjoms (2018): Royal jelly abrogates cadmium-induced oxidative challenge in mouse testes: involvement of the Nrf2 pathway. 19, 3979
Ansari MA, Raish M, Ahmad A, Alkharfy KM, Ahmad SF, Attia SM, Alsaad AM, Bakheet SAJEt, pharmacology (2017): Sinapic acid ameliorate cadmium-induced nephrotoxicity: in vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-κB downregulation. 51, 100 107
Antar SA, ElMahdy MK, Khodir AE (2021) A novel role of pirfenidone in attenuation acetic acid induced ulcerative colitis by modulation of TGF-β1/JNK1 pathway. Int Immunopharmacol 101:108289
Aoyagi T, Hayakawa K, Miyaji K, Ishikawa H, Hata MJIjou (2003): Cadmium nephrotoxicity and evacuation from the body in a rat modeled subchronic intoxication. 10, 332-338
Arafa MH, Mohammad NS, Atteia HHJE, pathology t (2014): Fenugreek seed powder mitigates cadmium-induced testicular damage and hepatotoxicity in male rats. 66, 293-300
Atzeni F, Sarzi-Puttini P, Cutolo M, Straub RHJHoSAD (2008): Modulation of hormone axes by anti-TNF therapy. 9, 301-308
Bashandy SAE-M, Omara EAA, Ebaid H, Amin MM, Soliman MSJAPJoTB (2016): Role of zinc as an antioxidant and anti-inflammatory to relieve cadmium oxidative stress induced testicular damage in rats. 6, 1056 1064
Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DTJTAjop (2008): Diabetes-enhanced tumor necrosis factor-α production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. 172, 1411–1418
Bustamante-Marín X, Quiroga C, Lavandero S, Reyes JG, Moreno RDJA 2012 Apoptosis, necrosis and autophagy are influenced by metabolic energy sources in cultured rat spermatocytes. 17, 539 550
Cameron L, Hinson JJJoE (1993): The role of nitric oxide derived from L-arginine in the control of steroidogenesis, and perfusion medium flow rate in the isolated perfused rat adrenal gland. 139, 415.423
Chadwick L, Zhao S, Mysler E, Moots RJJCRR (2018): Review of biosimilar trials and data on etanercept in rheumatoid arthritis. 20, 84
CHEN F-x, GONG P, JIN S, ZHOU A-nJLM, Research MM (2013): Histopathological Change of Mice Testis induced by Cadmium and Protection Effect of Anthocyanins from Blueberry. 03
Chuang S-Y, Lin C-H, Fang J-YJBri (2014): Natural compounds and aging: between autophagy and inflammasome. 2014
Ciarrocca M, Capozzella A, Tomei F, Tomei G, Caciari TJC (2013) Exposure to cadmium in male urban and rural workers and effects on FSH. LH and Testosterone 90:2077–2084
Circu ML, Aw TYJBeBA-MCR (2012): Glutathione and modulation of cell apoptosis. 1823, 1767-1777
Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Pizzorni C, Paolino S, Seriolo B, Felli L, Straub RHJAotNYAoS (2006): Anti‐TNF and Sex Hormones. 1069, 391-400
Djuric A, Begic A, Gobeljic B, Stanojevic I, Ninkovic M, Vojvodic D, Pantelic A, Zebic G, Prokic V, Dejanovic BJF, Toxicology C (2015): Oxidative stress, bioelements and androgen status in testes of rats subacutely exposed to cadmium. 86, 25–33
Ellenburg JL, Kolettis P, Drwiega JC, Posey AM, Goldberg M, Mehrad M, Giannico G, Gordetsky JJCP (2020): Formalin Versus Bouin Solution for Testis Biopsies: Which Is the Better Fixative? 13, 2632010X19897262
Elmallah MI, Elkhadragy MF, Al-Olayan EM, Abdel Moneim AEJIjoms (2017): Protective effect of Fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. 18, 957
Elwood RL, Pelszynski MM, Corman LIJTPidj (2003): Multifocal septic arthritis and osteomyelitis caused by group A Streptococcus in a patient receiving immunomodulating therapy with etanercept. 22, 286-288
Erboga M, Kanter M, Aktas C, Donmez YB, Erboga ZF, Aktas E, Gurel AJBter (2016): Anti-apoptotic and anti-oxidant effects of caffeic acid phenethyl ester on cadmium-induced testicular toxicity in rats. 171, 176-184
Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, Becerril S, Catalán V, Gómez-Ambrosi J, Silva CJTJoCE, Metabolism, (2019) Ghrelin Reduces TNF-α–Induced Human Hepatocyte Apoptosis Autophagy, and Pyroptosis. Role in Obesity-Associated NAFLD 104:21–37
Farombi E, Adedara I, Akinrinde S, Ojo O, Eboh A (2012) Protective effects of kolaviron and quercetin on cadmium-induced testicular damage and endocrine pathology in rats. Andrologia 44:273–284
Filomeni G, De Zio D, Cecconi FJCD, Differentiation (2015): Oxidative stress and autophagy: the clash between damage and metabolic needs. 22, 377-388
Fouad AA, Albuali WH, Jresat IJBter (2013): Simvastatin treatment ameliorates injury of rat testes induced by cadmium toxicity. 153, 269-278
Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Ali AAJT (2009): Protective effect of hemin against cadmium-induced testicular damage in rats. 257, 153-160
Fu Z, Xi S (2020) The effects of heavy metals on human metabolism. Toxicol Mech Methods 30:167–176
Goffe B, Cather JCJJotAAoD (2003): Etanercept: an overview. 49, 105–111
Gump JM, Thorburn AJTicb (2011): Autophagy and apoptosis: what is the connection? 21, 387–392
Habib R, Wahdan SA, Gad AM, Azab SS (2019a) Infliximab abrogates cadmium-induced testicular damage and spermiotoxicity via enhancement of steroidogenesis and suppression of inflammation and apoptosis mediators. Ecotoxicol Environ Saf 182:109398
Habib R, Wahdan SA, Gad AM, Azab SSJE, safety e (2019b): Infliximab abrogates cadmium-induced testicular damage and spermiotoxicity via enhancement of steroidogenesis and suppression of inflammation and apoptosis mediators. 182, 109398
Han C, Zhu Y, Yang Z, Fu S, Zhang W, Liu CJJoe (2020): Protective effect of Polygonatum sibiricum against cadmium-induced testicular injury in mice through inhibiting oxidative stress and mitochondria-mediated apoptosis. 261, 113060
Hong CY, Park JH, Ahn RS, Im SY, Choi H-S, Soh J, Mellon SH, Lee KJM, Biology C (2004): Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. 24, 2593-2604
Ilieva I, Sainova I, Yosifcheva K (2020) Toxic Effects of Heavy Metals (Lead and Cadmium) on Sperm Quality and Male Fertility. Acta Morphologica Et Anthropologica 27:3–4
Inoue K-i, Takano HJCpb (2013): Metallothionein as a negative regulator of pulmonary inflammation. 14, 414-419
Jiaxin S, Shengchen W, Yirong C, Shuting W, Shu LJF, immunology s (2020): Cadmium exposure induces apoptosis, inflammation and immunosuppression through CYPs activation and antioxidant dysfunction in common carp neutrophils. 99, 284–290
Kalogeris T, Baines CP, Krenz M, Korthuis RJJIroc, biology m (2012): Cell biology of ischemia/reperfusion injury. 298, 229-317
Kassab RB, Lokman MS, Daabo HM, Gaber DA, Habotta OA, Hafez MM, Zhery AS, Moneim AEA, Fouda MSJJoFB (2020): Ferulic acid influences Nrf2 activation to restore testicular tissue from cadmium‐induced oxidative challenge, inflammation, and apoptosis in rats. 44, e13505
Kim HW, Noh JW, Kim JJJoRD (2013): Rheumatoid Arthritis with Secondary Amyloidosis and Chronic Kidney Disease with a Good Response to Etanercept. 20, 389-392
Knight T, Luedtke D, Edwards H, Taub JW, Ge YJBp (2019): A delicate balance–The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. 162, 250–261
Kroemer G, Mariño G, Levine BJMc (2010): Autophagy and the integrated stress response. 40, 280-293
Lee V, McMahan RS, Hu X, Gao X, Faustman EM, Griffith WC, Kavanagh TJ, Eaton DL, McGuire JK, Parks WCJN (2015): Amphiphilic polymer-coated CdSe/ZnS quantum dots induce pro-inflammatory cytokine expression in mouse lung epithelial cells and macrophages. 9, 336-343
Ma Y, He X, Qi K, Wang T, Qi Y, Cui L, Wang F, Song MJJoES (2019): Effects of environmental contaminants on fertility and reproductive health. 77, 210-217
Mi Y, Xiao C, Du Q, Wu W, Qi G, Liu XJFRB, Medicine (2016): Momordin Ic couples apoptosis with autophagy in human hepatoblastoma cancer cells by reactive oxygen species (ROS)-mediated PI3K/Akt and MAPK signaling pathways. 90, 230–242
Michalova K, McKenney JK, Kristiansen G, Steiner P, Grossmann P, Putzova M, Martinek P, Chottova-Dvorakova M, Michal M, Hes OJVA (2020): Novel insights into the mixed germ cell-sex cord stromal tumor of the testis: detection of chromosomal aneuploidy and further morphological evidence supporting the neoplastic nature of the germ cell component. 477, 615-623
Morales A, Vicente-Sanchez C, Sandoval JS, Egido J, Mayoral P, Arévalo M, Fernández-Tagarro M, López-Novoa J, Pérez-Barriocanal FJF, Toxicology C (2006): Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. 44, 2092-2100
Morsy MA, Abdel-Aziz AM, Abdel-Hafez S, Venugopala KN, Nair AB, Abdel-Gaber SA (2020) The possible contribution of P-glycoprotein in the protective effect of paeonol against methotrexate-induced testicular injury in rats. Pharmaceuticals 13:223
Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SKJA (2014): Autophagy and apoptosis: where do they meet? 19, 555–566
Ni H-M, Bockus A, Wozniak AL, Jones K, Weinman S, Yin X-M, Ding W-XJA (2011): Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. 7, 188–204
Nna VU, Ujah GA, Mohamed M, Etim KB, Igba BO, Augustine ER, Osim EEJB, Pharmacotherapy (2017): Cadmium chloride–induced testicular toxicity in male wistar rats; prophylactic effect of quercetin, and assessment of testicular recovery following cadmium chloride withdrawal. 94, 109–123
Odewumi CO, Latinwo LM, Ruden ML, Badisa VL, Fils‐Aime S, Badisa RBJEt (2016): Modulation of cytokines and chemokines expression by NAC in cadmium chloride treated human lung cells. 31, 1612-1619
Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TYJF, toxicology c (2008): Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. 46, 3604-3611
Pascarelli NA, Fioravanti A, Moretti E, Guidelli GM, Mazzi L, Collodel GJR, Fertility, Development (2017): The effects in vitro of TNF-α and its antagonist ‘etanercept’on ejaculated human sperm. 29, 1169–1177
Ramonda R, Foresta C, Ortolan A, Bertoldo A, Oliviero F, Lorenzin M, Pizzol D, Punzi L, Garolla A (2014a) Influence of tumor necrosis factor α inhibitors on testicular function and semen in spondyloarthritis patients. Fertil Steril 101:359–365
Ramonda R, Foresta C, Ortolan A, Bertoldo A, Oliviero F, Lorenzin M, Pizzol D, Punzi L, Garolla AJF, sterility (2014b): Influence of tumor necrosis factor α inhibitors on testicular function and semen in spondyloarthritis patients. 101, 359-365
Renugadevi J, Prabu SMJE, Pathology T (2010): Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. 62, 471-481
Rumahlatu D, Duran-Corebima A, Amin M, Rohman F (2019) Effect of cadmium on the concentration and expression of TNF-α protein in sea urchin Diadema setosum (Leske, 1778). Hidrobiológica 29:181–188
Sadasivam M, Ramatchandirin B, Balakrishnan S, Prahalathan CJIR (2015): Tnf-α-mediated suppression of Leydig cell steroidogenesis involves DAX-1. 64, 549–556
Sadik NA (2008) Effects of diallyl sulfide and zinc on testicular steroidogenesis in cadmium-treated male rats. J Biochem Mol Toxicol 22:345–353
Scheinfeld NJJodt (2004): A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. 15, 280 294
Singh SS, Vats S, Chia AY-Q, Tan TZ, Deng S, Ong MS, Arfuso F, Yap CT, Goh BC, Sethi GJO (2018): Dual role of autophagy in hallmarks of cancer. 37, 1142-1158
Siu ER, Mruk DD, Porto CS, Cheng CYJT (2009) pharmacology a. Cadmium-Induced Testicular Injury 238:240–249
Sokanovic SJ, Baburski AZ, Janjic MM, Stojkov NJ, Bjelic MM, Lalosevic D, Andric SA, Stojilkovic SS, Kostic TSJE (2013): The opposing roles of nitric oxide and cGMP in the age-associated decline in rat testicular steroidogenesis. 154, 3914-3924
Son Y, Cheong Y-K, Kim N-H, Chung H-T, Kang DG, Pae H-OJJost (2011): Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? 2011
Terayama H, Naito M, Qu N, Hirai S, Kitaoka M, Ogawa Y, Itoh MJJoR, Development (2011): Intratesticular expression of mRNAs of both interferon γ and tumor necrosis factor α is significantly increased in experimental autoimmune orchitis in mice. 57, 296-302
Totoson P, Maguin-Gate K, Prigent-Tessier A, Monnier A, Verhoeven F, Marie C, Wendling D, Demougeot CJR (2016): Etanercept improves endothelial function via pleiotropic effects in rat adjuvant-induced arthritis. 55, 1308-1317
Wang Y, Yan J, Yin F, Li L, Qin Y, Meng C, Lu R, Guo LJH (2017b) toxicology e. Role of Autophagy in Cadmium-Induced Testicular Injury 36:1039–1048
Wang M-C, Wu A-G, Huang Y-Z, Shao G-L, Ji S-F, Wang R-W, Yuan H-J, Fan X-L, Zheng L-H, Jiao Q-LJIjoc, medicine e (2015): Autophagic regulation of cell growth by altered expression of Beclin 1 in triple-negative breast cancer. 8, 7049
Wang X-Y, Yang H, Wang M-G, Yang D-B, Wang Z-Y, Wang LJCd, disease (2017a): Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. 8, e3099-e3099
Yang Y, Zuo Z, Yang Z, Yin H, Wei L, Fang J, Guo H, Cui H, Ouyang P, Chen X (2021) Nickel chloride induces spermatogenesis disorder by testicular damage and hypothalamic-pituitary-testis axis disruption in mice. Ecotoxicol Environ Saf 225:112718
Yang M, Chen J, Zhao J, Meng MJPO (2014): Etanercept attenuates myocardial ischemia/reperfusion injury by decreasing inflammation and oxidative stress. 9, e108024
Ye JL, Mao WP, Wu AL, Zhao JM, Zhang C, Zhang NN, Jiang P, Tian TJFzxbswxbJomcb (2007): Cadmium induced apoptosis of HEK293 cells and its mitochondrial apoptosis pathway. 40, 7–16
Ye Q, Lin Y-N, Xie M-S, Yao Y-H, Tang S-M, Huang Y, Wang X-H, Zhu Y-HJIjoo (2019): Effects of etanercept on the apoptosis of ganglion cells and expression of Fas, TNF-α, caspase-8 in the retina of diabetic rats. 12, 1083
Yildirim Y, Cellad EG, Kara AV, Yilmaz Z, Kadiroglu AK, Bahadir MV, Gul M, Ketani MA, Yilmaz MEJOm, longevity c (2016): Effect of intraperitoneal etanercept on oxidative stress in rats with peritonitis. 2016
Yuan Y, Ding D, Zhang N, Xia Z, Wang J, Yang H, Guo F, Li BJCC (2018): TNF-α induces autophagy through ERK1/2 pathway to regulate apoptosis in neonatal necrotizing enterocolitis model cells IEC-6. 17, 1390–1402
Zou H, Zhuo L, Han T, Hu D, Yang X, Wang Y, Yuan Y, Gu J, Bian J, Liu XJB, communications br (2015): Autophagy and gap junctional intercellular communication inhibition are involved in cadmium-induced apoptosis in rat liver cells. 459, 713-719
Acknowledgements
The authors would like to express their gratitude to Dr. Mohamed El-Ghannam, Professor of Anatomy, Faculty of Veterinary, Kafr El-Sheikh University for his assistance. Dr. Walied Sobhy, Professor of Pathology, Faculty of Veterinary, Kafr El-Sheikh University for his assistance.
Funding
This study received no specific donations from funding organizations in the commercial, public, or nonprofit sectors. All drugs and laboratory reagents were purchased at authors’ expenses and without any donations from companies.
Author information
Authors and Affiliations
Contributions
S.A.A performed laboratory work, data analysis, software, and writing the first draft. M.A.E contributed to laboratory work, software, and writing the first draft. R.M.H done laboratory work and writing the first draft. Y.M.M was involved in supervision and editing the final manuscript. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol has been approved by the research ethics committee, Faculty of Pharmacy, Suez Canal University (Ismailia, Egypt) following the Canadian Council on Animal Care Guidelines (License number 201911MA3).
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declaration of competing interest
No conflict of interest to be declared.
Additional information
Communicated by Lotfi Aleya.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Antar, S.A., El-Gammal, M.A., Hazem, R.M. et al. Etanercept Mitigates Cadmium Chloride-induced Testicular Damage in Rats "An Insight into Autophagy, Apoptosis, Oxidative Stress and Inflammation". Environ Sci Pollut Res 29, 28194–28207 (2022). https://doi.org/10.1007/s11356-021-18401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18401-6