Abstract
Particulate matters (PMs) and their associated chemical compounds such as polycyclic aromatic hydrocarbons (PAHs) are important factors to evaluate air pollution and its health impacts particularly in developing countries. Source identification of these compounds can be used for air quality management. The aim of this study was to identify the sources of PM2.5-bound PAHs in Isfahan city, a metropolitan and industrialized area in central Iran. The PM2.5 samples were collected at 50 sites during 1 year. Source identification and apportionment of particle-bound PAHs were carried out using diagnostic ratios (DRs) of PAHs and positive matrix factorization (PMF) model. The results showed that the concentrations of PM2.5 ranged from 8 to 291 μg/m3 with an average of 60.2 ± 53.9 μg/m3, whereas the sum of concentrations of the 19 PAH compounds (ƩPAHs) ranged from 0.3 to 61.4 ng/m3 with an average of 4.65 ± 8.54 ng/m3. The PAH compounds showed their highest and lowest concentrations occurred in cold and warm seasons, respectively. The mean concentration of benzo[a]pyrene (1.357 ng m−3) in December–January, when inversion occured, was higher than the Iranian national standard value showing the risk of exposure to PM2.5-bound PAHs. Applying DRs suggested that the sources of the PAHs were mainly from fuel combustion. The main sources identified by the PMF model were gasoline combustion (23.8 to 33.1%) followed by diesel combustion (20.6 to 24.8%), natural gas combustion (9.5 to 28.4%), evaporative-uncombusted (9.5 to 23.0%), industrial activities (8.4 to 13.5%), and unknown sources (2.8 to 15.7%). It is concluded that transportation, industrial activities, and combustion of natural gas (both in residential-commercial and industrial sectors) as the main sources of PAHs in PM2.5 should be managed in the metropolitan area, particularly in cold seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is one of the world’s largest health and environmental problems. It includes a mixture of gases and solid or liquid particles suspended in the atmosphere. Among major air pollutants, particulate matters (PMs) are important as they significantly affect the human health, atmospheric chemistry, and global climate change (Li et al. 2020; Zong et al. 2016). PMs are classified by their size which smaller ones cause most health problems (Amil et al. 2016). Several studies have showed that PMs, especially PM2.5 (i.e., the particles with aerodynamic diameter smaller than 2.5 µm) can adversely affect human health (Ouyang et al. 2020; Pope et al. 2011). PMs contain various organic pollution such as hydrocarbons and inorganic pollutants such as heavy metals, whereas their chemical composition depends on the emission sources. Therefore, identification and quantification of PM sources and their associated compounds is necessary to improve strategies for effective air quality management (Waked et al. 2014).
Polycyclic aromatic hydrocarbons (PAHs) are the main group of organic pollutants associated with PMs which consist of two or more fused benzene rings (Luo et al. 2019; Wu et al. 2014; Hassanvand et al. 2015). PAHs both in gas and particle phase can emitted to the environment from natural processes such as incomplete combustion of organic matters, volcanic eruptions, and forest fires (Wang et al. 2008). However, in urban and industrial areas, these compounds are formed mainly through incomplete combustion of fossil fuels (Luo et al. 2019; Qin et al. 2015; Huang et al. 2015; Mostert et al. 2010; Lai et al. 2017). Due to adverse health effects, PAHs have attracted considerable attention by researchers and are considered as priority pollutants (Wang et al. 2020a, 2017). Therefore, for efficient control of air pollution caused by PAHs, identification of the sources of these compounds in the atmosphere is very important.
Diagnostic ratios (DRs) are a common tool for identifying the main PAHs sources (petrogenic or pyrogenic sources) in air, water, sediment, and soil samples as well as organism tissues (Tobiszewski and Namiesnik 2012). PAHs with a various range of molecular weight could be originated from pyrolysis process, while the lowest molecular weight compounds are dominated in petrogenic sources (Abdel-Shafy and Mansour 2016; Tobiszewski and Namiesnik 2012).
Multivariate receptor models are useful approach for identification and apportionment of pollutant sources at a receptor site. Among multivariate receptor modeling techniques, positive matrix factorization (PMF) is widely used for source apportionment. The model was developed by Paatero and Tapper (Paatero and Tapper 1994; Paatero 1997) and has been widely used for source apportionment of various environmental pollutants including organic and inorganic compounds (Sun et al. 2020; Li et al. 2020; Vlachou et al. 2019; Men et al. 2018; Karanasiou et al. 2009). One of the main advantages of this model is that the methodology can be applied without the need for data on source emission compositions. Ambient data of chemicals and their uncertainty are required for running the model. Another advantage of the model is to utilize a point-by-point least squares minimization scheme. Such a weighting scheme allows the inclusion of missing values, and noisy and uncertain data in the analysis through giving low weights (Khan et al. 2012).
Isfahan as a main metropolitan city of central Iran exhibits serious air pollution problems during the last three decades because of industrial development and increasing the population. According to the previous studies, PMs are the major air quality problems in Isfahan city (Norouzi and Khademi 2015; Norouzi et al. 2017; Soleimani et al. 2018) which are associated with other pollutants such as heavy metals and organic pollutants originated from anthropogenic and natural sources (Soleimani et al. 2018). To the best of our knowledge, no source apportionment studies have so far been performed to identify sources and quantify their contributions to ambient PMs and particle-bound PAHs in Isfahan city.
According to the previous studies, fine particulate matter (i.e., PM2.5) is the main factor affecting air quality of Isfahan city (Soleimani et al. 2018). Providing valuable information regarding to the concentration and sources of air pollutants such as PAHs is useful for air pollution management. It is therefore of outmost importance to investigate the associated pollutants PM-bound PAHs which can adversely affect the health of people living in the region. The main objective of this study was therefore to determine the concentrations, seasonal variations, and sources of PAHs in PM2.5 of an industrial metropolitan city in Iran as a developing country. This will do by the use of DRs in combination with PMF model. The results from this study revealing the potential sources of PAHs in particulate matters during cold and warm seasons may be used by managers and policy makers in action plan of air pollution control and management in the region.
Material and methods
Sampling sites
This study was conducted in Isfahan city, the second largest industrial and the third most populated city, in Iran with more than 2 million inhabitants (https://stat.mpo-es.ir). It located in central Iran (51° 39′ 40″ E, 32° 38′30″ N) with an average elevation of 1590 m (a.s.l.) covering an area about 482 km2 (Mansouri and Hamidian 2013).
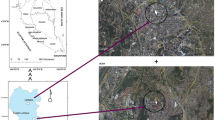
The climate of study area is principally characterized by hot, dry summers and cold winters. The mean annual temperature is 16.2 ºC with the warmest month in July (high average 29.3 ºC) and the coldest in January (low average 2.9 ºC) (Mansouri and Hamidian 2013). Isfahan is a receptor of air pollution released from different kinds of industries including steel and iron industries, petrochemical plants, oil refineries, cement and brick factories, power plants, and lead and zinc mines, within city and the surroundings. These industries also have a significant effect on the transportation and traffic capacity. Moreover, because of existence an arid area in north east of the city, it subject to frequent dust storm events during the year (Norouzi and Khademi 2015). Therefore, the city has severe air quality degradation due to the high levels of the atmospheric PMs. To have better understanding of the sources of PM2.5 in the study area, stratified and judgmental sampling approach was conducted according to guidance of US-EPA (2002) from December 2017 to September 2018 (including 4 seasons) and 200 samples were collected from 50 sites (Fig. 1) coverage of about 158 km2 based on population density, road traffic, and distance to industrial zones inside the study area. In addition to industrial plants around the city, several industrial zones inside the area were also considered in selection of sampling points. It should be noticed that the fall sampling period included the December–January months when the region was faced to the air temperature inversion, while in February–March period which was accounted as winter season, there was not the meteorological phenomenon. According to the European guide on air pollution source apportionment with PMF model, the number of samples should be ≥ 100 (Belis et al., 2014). However, according to the literature (Johnson et al., 2011; Watson et al., 2002), PMF model can also be used for source apportionment if a sufficient number of chemically characterized ambient samples is available (more than 50).
Sampling of PM2.5
The samples were collected during 24 h using a high-volume air sampler (TISCH, USA) running at 1.1 m3 min−1. Quartz filter fibers (with the size of 20.3 cm × 25.4 cm, Sigma-Aldrich) were used for sample collection. All filters were put inside a muffle furnace at 500 °C for 3 h prior to sampling for sure to be clean of any organic compounds. In order to minimize the influence of water adsorption, the filters were kept in a desiccator for 24 h before and after sampling. PM2.5 mass was measured using a 5 decimal digit balance. Then, the samples were stored at − 18 °C in a freezer prior to analysis (Khan et al. 2015).
Extraction of PAHs
Each sampled filter was cut into the same size pieces (with area of 3.7993 cm2) and three of them (determined by primary laboratory tests) including marginal and central parts were selected randomly and used for the extraction using a modified method of EPA-TO-13A (US-EPA, 1999). A sonication system was used for the extraction of PAHs using 5 mL of pentane/dichloromethane 90:10 for 10 min. Before extraction, 4 μL the lab PAH surrogate internal standard mixture (100 times diluted of stock standard) was added to the sample. After extraction, the filter was removed from the extract and 0.5 mL of isooctane was added to the extract and then the solvent volume was reduced to 1 mL. Then 4 μL PAH recovery standard mixture (1000 times diluted of stock standard) was added to the extract and then it was transferred to a GC vial. The list of internal and recovery standards for the quantification of PAHs is given in Table S1. The blank filters were also used in the field without sampling just to find any contamination of the sample transport to the laboratory. The analyzed PAHs were naphthalene (Nap), acenaphthene (Acy), acenaphthylene (Ace), fluorene (Flu), dibenzothiophene (Dbt), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fl), pyrene (Pyr), benzo(a)anthracene (B[a]A), chrycene (Chr), benzo(b)fluoranthene (B[b]F), benzo(k)fluoranthene (B[k]F), benzo(e)pyrene (B[e]P), benzo(a)pyrene (B[a]P), indeno[1,2,3-cd]pyrene (I[c]P), dibenzo[a,h]anthracene (D[ah]A), and benzo [g,h,i]perylene (B[ghi]P).
Analysis of PAHs
The extracts were analyzed for PAHs and alkyl PAHs by gas chromatography–mass spectrometry using an Agilent Technologies 5975C inert XL MSD with electron ionization operating in selected ion monitoring (SIM) mode. A 60-m HP-5 capillary column with 0.25-μm inner diameter and 0.25-μm film thickness was used. The injection port was kept at 300 °C and 1 µL sample was injected in the splitless mode. The flow rate of helium as carrier gas was 1.1 mL/min. The column temperature was initially held at 40 °C for 2 min, ramped with 25 °C/min to 100 °C, and then programmed at 5 °C/min to 315 °C and held for 14 min. The transfer line, ion source, and quadropole temperatures were 315 °C, 230 °C, and 150 °C, respectively. Values of 55 m/z were monitored in 12 groups with 13 m/z each, with a dwell time of 25 ms, according to Table S2 (Gallotta and Christensen 2012).
MassHunter Quantitative Analysis version B.07.00 was used for quantification of peaks (Agilent technologies, Inc.) and the data was quantified using the internal standards method and corrected for recoveries. Standard calibration curve in the range of 0.003–2 µg/mL was used for each target compound with internal and recovery standards. The overall recovery of the compounds ranged from 71 to 108%. The estimated limit of detections (LOD) for the PAH compounds (ranged from 0.01 to 0.21 ng m−3) are shown in Table S3 which were mostly in the range of previous researches (e.g., Hoseini et al., 2016).
Source apportionment techniques
Diagnostic ratio
In order to identify the source of PAHs, the ratio values of An/(An + Phe), (anthracene to anthracene plus phenanthrene), Flu/(Flu + Pyr), (fluoranthene to fluoranthene plus pyrene), BaA/(BaA + Chr), (benzo[a]anthracene to Benzo[a]anthracene plus chrysene), IP/(IP + Bghi), (Indeno[1,2,3-cd]pyrene to Indeno[1,2,3-cd]pyrene plus Benzo[ghi]perylene), and low molecular weight to high molecular weight (LMW/HMW) PAHs were widely used by previous researchers (Liang et al. 2019; Suman et al. 2016; Khan et al. 2015; Callén et al. 2011; Katsoyiannis et al. 2007). In this study, the diagnostic ratios were calculated and then compared with the standard values. Table S4 shows the standard values for diagnostic ratios of PAHs.
Positive matrix factorization
In this study, positive matrix factorization (PMF) 5.0 model (US EPA 2014) was applied to quantify the contribution of various PAH sources. Details of the model are described in EPA PMF 5.0 Fundamentals & User Guide (US EPA 2014; Norris et al. 2014). The user manual guideline was closely followed in this study. Briefly, two input files are required by the model: (1) the measured concentrations of the species and (2) the estimated uncertainty of the concentration. The quality of data was assessed based on the signal to noise ratio (S/N) and the percentage of samples above method detection limit (MDL). Those species which had S/N ≥ 2 were classified as strong and those with S/N between 0.2 and 2 and with S/N<0.2 were considered as weak and bad in quality, respectively. The bad quality values were excluded from further analyses.
The second data file was the uncertainty; for concentrations below and above the MDL, which was estimated by Eqs.(1) and (2), respectively (US EPA 2014).
The PAH concentrations and uncertainties were introduced to the model. In principle, the PMF model is a factor analysis algorithm based on the following equation:
where XIJ is a data matrix (the Jth species in the Ith sample), P is the number of factors, fkj is the source chemical composition (source profile), gik is the source contributions to each observation, and eij is the residual matrix that cannot be explained by the model.
The PMF model was used to minimize the object function, Q (E) through an iterative algorithm as shown in Eq. (4).
where Sij is the estimated uncertainty, n is the number of samples, and m is the number of species.
The dataset matrixes (200 samples × 25 PAHs) included concentrations and uncertainties of PAHs were loaded into the US-EPA PMF 5.0 model (US-EPA 2014). Then 100 bootstrap runs and a minimum correlation of 0.6 were performed to test the uncertainty and stability of the resolved profiles in the base run. A critical process in PMF modeling is the determination of the correct number of factors. The numbers of factors were chosen depending on the background understanding of the sources. During the PMF analysis, the model was run for different number of factors and finally, the six-factor solution gave the most stable results and the most easily interpretable factors. So, six factors (sources) of PAHs including diesel combustion, gasoline combustion, industrial activities, natural gas combustion, evaporative-uncombusted, and other sources were revealed by employing the model. The relevant source profiles of PAH species of the six factors related to the fall data have been shown in Fig. 2. The lowest or optimized Q value was selected as Q (robust) and Q (true) values were calculated including all points. The Q (robust) values for fall, winter, spring, and summer seasons were 945.240, 523.365, 355.038, and 309.242, respectively, and the correspondence Q (true) values were 952.040, 523.362, 354.968, and 309.193, respectively. More details are provided in Table S5. Upon running the PMF analysis, rotation by changing Fpeak values between − 1 and + 1 was conducted, but no significant variations or improvements in source profiles were found.
Health risk assessment
Inhalation is one of the main human exposure pathways to PAHs. In this study, the toxic equivalency factors (TEFs) were used for quantitative risk estimate from inhalation pathway. TEF is an estimate of the relative toxicity of a chemical compared to a reference chemical. Benzo[a]pyrene as a reference compound is the most appropriate and widely used indicator to assess the carcinogenic potential of PAHs through different exposure pathways. Therefore, the Benzo[a]pyrene equivalent concentration (BaPeq) was estimated based on the following equation:
where Ci is the concentration of the ith target PAH compound (ng/m3), TEFi is the toxic equivalency factor of the ith target compound. TEFi values of the PAHs were taken from the literature (Collins et al., 1998; Nisbet and Lagoy, 1992). The carcinogenic risk of each PAH as a lifetime lung cancer risk (LLCR) was calculated using Eq. (6).
BaPeq was estimated by Eq. (5), where UR[BaP] was the inhalation cancer unit risk factor of BaP. The recommended value of UR [BaP] is 8.7 × 10−5 (WHO, 2000) showing the insidence of 8.7 cases per 100,000 people at risk of cancer from inhalation a BaP equivalent concentration of 1 ng/m3 within their lifetime of 70 years.
Results and discussions
PM2.5 and PAH concentrations
The concentrations of PM2.5 and 19 individual PAH compounds in the collected samples were analyzed. Mean values of PM2.5 concentrations and individual PAH compounds in the four seasons are shown in Table 1. PM2.5 concentrations ranged from 8 to 291 μg/m3 with a mean concentration of 60.21 ± 53.96 μg/m3 and the total concentration of 19 PAH compounds ranged from 0.32 to 61.38 ng/m3 with a mean concentration of 4.65 ± 8.54 ng/m3.
The mean PM2.5 mass concentrations in all seasons exceeded target value of 25 µg/m3 proposed by the World Health Organization (WHO 2005), whereas the Iranian national standard value is 35 µg/m3.
Seasonal variations of the PAH concentrations during the sampling period showed the highest and the lowest concentrations in fall and summer, respectively. One of the main reasons of increasing concentration in fall season could be due to temperature inversion which usually enhance the diffusion of PM2.5 and consequently affect PAH concentrations (Chen et al. 2020).
The dependence of PAHs concentration on atmospheric temperature and the increase in particulate PAHs concentration during the cold season have been reported by others (Chen et al. 2020; Vlachou et al. 2019; Tsapakis and Stephanou 2005; Li et al. 2006). Seasonal variations of emission sources and meteorological conditions such as temperature and inversion that influence the difference of gas-to-particle partitioning may result in a difference of PAHs concentration in cold and warm seasons (Tan et al. 2006). Reduced atmospheric dispersion as well as reduced photochemical reactions in low atmospheric temperatures can lead to higher pollutant concentrations during cold season (Chen et al. 2020; Vlachou et al. 2019), as it is seen for PAH concentrations in the cold seasons in comparison to the warm seasons (Table 1). In contrast, the increase in ambient temperature during the warm season may result in an increased evaporation of the particle phase of PAHs to the gas phase. This could be the reason of significant decrease of LMW PAH concentrations (e.g., Nap, Acy, Ace, Flu, Dbt, and Phe) more than 10 times in summer than the fall (Table 1). In addition, the PAHs degradation by photochemical or thermal reactions in the atmosphere especially in the warmer seasons is well known (Dörr et al. 1996). Furthermore, increasing the fossil fuel consumption is another reason for the higher concentrations of PAHs in the cold season (Wu et al. 2014). It seems that increasing the fossil fuel combustion and temperature inversion during the cold seasons that influence the dispersion of air pollutants could have the main role of increasing the PAHs concentration. Since the particle-bound PAHs were analyzed in the current study and emission sources were mostly active throughout the year, meteorological conditions (e.g., inversion) might be the most important factor affecting PM2.5 and PAH concentrations.
Table 2 shows the mean concentration of PM2.5 and ƩPAHs in different cities worldwide. The mean concentration of PM2.5 in Isfahan were greater than those in Tehran, Iran (Ali-Taleshi et al. 2020; Kermani et al. 2017); Zaragoza, Spain (Callén et al. 2014); Kanazawa, Japan (Xing et al. 2020); São Paulo, Brazil (Bourotte et al. 2005); Venice, Italy (Masiol et al. 2012); Islamabad, Pakistan (Mehmood et al. 2020); Thessaloniki, Greece (Tolis et al. 2015); and Taiwan, China (Chen et al. 2016), but lower than those in Anshan, China (Wang et al. 2020b); Beijing, China (Feng et al. 2018); and Guangzhou, China (Liu et al. 2015). The concentration of ƩPAHs in Isfahan was higher than those in Bangi, Malaysia (Khan et al. 2015); Zaragoza, Spain (Callén et al. 2014); Kanazawa, Japan (Xing et al. 2020); and Taiwan, China (Chen et al. 2016), but lower than those in Tehran, Iran (Ali-Taleshi et al. 2020; Taghvaee et al. 2018; Kermani et al. 2017); Jinan, China (Zhang et al. 2019); Seoul, Korea (Kang et al. 2020); Anshan, China (Wang et al. 2020b); Beijing, China (Feng et al. 2018); Thessaloniki, Greece (Tolis et al. 2015); Islamabad, Pakistan (Mehmood et al. 2020); São Paulo, Brazil (Bourotte et al. 2005); Venice, Italy (Masiol et al. 2012); and Guangzhou, China (Liu et al. 2015).
Distribution of PAHs along seasons
According to the number of rings, PAHs are classified into five groups including 2-rings, 3-rings, 4-rings, 5-rings, and 6-rings. The analyzed PAHs in this study have been classified as follows: 2-rings (Nap); 3-rings (Acy, Ace, Flu, Dbt, Phe and Ant); 4-rings (Fl, Pyr, BaA and Chr); 5-rings (BbF, BkF, BeP, BaP, Per and DahA); and 6-rings (BghiP and Ind). They have been further divided into low molecular weight (LMW, 2- and 3-ring PAHs) and high molecular weight (HMW, 4-, 5-, and 6-ring PAHs). The concentration of high molecular weight (HMW) PAHs were significantly higher than of the low molecular weight (LMW) PAHs in all seasons (Fig. 3).
A similar composition pattern over the four seasons was observed with the highest contribution from the 5–6-ring (79.5%), followed by the 4-ring (18.5%) and the 3-ring PAHs (2%). The LMW PAHs can be formed in the pyrolysis of uncombusted fossil fuels, but the HMW PAHs mainly originate from high-temperature combustion processes such as vehicular exhaust (Dachs et al. 2002). Therefore, predomination of HMW PAHs in the study area indicated that PAHs mainly originate from combustion sources.
Since PAHs are semi-volatile organic compounds, they are partitioned between particle and gas phases. Low molecular weight PAHs tend to be more concentrated in the gas phase while the ones with higher molecular weight are often associated with particulates. So low concentrations of LMW PAHs in the samples of this study may just related to the particle-bound compounds not the total fractions including particle and gas phases.
Source apportionment of PAHs
Diagnostic ratios
The ratios of LMW/HMW, An/(An + Phe), (anthracene to anthracene plus phenanthrene), Flu/(Flu + Pyr), (fluoranthene to fluoranthene plus pyrene), BaA/(BaA + Chr), (Benzo[a]anthracene to Benzo[a]anthracene plus Chrysene), and IP/(IP + Bghi), (Indeno[1,2,3-cd]pyrene to Indeno[1,2,3-cd]pyrene plus Benzo[ghi]perylene) were used for identification of PAH sources. Figure 4 shows cross plots of PAHs ratios illustrating the different source types. Similar distribution patterns were observed along different seasons. For An/An + Ph, 0.10 is taken as a threshold to discriminate petrogenic from combustion sources. Values < 0.1 are usually considered as a petroleum source, while values > 0.1 indicate a combustion source (Yunker et al. 2002; Chen et al. 2013). The calculated ratio of An/(An + Ph) ranged from 0.09 to 0.34 with a mean value of 0.18. This means that the main source of PAHs in the city is from combustion process.
The Flu/Flu + Pyr ratio is another ratio that has been used to determine the emission source of PAHs. For this ratio, values less than 0.40 are distinctive of petroleum sources, values between 0.4 and 0.5 are distinctive of liquid fossil fuel combustion, and values above than 0.50 are distinctive of biomass or coal combustion (Yunker et al. 2002). These ratios have been also used to distinguish between gasoline and diesel emissions; where the values lower and higher than 0.5 refer to gasoline and diesel combustion, respectively. (Ravindra et al. 2006). In this study, the Flu/(Flu + Pyr) ratio was from 0.32 to 0.58 with a mean of 0.43. This shows the importance of gasoline and diesel emissions to Isfahan’s atmosphere.
The ratio BaA/BaA + Chr is also declarative of the PAH sources. For this ratio, values lower than 0.20 indicate a petroleum source; the range from 0.2 to 0.35 suggests either petroleum or combustion source and values higher than 0.35 imply a combustion source (Yunker et al. 2002; Akyuz and Cabuk 2010). Values for this ratio reported in the literature are 0.22–0.55 for gasoline, 0.38–0.64 for diesel (Simcik et al. 1997), and 0.5 for coal (Tang et al. 2005). The calculated ratio of BaA/(BaA + Chr) was from 0.17 to 0.43 with a mean of 0.34. This information reflects the significance of gasoline and diesel emissions to Isfahan city. Likewise, PAH sources may be identified by the ratio of IP/(IP + Bghi). For this ratio, values lower than 0.20 indicate a petrogenic source; the range from 0.20 to 0.50 suggests liquid fossil fuel combustion, and values greater than 0.50 indicates grass, wood, or coal combustion (Yunker et al., 2002; Chen et al. 2005). For this ratio, some researchers documented 0.21–0.22 values for gasoline emissions (Rogge et al. 1993) and 0.35–0.70 for diesel emissions (Pio et al. 2001; Alves et al. 2001; Tang et al. 2005; Grimmer et al. 1983). In this work, the IP/(IP + Bghi) ratio was from 0.22 to 0.41 with a mean of 0.31 which confirms the importance of gasoline and diesel emissions.
The ratio of LMW/HMW in all samples was less than 1 indicating overall combustion sources of PAHs. In conclusion of the PAH diagnostic ratios analysis, combustion of fossil fuels was found to be the main source of PAHs in ambient PM2.5 in Isfahan.
Obviously, diagnostic ratios are a useful technique to identify the sources of PAHs. But, due to difficulty to distinguish between different emission sources, this technique should be used with a great caution. Furthermore, degradation (e.g., photolysis) and reaction with other species in the atmosphere can alter distribution pattern of PAHs during their transfer from emission sources to receptor sites (Ravindra et al. 2008; Pongpiachan 2014). There should be awareness that coals and fossil fuels from diverse origins and countries can generate different proportions of PAHs (Masclet et al. 1987). However, interpretation of PAHs sources must be done based on such numerical data and on the knowledge available about the historical background of the study area.
Source identification using PMF model
The predicted and measured PAH concentration showed a significant correlation (r2 = 0.99, p < 0.01) (Fig. 5.). Regarding the factors used in PMF model, PAH compounds such as Flu, Chr, Pyr, BaA, BeP, BaP, Ind, and BghiP, which were related to industrial activities (Lin et al. 2011), were also considered in the current study to find the industrial sources. Among them, steel and iron industry can produce PAHs through different processes such as sintering, casting and cooling, and coke manufacturing (Yang et al. 2002). Ciaparra et al. (2009) demonstrated that low and moderate molecular weight PAHs (Flu, Phe, Ant, FluA, and Pyr) explained by coke making and HMW PAHs (BaP, IcdP, DahP, DacP, and BghiP) arising from the sintering process. As several types of industries, including the steel and iron industries, power plants, brick, and cement factories are located around the city, therefore, we attributed this factor to “Industrial activities.” LMW PAHs such as Nap, Flu, and Phe were introduced as markers of uncombusted petroleum (Liu et al. 2015, 2009; Marr et al. 1999). The higher contribution of this factor in the warm seasons and the association with LMW PAHs lead us to the conclusion that this factor arises from “evaporative-uncombusted” sources, mainly from the gasoline emissions from storage tanks and pumps in gasoline stations. HMW PAH compounds such as BghiP, Ind, Pyr, and BeP were reported as chemical tracers of gasoline combustion by some researchers (Khan et al. 2015; Guo et al. 2003; Schauer et al. 2002). Gasoline is widely used in Isfahan and gasoline-fueled cars and motorcycles have been found to be important sources of hydrocarbons in Isfahan. In recent decades, gasoline has been used primarily as a fuel in automobiles. Vehicles, especially those for private use, have increased since past few decades in Isfahan city. Due to ambiguous characteristics, the fourth factor was left as “other sources.” A mixture of minor sources such as wood, biomass and coal combustion, and natural dust seems to explain this factor. The dominant PAHs in factor of “diesel combustion” were BkF, BaP, Ind, and BghiP which have been reported as the tracers of this source (Fang et al. 2016; Harrison et al. 1996; Wang et al. 2009). BbF, BkF, BaP, and Ind have been reported as chemical tracers of diesel combustion in numerous studies (Yang et al. 2013; Chen et al. 2011; Lin et al. 2011; Khalili et al. 1995; Ravindra et al. 2008; Ma et al. 2014; Harrison et al. 1996; Wang et al. 2014, 2015). Diesel fuel has been widely used in trucks and public transportation in Isfahan city. Moreover, the influence of power plants, and steel and iron industries using diesel as fuel may be also reflected in this factor. The natural gas is not only the main source of heating and cooking in Isfahan city, but also used as fuel in various industries and power plants through the region. So considering the markers such as Phe, Flu, BaA, Chr, and Pyr, reported by Jamhari et al. (2014), Khan et al. (2017), and Simcik et al. (1999), this factor named “natural gas combustion” was also used as one of the source factors of the model.
The contributions of each PMF factor to the level of total PAHs in different seasons are shown in Fig. 6. The source fingerprints in different seasons are shown in Fig. S1. For the cold seasons including fall (December–January months) and winter (February–March), the contributions were respectively included by natural gas combustion (28.4 and 9.5%), gasoline combustion (23.8 and 33.1%), diesel combustion (21.9 and 22.3%), industrial activities (10.3 and 9.9%), evaporative-uncombusted (3.9 and 9.5%), and other or unidentified sources (11.7 and 15.7%). The source contributions in spring and summer were gasoline combustion (29.3 and 26.0%) diesel combustion (20.6 and 24.8%), evaporative-uncombusted (15.4 and 23.0%), natural gas combustion (14.1 and 15%), industrial activities (13.5 and 8.4%), and other or unidentified sources (7.1 and 2.8%), respectively. Since the natural gas are used in heating systems of residential-commercial sector and as a fuel in various industries and power plants particularly in cold seasons (fall and winter), it showed the higher contribution in comparison to warm seasons (spring and summer). The natural gas is mainly used by industrial plants as fuel in spring and summer in the region. The total natural gas consumption of Isfahan city in 2018 was reported more than 3000 million m3 where residential-industrial sectors consume more than 66 and 48% in cold (fall and winter) and warm (spring and summer) seasons, respectively (https://stat.mpo-es.ir). The power plants consume about 23 and 37% of the natural gas during cold and warm seasons, respectively (https://stat.mpo-es.ir). However, the consumption rate may change in different years. Since the main public transportation service in Isfahan city is provided by buses, they constitute together with trucks the major source of diesel combustion. It is estimated that more than 600,000 personal cars (as gasoline vehicles) and more than 1000 public transport buses (as diesel vehicles) are daily on road throughout the city (https://stat.mpo-es.ir).
To the best of our knowledge, no source apportionment studies have so far been performed to identify sources and quantify their contributions to ambient PMs in Isfahan. PAH source apportionment in respirable particles in Tehran, the capital of Iran, revealed five main sources including the following: diesel combustion (56.3%), gasoline combustion (15.5%), wood combustion and incineration (13.0%), industry (9.2%), and road soil particle (6.0%) (Moeinaddini et al. 2014). In other study in Tehran, source apportionment of PM2.5-bound PAHs by PMF model revealed five main sources including the following: diesel exhaust (22.3%), unburned petroleum (15.6%), industrial (7.5%), gasoline exhaust (30.9%), and coal/biomass and natural gas combustion (23.6%) (Ali-Taleshi et al. 2020).
Health risk of PAHs
According to concentration of particle-bound B[a]P (Table 1), the concentration of this compound in fall was higher than the national standard value (i.e., 1 ng m−3) of Iranian Department of Environment (Hoseini et al., 2016), while it was below the value in the other seasons showing the highest human exposure to PAHs and consequently the highest risk when inversion occurred (in December–January). The toxic equivalency factor (TEF), or relative potency, BaPeq of individual PAHs, and the lifetime lung cancer risk (LLCR) are shown in Table 3. The BaPeq of individual B[a]P accounted for more than 44% that shown by the total PAHs. Other studies reported that the carcinogenic potency of B[a]P was more than 50% (Khan et al., 2015), in the range of 81–85% (Cuadras et al., 2016) and in the range of 27–67% (Petry et al., 1996; Castellano et al., 2003) of the carcinogenic activity of total PAHs. The LLCR was in the range of 1.6 × 10−10 to 3.8 × 10−5 and for the overall data it was 8.5 × 10−5 which was very close to the critical value (8.7 × 10−5), showing the cancer risk to the residents on exposure to PM2.5-bound PAHs. However, the real PAH risk should be evaluated for both gas- and particle-phase PAHs in future researches and also should be investigated spatially and temporally for the people of different age groups living in the region.
Conclusion
The annual mean concentrations of PM2.5 and ƩPAHs were 60.2 ± 53.96 μg/m3 and 4.65 ± 8.54 ng/m3, respectively, in the study area. The mean PM2.5 mass concentrations exceeded target value of the WHO standard. Source apportionment of PAHs using diagnostic ratios confirmed that the PAHs predominated by high molecular weight originate mainly from combustion sources especially fuel oil combustion. PAH source apportionment by PMF model revealed that gasoline and diesel combustion had about 50% contribution of PAHs in PM2.5 showing the importance of air pollution control in transportation of the region. Furthermore, natural gas combustion mainly by residential-commercial and industrial sectors with contribution of about 9.5 to 28.4% in PAH production and also lifetime lung cancer risk of PM2.5-bound PAHs revealed the importance of air pollution control in Isfahan Metropolitan. Although the source apportionment techniques used in this study are useful, however, both DRs and PMF model have uncertainties such as changing the distribution pattern of the compounds from the sources to the receptors and subjective interpretation of source profiles. Total PAHs in the atmosphere including gas and particle phase should be investigated in future studies, specially for the health risk assessments.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet 25(1):107–123. https://doi.org/10.1016/j.ejpe.2015.03.011
Akyuz M, Cabuk H (2010) Gas-particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci Total Environ 408:5550–5558. https://doi.org/10.1016/j.scitotenv.2010.07.063
Ali-Taleshi MS, Moeinaddini M, Riyahi-Bakhtiyari A, Feiznia S, Squizzato S, Bourliva A (2020) A one-year monitoring of spatiotemporal variations of PM2.5-bound PAHs in Tehran, Iran: source apportionment, local and regional sources origins and source-specific cancer risk assessment. Environ Pollut 115883. https://doi.org/10.1016/j.envpol.2020.115883.
Alves C, Pio C, Duarte A (2001) Composition of extractable organic matter of air particles from rural and urban Portuguese area. Atmos Environ 35:5485–5496. https://doi.org/10.1016/S1352-2310(01)00243-6
Amil N, Latif MT, Khan MF, Mohamad M (2016) Seasonal variability of PM2.5 composition and sources in the Klang Valley urban-industrial environment. Atmos Chem Phys 16:5357–5381. https://doi.org/10.5194/acp-16-5357-2016
Belis CA, Larsen BR, Amato F, Haddad IE, Favez O, Harrison RM, Hopke PK, Nava S, Paatero P, Prevot ASH, Quass U, Vecchi R, Viana M (2014) European Guide on Air Pollution Source Apportionment with Receptor Models. JRC References Report. https://doi.org/10.2788/9307
Bourotte C, Forti MC, Taniguchi S, Bícego MC, Lotufo PA (2005) A wintertime study of PAHs in fine and coarse aerosols in São Paulo city. Brazil Atmos Environ 39(21):3799–3811. https://doi.org/10.1016/j.atmosenv.2005.02.054
Callén MS, de la Cruz MT, López JM, Mastral AM (2011) PAH in airborne particulate matter. Carcinogenic character of PM10 samples and assessment of the energy generation impact. Fuel Process Technol 92:176–182. https://doi.org/10.1016/j.fuproc.2010.05.019
Callén MS, Iturmendi A, López JM (2014) Source apportionment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons by a PMF receptor model, Assessment of potential risk for human health. Environ Pollut 195:167–177. https://doi.org/10.1016/j.envpol.2014.08.025
Castellano AV, Cancio JL, Aleman PS, Rodríguez JS (2003) Polycyclic aromatic hydrocarbons in ambient air particles in the city of Las Palmas de Gran Canaria. Environ Int 29:475–480. https://doi.org/10.1016/s0160-4120(03)00003-5
Chen C, Chen C, Dong C, Kao C (2013) Assessment of toxicity of polycyclic aromatic hydrocarbons in sediments of Kaohsiung Harbor. Taiwan Sci Total Environ 463:1174–1181. https://doi.org/10.1016/j.scitotenv.2012.06.101
Chen LG, Ran Y, Xing BS (2005) Contents and sources of polycyclic aromatic hydrocarbons and organochlorine pesticides in vegetable soils of Guangzhou, China. Chemosphere 60:879–890. https://doi.org/10.1016/j.chemosphere.2005.01.011
Chen YC, Chiang HC, Hsu CY, Yang TT, Wu YS (2016) Ambient PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Changhua county, central Taiwan: seasonal variation, source apportionment and cancer risk assessment. Environ Pollut 218:372–382. https://doi.org/10.1016/j.envpol.2016.07.016
Chen YJ, Feng YL, Xiong SC, Liu DY, Wang G, Sheng GY, Fu JM (2011) Polycyclic aromatic hydrocarbons in the atmosphere of Shanghai. China Environ Monit Assess 172(1–4):235–247. https://doi.org/10.1007/s10661-010-1330-x
Chen Z, Chen D, Zhao C, Kwan M, Cai J, Zhuang Y, Zhao B, Wang X, Chen B, Yang J, Li R, He B, Gao B, Wang K, Xu B (2020) Influence of meteorological conditions on PM2.5 concentrations across China: a review of methodology and mechanism. Environ Int 139:105558. https://doi.org/10.1016/j.envint.2020.105558.
Ciaparra D, Aries E, Booth MJ, Andersen DR, Almeida SM, Harrad S (2009) Characterisation of volatile organic compounds and polycyclic aromatic hydrocarbons in the ambient air of steelworks. Atmos Environ 43:2070–2079. https://doi.org/10.1016/j.atmosenv.2008.09.078
Collins J, Brown J, Alexeeff G, Salmon A (1998) Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul Toxicol Pharmacol 28:45–54. https://doi.org/10.1006/rtph.1998.1235
Cuadras A, Rovira E, Marce RM, Borrull F (2016) Lung cancer risk by polycyclic aromatic hydrocarbons in a Mediterranean industrialized area. Environ Sci Pollut Res 23:23215–23227. https://doi.org/10.1007/s11356-016-7566-4
Dachs J, Glenn TR, Gigliotti CL, Brunciak P, Totten LA, Nelson ED, Franz TP, Eisenreich SJ (2002) Processes driving the short-term variability of polycyclic aromatic hydrocarbons in the Baltimore and northern Chesapeake Bay atmosphere, USA. Atmos Environ 36(14):2281–2295. https://doi.org/10.1016/S1352-2310(02)00236-4
Dörr G, Hippelein M, Kaupp H, Hutzinger O (1996) Baseline contamination assessment for a new resource recovery facility in Germany: part VI: levels and profiles of polycyclic aromatic hydrocarbons (PAH) in ambient air. Chemosphere 33(8):1569–1578. https://doi.org/10.1016/0045-6535(96)00276-7
Fang Y, Chen Y, Tian C, Lin T, Hu L, Li J, Zhang G (2016) Application of PMF receptor model merging with PAHs signatures for source apportionment of black carbon in the continental shelf surface sediments of the Bohai and Yellow Seas, China. J Geophys Res Oceans 121:1346–1359. https://doi.org/10.1002/2015JC011214
Feng BH, Li LJ, Xu HB, Wang T, Wu RS, Chen J, Zhang Y, Liu S, Ho SSH, Cao JJ, Huang W (2018) PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Beijing: seasonal variations, sources, and risk assessment. J Environ Sci 77:11–19. https://doi.org/10.1016/j.jes.2017.12.025
Gallotta FDC, Christensen JH (2012) Hydrocarbons in soil and sediments from Iguaçu River Watershed, Paraná, Brazil using the CHEMSIC method (CHEMometric analysis of selected ion chromatograms). J Chromatogr A 1235(27):149–158. https://doi.org/10.1016/j.chroma.2012.02.041
Grimmer G, Jacob J, Naujack KW (1983) Profile of the polycyclic aromatic compounds from crude oils-inventory by GC, GC-MS. PAH in environmental materials, part 3.
Guo H, Lee SC, Ho KF, Wang XM, Zou SC (2003) Particle associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos Environ 37:5307–5317. https://doi.org/10.1016/j.atmosenv.2003.09.011
Harrison RM, Smith DJT, Luhana L (1996) Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham. UK Environ Sci Technol 30(3):825–832. https://doi.org/10.1021/es950252d
Hassanvand MS, Naddafi K, Faridi S, Nabizadeh R, Sowlat MH, Momeniha F, Gholampour A, Arhami M, Kashani H, Zare A, Niazi S, Rastkari N, Nazmara S, Ghani M, Yunesian M (2015) Characterization of PAHs and metals in indoor/outdoor PM10/PM2.5/PM1 in a retirement home and a school dormitory. Sci Total Environ 527–528:100–110. https://doi.org/10.1016/j.scitotenv.2015.05.001
Hoseini M, Yunesian M, Nabizadeh R, Yaghmaeian K, Ahmadkhaniha R, Rastkari N, Parmy S, Faridi S, Rafiee A, Naddafi K (2016) Characterization and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban atmospheric particulate of Tehran. Iran Environ Sci Pollut Res 23(2):1820–1832. https://doi.org/10.1007/s11356-015-5355-0
Huang KL, Tsai TH, Tsai JH, Chen SJ, Lee WJ (2015) Emission of PAHs from a single hydrogenoxygen PEM fuel cell: in relation to fuel cell carbon materials. Aerosol Air Qual Res 15:2654–2667. https://doi.org/10.4209/aaqr.2015.08.0489
Jamhari AA, Sahani M, Latif MT, Chan KM, Tan HS, Khan MF, Mohd TN (2014) Concentration and source identification of polycyclic aromatic hydrocarbons (PAHs) in PM10 of urban, industrial and semi-urban areas in Malaysia. Atmos Environ 86:16–27. https://doi.org/10.1016/j.atmosenv.2013.12.019
Johnson T, Guttikunda S, Wells GJ, Artaxo P, Bond TC, Russell, AG, et al. (2011) Tools for improving air quality management: a review of top- down source apportionment techniques and their application in developing countries. Report No 339/11. Washington, DE: ESMAP.
Kang M, Kim K, Choi n, Kim YP, Lee JY (2020) Recent occurrence of PAHs and n-alkanes in pm2.5 in seoul, korea and characteristics of their sources and toxicity. Int J Environ Res Public Health 17(4):1397. https://doi.org/10.3390/ijerph17041397.
Karanasiou AA, Siskos PA, Eleftheriadis K (2009) Assessment of source apportionment by positive matrix factorization analysis on fine and coarse urban aerosol size fractions. Atmos Environ 43:3385–3395. https://doi.org/10.1016/j.atmosenv.2009.03.051
Katsoyiannis A, Terzi E, Cai QY (2007) On the use of PAH molecular diagnostic ratios in sewage sludge for the understanding of the PAH sources. Is this use appropriate? Chemosphere 69:1337–1339. https://doi.org/10.1016/j.chemosphere.2007.05.084
Kermani M, Asadgol Z, Arfaeinia H (2017) A study of polycyclic aromatic hydrocarbons (PAHs) and trace elements in ambient air PM2.5 in an urban site of Tehran, Iran. Iran J Health Saf Environ 5(2):947–956. Available at: http://www.ijhse.ir/index.php/IJHSE/article/view/303/0
Khalili NR, Scheff PA, Holsen TM (1995) PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmos Environ 29:533–542. https://doi.org/10.1016/1352-2310(94)00275-P
Khan MF, Hwa SW, Hou LC, Mustaffa NIH, Amil N, Mohamad N, Sahani M, Jaafar SA, Nadzir MSM, Latif MT (2017) Influences of inorganic and polycyclic aromatic hydrocarbons on the sources of PM2.5 in the Southeast Asian urban sites. Air Qual Atmos Health 10:999–1013. https://doi.org/10.1007/s11869-017-0489-5
Khan MF, Latif MT, Lim CH, Amil N, Jaafar SA, Dominick D, Nadzir MSM, Sahani M, Tahir NM (2015) Seasonal effect and source apportionment of polycyclic aromatic hydrocarbons in PM2.5. Atmos Environ 106:178–190. https://doi.org/10.1016/j.atmosenv.2015.01.077
Khan MF, Hirano K, Masunaga S (2012) Assessment of the sources of suspended particulate matter aerosol using US EPA PMF 3.0. Environ Monit Assess 184:1063–1083. https://doi.org/10.1007/s10661-011-2021-y
Lai YC, Tsai CH, Chen YL, Chang CGP (2017) Distribution and sources of atmospheric polycyclic aromatic hydrocarbons at an industrial region in Kaohsiung. Taiwan Aerosol Air Qual Res 17:776–787. https://doi.org/10.4209/aaqr.2016.11.0482
Li J, Zhang G, Li XD, Qi SH, Liu GQ, Peng XZ (2006) Source seasonality of polycyclic aromatic hydrocarbons (PAHs) in a subtropical city, Guangzhou. South China Sci Total Environ 355(1–3):145–155. https://doi.org/10.1016/j.scitotenv.2005.02.042
Li T, Li J, Jiang H, Chen D, Zong Z, Tian C, Zhang G (2020) Source apportionment of PM2.5 in Guangzhou based on an approach of combining positive matrix factorization with the Bayesian mixing model and radiocarbon. Atmosphere 11(5):512. https://doi.org/10.3390/atmos11050512.
Liang M, Liang H, Rao Z, Hong X (2019) Characterization of polycyclic aromatic hydrocarbons in urban-rural integration area soil, North China: spatial distribution, sources and potential human health risk assessment. Chemosphere 234:875–884. https://doi.org/10.1016/j.chemosphere.2019.06.119
Lin T, Hu LM, Guo ZG, Qin YW, Yang ZS, Zhang G, Zheng M (2011) Sources of polycyclic aromatic hydrocarbons to sediments of the Bohai and Yellow Seas in East Asia. J Geophys Res 116:D23305. https://doi.org/10.1029/2011JD015722
Liu J, Man R, Ma S, Li J, Wu Q, Peng J (2015) Atmospheric levels and health risk of polycyclic aromatic hydrocarbons (PAHs) bound to PM2.5 in Guangzhou, China. Mar Pollut Bull 100(1):134–143. https://doi.org/10.1016/j.marpolbul.2015.09.014.
Liu Y, Chen L, Huang QH, Li WY, Tang YJ, Zhao JF (2009) Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Huangpu River, Shanghai, China. Sci Total Environ 407:2931–2938. https://doi.org/10.1016/j.scitotenv.2008.12.046
Luo Q, Gu L, Shan Y, Wang H, Sun L (2019) Distribution, source apportionment, and health risk assessment of polycyclic aromatic hydrocarbons in urban soils from Shenyang. China Environ Geochem Health 42:1817–1832. https://doi.org/10.1007/s10653-019-00451-y
Ma CL, Ye SY, Lin T, Ding XG, Yuan HM, Guo ZG (2014) Source apportionment of polycyclic aromatic hydrocarbons in soils of wetlands in the Liao River Delta. Northeast China Mar Pollut Bull 80(1–2):160–167. https://doi.org/10.1016/j.marpolbul.2014.01.019
Mansouri B, Hamidian AH (2013) Assessment of the air quality of Isfahan city, Iran, using selected air quality parameters. Iran J Toxicol 7(21):842–848. Available at: http://ijt.arakmu.ac.ir/article-1-208-en.html.
Marr LC, Kirchstetter TW, Harley RA, Miguel AH, Hering SV, Hammond SK (1999) Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environ Sci Technol 33:3091–3099. https://doi.org/10.1021/es981227l
Masclet P, Bresson MA, Mouvier G (1987) Polycyclic aromatic hydrocarbons emitted by power stations, and influence of combustion conditions. Fuel 66:556–562. https://doi.org/10.1016/0016-2361(87)90163-3
Masiol M, Hofer A, Squizzato S, Piazza R, Rampazzo G, Pavoni B (2012) Carcinogenic and mutagenic risk associated to airborne particle-phase polycyclic aromatic hydrocarbons: a source apportionment. Atmos Environ 60:375–382. https://doi.org/10.1016/j.atmosenv.2012.06.073
Mehmood T, Zhu T, Ahmad I, Li X (2020) Ambient PM2.5 and PM10 bound PAHs in Islamabad, Pakistan: concentration, source and health risk assessment. Chemosphere 257:127187. https://doi.org/10.1016/j.chemosphere.2020.127187.
Men C, Liu RM, Xu F, Wang QR, Guo LJ, Shen ZY (2018) Pollution characteristics, risk assessment, and source apportionment of heavy metals in road dust in Beijing, China. Sci Total Environ 612:138–147. https://doi.org/10.1016/j.scitotenv.2017.08.123
Moeinaddini M, Esmaili-Sari A, Riyahi-bakhtiari A, Yiu-Chung Chan A, Taghavi SM, Hawker D, Connell D (2014) Source apportionment of PAHs and n-alkanes in respirable particles in Tehran, Iran by wind sector and vertical profile. Environ Sci Pollut Res 21:7757–7772. https://doi.org/10.1007/s11356-014-2694-1
Mostert MMR, Ayoko GA, Kokot S (2010) Application of chemometrics to analysis of soil pollutants. Trac-Trend Anal Chem 29:430–445. https://doi.org/10.1016/j.trac.2010.02.009
Nisbet ICT, Lagoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300. 10.10160273–2300(92)90009-x.
Norouzi S, Khademi H (2015) Source identification of heavy metals in atmospheric dust using Platanus orientalis L. leaves as bioindicator. Eurasian J Soil Sci 4(3):144–152. https://doi.org/10.18393/ejss.2015.3.144-152.
Norouzi S, Khademi H, Ayoubi S, Cano AF, Acosta JA (2017) Seasonal and spatial variations in dust deposition rate and concentrations of dust borne heavy metals, a case study from Isfahan, central Iran. Atmos Pollut Res 8:686–699. https://doi.org/10.1016/j.apr.2016.12.015
Norris G, Duvall R, Brown S, Bai S (2014) EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals & User Guide. Prepared for the US Environmental Protection Agency, Washington, DC. National Exposure Research Laboratory, Research Triangle Park, USA. https://www.epa.gov/sites/production/files/2015-02/documents/pmf_5.0_user_guide.pdf.
Ouyang R, Yang S, Xu L (2020) Analysis and risk assessment of PM2.5-bound PAHs in a comparison of indoor and outdoor environments in a middle school: a case study in Beijing, China. Atmosphere 11(9):904. https://doi.org/10.3390/atmos11090904.
Paatero P (1997) Least square formulation of robust non-negative factor analysis. Chemometr Intell Lab Syst 37:23–35. https://doi.org/10.1016/S0169-7439(96)00044-5
Paatero P, Tapper U (1994) Positive Matrix Factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5:111–126. https://doi.org/10.1002/env.3170050203
Petry T, Schmid P, Schlatter C (1996) The use of toxic equivalency factors in assessing occupational and environmental health risk associated with exposure to airborne mixtures of polycyclic aromatic hydrocarbons (PAHs). Chemosphere 32:639–648. https://doi.org/10.1016/0045-6535(95)00348-7
Pio CA, Alves CA, Duarte AC (2001) Identification, abundance and origin of atmospheric organic particulate matter in a Portuguese rural area. Atmos Environ 35:1365–1375. https://doi.org/10.1016/S1352-2310(00)00391-5
Pongpiachan S (2014) Application of binary diagnostic ratios of polycyclic aromatic hydrocarbons for identification of tsunami 2004 backwash sediments in Khao Lak Thailand. Sci World J 2014:1–14. https://doi.org/10.1155/2014/485068
Pope CA, Burnett RT, Turner MC, Cohen AJ, Krewski D, Jerrett M, Gapstur SM, Thun MJ (2011) Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect 119(11):1616–1621. https://doi.org/10.1289/ehp.1103639
Qin L, Zhang Y, Han J, Chen W (2015) Influences of waste iron residue on combustion efficiency and polycyclic aromatic hydrocarbons release during coal catalytic combustion. Aerosol Air Qual Res 15:2720–2729. https://doi.org/10.4209/aaqr.2015.04.0227
Ravindra K, Bencs L, Wauters E, Hoog J, Deutsch F, Roeken E, Bleux N, Berghmans P, Grieken RV (2006) Seasonal and site specific variation in vapor and aerosol phase PAHs over Flanders (Belgium) and their relation with anthropogenic activities. Atmos Environ 40:771–785. https://doi.org/10.1016/j.atmosenv.2005.10.011
Ravindra K, Sokhi R, Grieken RV (2008) Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ 42:2895–2921. https://doi.org/10.1016/j.atmosenv.2007.12.010
Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT (1993) Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environ Sci Technol 27(4):636–651. https://doi.org/10.1021/es00041a007.
Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT (2002) Measurement of emissions from air pollution sources. 5. C1–C32 organic compounds from gasoline-powered motor vehicles. Environ Sci Technol 36:1169–1180. https://doi.org/10.1021/es0108077
Simcik MF, Eisenreich SJ, Lioy PJ (1999) Source apportionment and source/sink relationships of PAHs in the coastal atmosphere of Chicago and Lake Michigan. Atmos Environ 33:5071–5079. https://doi.org/10.1016/S1352-2310(99)00233-2
Simcik MF, Zhang H, Eisenreich SJ, Franz TP (1997) Urban contamination of the Chicago/Coastal Lake Michigan atmosphere by PCBs and PAHs during AEOLOS. Environ Sci Technol 31(7):2141–2147. https://doi.org/10.1021/es9609765
Soleimani M, Amini N, Sadeghian B, Wang D, Fang L (2018) Heavy metals and their source identification in particulate matter (PM2.5) in Isfahan City. Iran J Environ Sci 72:166–175. https://doi.org/10.1016/j.jes.2018.01.002
Suman S, Sinha A, Tarafdar A (2016) Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad. India Sci Total Environ 353:545–546. https://doi.org/10.1016/j.scitotenv.2015.12.061
Sun X, Wang H, Guo Z, Lu P, Song F, Liu L, Liu J, Rose NL, Wang F (2020) Positive matrix factorization on source apportionment for typical pollutants in different environmental media: a review. Environ Sci Process Impacts 22(2):239–255. https://doi.org/10.1039/c9em00529c
Taghvaee S, Sowlat MH, Hassanvand MS, Yunesian M, Naddafi K, Sioutas C (2018) Source specific lung cancer risk assessment of ambient PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in central Tehran. Environ Int 120:321–332. https://doi.org/10.1016/j.envint.2018.08.003
Tan JH, Bi XH, Duan JC, Rahn KA, Sheng GY, Fu JM (2006) Seasonal variation of particulate polycyclic aromatic hydrocarbons associated with PM10 in Guangzhou. China Atmos Res 80(4):250–262. https://doi.org/10.1016/j.atmosres.2005.09.004
Tang N, Hattori T, Taga R, Igarashi K, Yang X, Tamura K, Kakimoto H, Mishukov VF, Toriba A, Kizu R, Hayakawa K (2005) Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in urban air particulates and their relationship to emission sources in the Pan-Japan Sea countries. Atmos Environ 39(32):5817–5826. https://doi.org/10.1016/j.atmosenv.2005.06.018
Tobiszewski M, Namiesnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119. https://doi.org/10.1016/j.envpol.2011.10.025
Tolis EI, Saraga DE, Lytra MK, Papathanasiou AC, Bougaidis PN, Prekas-Patronakis OE, Ioannidis II, Bartzis JG (2015) Concentration and chemical composition of PM2.5 for a one-year period at Thessaloniki, Greece: a comparison between city and port area. Atmos Environ 113:197–207. https://doi.org/10.1016/j.atmosenv.2015.05.014
Tsapakis M, Stephanou EG (2005) Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environ Pollut 133(1):147–156. https://doi.org/10.1016/j.envpol.2004.05.012
US-EPA (2014) EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals & User Guide. Online available at: http://www.epa.gov/heasd/research/pmf.html.
US-EPA (2002) Guidance for choosing a sampling design for environmental data collection (QA/G-5S). (EPA/240/R-02/005). Washington, DC.
US-EPA (1999) Compendium method TO-13A: determination of polycyclic aromatic hydrocarbons (PAHs) in ambient air using gas chro- matography/mass spectrometry (GC/MS) (EPA/625/R-96/010b). Office of Research and Development, Washington, DC
Vlachou A, Tobler A, Lamkaddam H, Canonaco F, Daellenbach KR, Jaffrezo JL, Minguillón MC, Maasikmets M, Teinemaa E, Baltensperger U, El Haddad I, Prevot ASH (2019) Development of a versatile source apportionment analysis based on positive matrix factorization: a case study of the seasonal variation of organic aerosol sources in Estonia. Atmos Chem Phys 19:7279–7295. https://doi.org/10.5194/acp-19-7279-2019
Waked A, Favez O, Alleman LY, Piot C, Petit JE, Delaunay T, Verlinden E, Golly B, Besombes JL, Jaffrezo JL, Leoz Garziandia E (2014) Source apportionment of PM10 in a north-western Europe regional urban background site (Lens, France) using positive matrix factorization and including primary biogenic emissions. Atmos Chem Phys 14:3325–3346. https://doi.org/10.5194/acp-14-3325-2014
Wang C, Zou Y, Yu l, Lu Y, (2020a) Potential source contributions and risk assessment of PAHs in sediments from the tail reaches of the Yellow River Estuary, China: PCA model, PMF model, and mean ERM quotient analysis. Environ Sci Pollut Res 27:9780–9789. https://doi.org/10.1007/s11356-019-07530-8
Wang DG, Tian FL, Yang M, Liu CL, Li YF (2009) Application of positive matrix factorization to identify potential sources of PAHs in soil of Dalian. China Environ Pollut 157(5):1559–1564. https://doi.org/10.1016/j.envpol.2009.01.003
Wang FW, Lin T, Feng JL, Fu HY, Guo ZG (2015) Source apportionment of polycyclic aromatic hydrocarbons in PM2.5 using positive matrix factorization modeling in Shanghai, China. Environ Sci Process Impacts 17(1):197–205. https://doi.org/10.1039/c4em00570h.
Wang FW, Lin T, Li YY, Ji TY, Ma CL, Guo ZG (2014) Sources of polycyclic aromatic hydrocarbons in PM2.5 over the East China Sea, a downwind domain of East Asian continental outflow. Atmos Environ 92:484–492. https://doi.org/10.1016/j.atmosenv.2014.05.003
Wang M, Wang CY, Li YW (2017) Petroleum hydrocarbons in a water sediment system from Yellow River estuary and adjacent coastal area, China: distribution pattern, risk assessment and sources. Mar Pollut Bull 122:139–148. https://doi.org/10.1016/j.marpolbul.2017.06.037
Wang S, Ji Y, Zhou J, Lin Y, Lin Z (2020b) Source apportionment and toxicity assessment of PM2.5-bound PAHs in a typical iron-steel industry city in northeast China by PMF-ILCR. Sci Total Environ 15(713):136428. https://doi.org/10.1016/j.scitotenv.2019.136428.
Wang X, Cheng H, Xu X, Zhuang G, Zhao C (2008) A wintertime study of polycyclic aromatic hydrocarbons in PM2.5 and PM2.5–10 in Beijing: assessment of energy structure conversion. J Hazard Mater 157(1):47- 56. https://doi.org/10.1016/j.jhazmat.2007.12.092.
Watson JG, Chow JC, Lowenthal DH, Robinson NF, Cahill CF, Blumenthal DL (2002) Simulating changes in source profiles from coal-fired power stations: use in chemical mass balance of PM2.5 in the Mt. Zirkel Wilderness. Energy & Fuels, 16(2):311–324. https://doi.org/10.1021/ef010202w.
WHO (2005) WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide Global update 2005 Summary of risk assessment.
WHO (2000) Polycyclic aromatic hydrocarbons. In: Air Quality Guidelines for Europe WHO Regional Office for Europe (WHO Regional Publications, European Series, No. 91), Second ed. Copenhagen.
Wu SP, Yang BY, Wang XH, Yuan CS, Hong HS (2014) Polycyclic Aromatic hydrocarbons in the atmosphere of two subtropical cities in southeast china: seasonal variation and gas/particle partitioning. Aerosol Air Qual Res 14:1232–1246. https://doi.org/10.4209/aaqr.2013.01.0015
Xing W, Zhang L, Yang L, Zhou Q, Zhang X, Toriba A, Hayakawa K, Tang N (2020) Characteristics of PM2.5-bound polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons at a roadside air pollution monitoring station in Kanazawa, Japan. Int J Environ Res Public Health 17(3):805. https://doi.org/10.3390/ijerph17030805.
Yang B, Zhou L, Xue N, Li F, Li Y, Vogt RD, Cong X, Yan Y, Liu B (2013) Source apportionment of polycyclic aromatic hydrocarbons in soils of Huanghuai Plain, China: comparison of three receptor models. Sci Total Environ 443:31–39. https://doi.org/10.1016/j.scitotenv.2012.10.094
Yang HH, Lai SO, Hsieh LT, Hsueh HJ, Chi TW (2002) Profiles of PAH emission from steel and iron industries. Chemosphere 48:1061–1074. https://doi.org/10.1016/S0045-6535(02)00175-3
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515. https://doi.org/10.1016/S0146-6380(02)00002-5
Zhang Y, Yang L, Zhang X, Li J, Zhao T, Gao Y, Jiang P, Li Y, Chen X, Wang W (2019) Characteristics of PM2.5-bound PAHs at an Urban Site and a Suburban Site in Jinan in North China Plain. Aerosol Air Qual Res 19:871–884. https://doi.org/10.4209/aaqr.2018.09.0353
Zong Z, Wang X, Tian C, Chen Y, Qu L, Ji L, Zhi G, Li J, Zhang G (2016) Source apportionment of PM2.5 at a regional background site in North China using PMF linked with radiocarbon analysis: insight into the contribution of biomass burning. Atmos Chem Phys 16:11249–11265. https://doi.org/10.5194/acp-16-11249-2016
Acknowledgements
The authors would like to appreciate Ali Ashrafi for help with the field work and Majbrit Dela Cruze, Jette Petersen, and Peter Christensen for help with the chemical analyses.
Funding
The financial support of the project was from Isfahan Municipality, Department of Planning and Human Capital Development (Contract No. 96/3439) and Isfahan University of Technology which is appreciated
Author information
Authors and Affiliations
Contributions
Mohsen Soleimani: Conceptualization, methodology, resources, validation, writing original draft, writing-review and editing, supervision, project administration, funding acquisition.
Zohreh Ebrahimi: methodology, formal analysis, investigation, writing original draft, writing-review and editing.
Nourollah Mirghaffar: methodology, writing and editing, supervision,
Hossein Moradi: writing and editing, advisor.
Nasibeh Amini: methodology, formal analysis, investigation, validation, writing,
Kristoffer Gulmark Poulsen: methodology, validation, writing and editing.
Jan H. Christensen: resources, validation, writing-review and editing, funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Constantini Samara
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soleimani, M., Ebrahimi, Z., Mirghaffari, N. et al. Seasonal trend and source identification of polycyclic aromatic hydrocarbons associated with fine particulate matters (PM2.5) in Isfahan City, Iran, using diagnostic ratio and PMF model. Environ Sci Pollut Res 29, 26449–26464 (2022). https://doi.org/10.1007/s11356-021-17635-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17635-8