Abstract
The recent focus of scientific communities relay on assessing marine environment using different bio-indicators. The Cuddalore coastal water receives different effluents from SIPCOT industrial area through the Uppanar estuary. In this background, the present study deals with the hypothesis that polychetes can be a bio-indicator for assessing environmental conditions of Cuddalore coastal waters. The result revealed that Cluster and MDS plots showed that stations I, II and III, IV grouped together in respect to species composition. Abundance Biomass Curve (ABC) indicates stations I and II are polluted because of the dominance of indicator species of an ecological group (EG-IV & V). These stations (I & II) has been fixed at the confluence point of fishing harbor and Uppanar back waters while other stations (III & IV) in off-shore of the coastal waters. Thus, this study proved that polychaetes having the great potential for assessing the nature of the ecosystem receiving industrial effluents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, coastal and marine ecosystems are significantly disturbed by a variety of human activities. As far as Indian coastal line significant impacts from nearby industrial effluents and assessment of these coastal environments are essential (Sivaraj et al. 2014). Scientific communities used various indicator species to assess the coastal waters (Abhiroop and Subodh Kumar 2016) with the view of protecting the healthiness of an ecosystem. Polychaetes are one among them and they constitute the most abundant taxon in macrobenthic communities in terms of number and abundance. Unlike nektonic or crawling organisms, the polychaetes are usually live within the sediments or attached to the hard substrate, and their immobility facilitates chronic exposure to any pollutants in the environment rather than free-moving organisms. Therefore, any changes in the environment it seems to be evident through the polychaetes community (Papageorgiou et al. 2006; Belan 2003; Omena and Creed 2004; Tomassetti and Porrello 2005).
The coastal waters might be polluted through sewage and effluent runoff in terms of the input of organic matter and it’s broken down, the oxygen levels may be reduced in the benthic realm leading to anoxic conditions. The species composition and population density may vary in that ecosystem. As proved by Pearson and Rosenberg (1978) described a model of macrobenthic successional change in benthic communities due to organic matter increases in the environment. In this model showed that the high organic community made up of a few resistant species such as Capitella capitata and Malacoceros fuliginosus present in high abundance whereas intermediate zone with reduced organic input characterized by moderate pollution tolerant species like Nereis caudate and Cirriformia tentaculata (Méndez 2006; Harlan 2008). The point away from the source of organic input would be found species usually present in the non-polluted region. Based on this observation a list of species was categorized in terms of highly resistant, tolerant and sensitive species (Ait Alla et al. 2006; Granberg et al. 2008). Besides, they have both k-selected and r-selected species with the gradient from very pristine to polluted habitats and used as bioassay/pollution indicators at species, population and community levels (Elias et al. 2003; Surugiu 2005; Joydas et al. 2011).
The utility of polychaetes in bio-monitoring studies having special value; they are extremely responsive to changes in environmental conditions over spatial and temporal scales. Especially, the mass proliferation of certain polychaete families provides a good ‘snapshot’ of the health of the benthic habitats. As evidence, some polychaetes families Capitellidae (e.g. Capitella capitata) and Spionidae (e.g. Polydora cornuta, Streblospio benedicti, Prionospio cirrobranchiata, Malacoceros fuliginosa etc.,) have been widely accepted as indicators of organic pollution (Levin 2000; Vallarino et al. 2002; Ajmal Khan et al. 2004; Sivadas 2009). Based on the above said credential, many biotic tools are available using polychaetes enabling better assessment of the health of an ecosystem (Borja et al. 2000; Adams 2002; Borja 2005; Salas et al. 2006; Labrune et al. 2006; Sivaraj et al. 2015).
Owing to their acute sensitivity to environmental changes, many studies had been conducted using polychaetes in environmental impact assessment studies elsewhere (Harlan 2008; Feebarani and Damodaran 2014; Thamer et al. 2018). The reasons are; (a) they constitute more than half of total benthic population, which are readily available and easier to sample (b) they include a great diversity of reproductive/rejuvenate strategies and (c) they respond to disturbance induced by different kinds of pollution exhibiting detectable changes in the community structure of benthic biodiversity (Murugesan et al. 2011). From the credential point of view, this study tries to ascertain the impact of effluents on Cuddalore coastal waters using polychaetes as a bio-indicator.
Materials and Methods
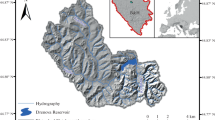
The State Industries Promotion Corporation of Tamil Nadu Limited (SIPCOT) is located on the northern bank of Uppanar estuary covering an area of 520 acres with 44 industries, dealing with chemicals, petrochemicals, fertilizers, pharmaceuticals, dyes, soap, detergents, packing materials, resins, beverages, pesticides, drugs, antibiotics, etc. The untreated effluents from the SIPCOT industrial complex are direct discharges into the Uppanar estuary for many years. Hence, it is considered as high polluted estuary ran along the southeast coast of India. During monsoonal seasons, the anthropogenic wastes enter into the estuary and they disposed into coastal waters along with industrial effluents. In this backdrop, four stations were selected in the inshore waters of Cuddalore, southeast coast of India. Stations I was fixed near the inner harbour (mouth of the estuary) while other stations (St-II, III & IV) were fixed moving towards the coastal waters with 500 m intervals between the stations (Fig. 1). Monthly sampling was made from February 2015 to January 2017. For the sake of interpretation of data, the monthly data were pooled for seasons-wise (Postmonsoon, Summer, Premonsoon and Monsoon).
Sample Collection
Three replicate sediment samples were collected at each station using van Veen grab, which covered an area of 0.1 m2. The larger organisms were handpicked immediately from the sediments and then sieved through 0.5 mm mesh screen. The methodology adopted for benthic sample collection by Mackie (1994) and identification using Day (1967). Physico-chemical parameters such as temperature was measured in-situ using thermometer with ±0.50 C accuracy; salinity by Hand Refractometer (Atago co. Ltd., Japan with ±0.2%); pH by pH pen (±0.2) (Eutech Instruments, Singapore), and total organic carbon (TOC) by El Wakeel and Riley (1956) and dissolved oxygen (DO) using Winkler’s method (Strickland and Parsons 1972). The percentage composition of sand, silt, and clay was analyzed standard methodology proposed by Krumbein and Pettijohn (1938).
Statistical Analysis
Physical/biological data were treated with various statistical methods namely Shannon-Wiener index, Margalef index, and Pielou’s index. k – dominance curve was originally described by Warwick (1986) which involves the plotting of separate k - dominance curves (Lambshead et al. 1983) for species abundance (species diversity) of various stations on the same graph and making a comparison of these curves. The species are ranked in order of importance in terms of abundance on the X axis (logarithmic scale) with percentage dominance on the Y axis (cumulative scale). The cumulative plot is often referred to as a k - dominance curve.
Abundance Biomass Curve – plot containing both abundance and biomass curves. The plot interpreted an ‘undisturbed’ community indicating the biomass curve is above the abundance curve, ‘gross disturbance’ the abundance curve lies above the biomass curve and ‘moderate disturbance’ the two curves largely intersect. This is based on the observation of soft-sediment macrobenthos, the biomass dominants are large-bodied with low abundance, and are amongst the more susceptible species to environmental impact classified as undisturbed whereas gross disturbance (especially from organic enrichment) is often characterized by large numbers of individuals of a few, small-bodied ‘opportunist’ species.
Cluster and MDS analysis were done to find out the similarities between the samples collected in various stations. The most commonly used clustering technique is the hierarchical agglomerative method. The results are represented by a tree diagram or dendrogram with the x-axis representing the full set of samples and the y-axis defining the similarity level at which the samples or groups are fused. Bray – Curtis coefficient (Bray and Curtis 1957) was used to produce the dendrogram. MDS method was used to find out the similarities (or dissimilarities) between each pair of entities to produce a map.
BIO-ENV procedure (biota-environmental variables matching) was in order to ascertain the relationship between biological and environmental variables (Clark and Ainsworth 1993). The basic principle behind this is to measure the agreement between the rank correlations of the biological (Bray-Curtis similarity) and environmental (Euclidean distance) matrices. A weighted Spearman rank correlation coefficient (ρω) was used to determine the harmonic rank correlation between the biological matrix and all possible combinations of the environmental variables: Further, the species belong to the Ecological Groups (EG) can be classified based on the very sensitive to opportunistic species through AMBI indices followed by Borja et al. (2000).
Results
Environmental Variables
Temperature varied from 27 to 32 °C (28.9 ± 2.9) with higher during summer (May) at station III and lower during monsoon (November) at station I. Salinity from 28 to 36 psu (32.6 ± 2.8) with lower during monsoon (December) at station I and higher in summer (May) at station III; Dissolved oxygen varied from 3.4 to 6.5 mg/l (5.1 ± 1.1) with higher during monsoon (November) at station III and lower during summer (May) at station I. pH values were from 7.8 to 8.3 (8.2 ± 0.2) with lower during monsoon (December) at station III and higher in summer (May) at station I. Total organic carbon varied from 2.11 to 9.72mgC/g (6.4 ± 2.4) with higher value during monsoon (October) at station I and lower during summer (May) at station IV (Fig. 2). Two-way ANOVA showed significant seasonal variations in temperature, salinity, DO and TOC (P < 0.05) and the variations between stations were also found to be significant. The sediment textural characteristic in the study stations were broadly classified into sand, silt and clay. The result revealed that sediment texture were significantly varied between the stations and also in different seasons.
Benthic Assemblages
As regards benthic fauna, as many as 54 species belonging to 22 families were recorded (Table 1). Species composition and density showed pronounced variation among the sampling stations. Based on AMBI classifications, out of 54 polychaetes, 19 species (35.19%) was fit into EG-I (very sensitive to disturbance), 21 species (38.89%) into EG-II (indifferent to disturbance), 5 species (9.26%) into EG-III (tolerant to disturbance), 8 species (14.81%) to EG-IV (second order opportunistic species) and one species (1.85%) to EG-V (first order opportunistic species). The abundance of polychaetes varied from 2769 to 6228 nos/m−2 with the minimum at a station IV during monsoon and maximum at the station I during premonsoon. Among the total species, top ten bio-indicator species (Plate 1) were selected based on the predominant occurrence like C. capitata (199 to 1832 nos/m−2), Sternaspis scutata (40 to 752 nos/m−2), Cossura coasta (709 to 1515 nos/m−2), Prionospio sexoculata (88 to 768 nos/m−2), Ancistrocillis parva (80 to 234 nos/m−2), Cirratulus cirratus (45 to 667 nos/m−2), Nereis sp. (304 to 706 nos/m−2), Glycera longipinnis (46 to 208 nos/m−2), Cirriformia tentaculata (64 to 588 nos/m−2) and Exogone clavator (54 to 549 nos/m−2). With regard to biomass (wet weight), minimum (42.8 ww/g−2) was recorded at the station I dominated by mostly opportunistic species (very short lifespan; species too small; many reproductive stages per year) and maximum (80.2 ww/g−2) at station III having conservative species (long lifespan; species larger in size; one reproductive stages per year). Seasonally, maximum biomass was recorded during premonsoon and minimum in monsoon. Two-way ANOVA calculated for the differences in polychaetes biomass were found to be significant between seasons (P < 0.05) and not between stations.
Multivariate Results
Shannon diversity index showed lower (2.16) at station II during monsoon and higher (3.86) at station III during summer. Margalef’s species richness showed higher (5.29) at station II in summer and lower (2.95) at station I in monsoon. Species evenness varied between 0.85 and 0.92 with higher at station III during summer and lower at station II during postmonsoon. Dominance index ranged from 0.67 to 0.89 with the maximum in the station I during summer and minimum in station IV during monsoon. The results of Two-way ANOVA calculated for the differences in Shannon-Wiener diversity were found to be significant (P < 0.025) between seasons and not between stations and also species richness and evenness (P > 0.05).
Cluster/MDS Analysis/ BIO-ENV
The dendrogram showed that the stations I, II and III, IV formed separate groups. Among the stations, I and II got linked at the highest level of similarity (96%) compared to stations III and IV. This fact was confirmed through MDS which was also revealed the same pattern of groupings as observed in cluster analysis was formed (Fig. 3). Biological (Bray-Curtis similarity) and environmental (Euclidean distance) matrices were allowed to match the biota. The results showed (salinity, DO, silt, clay, and TOC) were featured as the major variables explaining the best match (0.94) with faunal distributions (Table 2).
K-Dominance Plot
This plot was drawn in order to confirm the trend observed in the diversity indices, curves of stations III and IV were found to lie lower than the other curves which indicate higher diversity whereas stations I and II lie above the curves of stations III and IV due to the greater abundance of a few species. Thus, this plot also proved the rich diversity in the stations III and IV compared to stations I and II (Fig. 4).
ABC Plot
The basic principles of this plot interpreted ‘undisturbed’ community indicating the biomass curve lies above the abundance curve, ‘gross disturbance’ the abundance curve lies above the biomass curve. Based on the principles, curves of stations I and II were found to lie above the biomass curve indicating polluted nature owing to opportunistic species (very short lifespan; species too small; many reproductive stages per year). The curves of stations III and IV were found to lies above the abundance curve (Fig. 5) indicating more conservative species (long lifespan; species larger in size; one reproductive stage per year) reflecting pristine in nature.
Bubbles Plot
Top ten bio-indicator species Capitella capitata,Sternaspis scutata, Cossura coasta,Prionospio sexoculata, Ancistrocillis parva, Cirratulus cirratus, Nereis sp., Glycera longipinnis, Cirriformia tentaculata, and Exogone clavator were selected and abundance of these species superimposed in the bubbles plot. The stations I and II of bubbles were big in size indicating the maximum abundance while stations III and IV showed small in size indicating the lesser abundance of those species (Fig. 6).
Discussion
The physico-chemical parameters like temperature, pH, salinity and DO were fluctuated owing to the seasonal pattern. The maximum total organic content was found in stations I and II (monsoon) and minimum in stations III and IV (summer). Levin et al. (1991) noted that organic carbon frequently viewed as the more significant parameter in the effluent stressed ecosystem. As evidence in the above, maximum TOC content was recorded in stations I & II. These stations are located near the mouth; it means confluence point of fishing harbor and Uppanar back and it can be the reason of maximum TOC recorded in those stations. Besides, the continuous movement of fishing vessels and dredging activities take place in those stations in order to maintain the navigation channel. This leads to affect the benthic faunal distribution in the present study which resulted in lesser diversity. Auster et al. (1996) reported that minimum benthic diversity in the ecosystem receiving anthropogenic runoff and industrial effluents.
Bio-indicator species such as Prionospio sexoculata in Spionidae; Capitella capitata in Capitellidae; Ancistrocillis parva in Pilargidae and Cossura coasta in Cossuridae and Cirriformia tentaculata in Cirratuladae were found remarkably increase in stations I and II owing to high organic load. These species are adapted themselves in those stations and then proliferate into a number of individuals. These sorts of polychaetes with high abundance were recorded earlier by many researchers elsewhere (Pocklington and Wells 1992; Belan 2003; Rivero et al. 2005; Sivadas et al. 2010).
AMBI indices were applied to Cuddalore coastal waters in order to assess the station having pollution indicator species. Based on that, the stations I & II are being dominated by opportunistic species such as Capitella capitata, Sternaspis scutata, Cossura coasta, Prionospio sexoculata, Cirratulus cirratus and Cirriformia tentaculata which belongs to ecological groups (EG III-V). Grall and Glemarec (1997) and Sivaraj et al. (2014) reported that these group of species are able to proliferate in reduce sediment and also tolerate in toxic conditions.
Studies conducted elsewhere reported that C. capitata was first-order opportunistic species, and preferred a highly organic polluted site (Ajao 1990; Mendez et al. 1998). In our study, the richness of C. capitata, Prionospio pinnata, and Sternaspis scutata were found in higher abundance in the top ten species belongs to the stations (I & II). Studies conducted in foreign waters (Levin 2000; Rivero et al. 2005), where the organic load was extremely high, in those circumstances, species namely Prionospio, Capitella, Streblospio, and Mediomastus were recorded increase in number and identified as bio-indicator species and it is a testimony to the results of the present study.
Generally, in a healthy environment, the shannon diversity ranged between 3.0 and above in the coastal environment (Magurran 1988). In the present study, data were treated in all the station/seasons; the Shannon diversity was in the range of 2.16–3.86 with the maximum in station III and minimum in II. Sivadas (2009) reported in Mormugao harbor, the diversity was more in the stations situated in outer harbour and less in the inner harbor. Likewise, in the present study also, stations III and IV which is situated away from the mouth of Uppanar estuary (outer harbour) had maximum diversity while stations I and II situated quite close to the mouth of the estuary (inner harbour) showed minimum diversity. This fact was further confirmed through k- dominance curves, the results showed that the curves of stations III and IV were found lying above the other curves indicating more diversity (Harkantra et al. 1980).
In cluster analysis, the stations I, II and III, IV got grouped together and further confirmed through MDS plot which was also revealed the same pattern of grouping as recognized in the cluster analysis was formed (Muthuvelu et al. 2013; Sivaraj et al. 2015). In BIO – ENV procedure, the combination of eight variables was allowed to match the biota. The result showed that salinity, DO, silt, clay, and TOC showed the important variables influencing the faunal distribution (ρω-0.94). Likewise, studies conducted elsewhere; Mackie et al. (1997) in the Irish Sea. Murugesan et al. (2011) in Tuticorin waters and Manokaran (2011) in the southeast coast of India recorded similar combinations of parameters influencing faunal distribution.
The ABC – plot is drawn clearly demonstrated that the stations I and II are polluted due to the abundance curves of this two stations were found to lie above the biomass curve while the abundance curves of stations III and IV were found to lie below the biomass curve showing undisturbed situation. In addition, W – statistic values were also on the negative side for stations I and II indicating the polluted nature while other stations III and IV on the positive side indicates pristine in nature. Sivaraj et al. (2015) conducted a study using AMBI indices and multivariate approach to assess the Vellar-Coleroon estuary, in which they clearly elucidated that the abundance curve of the polluted station was lying above the biomass curves.
Conclusion
Nowadays researchers are focusing on the use of bio-indicator species for assessing the health of the coastal ecosystem. In the present study, we made an attempt by using polychaetes as bio-indicator to assess effluent stressed ecosystem of Cuddalore coastal waters by applying multivariate statistical tools. In cluster and MDS analysis stations, I and II form a separate cluster indicating polluted nature. As well in ABC plot, abundance curves lie above the biomass curves indicating pollution of the station I and II, and even so in a bubble plot, indicator species predominantly occur in those stations. To sum up, polychaetes as a good bio-indicator, hence they had great potential to assess the coastal environment facing industrial activities and man-made pressure.
References
Abhiroop C, Subodh Kumar M (2016) Identification of metal tolerant plant species in mangrove ecosystem by using community study and multivariate analysis: a case study from Indian Sunderban. Environ Earth Sci 75:744
Adams SM (2002) Biological indicators of aquatic ecosystem stress: introduction and overview. In: Adams SM (ed) Biological indicators of aquatic ecosystem stress. American Fisheries Society, Bethesda, Maryland, pp 1–11
Ait Alla A, Mouneyrac C, Durou C, Moukrim A, Pellerin A (2006) Tolerance and biomarkers as useful tools for assessing environmental quality in the Oued Souss estuary (bay of Agadir, Morocco). Comp Biochem Physiol 143:23–29
Ajao E A (1990) The influence of domestic and industrial effluents on populations of sessile and benthic organisms in Lagos Lagoon Ph D, Thesis (pp 411) University of Lagos
Ajmal Khan S, Murugesan P, Lyla PS, Jeganathan S (2004) A new indicator macro invertebrate of pollution and utility of graphical tool and diversity indices in pollution monitoring studies. Curr Sci 87:1508–1510
Auster PJ, Malatesta RJ, Langton RW, Watling L, Valentine PC, Donaldson CL, Langton EW, Shepard AN, Babb IG (1996) The impacts of mobile fishing gear on seafloor habitats in the Gulf of Maine (Northwest Atlantic): implications for conservation of fish populations. Rev Fish Sci 4:185–202
Belan TA (2003) Benthos abundance patterns and species composition in conditions of pollution in Amursky Bay (the Peter the Great Bay, the sea of Japan). Mar Pollut Bull 46:1111–1119
Borja A (2005) The European water framework directive: a challenge for nearshore, coastal and continental shelf research. Cont Shelf Res 25:1768–1783
Borja A, Franco J, Perez V (2000) A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar Pollut Bull 40:1100–1114
Bray JR, Curtis JT (1957) An introduction of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Clark K, Ainsworth M (1993) A method linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
Day J H, (1967) A monograph on the polychaeta of Southern Africa Parts I Errantia & II Sedentaria (p 878) Trustees of The British Museum (Natural history) London
El Wakeel S K, Riley J P (1956) The determination of organic carbon in marine mud Paper board / International Council for the Exploration of the Sea, 22: 180–183
Elias R, Rivero MS, Vallarino EA (2003) Sewage impact on the composition and distribution of polychaeta associated to intertidal mussel beds of the Mar del Plata rocky shore, Argentina. Thuruk Zool Sur 93:309–318
Feebarani J, Damodaran R (2014) Environmental impact assessment based on polychaete species in cochin backwaters (southwest coast of India). J Aqua Biol Fish:148–154
Grall J, Glemarec M (1997) Using bioti indices to estimate macrobenthic community perturbations in the bay of Brest. Estuar Coast Shelf Sci 44:43–53
Granberg ME, Gunnarsson JS, Hedman JE, Rosenberg R, Jonsson P (2008) Bioturbation-driven release of organic contaminants from Baltic Sea sediments mediated by the invading polychaete Marenzelleria neglecta. Environ Sci Technol 42:1058–1065
Harkantra SN, Ayyappan N, Anasari ZA, Parulekar AH (1980) Benthos of the shelf region along the west coast of India Ind. J Mar Sci 9:106–110
Harlan KD (2008) The use of polychaetes (Annelida) as indicator speciesof marine pollution: a review. Rev Biol Trop Int J Trop Biol 56(4):11–38
Joydas TV, Krishnakumar PK, Mohammad AQ, Said MA, Abdulaziz AS, Khaled AA (2011) Status of macrobenthic community of Manifa–Tanajib Bay system of Saudi Arabia based on a once-off sampling event. Mar Pollut Bull 62:1249–1260
Krumbein WC, Pettijohn FJ (1938) Manual of Sedimentary Petrology. Century and Crofts, New York, p 549
Labrune C, Amouroux JM, Sarda R, Dutrieux E, Thorin S, Rosenberg R, Gremare A (2006) Characterization of the ecological quality of the coastal gulf of lions (NW Mediterranean) a comparative approach based on three biotic indices. Mar Pollut Bull 52:34–47
Lambshead PJD, Platt HM, Shaw KM (1983) The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. J Nat Hist 17:859–874
Levin LA (2000) Polychaetes as environmental indicators: response to low oxygen and organic enrichment. Bull Mar Sci 67:668–683
Levin LA, Huggett CL, Wishner KF (1991) Control of deep-sea benthic community structure by oxygen and organic-matter gradients in the eastern Pacific Ocean. J Mar Res 49:763–800
Mackie A S Y (1994) Collecting and preserving polychaetes Polychaete Research, 16: 7–9
Mackie ASY, Parmiter C, Tong LKY (1997) Distribution and diversity of polychaeta in the southern Irish Sea. Bull Mar Sci 60(2):467–481
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, New Jersey, p 179
Manokaran S (2011) Diversity and trophic relationship of shelf macrobenthos of Southeast coast of India Ph D, Thesis (pp 318) Annamalai University, India
Méndez N (2006) Effects of teflubenzuron on sediment processing by members of the Capitella species complex. Environ Pollut 139:118–124
Mendez N, Flos J, Romero J (1998) Littoral soft-bottom polychaete communities in a pollution gradient in front of Barcelona (Western Mediterranean, Spain). Bull Mar Sci 63:167–178
Murugesan P, Muniasamy M, Muthuvelu S, Vijayalakshmi S, Balasubramanian T (2011) Utility of benthic diversity in assessing the health of an ecosystem Ind. J Mar Sci 40:783–793
Muthuvelu S, Murugesan P, Muniasamy M, Vijayalakshmi S, Balasubramanian T (2013) Changes in benthic macrofaunal assemblages in relation to bottom-trawling in Cuddalore and Parangipettai coastal waters, southeast coast of India. Ocean Sci J 48(2):183–194
Omena E, Creed JC (2004) Polychaete fauna of sea grass beds (Halodule wrightii Ascherson) along the coast of Rio de Janeiro (Southeast Brazil). Mar Ecol 25:273–288
Papageorgiou N, Arvanitidis C, Eleftheriou A (2006) Multi causal environmental severity: a flexible framework for microtidal sandy beaches and the role of polychaetes as an indicator taxon. Estuar Coast Shelf Sci 70:643–653
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol Annu Rev 16:229–311
Pocklington P, Wells PG (1992) Polychaetes - key taxa for marine environmental quality monitoring. Mar Pollut Bull 24:593–598
Rivero MS, Elías R, Vallarino EA (2005) First survey of macrofauna in the Mar del Plata harbour (Argentina), and the use of polychaetes as pollution indicators. J Mar Bol Oceanogr 40:101–108
Salas F, Marcos C, Neto JM, Patrício J, Pérez-Ruzafa A, Marques JC (2006) User friendly guide for using benthic ecological indicators in coastal and marine quality assessment. Ocean Coast Manag 49:308–331
Sivadas S (2009) Impact of anthropogenic activities on macro benthic communities of west coast of India PhD, thesis, Goa University
Sivadas S, Ingole B, Nanajkumar M (2010) Benthic polychaetes as good indicator of anthropogenic impacts Ind. J Mar Sci 39:201–211
Sivaraj S, Murugesan P, Muthuvelu S, Vivekanandan KE, Vijayalakshmi S (2014) AMBI and M-AMBI indices as a robust tool for assessing the effluent stressed ecosystem in Nandgaon coastal waters, Maharashtra, India. Estuar Coast Shelf Sci 146:60–67
Sivaraj S, Murugesan P, Silambarasan A, Preetha Mini Jose HM, Bharathidasan V (2015) AMBI indices and multivariate approach to assess the ecological health of Vellar-Coleroon estuarine system undergoing various human activities. Mar Pollut Bull 100:334–343
Strickland J D H, Parsons T R, (1972) A practical handbook of seawater analysis Bull Fish Res Canada, 167: 310
Surugiu V (2005) The use of polychaetes as indicators of eutrophication and organic enrichment of coastal waters: a study case – Romanian Black Sea coast scientific annals of Alexandru Ioan Cuza University of Iasi, new series. Section I Animal Biol 51:55–62
Thamer SA, Sarah AD, Farah AD (2018) Univariate analysis of benthic Infaunal biodiversity in the Kingdom of Bahrain. Biol App Environ Res 2(2):111–129 2018
Tomassetti P, Porrello S (2005) Polychaetes as indicators of marine fish farm organic enrichment. Aquac Int 13:109–128
Vallarino EA, Rivero S, Elias R (2002) The community-level response to sewage impact in intertidal mytilid beds of the Southwestern Atlantic and the use of the Shannon index to assess pollution. J Mar Biol Oceanogr 37:25–33
Warwick RM (1986) A new method for detecting pollution effects on marine macrobenthic communities. Mar Biol 92:557–562
Acknowledgments
The authors are very much thankful to Prof. K. Kathiresan, Director & Dean, Centre of Advanced Study in Marine Biology for the constant encouragement and authorities of Annamalai University for providing the facilities. They also acknowledge Science and Engineering Research Board (SERB) (File No.PDF/2016/001096) Govt. of India for funding support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Regional Terms: Cuddalore Coastal waters; India
Geographical Co-ordinates: 11° 42' 354˝ N; 79° 47' 310" E
Rights and permissions
About this article
Cite this article
Sigamani, S., Samikannu, M. & Alagiri, T.G. Assessment of Effluent Stressed Ecosystem of Cuddalore Coastal Waters – a Bio-Indicator Approach. Thalassas 35, 437–449 (2019). https://doi.org/10.1007/s41208-019-00128-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-019-00128-4