Abstract
PM2.5 released from urban sources and regional biomass fire is of great concern due to the deleterious effect on human health. This study was conducted to determine the chemical compositions andsource apportionment of PM2.5. Twenty-four-hour PM2.5 samples were collected at two urban monitoring sites in Kuala Lumpur, Malaysia, from 12 November 2013 to 15 January 2014 using a high volume air sampler (HVS). The source apportionment of PM2.5 was determined using positive matrix factorization (PMF) version 5.0. Overall, the PM2.5 mean concentrations ranged from 16 to 55 μg m−3 with a mean of 23 ± 9 μg m−3. The results of enrichment factor (EF) analysis showed that Zn, Pb, As, Cu, Cr, V, Ni, and Cs mainly originated from non-crustal sources. The dominant polycyclic aromatic hydrocarbons (PAHs) were benzo[b]fluoranthene (B[b]F), benzo[ghi]perylene (B[ghi]P), indeno[1,2,3-cd]pyrene (I[cd]P), benzo[a]pyrene (B[a]P) and benzo[k]fluoranthene (B[k]F). PMF 5.0 results showed that the secondary aerosol coupled with biomass burning was the largest contributor followed by combustion of fuel oil and road dust, soil dust source and sea salt and nitrate aerosol, accounting for 34, 25, 24 and 17% of PM2.5 mass, respectively. On the other hand, biomass and wood burning (42%) was the predominant source of PAHs followed by combustion of fossil fuel (36%) and natural gas and coal burning (22%). The broad overview of the PM2.5 sources will help to adopt adequate mitigation measures in the management of future urban air quality in this region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Airborne particles with an aerodynamic diameter of ≤2.5 μm (PM2.5) are of great concern as they can affect human health. The size of the particles enables them to move along the respiratory tract into alveoli. A previous study has linked the concentration of PM2.5 with several diseases related to the respiratory system such as asthma and chronic bronchitis (Romieu et al. 1996). In addition, PM2.5 has effect on cardiovascular disease and lung cancer as well as hospital admissions for haemorrhagic stroke. A previous study provided evidence that each 10 μg m−3 increase in the concentration of PM2.5 is associated with an increase in daily mortality (Laden et al. 2000).
The composition of PM2.5 is interrelated with the sources. The combustion-related sources, particularly from motor vehicles, were found to contribute considerably to the amount of NH4 + and SO4 2− in PM2.5. A study by Alves et al. (2015) showed that trace metals such as Fe, Ba, Zn, Cu, Sb and Sn were likely to be associated with the mechanical wear of different parts of the vehicles and dominate in particles while Ca, Al, K, Sr and Ti typically originated from the re-suspension of dust (including pavement wear). The composition of PM2.5 is also dominated by organic substances such as polycyclic aromatic hydrocarbons (PAHs) compared to coarse fraction of particulate matter (Di Filippo et al. 2010). The United States Environmental Protection Agency (US EPA) has listed 16 PAHs as a priority and seven are categorised in the B2 group: possible human carcinogenic PAHs. The seven PAHs in the B2 group are benzo[a]anthracene (B[a]A), benzo[a]pyrene (B[a]P), benzo[b]fluoranthene (B[b]F), benzo[k]fluoranthene (B[k]F), chrysene (CHR), dibenzo[a,h]anthracene (D[ah]A) and indeno[1,2,3-c,d]pyrene (I[cd]P) (USEPA 2016). In 2012, B[a]P was classified as a highly genotoxic compound. According to the International Agency for Research on Cancer (IARC), B[a]P belongs to group I which is carcinogenic to humans. Moreover, some products containing B[a]P, e.g., particulate matter in outdoor air pollution, tobacco smoke, exhaust from coal combustion and diesel exhaust, are also classified as human carcinogens (IARC 2013). PAHs in PM2.5 are generally associated with fossil fuel combustion by traffic as well as fuel oil, coal combustion and incineration as suggested by Harrison et al. (1996). The investigation of PAHs will help to examine the level of pollution as well as be used as a signature to evaluate the potential sources of PM2.5. Diagnostic ratios (DRs) can commonly be used as conventional source apportionment method in determining the potential sources of PAHs congeners.
Receptor modelling has been used as a chemometric tool to apportion appropriately the sources of airborne particulate matter at a receptor site (Kim et al. 2016). The number, compositions and the contribution of the fingerprints in each sample can be determined by applying the multivariate receptor models. Among the receptor modelling techniques, positive matrix factorization (PMF) has proven to be a trusted and robust technique and has become popular and widely applied in the prediction of the relative contribution of sources of PM2.5 and its compositions. This model has several advantages and strong points to be consider: (a) missing data or that below detection limit can be treated and retained for the use of the model with an adjustment of the associated uncertainty, (b) it optimises the uncertainty of the results, (c) it produces state-of-the-art graphical output (d) priori source information does not require, (e) works with the number of samples that should be more than the number of species, (f) suitable mainly with the ambient measurement data of PM2.5 (g) no negative contribution of the identified source and (h) it can predict the contributing sources of pollutants using single or multiple point data (Norris et al. 2014; Paatero et al. 2014; Khan et al. 2016b). The PMF technique was introduced by Paatero and Tapper (1994) and Paatero (1997) to undertake source apportionment using station data. The software has been further customised and updated with an improved version by the US EPA with latest version of PMF 5.0 (Norris et al. 2014; Paatero et al. 2014). Several researchers have successfully applied this version to the source apportionment of PAHs and inorganic in the fine particulate matter (Wang and Hopke 2014; Khan et al. 2015, 2016a, b). Therefore, PMF 5.0 was chosen to carry out the source apportionment of PM2.5 based on inorganic compositions and polycyclic aromatic hydrocarbons.
As the capital and the largest city in Malaysia, the Kuala Lumpur metropolitan environment is rapidly developing. The population and public/private transport in this city area are steadily increasing and thus, the exposure to various sources of air pollution both from local and trans-boundary emissions is of deep concern. As a result of agricultural activities, mainly by the palm oil growers in Indonesia, as well as the high density of fire hotspots in mainland China, Malaysia often experiences extreme air pollution episodes (Sulong et al. 2017; Zhang et al. 2015). This seasonal pollution greatly influences on the concentration of inorganic and organic compounds in particulate matter (Abas and Simoneit 1996; Omar et al. 2006; Khan et al. 2015, 2016a). Therefore, this study was carried out to determine the PM2.5 concentration and its composition covering both inorganic and organic constituents, particularly during the northeast monsoon (November, December and January) in Southeast Asia. In addition, the potential sources of PM2.5 in Kuala Lumpur were identified by applying US EPA PMF 5.0.
Methodology
Sample and chemical analysis
Sampling description
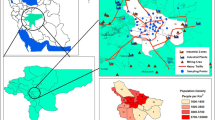
The sampling campaign for 24-h PM2.5 was conducted in the Kuala Lumpur urban environment at two city campuses of Universiti Kebangsaan Malaysia (UKM), namely the UKM Cheras Campus (HUKM) (S1) (3°5′59.6112′′ N, 101°43′33.153′′ E) and the UKM Kuala Lumpur Campus (UKMKL) (S2) (3°10′6.117′′ N, 101°42′4.91′′ E) (Fig. 1), from 12 November 2013 to 15 January 2014 (during the northeast monsoon). S1 is located in an urban area surrounded by residential complexes while S2 is located at the centre of the Kuala Lumpur metropolitan area, an area affected by heavy traffic. The distance between the two stations is about 9 km. The overall local meteorological conditions at the two sites were humid with heavy rainfall due to the prevailing conditions during the sampling period (the wet season). The temperature of the wet season is 28 °C (21–38 °C) and the relative humidity is 74% (20–100%) during the northeast monsoon around the Kuala Lumpur area (Khan et al. 2015). PM2.5 mass samples were collected on quartz microfiber filters (QM-A, 20.3 × 25.4 cm, Whatman, UK) using a HVS PM2.5 sampler (Staplex, USA) at a flow rate of 1.13 m3 min−1 for 24-h. A total of 18 samples, 9 from each of the sites, were collected during the whole sampling period. Before sampling, all filters were wrapped in aluminium foil and pre-fired at 500 °C for 3 h in a muffle furnace (ELF 11/23, Carbolite, UK). Then, the filters were placed in a desiccator for at least 24-h prior to weighing and were weighed both before and after sampling. PM2.5 mass concentration was determined gravimetrically using a five-digit electronic balance (A&D GR-202, USA). Samples were then stored in a freezer at −18 °C prior to extraction for further analysis. The sampling and the subsequent analytical procedures were corroborated in Fig. SI.
Water-soluble ion analysis
For sample extraction, the ultrasonic-shaking digestion method was used following a procedure described by Khan et al. (2016a, b). A strip of the filter was cut into small pieces (~1 cm2) directly into a 50 mL centrifuge tube and filled with 20 mL of 18.2 MΩ ultra-pure water (UPW). The first step of extraction, 45 min of sonication, took place in an ultrasonic bath (Elmasonic S40, Elma GmbH, Germany). Next, centrifugation extraction was carried out using a centrifuge (Kubota 5100, Japan) at 35 rpm for the same period. The solution was subsequently filtered through a syringe filter (Acrodisc®, 0.2 μm 25 mm, Pall Gelman Laboratory, MI, USA) using a 20-cm3 mL-1 Terumo syringe (Terumo, Tokyo, Japan) directly into a 50-mL volumetric flask and topped up to the mark with UPW. This solution was then transferred into a 12-mL low-density polyethylene (LDPE) tube and stored at 4 °C inside a refrigerator prior to analysis. For the detection of cations and anions, metrosep A-Supp 5-150/4.0 and C4-100/4.0 columns were used, respectively. Nitric (1.7 mmol L−1) and 0.7 mmol L−1 dipicolinic acid (Merck KGaA, Germany) were used as eluents for cations. As eluents for anions, 6.4 mmol L−1 sodium carbonate (Na2CO3) (Merck KGaA, Germany) and 2.0 mmol L−1 sodium bicarbonate (NaHCO3) (Merck KGaA, Germany) were used. The flow rate was maintained at the rate of 0.7 mL min−1. For suppressor regenerant, 100 mmol L−1 Suprapur® sulphuric acid (H2SO4) (Merck KGaA, Germany) was used and ions were detected by a conductivity detector. The concentrations of selected ions (NH4 +, K+, Mg2+, NO3 − and SO4 2−) were determined using ion chromatography (Metrohm 850 model 881 Compact IC Pro, Switzerland) within 48 h of extraction which resulted in good standard recoveries of major ions ranging between 93 and 129% (Table 1). The concentrations of ions were corrected from the filter blank samples.
Trace element analysis
A procedure modified from Khan et al. (2016a) was employed for the determination of trace elements. Specifically, microwave-assisted extraction was used for the extraction. Firstly, a 1/32 portion of the filter paper was cut directly into a 100-mL teflon vessel before adding a 4:1 ratio of HNO3 (65%, Merck KGaA, Germany) and H2O2 (40%, Merck KGaA, Germany). The vessel was then placed inside the microwave (MLS-1200-240 Mega, Milestone). Operated at 1000 W, the extraction process involved three steps: (1) 20 min of ramping to 220 °C; (2) 20 min of steady state at 220 °C; and (3) 10 min of cooling down to 60 °C. Upon completion, the vessel was taken out and placed inside a basin filled with tap water to cool down in order to reduce the residual pressure so the vessel was safe to open. The solution was filtered through a 0.2-μm and 25-mm of size Acrodisc® filter (Pall Gelman Laboratory, MI, USA) using a 20-cm3 mL-1 Terumo syringe directly into a 50-mL volumetric flask before dilution with 18.2 MΩ UPW to the mark. The solution was then transferred into high density polyethylene (HDPE) bottles and stored inside a refrigerator at 4 °C prior to analysis. Inductively coupled plasma mass spectrometry (ICP-MS) (PerkinElmer, ELAN 9000, USA) was employed for trace element determination. The standard reference material Urban Particulate Matter (SRM 1648a) from the National Institute of Standards and Technology (NIST), USA, was used for quality assurance purposes. Based on the SRM 1648a, two modes of ICP-MS analysis were carried out: (1) one set of trace elements with high concentrations; and (2) one set of metals with low concentrations. During samples analysis, the concentration of trace metals was corrected from reagent blank and filter blank. Overall, good recoveries detected for all elements ranged between 57 and 106% (Table 1). The method detection limit (MDL) was calculated as three times of the standard deviation of four blank samples. The MDL values were ranged as 0.01 ng m−3 (Cs and Bi) to 22.67 ng m−3 (Al).
PAHs analysis
Solvent extraction with an ultrasonic agitation followed by solid phase extraction (SPE) was employed to extract the PAHs before determination using GCMS. A slightly modified method from Sun et al. (1998) was used in this study as described by Khan et al. (2015). In brief, a portion of the filter sample was mixed with 20 mL of dichloromethane (DCM) before adding spikes of three standard solutions, 0.5 ppb each of Acenaphthelene-D10 (Sigma-Aldrich, USA), Chrysene-D12 (Supelco, USA) and Perylene-D12 (Supelco, USA) as surrogate standards. Next, the mixture was subjected to an ultrasonic agitation process for 10 min (Elmasonic S70H, Elma, Germany) followed by 10 min of centrifuge (Kubota 5100, Japan) at 2500 rpm. After filtration with glass microfiber (Whatman, UK), a volume suppression process was undertaken to reduce the solution volume to ~200 μL under gentle stream of nitrogen gas (N2). The SPE procedure was undertaken using a silica-based sorbent (C18 Cartridge, Lichrolut® RP-18, Merck, Germany) with regard to the clean-up and pre-concentration of PAH samples.
Prior to use, the C18 cartridge was conditioned with 10 mL of n-hexane (Friendemann Schmidt, Germany). Once ready, the extraction solutions were loaded and passed through the cartridge under a gentle vacuum. The C18 cartridge was then eluted with 1:9 of DCM/n-hexane at 1 mL min−1 and the pure PAH sample solution (eluate) was collected in a 20-mL glass test tube. The eluents were again concentrated under a gentle stream of nitrogen gas to ~500 μL. Using a dropper, this 500 μL sample was then transferred to a 1.5-mL GCMS vial before adding n-hexane. Samples were then sent for GCMS (Agilent, 5975C, USA) analysis using a capillary column (HP-5MS), internal diameter (id) 0.25 mm, length of 30 m and thickness of 2.25 μm for the determination of 16 PAHs with the use of selected ion monitoring (SIM) mode and external calibration (EPA 610 PAH Mix, Supelco, USA). The full names of the 16 US EPA-recommended PAHs are as follows: naphthalene (NAP); acenaphthene (ACE); acenaphthylene (ACY); anthracene (ANT); fluorene (FLU); phenanthrene (PHE); fluoranthene (FLN); pyrene (PYR); B[a]A; CHR; B[b]F; B[k]F; B[a]P; I[cd]P; D[ah]A and benzo[g,h,i] perylene (B[ghi]P). The limit of detection (ng m−3) was estimated as three times of standard deviation of each PAH in the blank samples and were reported as 0.109 for NAP, 0.033 for ACE, 0.017 for ACY, 0.013 for FLU, 0.003 for ANT, 0.003 for PHE, 0.005 for PYR, 0.023 for FLN, 0.001 for B[a]A, 0.002 for CHR, 0.004 for B[k]F, 0.005 for B[a]P, 0.008 for B[b]F, 0.008 for I[cd]P, 0.005 for D[ah]A and 0.003 for B[ghi]P. For quality assurance purposes, glassware was soaked in 6% v/v Decon 90 for about 24 h, then rinsed five times with tap water followed by three times with UPW. After that, the glassware was rinsed with n-hexane and dried overnight in an oven at a temperature of 100 °C. The concentrations of PAHs were corrected from the filter blank samples.
PMF data analysis
For the purpose of source identification, PMF 5.0 (US EPA) was applied to both the inorganic content and PAHs as explained by Norris et al. (2014). All other statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) (version 14, Chicago, IL, USA). Figures were plotted using the graphical software, IGOR Pro 6.0.1 (WaveMetrics, OR, USA). The detailed of the PMF 5.0 procedures were considered from Paatero and Tapper (1994) and Paatero (1997). While estimating the uncertainty involved to each of the variable in the samples, we evaluated the empirical equations proposed by Ogulei et al. (2006a, b) (Eq. 1)
where σ ij is the estimated measurement error for jth species in the ith sample, X ij is the observed PM2.5 compositions or PAHs concentration and \( \overset{-}{X_j} \) is the mean value of each PM2.5 composition or PAH. The factor 0.01 was determined through trial and error procedures. Ogulei et al. (2006a, b) uses this method in estimation of uncertainty. Thus, the measurement of uncertainty (S ij ) can be computed with the following (Eq. 2):
where σ ij is the estimation of measurement error (Eq. 2) and C is a constant. This empirical procedure was used to estimate the uncertainty of variables if there were measurements or methodological data to estimate errors (Ogulei et al. 2006a, b). This procedure of uncertainty estimation was applied by Khan et al. (2016b). However, we considered and selected the variability of C factor (Eq. 2 in the methodology of PMF 5.0) value of 0.2 for C as the end calculation was optimised with lower error (%) observed by Khan et al. (2015). An additional 5% uncertainty was added to account for methodological errors in the preparation of filter papers, gravimetric mass measurements and preparing the calibration curves.
The model output of source contribution is provided as normalised or dimensionless (average of each factor contribution is one). Therefore, the mass concentrations of the identified sources were scaled by using the following multiple linear regression (MLR) analysis (Eq. 3)
where M i is the total concentration of PAHs or PM2.5 mass in ith sample, S k is the scaling constant and g ik the normalised formed of source contribution found in the result of PMF modelling. Several other researchers have successfully applied the MLR approach to express the output of PMF (Amil et al. 2016; Khan et al. 2016b).
Backward trajectory model
Backward trajectories were calculated for S1 (HUKM) and S2 (UKMKL). The Hybrid Single Particle Lagrangian Integrated Trajectory (HYSPLIT 4.9) was used to calculate the air mass back trajectories (Stein et al. 2015). The backward trajectories were re-plotted integrating the pressure gradient using Igor Pro, a graphical software (Wavemetrics, USA). The trajectories were calculated for 6 h intervals and a releasing height of 500 m for 120 h. The starting time was selected 0:00, 06:00, 12:00, 18:00 UTC. From Fig. 2, it was observed that the air mass originated from the mainland of China. The gradient of pressure shows that it was governing the transport of air mass to the current locations. Thus, the outflow during northeast monsoon from mainland China can influence significantly on the concentration of PM2.5.
Statistical analysis
SPSS (Inc, version 18 for Windows, Chicago, IL, USA) was used for all related statistical analysis. A paired t test was conducted to see if there were any statistically significant differences to the data obtained from the two sites. Due to the small number of data points and the non- normally distributed data, a Spearman Rank Order correlation, as a nonparametric statistical treatment, was undertaken among the data variables. Moreover, a Mann-Whitney test was applied to the data obtained from the two sites. The two set of the data were not significantly correlated (0.085, p > 0.05). Thus, nonparametric statistical analysis was justified. MLR analysis was also taken into consideration to estimate the contribution of each source identified by the above PMF 5.0 procedure.
Results and discussion
PM2.5 mass concentration
Table 1 shows a summary of PM2.5 and the inorganic components of the samples collected at HUKM (S1) and UKMKL (S2). S2 showed a higher PM2.5 concentration with an average of 25 ± 12 μg m−3 (ranging from 18 to 55 μg m−3) than S1 with an average of 21 ± 4 μg m−3 (ranging from 16 to 28 μg m−3). The t test showed no significant differences (p > 0.05) between PM2.5 concentrations from both stations. Overall, the range of PM2.5 mass concentration varied between 16 and 55 μg m−3, with an average of 23 ± 9 μg m−3. The overall PM2.5 average value is lower than suggested guideline value by World Health Organisation (WHO) PM2.5 which is 25 μg m−3 in a 24-h period, and by far low compared to the US EPA National Ambient Air Quality Standard (NAAQS) of 35 μg m−3 in a 24-h period. The average PM2.5 concentrations in this study were also lower compared to the concentrations measured by Amil et al. (2016) in Petaling Jaya, Malaysia, in 2011–2012 (28 ± 18 μg m−3) but a similar concentration in UKM Bangi, Malaysia, in 2013–2014 (25.13 ± 9.21 μg m−3) and in 2014 (18.3 ± 11.8 μg m−3) was reported by Khan et al. (2016a, b), respectively. However, our value was higher compared to the concentration measured by Mohd Tahir et al. (2013) on the east coast of Malaysia in 2006–2007 (14.3 μg m−3).
Chemical compositions
Referring to Table 1, the overall results for Kuala Lumpur anions show the dominance of SO4 2− (1.90 ± 0.80 μg m−3), followed by NO3 − (0.86 ± 0.26 μg m−3), PO4 3− (0.28 ± 0.13 μg m−3) and Cl− (0.05 ± 0.05 μg m−3). SO4 2− and NO3 − accounted for 9.4 and 4.3% of the total PM2.5 mass, respectively, where the sampling sites showed similar dominance of both ions. Overall in Kuala Lumpur, NH4 + accounted for 2.2% of the total PM2.5 mass and was revealed as the domi nant cation species with an average of 0.45 ± 0.19 μg m−3 (ranging from 0.15 to 0.82 μg m−3). Ca2+ and Na+ were detected at average concentrations of 0.33 ± 0.15 and 0.27 ± 0.13 μg m−3, respectively, and Mg2+ was found as the lowest cation species in both stations with a range of 0.01 to 0.12 μg m−3. Similar trends of cations (NH4 + > Ca2+ > Na+ > K+ > Mg2+) were recorded for both stations. As both stations are located in high traffic density areas, high concentrations of SO4 2− and NO3 − could be associated with motor vehicle emissions. According to Kim et al. (2016), oxidation processes of SO2 and NOx from motor vehicle emissions can in turn increase the concentration of SO4 2− and NO3 − in the atmosphere, particularly in urban and high traffic density areas. Meanwhile, another possible source of NH4 + is biogenic emissions, such as the neutralisation of ammonia gas and atmospheric nitric acid or acidic sulphate particles. Secondary, SO4 2− aerosol is thermally stable and accumulates in the atmosphere and thus influences to increase the concentration (Fang et al. 2011).
Elemental composition
Twenty-one trace elements (Al, Ca, Fe, K, Mg, Na, Pb, Zn, Ag, As, Cd, Cr, Cs, Co, Cu, Mn, Ni, Rb, Se, Sr and V) were measured from the PM2.5 samples and their statistical data are shown in Table 1. The trend of average concentrations of trace elements was ranked as follows: Na > Al > K > Mg > Ca (=Fe) > Zn > Pb > other elements. Overall, metallic elements such as Zn (10.69 ± 2.60 ng m−3) and Pb (9.90 ± 4.89 ng m−3) were abundant in PM2.5 samples. Zn concentrations at both sites were almost the same while Pb concentrations at S2 (heavy traffic) were slightly higher but not statistically significant. Both Zn and Pb are related to traffic, i.e. the brake and tyre wear (Sternbeck et al. 2002). Anthropogenic elements such as Cu, V, Cr, Mn and Ni were found at average concentrations of 8.84 ± 7.06, 5.54 ± 6.16, 5.47 ± 3.37, 5.06 ± 2.65 and 1.57 ± 1.45 ng m−3, respectively.
To obtain a first indication of source contributions, the EF was calculated for each element using the following equation as described by Taylor (1964).
EFcrust = (Ex/Al)sample/(Ex/Al)crust (4)
Ex refers to a median concentration of each element. (Ex/Al)sample is the concentration ratio of element X to Al in aerosols samples and (Ex/Al)crust is the concentration ratio of X to Al in the crustal material. Al was used as the reference element elsewhere (Taylor 1964; Khan et al. 2010). A cut-off of 10 was proposed to differentiate between crustal and natural and anthropogenic origins of trace metals as referred by several researchers (Khan et al. 2010; Cheung et al. 2012). Therefore, we choose EF = 10 as the cut-off point.
Based on the EF profiles (Fig. 3), elements such as Ni, Cs, V, Cr, Zn, Cu, Ag, As, Pb, Cd and Se have EFs of greater than 10, indicating those elements mainly originated from non-crustal sources such as motor vehicles and industrial emissions. Whereas the EF values for the elements Na, Rb, Mn, K, Mg, Co, Sr, Ca, Al and Fe were below 10 suggesting that these elements were mainly contributed to by crustal sources.
Polycyclic aromatic hydrocarbons
Table 2 summarises the statistical data of the 16 PAHs, mean concentrations and their standard deviations at both sampling stations. Overall, the total PAHs concentration was found to be 3.10 ± 1.25 ng m−3 (1.56 to 6.52 ng m−3). The individual PAH concentration (ng m−3) were reported as 0.10 for NAP, 0.16 for ACE, 0.07 for ACY, 0.11 for FLU, 0.06 for ANT, 0.07 for PHE, 0.08 for PYR, 0.07 for FLN, 0.06 for B[a]A, 0.11 for CHR, 0.26 for B[k]F, 0.29 for B[a]P, 0.58 for B[b]F, 0.34 for I[cd]P, 0.19 for D[ah]A and 0.56 for B[ghi]P. S1 (HUKM station) (3.39 ± 1.63 ng m−3) showed a slightly higher total PAHs concentration compared to S2 (UKMKL station) (2.82 ± 0.67 ng m−3) but the difference was not statistically significant (p > 0.05). Although both sites have high traffic density, different sampling dates with different background activities and meteorological conditions might influence the amount of pollutants released and therefore affect the concentration of PAHs at both stations. The high molecular weight (HMW) PAHs, B[b]F, B[ghi]P, I[cd]P, B[a]P and B[k]F, dominated at both stations with concentrations ranging from 0.12 to 1.41 ng m−3. The lower molecular weight (LMW) PAHs such as NAP, ACE, ACY, ANT, PHE, PYR and FLN were only detected at low concentrations, ranging between 0.02 and 0.23 ng m−3. The results concurred with Yunker et al. (2002) who suggested that the HMW PAHs are more likely to be portioned in PM2.5 compared to LMW PAHs. Among the 16 PAHs analysed, B[b]F and B[ghi]P were the most abundant compounds with average concentrations of 0.58 ± 0.30 and 0.56 ± 0.29 ng m−3, respectively. A high abundance of B[b]F and B[ghi]P has been reported to be a marker of vehicle emissions. B[ghi]P is known to be a characteristic of gasoline engines (Miguel et al. 1998). On the other hand, B[b]F and B[ghi]P are indicators of diesel vehicles (Harrison et al. 1996)
Spearman rank order correlations among chemical components
We conducted a Spearman Rank Order correlation analysis as shown in Tables SI, SII and SIII to investigate the relationships among the chemical components. We focused only on correlation coefficients (r) of ≥ 0.70 where significant values are highlighted for p < 0.05 and p < 0.01. The correlation coefficients among the major ionic compounds revealed strong correlations between PM2.5 and K+ (r = 0.73, p < 0.01). Na+ and Cl− are strongly and significantly correlated to each other (r = 0.90) (Table SI). Similarly, the strong and significant correlations were also shown between NH4 + and K+, K+ and Cl−, Ca2+ and Mg2+, SO4 2− and Cl−, and SO4 2− and NO3 −. The results of the trace elements correlation analysis using the Spearman Rank Order showed that strong correlation coefficients were found among the several paired elements. It was noteworthy that the elements representing Earth’s crust, namely Al, Ca, Fe and Mg, are strongly correlated. The correlation results of As, Cr and Cs suggest that these elements might originate from the coal processing facilities. The r values among the pairs of Ni, V, Cu and Mn are significant, indicating that these elements are emitted from a similar source. The Cu-Pb pair shows a strong and significant correlation (r = 0.78, p < 0.01). The results showing strong correlations with r ≥ 0.70 coefficient values were all positive correlations as shown in Table SII. The results of strong correlations among the pairs of variables indicate that these pairs have similar origins. For example, the potential sources of Ca2+ at these locations are mineral dust and construction materials. Na+ and Cl− are potentially emitted from the marine sea salt source. Ni and V are tracers of fuel oil burning in combustion engines. Cs, Cr and As mainly originate from the coal processing sites. Thus, mineral dust, construction activities, marine sea salt, oil burning and coal combustion are significant sources of the chemical components analysed in PM2.5. Similarly, the results of the PAH correlations showed that the lighter molecular weight PAH compounds correlated strongly among themselves and the heavier molecular weight PAH compounds also correlated strongly among themselves. The poor correlation coefficient from the Spearman correlation showed that there is a visible separation of LMW and HMW PAHs.
Diagnostic ratios of polycyclic aromatic hydrocarbons
Table 3 shows the DR values of few selected PAHs. The DR of ANT to ANT + PHE is about 0.45 and 0.46 for site S1 and site S2, respectively, which are showing a strong pyrogenic effect. Khan et al. (2015) reported a similar ratio value at a semi urban area of a nearby city in Bangi. Fossil fuel combustion was identified based on the DR value of FLT to FLT + PYR at each location as also suggested by Rogula-Kozłowska (2016). The DR of B[a]A to B[a]A + CHR suggesting the influence of pyrogenic coal combustion source as referred by Yunker et al. (2002) and Manoli et al. (2004). Combustion of gasoline petroleum was revealed as the DR value of I[cd]P to I[cd]P + B[ghi]P was about 0.3–0.4 and combustion from traffic source was identified based on the DR value of B[a]P to B[ghi]P (Yunker et al. 2002). The B[a]A and CHR were released from the industrial source as suggested by Dickhut et al. (2000). The results of the ratio values for B[a]A/B[a]P suggest that this pair of PAHs can emit from light duty traffic source (Błaszczyk et al. 2017).
Positive matrix factorization (PMF)
By employing US EPA PMF 5.0, four sources of inorganic compositions (18 samples × 17 inorganic compositions matrix) and three sources of PAHs (18 samples × 16 PAHs matrix) in PM2.5 were revealed as shown in Fig. 4 and 5. The source contribution by each factor was summed up to estimate the predicted mass of PM2.5 and total PAHs. A regression of the predicted and measured PM2.5 for the inorganic constituent source apportionment analysis showed that the PM2.5 had been significantly reproduced by PMF 5.0 with an error or overestimation of only 1% (Fig. 6a) (r 2 = 0.88, p < 0.01). Similarly, a correlation of the predicted and the measured PAHs concentration showed a strong and significant correlation (r 2 = 0.99, p < 0.01) (Fig. 6b).
The factor profiles of the inorganic constituents of PM2.5 are listed in Fig. 4 which shows the mass concentration as well as the percentage that each of the variables contribute to each component. The factor components were represented by soil dust (factor 1), sea salt and nitrate aerosol (factor 2), combustion of fuel oil and road dust (factor 3) and secondary aerosol coupled with biomass burning (factor 4).
Soil dust source
Soil dust accounted for 24% of the PM2.5 mass as shown in Fig. 4 and Fig. SII. The factor component 1 featured by Al (90% of Al mass), Ca (93% of Ca mass) and Mg (99% of Mg mass) giving an indication that these tracers are related to soil dust source. Al, Ca and Mg represent a natural soil dust source. Soil and windblown dust particles are mainly released from farm land, pasture land and unpaved road and Al, Ca and Mg were used as signature components of soil dust source (Gaita et al. 2014).
Sea salt and nitrate aerosol
Sea salt and nitrate aerosol accounted for 17% of the total PM2.5 mass concentration (Fig. 4). The major compositions of this source were Cl− (77% of Cl− mass), Na+ (32% of Na+ mass) and NO3 − (54% of NO3 − mass) which define the component as fresh sea salt and nitrate aerosol. As suggested by Hasheminassab et al. (2014), the dominant tracers of aged sea salts are Na+, SO4 2− and NO3 − , and chlorine has negligible or nearly zero-contribution to the aged sea salt source. Chlorine depletion commonly occurs due to the reaction of sea salt emits through the well-defined mechanism of bubble bursting and atmospheric acidic gases during their long transport from sources (Song and Carmichael 1999). This source is also largely affected by nitrate aerosol.
Combustion of fuel oil and road dust
Combustion of fuel oil and road dust accounted for 25% of the PM2.5 mass (Fig. 4). The highest percentages of mass contributing to this source profile were V (90% of V mass), As (60% of As mass), Pb (53% of Pb mass), Ni (50% of Ni mass), Mn (48% of Mn mass) and Cd (34% of Cd mass). Combustion of fuel oil and road dust is commonly characterised by a good number of tracers from the heavy metal group. Ni and V are well recognised as specific markers released from the combustion of fuel oil. A number of studies described the V and Ni as representative of fuel oil combustion (Vallius et al. 2005). The brake-wear dust of motor vehicles contains Pb and release as non-exhaust traffic emission re-suspended as road dust (Pant and Harrison 2013). Zn has been used in tyres and also as an additive in car engine oil as a lubricant (Dall’Osto et al. 2013). Ewen et al. (2009) suggested that along with the wear and tear of tyres, Cd is mainly emitted from the combustion of diesel fuel and oil or lubricants. Khan et al. (2016a) identified a vehicle source based on the significant contribution of Mn to the respective source profile.
Secondary aerosol coupled with biomass burning
Secondary aerosol coupled with biomass burning accounted for 34% of the PM2.5 mass (Fig. 4 and Fig. SII). This factor profile was dominated by the tracers of SO4 2, K+, Cd, NH4 + and NO3 −. The secondary aerosol source is identified based on the predominant concentration of SO4 2− (84% of SO4 2− mass), Cd (54% of Cd), NH4 + (50% of NH4 +) and NO3 − (47% of NO3 −). K+ is widely considered a marker of biomass burning and accounted for K+ (51% of K+). A study by Echalar et al. (1995) established a considerable relationship between K+ and a biomass burning source. Similarly, K+ was seen in other literature as a marker of biomass origin (Khan et al. 2016b).
Figure 5 shows the profiles of the sources of PAHs in the mass concentration and the percentage of the variables. Three factor profiles of PAHs samples were determined using PMF 5.0. These factors were explained by combustion of fossil fuel (factor 1), natural gas and coal burning (factor 2) and biomass and wood burning (factor 3) sources.
Combustion of fossil fuel
The factor profile 1 showed the predominant tracers B[k]F (46% of B[k]F mass), B[a]P (47% of B[a]P mass), I[cd]P (51% of I[cd]P mass) and B[ghi]P (50% of B[ghi]P mass) and could be attributed to the combustion of fossil fuel. These PAHs are widely known as biomarkers related to the combustion of fuel from traffic (Jamhari et al. 2014). Simcik et al. (1999) also suggested that the B[ghi]P release from traffic combustion source. This source was accounted for 36% of total PAH mass (Fig. SIII).
Natural gas and coal burning
Natural gas and coal burning represented 22% of the total PAHs (Fig. 5). The molecular markers of PAHs that dominated this factor profile were NAP (81% of NAP mass), ACY (54% of ACY mass), ACE (56% of ACE mass), FLR (50% of FLR mass), PHE (43% of PHE mass), FLN (43% of FLN mass) and PYR (42% of PYR mass). The presence of these LMW PAHs could indicate a natural gas and coal burning source. FLR and PYR are referred to as molecular markers of coal combustion (Harrison et al. 1996). LMW PAHs, particularly NAP, are related to ground evaporation or unburned fuel (Khairy and Lohmann 2013). PHE, FLN and PYR were used as markers to identify natural gas and coal burning sources. Jamhari et al. (2014) also applied the above molecular markers to identify the emission source of natural gas and coal burning.
Biomass and wood burning
Biomass and wood burning accounted for 42% of total PAHs and included ANT (46% of ANT mass), PHE (48% of PHE mass), PYR (46% of PYR mass), CHR (38% of CHR mass), and B[b]F (66% of B[b]F mass) (Figs. 5 and SIII). Other researchers have referred to FLR and PYR as tracers of wood burning (Yunker et al. 2002). Rajput et al. (2011) identified FLR, B[b]F and B[k]F as major markers representing the burning of agriculture refuse. The HMW PAHs were dominant during the biomass burning event observed by Phoothiwut and Junyapoon (2013). In Malaysia, the burning of agricultural refuse is common practice. This, along with other means of waste management, can lead to atmospheric pollution including PAHs through direct or secondary pathways. Thus, factor 3 might be classified as a biomass and wood burning source.
Conclusions
Our results on the determination of PM2.5 mass and its constituents (inorganic compounds and PAHs) during the northeast monsoon showed that the average PM2.5 mass concentration was lower than the WHO and US EPA 24 h standards. For inorganic constituents, SO4 2−, NO3 − and NH4 + dominated the water-soluble ions at both stations. High concentrations of these ions could be associated with motor vehicle emissions with an addition of biogenic emissions as the NH4 + contributor, as both stations are located in areas with high traffic density. The trend of average concentrations of trace elements was Na > Al > K > Mg > Ca (= Fe) > Zn > Pb > other elements. The EFs indicated that Ni, Cs, V, Cr, Zn, Cu, Ag, As, Pb, Cd and Se mainly originated from non-crustal sources such as motor vehicles and industrial emissions, where As, Pb, Cd and Se were the most abundant elements.
The PM2.5-bound PAHs showed that the total concentration of 16 PAHs was slightly higher at the HUKM site (S1) compared to UKMKL (S2) but statistically not significant (p > 0.05). Among the 16 PAHs, the HMW PAHs, e.g. B[b]F and B[ghi]P, were the most abundant analysed at both stations. These are known as indicators of emissions from diesel vehicles. The other HMW PAHs, e.g. I[cd]P, B[a]P and B[k]F, also dominated both stations. Pearson correlations further revealed the visible separation of LMW and HMW PAHs with poor correlation between these groups. DRs were employed to determine the potential sources of PAHs congeners to enhance source apportionment result interpretations. Among them were strong pyrogenic effect, fossil fuel combustion, pyrogenic coal combustion source, combustion of gasoline petroleum, combustion from traffic source and the industrial source.
To further understand the PM2.5 constituents, source apportionment analysis was carried out for both the inorganic and PAHs datasets by employing US EPA PMF 5.0. Four sources were determined for inorganic constituents while three sources were revealed for the PAHs. Secondary aerosol coupled with biomass burning was found to be the major source (34%) for the inorganic constituents in PM2.5 in Kuala Lumpur, with abundance of SO4 2, K+, NH4 + Cd and NO3 −. The other three factors were combustion of fuel oil and road dust, soil dust source and sea salt and nitrate aerosol. On the other hand, for the 16 PAHs, biomass and wood burning was the major source for Kuala Lumpur, contributing to 42% of the total PAHs. Second and third sources identified for the 16 PAHs were combustion of fossil fuel, and natural gas and coal burning. Both results from the PMF 5.0 show strong and significant correlations (r 2 = 0.88 (PM2.5), r 2= 0.99 (PAHs), p < 0.01) between predicted and actual mass indicating that our techniques and output are reliable for future comprehensive investigation and air quality management purposes.
References
Abas MR, Simoneit BRT (1996) Composition of extractable organic matter of air particles from Malaysia. Initial Stud Atmos Environ 30:2779–2793. doi:10.1016/1352-2310(95)00336-3
Alves CA., Gomes J, Nunes T, Duarte M, Calvo A, Custódio D, Pio C, Karanasiou A, Querol X (2015) Size-segregated particulate matter and gaseous emissions from motor vehicles in a road tunnel. Atmos Res 153:134–144
Amil N, Latif MT, Khan MF, Mohamad M (2016) Seasonal variability of PM2.5 composition and sources in the Klang Valley urban-industrial environment. Atmos Chem Phys 16:5357–5381. doi:10.5194/acp-16-5357-2016
Akyüz M, Çabuk H (2010) Gas-particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci Total Environ 408(22):5550–5558
Błaszczyk E, Rogula-Kozłowska W, Klejnowski K, Fulara I, Mielżyńska-Švach D (2017) Polycyclic aromatic hydrocarbons bound to outdoor and indoor airborne particles (PM2.5) and their mutagenicity and carcinogenicity in Silesian kindergartens. Poland Air Qual Atmos Health 10:389–400. doi:10.1007/s11869-016-0457-5
Brandli RC, Bucheli TD, Ammann S, Desaules A, Keller A, Blum F, Stahel WA (2008) Critical evaluation of PAH source apportionment tools using data from the Swiss soil monitoring network. J Environ Monit 10(11):1278
Cheung K, Shafer MM, Schauer JJ, Sioutas C (2012) Historical trends in the mass and chemical species concentrations of coarse particulate matter in the Los Angeles Basin and relation to sources and air quality regulations. J Air Waste Mange Assoc 62:541–556. doi:10.1080/10962247.2012.661382
Dall'Osto M, Querol X, Amato F, Karanasiou A, Lucarelli F, Nava S, Calzolai G, Chiari M (2013) Hourly elemental concentrations in PM2.5 aerosols sampled simultaneously at urban background and road site during SAPUSS—diurnal variations and PMF receptor modelling. Atmos Chem Phys 13:4375–4392. doi:10.5194/acp-13-4375-2013
De La Torre-Roche RJ, Lee WY, Campos-Díaz SI (2009) Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: Analysis of a potential problem in the United States/Mexico border region. J Hazard Mater 163(2–3):946–958
Di Filippo P, Riccardi C, Pomata D, Buiarelli F (2010) Concentrations of PAHs, and nitro- and methyl- derivatives associated with a size-segregated urban aerosol. Atmos Environ 44:2742–2749. doi:10.1016/j.atmosenv.2010.04.035
Dickhut RM, Canuel EA, Gustafson KE, Liu K, Arzayus KM, Walker SE, Edgecombe G, Gaylor MO, MacDonald EH (2000) Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region. Environ Sci Technol 34:4635–4640. doi:10.1021/es000971e
Echalar F, Gaudichet A, Cachier H, Artaxo P (1995) Aerosol emissions by tropical forest and savanna biomass burning: characteristic trace elements and fluxes. Geophys Res Lett 22:3039–3042. doi:10.1029/95GL03170
Ewen C, Anagnostopoulou M, Ward N (2009) Monitoring of heavy metal levels in roadside dusts of Thessaloniki, Greece in relation to motor vehicle traffic density and flow. Environ Monit Assess 157:483–498. doi:10.1007/s10661-008-0550-9
Fang G-C, Lin S-C, Chang S-Y, Lin C-Y, Chou C-CK, Wu Y-J, Chen Y-C, Chen W-T, Wu T-L (2011) Characteristics of major secondary ions in typical polluted atmospheric aerosols during autumn in central Taiwan. J Environ Manag 92:1520–1527. doi:10.1016/j.jenvman.2011.01.011
Gaita SM, Boman J, Gatari MJ, Pettersson JBC, Janhäll S (2014) Source apportionment and seasonal variation of PM2.5 in a Sub-Saharan African city: Nairobi, Kenya. Atmos Chem Phys 14:9977–9991. doi:10.5194/acp-14-9977-2014
Harrison RM, Smith DJT, Luhana L (1996) Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, U.K. Environ Sci Technol 30:825–832. doi:10.1021/es950252d
Hasheminassab S, Daher N, Saffari A, Wang D, Ostro BD, Sioutas C (2014) Spatial and temporal variability of sources of ambient fine particulate matter (PM2.5) in California. Atmos Chem Phys 14:12085–12097. doi:10.5194/acp-14-12085-2014
IARC (2013) International Agency for Research on Cancer. Air pollution and cancer/editors, K. Straif, A. Cohen, J. Samet.
Jamhari AA, Sahani M, Latif MT, Chan KM, Tan HS, Khan MF, Mohd Tahir N (2014) Concentration and source identification of polycyclic aromatic hydrocarbons (PAHs) in PM10 of urban, industrial and semi-urban areas in Malaysia. Atmos Environ 86:16–27. doi:10.1016/j.atmosenv.2013.12.019
Khairy MA, Lohmann R (2013) Source apportionment and risk assessment of polycyclic aromatic hydrocarbons in the atmospheric environment of Alexandria, Egypt. Chemosphere 91:895–903. doi:10.1016/j.chemosphere.2013.02.018
Khan MF, Latif MT, Lim CH, Amil N, Jaafar SA, Dominick D, Mohd Nadzir MS, Sahani M, Tahir NM (2015) Seasonal effect and source apportionment of polycyclic aromatic hydrocarbons in PM2.5. Atmos Environ 106:178–190. doi:10.1016/j.atmosenv.2015.01.077
Khan MF, Latif MT, Saw WH, Amil N, Nadzir MSM, Sahani M, Tahir NM, Chung JX (2016a) Fine particulate matter in the tropical environment: monsoonal effects, source apportionment, and health risk assessment. Atmos Chem Phys 16:597–617. doi:10.5194/acp-16-597-2016
Khan MF, Shirasuna Y, Hirano K, Masunaga S (2010) Urban and suburban aerosol in Yokohama, Japan: a comprehensive chemical characterization. Environ Monit Assess 171:441–456. doi:10.1007/s10661-009-1290-1
Khan MF, Sulong NA, Latif MT, Nadzir MSM, Amil N, Hussain DFM, Lee V, Hosaini PN, Shaharom SY, Nur Amira YM, Hoque HMS, Chung JX, Sahani M, Mohd Tahir N, Juneng L, Maulud KNA, Abdullah SMS, Fujii Y, Tohno S and Mizohata A (2016b) Comprehensive assessment of PM2.5 physicochemical properties during the Southeast Asia dry season (southwest monsoon). J Geophys Res Atmos 121:2016JD025894. doi:10.1002/2016JD025894
Kim BM, Lee S-B, Kim JY, Kim S, Seo J, Bae G-N, Lee JY (2016) A multivariate receptor modeling study of air-borne particulate PAHs: regional contributions in a roadside environment. Chemosphere 144:1270–1279. doi:10.1016/j.chemosphere.2015.09.087
Laden F, Neas LM, Dockery DW, Schwartz J (2000) Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Persp 108:941–947
Manoli E, Kouras A, Samara C (2004) Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere 56:867–878. doi:10.1016/j.chemosphere.2004.03.013
Miguel AH, Kirchstetter TW, Harley RA, Hering SV (1998) On-road emissions of particulate polycyclic aromatic hydrocarbons and black carbon from gasoline and diesel vehicles. Environ Sci Technol 32:450–455. doi:10.1021/es970566w
Mohd Tahir N, Suratman S, Fong FT, Hamzah MS, Latif MT (2013) Temporal distribution and chemical characterization of atmospheric particulate matter in the eastern coast of peninsular Malaysia. Aerosol Air Qual Res 13:584–595
Norris G, Duvall R, Brown S, Bai S (2014) EPA positive matrix factorization (PMF) 5.0 fundamentals & user guide. Prepared for the US Environmental Protection Agency, Washington, DC, by the National Exposure Research Laboratory, Research Triangle Park.
Ogulei D, Hopke PK, Wallace LA (2006a) Analysis of indoor particle size distributions in an occupied townhouse using positive matrix factorization. Indoor Air 16:204–215. doi:10.1111/j.1600-0668.2006.00418.x
Ogulei D, Hopke PK, Zhou L, Patrick Pancras J, Nair N, Ondov JM (2006b) Source apportionment of Baltimore aerosol from combined size distribution and chemical composition data. Atmos Environ 40(Supplement 2):396–410. doi:10.1016/j.atmosenv.2005.11.075
Omar NYMJ, Mon TC, Rahman NA, Abas MRB (2006) Distributions and health risks of polycyclic aromatic hydrocarbons (PAHs) in atmospheric aerosols of Kuala Lumpur, Malaysia. Sci Total Environ 369:76–81. doi:10.1016/j.scitotenv.2006.04.032
Paatero P (1997) Least squares formulation of robust non-negative factor analysis. Chemometr Intell Lab Syst 37:23–35. doi:10.1016/S0169-7439(96)00044-5
Paatero P, Eberly S, Brown SG, Norris GA (2014) Methods for estimating uncertainty in factor analytic solutions. Atmos Meas Tech 7:781–797. doi:10.5194/amt-7-781-2014
Paatero P, Tapper U (1994) Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5:111–126. doi:10.1002/env.3170050203
Pant P, Harrison RM (2013) Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: a review. Atmos Environ 77:78–97. doi:10.1016/j.atmosenv.2013.04.028
Pies C, Hoffmann B, Petrowsky J, Yang Y, Ternes TA, Hofmann T (2008) Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 72(10):1594–1601
Phoothiwut S, Junyapoon S (2013) Size distribution of atmospheric particulates and particulate-bound polycyclic aromatic hydrocarbons and characteristics of PAHs during haze period in Lampang Province, Northern Thailand. Air Qual Atmos Health 6:397–405. doi:10.1007/s11869-012-0194-3
Rajput P, Sarin MM, Rengarajan R, Singh D (2011) Atmospheric polycyclic aromatic hydrocarbons (PAHs) from post-harvest biomass burning emissions in the Indo-Gangetic Plain: isomer ratios and temporal trends. Atmos Environ 45:6732–6740. doi:10.1016/j.atmosenv.2011.08.018
Rogula-Kozłowska W (2016) Size-segregated urban particulate matter: mass closure, chemical composition, and primary and secondary matter content. Air Qual Atmos Health 9:533–550. doi:10.1007/s11869-015-0359-y
Romieu I, Meneses F, Ruiz S, Sienra JJ, Huerta J, White MC, Etzel RA (1996) Effects of air pollution on the respiratory health of asthmatic children living in Mexico City. Am J Resp Crit Care 154:300–307. doi:10.1164/ajrccm.154.2.8756798
Simcik MF, Eisenreich SJ, Lioy PJ (1999) Source apportionment and source/sink relationships of PAHs in the coastal atmosphere of Chicago and Lake Michigan. Atmos Environ 33:5071–5079. doi:10.1016/S1352-2310(99)00233-2
Song CH, Carmichael GR (1999) The aging process of naturally emitted aerosol (sea-salt and mineral aerosol) during long range transport. Atmos Environ 33:2203–2218. doi:10.1016/S1352-2310(98)00301-X
Stein AF, Draxler RR, Rolph GD, Stunder BJB, Cohen MD, Ngan F (2015) NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull Am Meteorol Soc 96:2059–2077. doi:10.1175/bams-d-14-00110.1
Sternbeck J, Sjödin Å, Andréasson K (2002) Metal emissions from road traffic and the influence of resuspension—results from two tunnel studies. Atmos Environ 36:4735–4744. doi:10.1016/S1352-2310(02)00561-7
Sulong NA, Latif MT, Khan MF, Amil N, Ashfold MJ, Wahab MIA, Chan KM, Sahani M (2017) Source apportionment and health risk assessment among specific age groups during haze and non-haze episodes in Kuala Lumpur, Malaysia. Sci Total Environ 601–602:556–570
Sun F, Littlejohn D, David Gibson M (1998) Ultrasonication extraction and solid phase extraction clean-up for determination of US EPA 16 priority pollutant polycyclic aromatic hydrocarbons in soils by reversed-phase liquid chromatography with ultraviolet absorption detection1. Anal Chim Acta 364:1–11. doi:10.1016/S0003-2670(98)00186-X
Taylor SR (1964) Abundance of chemical elements in the continental crust: a new table. Geochim Cosmochim Acta 28:1273–1285. doi:10.1016/0016-7037(64)90129-2
USEPA (2016) Polycyclic organic matter. http://www3.epa.gov/airtoxics/hlthef/polycycl.html. Accessed 27/01/2016
Vallius M, Janssen NAH, Heinrich J, Hoek G, Ruuskanen J, Cyrys J, Van Grieken R, de Hartog JJ, Kreyling WG, Pekkanen J (2005) Sources and elemental composition of ambient PM2.5 in three European cities. Sci Total Environ 337:147–162. doi:10.1016/j.scitotenv.2004.06.018
Wang Y, Hopke PK (2014) Is Alaska truly the great escape from air pollution?–long term source apportionment of fine particulate matter in Fairbanks, Alaska. Aerosol Air Qual Res 14:1875–U1101
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515. doi:10.1016/S0146-6380(02)00002-5
Zhang Z, Gao J, Engling G, Tao J, Chai F, Zhang L, Zhang R, Sang X, Chan C-y, Lin Z, Cao J (2015) Characteristics and applications of size-segregated biomass burning tracers in China’s Pearl River Delta region. Atmos Environ 102:290–301
Acknowledgements
The authors would like to thank Universiti Kebangsaan Malaysia for Research University Grants (DIP-2016-015 and GGPM-2016-034) and the Ministry of Education for the Fundamental Research Grant (FRGS/1/2015/WAB03/UKM/01/1). The meteorological data applied to the HYSPLIT model were accessible at ftp://arlftp.arlhq.noaa.gov/pub/archives/reanalysis. Special thanks go to Dr. Rose Norman for proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research Highlights
• We determined the source apportionment analysis based on PM2.5 composition
• SO4 2−, NO3 −, NH4 +, Na, Al, K and Mg were major inorganic elements in PM2.5
• B[b]F and B[ghi]P were the most abundant PAHs in atmospheric PM2.5
• Secondary/biomass, fuel oil/road dust and soil were the predominant PM2.5 sources
• Biomass and wood burning were the predominant sources of PAHs
Electronic supplementary material
ESM 1
(DOCX 2451 kb)
Rights and permissions
About this article
Cite this article
Khan, M.F., Hwa, S.W., Hou, L.C. et al. Influences of inorganic and polycyclic aromatic hydrocarbons on the sources of PM2.5 in the Southeast Asian urban sites. Air Qual Atmos Health 10, 999–1013 (2017). https://doi.org/10.1007/s11869-017-0489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-017-0489-5