Abstract
The fatty acid composition of top-selling fish oil dietary supplements in the markets was compared with the content stated on product label, and their oxidative qualities and heavy metal contents were evaluated in this study. While all the capsule groups (C) confirmed the label information, it was observed that one-third of the syrup groups (S) had less than the specified content. Capsule groups generally had richer EPA and DHA contents than syrup groups in the samples examined. The peroxide values (PV) of all fish oil capsules and syrups were found in the range of 1.97–2.89 mEq/kg and 2.22–18.30 mEq/kg, respectively. As for free fatty acids (FFA) values, the C4, S6, S9, and S10 groups were above the 3% oleic acid limit recommended for high-quality oils. However, thiobarbituric acid reactive substances (TBARs) values were found below 1 mg MA/kg in all groups. All fish oil supplements were within the limits specified in terms of As (0.50–4.19 µg/g), Cd (0.14 µg/g detected for one group, C5), Cu (not detected), Fe (0.32–15.7 µg/g), and Hg (≤ 0.1 µg/g). On the other hand, two fish oil supplements from the capsule group (0.17 for C6 and 1.01 µg/g for C8) and one group from the syrup group (0.29 µg/g for S10) exceeded the recommended limit in terms of Pb (0.1 mg/kg). As a result of the research, it can be concluded that the chemical quality of fish oils in syrup form needs to be improved and their reliability in terms of fatty acid content should be increased. Considering the heavy metals, it seems significant to follow up the fish oil products more strictly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The long-chain polyunsaturated fatty acids (PUFAs), especially eicosapentaenoic acid (EPA, 20:5ω3) and docosahexaenoic acid (DHA, 22:6ω3) found in fish, have been recognized with their health benefits. These fatty acids are found in many parts of the body, including cell membranes, and are involved in anti-inflammatory processes and the viscosity of cell membranes (Lazzarin et al. 2009; Smith et al. 2011). It is known that EPA and DHA are essential for proper fetal development and healthy aging. EPA and DHA, also precursors of several metabolites, are considered by many researchers to be useful in the prevention or treatment of various diseases (Serhan et al. 2008). The existing literature remarks that the deficiency of EPA and DHA has contributed to the increasing incidence of atherosclerosis, obesity, coronary heart disease (CHD), hypertension, metabolic syndrome, immune system disorders, collagen vascular diseases, and possibly cancer (Kremmyda et al. 2011; Swanson et al. 2012; Ceylan et al. 2018; 2020; Cetinkaya et al. 2021).
Alpha-linolenic acid (ALA, 18:3ω3), a shorter-chain ω3 fatty acid, is a prominent constituent in our diets because it is found in many commonly consumed terrestrial plants, but it does not provide the health benefits seen with EPA and DHA. While it is possible for the body to convert ALA to EPA and DHA with the elongase and desaturase enzymes, the researches indicated that only a small amount can be synthesized (Neff et al. 2011). Chiu et al. (2008) found that only 2–10% of ALA was converted to EPA or DHA. Meanwhile, it was also observed that the conversion of ALA to EPA was 0.3% and to DHA was < 0.01% (Hussein et al. 2005). It was reported that the conversion of ALA to these fatty acids is a little better in women than men, possibly in consequence of an upregulatory effect of estrogen (Givens and Gibbs 2008). Because the body is able to slowly convert the shorter-chain ALA to the more active long chain, especially in men, it can be challenging to get adequate intake of EPA and DHA through land-based diets alone. EPA and DHA are found in reasonably high quantities in most seafood, especially in fatty fish. Therefore, the intake of these fatty acids is directly influenced by fish consumption. The Food and Agriculture Organization (FAO) and World Health Organization (WHO) recommended that people who have a balanced and healthy diet should consume 0.25–2 g EPA + DHA per day (FAO-WHO 2010). Because the higher intake is needed to meaningfully modify many of the cardiovascular diseases (CVD) risk factors, patients with CVD should be encouraged to increase their consumption of EPA and DHA (Kris-Etherton et al. 2002).
The major changes in modern diet over the last century have led to a decrease in the general consumption of ω3 fatty acids. The daily intake of EPA and DHA in most Western diet is considerably below the recommended ratio. Therefore, dietary supplementation of these fatty acids appears to be an alternative way to meet of ω3 PUFAs by many consumers. Increasing consumer demand for EPA- and DHA-enriched foods has resulted in consistent growth of the market. The global ω3 supplements market size was valued at USD 5.18 billion in 2019 and is estimated to expand at a compound annual growth rate of 8.4% from 2020 to 2027 (GVR 2021). By geographic region, North America (USA and Canada) accounted for approximately 43% of these consumer sales, while Europe and Asia–Pacific each demanded approximately 27%, and the rest of the world also accounts for 5% of total spending (Packaged Facts 2012). With a ratio of approximately 13%, nutritional supplements are an important part of this market after enriched baby foods (40%) and enriched food and beverages (31%) (Packaged Facts 2012).
It is clear that EPA and DHA levels in fish oils change due to intrinsic (species, size, age, and sexual maturity) and extrinsic factors (food sources, fishing season, and area), but the level of these fatty acids should be accurately displayed on the product labels. Mislabelling would be deceptive for the consumers who expect health benefits. Due to their high content of unsaturated fatty acids, fish oils are easily oxidized even at room temperature, causing undesirable flavors and loss of nutritional quality. During the extraction of fish oil by wet pressing which is the most commonly used method for production on an industrial scale, fish is heated (100–145 °C), pressed, decanted, and centrifuged (Bonilla-Méndez and Hoyos-Concha 2018). Drastic temperature and pressure conditions used for protein coagulation and subsequent oil release can cause hydrolysis and/or oxidation of the PUFAs in fish oil. Although antioxidant addition is widely used to prevent oxidation during storage, some oxidized products can still occur in fish oil products (Shukla and Perkins 1998; Kolanowski 2010). In addition to that, it may not even be detected by consumers because of gelatine coat or aroma ingredients. Studies about the ω3 series fatty acid content and the chemical integrity of fish oil dietary supplements in recent years cause concern. Ritter et al. (2013) found that in the 16 top-selling fish oil dietary supplements in North America, many products had unacceptably high peroxide levels, and more than half did not meet label claims for EPA and DHA content. Jackowski et al. (2015) observed that 50% of the fish oil dietary supplements tested (171 samples) exceeded the recommended levels for oxidation markers. Ingestion of oxidized lipids with dietary supplements can lead to an increase in circulating oxidized lipid levels (Turner et al. 2006), and elevated oxidized lipid levels are associated with increased cardiovascular risk in patients with coronary disease (Walter et al. 2008). Furthermore, oxidized lipids have a main role in atherogenesis and may play a role in both vascular damage and insulin resistance (Berliner and Watson 2005). For instance, HNE (4-hydroxy-2-nonenal), a major reactive aldehyde formed by the peroxidation of ω-6 PUFAs, can cause diseases such as atherosclerosis, neurodegenerative diseases, and cancer (Rosenfeld et al. 1990; Yoritaka et al. 1996; Shibata et al. 2001; Zhong and Yin 2015).
Heavy metals released in aquatic ecosystems enter the water and sediment phases and also have the potential to bioaccumulate in biota (phytoplankton, zooplankton, nektons, mollusks, benthos, and fish). People at the top of the food chain are more likely to be affected by metal contamination through the food intake of aquatic foods such as fish, mollusks, and shrimp (Kumari et al. 2018). Metal contaminants found in fish oils produced from fish caught from industrially polluted waters are another important health risk for the consumer (Zohra and Habib 2016; Das et al. 2017). Although some heavy metals such as zinc, iron, cobalt, and copper are essential for enzymatic activity and other biological processes at low levels, they become toxic when certain limits are exceeded (Yi and Zhang 2012). However, some elements such as lead, cadmium, and mercury do not have a known role in metabolism and are toxic even when taken in low concentrations. It has been observed that the accumulation of heavy metals in fish is predominantly in the liver, while the least accumulation is in the muscle tissues (Kargın and Erdem 1992; Kosker et al. 2019). For this reason, it is especially important to know the heavy metal content of fish oil products obtained from the liver. In a few studies on this respect, negligible levels of heavy metals were found in fish oil preparations (Güzelsoy and İzgi 2015; Lee et al. 2016). It is thought that heavy metal content and oxidative modification of ω3 fatty acids in dietary supplements may interfere with their intended biological or clinical benefits (Garcia-Hernandez et al. 2013; Nogueira et al. 2016). The increase in the use of food supplements reveals the necessity of focusing on these products that may pose serious health risks. Therefore, the aim of this study was to determine the fatty acid content (e.g., saturated fat, EPA, DHA) of top-selling fish oil dietary supplements in the market for comparison with the claimed contents on the product labels and to assess the oxidative qualities and heavy metal contents.
Materials and methods
Samples
In order to “assess the safety of dietary fish oil supplements in terms of content and quality,” local drugstores (Adana, Turkey) were searched to identify top-selling fish oil supplements. Marine oils from other sources (such as krill, squid, or algae) were not included in the scope of this study because of significant differences in fatty acid composition. Ten brands of fish oil capsules (C) and ten brands of fish oil syrups (S) were randomly purchased among the top-selling ones during December 2019–January 2020. All the purchased products had shelf life left at the time of sampling and analysis. The details of all samples (label claim for EPA, DHA, and total ω3 contents, remaining shelf life, price, and additional information) are presented in Table 1. All products were subsequently tested for the fatty acid composition, peroxide value (PV), free fatty acids (FFA), thiobarbituric acid reactive substances (TBARs), and heavy metals (As, Cd, Cu, Fe, Pb, and Hg). All used chemicals were obtained from Merck (Darmstadt, Germany).
Fatty acid methyl ester analyses (FAME)
Lipids were derivatized to fatty acid methyl esters (FAME) according to the method of Ichihara et al. (1996) with minor modifications. Briefly, extracted lipid sample (25 mg) was dissolved in 2 ml of n-heptane followed by 4 ml of 2 M methanolic KOH. The tube was vortexed for 2 min at room temperature and centrifuged at 4000 RPM for 10 min. After centrifugation, the n-heptane layer containing the FAME was taken for gas chromatography analyses (GC).
Gas chromatography condition for FAME
The fatty acid composition was analyzed using a gas chromatography (GC) Clarus 500 device (PerkinElmer, USA), equipped with a flame ionization detector and a fused silica capillary SGE column (60 m × 0.32 mm ID BPX70 × 0.25 μm, USA or Australia). The oven temperature was 140 °C held for 8 min and raised to 220 °C at a rate of 4 °C/min and then to 230 °C at a rate of 1 °C/min, while the injector and detector temperatures were maintained at 260 and 230 °C, respectively. Helium was used as a carrier gas and had a flow of 40 ml/min (1:40), with a constant pressure of 16 ps. During the analysis, 1 μl of the sample was injected. Fatty acids were identified by comparing the retention times of FAME (Supelco, Catalogue No: 18919) with the standard 37-component FAME mixture. Three replicates of GC analyses were carried out, and the results were expressed in GC area % as mean value standard deviation (SD).
Lipid stability analysis
Peroxide values (PV) of fish oil capsules and syrups were determined according to the official AOCS PV method Cd 8–53 (AOCS 1994) and stated as mEq of peroxide O2 per kg oil. Approximately 2 g of lipid sample was combined with acetic acid:chloroform (3/2, v/v) and constantly stirred to dissolve the fish lipids. Then, the flask was filled with potassium iodide (KI) solution and left to stand for 1 min with occasional agitation. Sodium thiosulfate, with starch as an indicator, was used to titrate the liberated iodine after being added to distilled water. The blank was calculated by titration of samples that did not contain fish lipids.
Determination of free fatty acids (FFA), expressed as percentage of oleic acid, was performed by AOCS FFA method Cd 5a-40 (AOCS 1994). FFA determination is based on a titration method with a standard alkali (0.1 M NaOH) using phenolphthalein as an indicator.
Thiobarbituric acid (TBA) value of samples was detected according to AOCS method Cd 19–90 ( 1998). Oil samples were dissolved in 1-butanol, mixed with TBA (0.02% in 1-butanol), and incubated for 2 h in thermostatic water bath (95 °C). Then, the absorbance was measured at 532 nm compared with corresponding blank. For the determination of standard curve, 0.2 mM 1,1,3,3-tetraethoxypropane prepared in 1-butanol was used. TBA values were expressed as mg MA/g of oil.
Heavy metal analyses
Analyses were performed at the Cukurova University, Central Research Laboratory (CUMERLAB). Homogenized samples were weighed and digested in a microwave oven with 2 ml of hydrogen peroxide solution (35%, Merck) and 8 ml of nitric acid (65%, Merck). The digested samples were then diluted to a final volume of 50 ml with ultrapure water. For the identification of the metal levels (As, Cd, Cu, Fe, Pb, and Hg), inductively coupled plasma mass spectrometer (ICP-MS, PerkinElmer, 2000P Model, USA) was used. Instrumental conditions used were cyclonic chamber; MEINHARD concentric quartz nebulizer; Glass High Sensitivity Spray Chamber with Matrix Gas Port, 2.0-mm Injector Quartz Torch; signal integration time of 1 s; nebulizer gas flow rate of 0.97 ml min−1; auxiliary gas flow rate of 1.35 l min−1; plasma gas flow rate of 15.0 l min−1; nebulizer pump (rpm), 35; and radiofrequency power of 1600 W. Triplicate analyses were performed for each metal. Calibration standards were obtained from High-Purity Multi Standard (PerkinElmer, PE N9300233). The instrument was calibrated using five aqueous standard solutions of concentrations 1, 10, 25, 50, and 100 µg/l prepared using ultrapure water in 2.5% (v/v) HNO3 (purity 65%); the calibration curves obtained were analyzed using linear regression with a minimum R2 of 0.999. After every ten-sample analysis, a standard solution was analyzed for calibration control. In and Ge were added to each standard, blank solution, and sample as an internal standard.
Statistical analysis
Analyses per sample were carried out in triplicate, and the results are shown as the average and standard deviation. The data obtained from the study were evaluated for normality and homogeneity test prior to one-way analysis of variance (ANOVA). Significant differences among the means were processed by means of Duncan’s multiple range test. Significant differences were defined as p < 0.05.
Results and discussion
Fatty acid composition
The fatty acid composition of the fish oil capsules and syrups examined in this study is shown in Tables 2 and 3. The essential fatty acids of the fish oil supplements in capsule form were generally palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1ω9), linoleic acid (C18:2ω6), eicosapentaenoic acid (EPA, C20:5ω3), and docosahexaenoic acid (DHA, C22:6ω3). In fish oil supplements in the form of syrup, the main fatty acids were found as myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0), palmitoleic acid (C16:1), oleic acid (C18:1ω9), vaccenic acid (C18:1ω7), eicosapentaenoic acid (EPA, C20:5ω3), and docosahexaenoic acid (DHA, C22:6ω3). It was observed that the content of saturated fatty acid (SFA) components in capsule groups was in the range of 2.75–10.31% except for C6 group. The highest total SFA rate in the capsule group was 32.86% in the C6 group. The main saturated fatty acids in these products were palmitic and stearic fatty acids. In syrup groups, the lowest and highest total SFA rates were found in S2 (20.22%) and S5 (35.02%) groups, respectively. Fish oils are distinguished from other oils because of their high total unsaturated fatty acids and lower saturated fatty acids. Therefore, it can be said that fish oil supplements in capsule form examined in this study were more reflective of the general characteristics of fish oils and were more suitable for healthy nutrition.
In terms of monounsaturated fatty acid (MUFA) components, MUFA was found to be the lowest in the C4 group (3.33%), the highest in the C6 group (23.47%), and 4.06–15.18% in the rest of capsule groups. In general, oleic acid (C18:1ω9) was the dominant MUFA in all capsule groups, while a significant amount of palmitoleic acid (C16:1) content was also observed in the C6 group. It was determined that the fish oil supplements in the form of syrup generally contained higher MUFA (20.98–27.12%) than the capsule groups. It was due to the high content of palmitoleic acid (C16:1, 8.02–8.69%, except S2) and vaccenic acid (C18:1ω7, 1.70–3.26%) in addition to the oleic acid content in syrup groups. Özyurt et al. (2013) investigated the fatty acid composition of five brands of fish oil capsule and four brands of fish oil syrup in a study. They determined the MUFA content at 10.71–50.46% in fish oil supplements, and the highest MUFA components were determined in syrup groups (49.83–50.46%). The lipids of marine fish species are characterized by low levels of linoleic acid (C18:2ω6) and linolenic acid (C18:3ω3) and high levels of long-chain ω3 polyunsaturated fatty acids (Steffens 1997). It is well known that the dominant fatty acids in the PUFA content of fish oil are EPA (C20:5ω3) and DHA (C22:6ω3) (Ackman 1989; Gamez-Meza et al. 1999; Özyurt et al. 2005). It was observed that the PUFAs of all groups in capsule form were convenient for this definition. Although the syrup groups showed similar characteristics, the S2 group was not reflecting this definition. In S2 group, linoleic acid (C18:2ω6) was 37.76%, and EPA and DHA were 6.01% and 3.62%, respectively. Thus, it can be suspected that oils obtained from oilseed plants such as sunflower and soybean were added to fish oil in this group.
In the capsule groups, the highest EPA content was found in the C2 (40.06%) and C9 (41.11%) groups, and the lowest EPA content was found in the C6 (16.84%) group (p < 0.05). It was determined that the richest group in terms of DHA content was C4 (58.85%), followed by C2 (27.12%) and C9 (25.99%). Although the lowest DHA content in the capsule groups was detected as 11.37% in the C6 group, this value was generally similar to those of the syrup groups examined in this study. EPA was determined as 16.62–17.70%, and DHA was determined in the range of 10.84–12.77% in all syrup groups except for S2 group. According to these results, it can be concluded that capsule groups generally had richer EPA and DHA contents than syrup groups. PUFA and ω3 fatty acid ratios were also determined as 31.79–77.27% and 28.97–76.26%, respectively, in capsule form and 30.39–49.23% and 10.82–30.57%, respectively, in syrup groups.
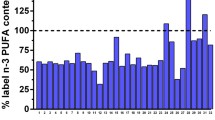
A comparison of the label information with the results obtained from this study is shown in Table 4. The label information of fish oil dietary supplements in capsule form was mostly correlated with fatty acid composition analysis data. It was supposed that the minor differences between label and experimental values may be due to sampling. However, similar accordance was not observed in fish oils in syrup form (Table 4). In this group, while the label information for the S3, S7, S8, and S9 groups was verified, the S1, S4, S5, and S6 groups could not be evaluated because the label information was not sufficient. In groups S1, S4, and S5, it was stated that there was 390 mg of EPA, 260 mg of DHA, and 820 mg of total ω3 in 10 ml of syrup as label information. In the S6 group, it was informed that there was 200 mg total ω3 in 10 ml. However, since the amount of fish oil in 10 ml syrup in these groups was not specified, their contents could not be confirmed. However, the S2 and S10 groups had lower levels of EPA, DHA, and ω3 content than claimed label information. Excluding the insufficiently labelled groups, 33% (1 out of 3) of the syrups were not labelled correctly. Albert et al. (2015) reported that only 3 of 32 fish oil supplements contained quantities of EPA and DHA that were equal or higher than labelled content, with most products tested (69%) containing < 67%. Similarly, it was found that more than half of the ω3 supplements available on the South African market contained less than 90% of the claimed content of EPA and/or DHA as stated on the product labels (Opperman et al. 2011). However, Bannenberg et al. (2017) investigated the EPA/DHA content of 47 fish oil dietary supplements sold on the New Zealand market, and they found that 91% of the fish oil products tested complied with EPA/DHA content claims. Tatarczyk et al. (2007) reported that eight of the commercially available fish oil supplements in Austria contained either equal or significantly greater amounts of long-chain ω3 PUFA than denoted by the manufacturer (one sample did not provide any information). On the other hand, Fierens and Corthout (2007) reported that 7 out of 16 fish oil products sold commercially in Europe did not meet the label claims for EPA and/or DHA. In addition, Ritter et al. (2013) found that only 9 out of 16 fish oil sold in the USA met the label information. Label claims for total ω3, EPA, and DHA in Turkey presented generally reasonable accuracy for the products examined, but some of the groups showed considerable difference with the label (Özyurt et al. 2013). In this study, all the capsule groups examined confirmed the label information, but one-third of the syrup groups had less content than claimed.
Lipid stability
The PV of fish oil capsules and syrups are shown in Fig. 1. PV of all fish oil capsules were found in the range of 1.97–2.89 mEq/kg. PV of the fish oil syrups were in the range of 2.22–3.16 mEq/kg for the S1, S2, S3, S4, S5, S7, and S8 groups; however, the PV for the S6, S9, and S10 groups were 5.70, 10.39, and 18.30 mEq/kg, respectively. Poor processing and storage conditions cause high PV in crude oils (over 10 mEq/kg) (EFSA 2010). Based on this limit value, it was observed that the S9 and S10 groups exceeded the recommended limit value. Albert et al. (2015) found that 30 of the 36 fish oil supplements sold in New Zealand exceeded the recommended limit values in terms of PV value (83% product), 9 in terms of anisidine value (AV) (25% product), and 18 in terms of totox value (50% product). Bannenberg et al. (2017) also determined that 28% of the fish oil supplements sold in New Zealand exceeded 5 mEq/kg. On the other hand, they stated that 72% of the fish oils examined in their studies met the maximum allowable limits in terms of primary oxidation products and 86% in terms of secondary oxidation products. Ritter et al. (2013) investigated that the 16-liquid ω3 dietary fish oil supplements from nine manufacturers represent the best-selling brands in the US market. The researchers determined that the PV of the fish oil supplements were in the range of 1.0–14.8 mEq/kg, and 5 of them exceeded the peroxide value of 5 mEq/kg. Özyurt et al. (2013) found the PV in the range of 0.86–4.72 mEq/kg in the capsule group of the fish oil supplements and between 5.03 and 6.44 mEq/kg in the syrup group, and they stated that the oxidative stability of fish oil capsules was safer than the syrups. Similarly, in this study, it was observed that the PV of fish oils sold in capsule form (1.97–2.89 mEq/kg) were generally lower than fish oils sold in syrup form (2.22–18.30 mEq/kg).
The International Fish Meal and Oil Producers Association (IFOMA) stated that the allowable limit of FFA value for crude fish oil is in the range of 1–7% (usually 2–5%) of oleic acid (Bimbo 1998), but the general recommendation is FFA values of oils should be below 3% (Özyurt et al. 2013; Soldo et al. 2019). As for FFA values, it was observed that the C4, S6, S9, and S10 groups were above the 3% oleic acid limit recommended for high-quality oils (Fig. 2). García-Moreno et al. (2014) noted that fish oils extracted at temperatures above 45 °C had low FFA values and this may be due to the instability of lipases. Soldo et al. (2019) indicated that there was a decrease in the FFA value after the deodorization and neutralization stages in the refining stages of crude fish oil. Crexi et al. (2010) reported that crude fish oil with 3.35% oleic acid FFA content initially was 5.31%, 0.56%, 0.45%, 0.47%, and 0.08% oleic acid after degumming, neutralization, bleaching, overwintering, and deodorization, respectively. Therefore, it should be noted that the effect of the processing method on the chemical quality of fish oils is as important as the storage conditions of fish oils.
TBARs values of fish oils in capsule form were determined as 0.76–1.29 mg MA/kg, and TBARs values of fish oils in syrup form were determined as 0.24–1.24 mg MA/kg in this study (Fig. 3). In parallel with the high PV and FFA values determined in the S6, S9, and S10 groups of fish oils in syrup form, relatively high TBARs values were also determined in the same groups. An acceptable TBARs value for good-quality crude fish oils is lower than 3 mg MA/kg (Schormüller 1969). In this study, TBAR values were found to be around or below 1 mg MA/kg in all groups. Therefore, it can be concluded that all fish oil supplements were of good quality in terms of TBARs value, which expresses the secondary lipid oxidation content.
Heavy metal content
Fish and processed seafood may contain significant percentages of heavy metals such as As, Cd, Pb, and Hg. Consumption of these elements for a long period, even at low rates, can have toxic effects. As, Cd, Cu, Fe, Pb, and Hg content of the fish oil capsules and syrups examined in this study is shown in Table 5. In capsule form, the As content was the lowest in the C6 group (0.56 µg/g) and the highest in the C4 group (2.29 µg/g). In syrup form, the lowest (0.50 µg/g) and the highest (4.19 µg/g) As contents were determined in the S4 and S2 groups, respectively. Dobrzański et al. (2002) determined As levels in fish oils obtained from the fish processing industry in the range of 1.39–5.21 mg/kg. Usydus et al. (2009) reported that the amount of As, which they determined as 6.04–9.42 mg/kg, decreased to 0.45–1.2 mg/kg (approximately 62%) after the purification of the fish oil because of the activated carbon used during the refining process. Gomez-Caminero et al. (2001) reported that the organic form of As found in fish is less toxic than the inorganic form found in other foods. According to the Joint FAO/WHO Expert Committee on Food Additives, the tolerable As level is 0.015 mg/kg body weight/week for its inorganic form and 0.05 mg/kg body weight/week for organic arsenic compounds (WHO 2011). The Committee noted that organic arsenic found in seafood requires a different evaluation than inorganic arsenic in water. There were no reports of adverse effects among populations consuming large amounts of fish resulting in an intake of approximately 0.05 mg of organoarsenic per kg of body weight per day. According to the recommended limit (0.05 mg per kg body weight), the daily amount of As for children aged 6–12 years with an average weight of 20–45 kg is 1–2.25 mg. The maximum amount of As that could be taken was 0.002 mg for the capsule group and 0.004 mg for the syrup group, if maximum of 1000 mg fish oil supplements were consumed per day (Table 5). As a result, it could be said that the fish oil food supplements examined in this study were safe in terms of As, according to the reports of the World Health Organization.
Cd and Cu elements were not detected in all fish oil food supplements (except the C5 group) examined in this study. The Cd level in the C5 group was 0.14 µg/g. It was emphasized that Cd is not particularly bio-condensed in fish species (Saiki et al. 1995) but accumulates more readily in invertebrates (Satarug et al. 2003). Türkmen et al. (2008) found that the lowest and highest Cd concentrations from Turkish seas were 0.02–0.37 mg/kg for muscles and 0.13–0.47 mg/kg for livers. According to the European Commission (1997), the maximum allowable concentration for Cd in fish is 0.5 mg/kg.
The lowest Fe content was found in the C9 group (0.32 µg/g), while the highest iron concentration was found in the C8 group (15.7 µg/g) in fish oils in capsule form. Similarly, Fe contents of fish oils sold in syrup form were determined in the range of 2.05–15.1 µg/g, and significant differences were observed between groups in terms of Fe content (p < 0.05). Dobrzański et al. (2002) determined 14.50–17.38 µg/g Fe content in fish oils obtained from the fish processing industry. Ikem and Egiebor (2005) determined the Fe content in the range of 0.01–88.4 µg/g in canned fish sold in the USA. Mol (2011) stated that the Fe content of canned tuna products sold in Turkey was in the range of 20.2–38.7 µg/g; however, the upper limit recommended for Fe in canned foods according to the Turkish Food Codex was 15 µg/g. Mendil et al. (2009) determined the Fe content in different vegetable oils (olive oil, hazelnut oil, sunflower oil, margarine, butter, and corn oil) in the range of 52.0–291 µg/g. Similarly, Zhu et al. (2011) found 16.2–45.3 µg/g Fe levels in edible vegetable oils. Although Fe is an essential nutrient for the human body, it is known that excessive amounts of Fe can cause diseases such as breast cancer, colorectal cancer, prostate cancer, lung cancer, and eventually death (Zhou et al. 2005; Naz et al. 2020; Chowdhury et al. 2021). Therefore, the tolerable upper Fe intake level in children (0 months–8 years) and men/women (14–70 years) has been recommended as 40 and 45 mg/day, respectively (Institute of Medicine 2003). All fish oils examined in this study were detected to be safe according to these recommended limits for Fe.
Among the fish oil food supplements examined in this study, Pb content was determined in 4 samples (0.02–1.01 µg/g for C1, C3, C6, and C8) in the capsule group and in only 1 sample (0.29 µg/g for S10) in the syrup group. In the Turkish food codex, 0.30 for fish meat, 0.50 for crustaceans, and 1.5 mg/kg for bivalve mollusks are allowed as maximum limits of Pb (Anonymous 2002). For fresh fish, the legal limit of Pb is 5 mg/kg in India, 2 mg/kg in New Zealand and Chile, 0.5 mg/kg in China and the Philippines, and 0.3 mg/kg in South Korea (Anonymous 2011). The FAO/WHO (2011) Joint Committee of Experts also recommended a maximum Pb level of 0.3 mg/kg for fish and 0.1 mg/kg for edible oils. Similarly, GOED (2012) recommended 0.1 mg/kg Pb level as the maximum value for fish oils. Güzelsoy and İzgi (2015) reported that the highest Pb level was 0.01 mg/kg in 33 fish oil supplements they examined. The Pb content in canned fish was reported as 0.09–0.045 mg/kg by Mol (2011), 0.03–0.52 mg/kg by Ashraf et al. (2006), and a maximum of 0.12 mg/kg by Akalın et al. (2020). Although Pb element could not be detected in only 15 groups out of 20 groups examined in total, C6, C8, and S10 groups, which are among the detected groups, exceeded the recommended limit for high-quality fish oil (0.1 mg/kg). Considering that Pb is a heavy metal with negative effects on health and among the ten most toxic metals, it should be taken into account that some fish oil supplements may carry a risk for Pb.
Seafood is an important exposure route for mercury, especially methyl mercury (Yu et al., 2020). GOED (2012) recommended a maximum Hg level of 0.1 mg/kg for fish oil. In this study, in fish oil supplements in capsule form, Hg was ≤ 0.04 µg/g in all groups except C1, and Hg was ≤ 0.01 in the syrup groups (Table 5). In the C1 group, the Hg content detected at the level of 0.1 µg/g was within the recommended limit. Kürklü et al. (2020) determined that the Hg level of krill oils sold in Turkey was < 0.2 µg/kg. Güzelsoy and İzgi (2015) found < 0.1 µg/kg Hg level in fish oil food supplements. In this study, it can be concluded that the examined fish oils were safe in terms of mercury content, because all groups contain very low levels of Hg except C1 group which was at the limit level for good quality oil.
Conclusion
Fish oil supplements in capsule form confirmed the label information in terms of fatty acid compositions, but one-third of the syrup groups had less ω3 content than claimed. In regard to the lipid quality, it was also seen that fish oil supplements in capsule form were safer in terms of PV and FFAs than syrup form. Considering all these aspects, it is clear that the chemical quality of fish oils in syrup form needs to be improved and their reliability in terms of content should be increased. As highlighted in the previous research, the progress of lipid oxidation may result from poor storage conditions, the production process, and the use of poor-quality starting material. However, due to the possible negative effects of these oxidation products on human health, it is very important to take the necessary precautions both in the production process and in the storage process of these fish oils. It was determined that the fish oil supplements examined in this study were within the limits specified in terms of heavy metals As, Cd, Cu, Fe, and Hg, but two groups from the capsule group and one group from the syrup group exceeded the recommended limit in terms of Pb. Considering the heavy metals, it seems important to follow up the fish oil products more strictly. Not only the manufacturers should know that their products on the market are monitored, but also the consumers should be aware of the content of the products purchased. Continuous and regular monitoring of fish oil supplements is important to ensure the safety of the products and to advise consumers at risk and seeking health benefits, such as pregnant women and patients with cardiovascular diseases. In general, since food supplements are not offered for sale under a certain control, it would be beneficial to evaluate the risks by academic studies for these foods.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ackman RG (1989) Nutritional composition of fats in sea foods. Prog Food Nutr Sci 13:161–241
Akalın S, Eroğlu Eİ, Güleç A, Ayaz A (2020). Yetişkinlerde konserve balık tüketimine bağlı ağır metal maruziyet riskinin değerlendirilmesi. Beslenme ve Diyet Dergisi. 48(2):15–23. https://doi.org/10.33076/2020.BDD.1298
Albert BB, Derraik JG, Cameron-Smith D, Hofman PL, Tumanov S, Villas-Boas SG, Garg ML, Cutfield WS (2015) Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep 5:7928. https://doi.org/10.1038/srep07928
Anonymous (2002). Gıda maddelerinde belirli bulaşanların maksimum seviyelerinin belirlenmesi hakkında tebliğ. 23.09.2002–24885
Anonymous (2011). www.inspection.gc.ca/english/fssa/fispoi/export/coupaye.shtml#aus. Accessed August 2021.
AOCS (1994). AOCS Official Method Cd 8–53 and Cd 5a-40. Official methods and recommended practices of the American Oil Chemists Society. Champaign, IL: American Oil Chemistry Society.
AOCS (1998). AOCS Official Method Cd 19–90. 2-Thiobarbituric acid value. Direct Method. In: Official Methods and Recommended Practices of the American Oil Chemists’ Society, Firestone, D. (Ed.), AOCS, Champaign, III
Ashraf W, Seddigi Z, Abulkibash A, Khalid M (2006) Levels of selected metals in canned fish consumed in Kingdom of Saudi Arabia. Env Monit Asses 117(1–3):271–279. https://doi.org/10.1007/s10661-006-0989-5
Bannenberg G, Mallon C, Edwards H, Yeadon D, Yan K, Johnson H, Ismail A (2017) Omega-3 long-chain polyunsaturated fatty acid content and oxidation state of fish oil supplements in New Zealand. Sci Rep 7(1):1–13. https://doi.org/10.1038/s41598-017-01470-4
Berliner JA, Watson AD (2005). A role for oxidized phospholipids in atherosclerosis. N Engl J Med 353:9e11. https://doi.org/10.1056/NEJMp058118
Bimbo AP (1998). Guidelines for characterizing food-grade fish oils. Inform, 9(5).
Bonilla-Méndez JR, Hoyos-Concha JL (2018). Methods of extraction refining and concentration of fish oil as a source of omega-3 fatty acids. Ciencia y Tecnología Agropecuaria. 19(3):645–668. https://doi.org/10.21930/rcta.vol19_num2_art:684
Cetinkaya T, Mendes AC, Jacobsen C, Ceylan Z, Chronakis IS, Bean SR, García-Moreno PJ (2021) Development of kafirin-based nanocapsules by electrospraying for encapsulation of fish oil. LWT 136:110297. https://doi.org/10.1016/j.lwt.2020.110297
Ceylan Z, Meral R, Cavidoglu I, Yagmur Karakas C, Tahsin Yilmaz M (2018) A new application on fatty acid stability of fish fillets: coating with probiotic bacteria-loaded polymer-based characterized nanofibers. J Food Saf 38(6):e12547. https://doi.org/10.1111/jfs.12547
Ceylan Z, Meral R, Kose YE, Cavidoglu I (2020) Wheat germ oil nanoemulsion for oil stability of the cooked fish fillets stored at 4°C. J Food Sci Technol 57(5):1798–1806. https://doi.org/10.1007/s13197-019-04213-7
Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY (2008) The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double blind placebo-controlled study Prog. Neuropsychopharmacol Biol Psychiatry 32:1538–1544. https://doi.org/10.1016/j.pnpbp.2008.05.015
Chowdhury A, Naz A, Maiti SK (2021) Bioaccumulation of potentially toxic elements in three mangrove species and human health risk due to their ethnobotanical uses. Environ Sci Pollut Res 28:33042–33059. https://doi.org/10.1007/s11356-021-12566-w
Crexi VT, Monte ML, de Souza Soares LA, Pinto LAA (2010) Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chem 119(3):945–950. https://doi.org/10.1016/j.foodchem.2009.07.050
Das D, Moniruzzaman M, Sarbajna A, Chakraborty SB (2017) Effect of heavy metals on tissue-specific antioxidant response in Indian major carps. Environ Sci Pollut Res 24(22):18010–18024. https://doi.org/10.1007/s11356-017-9415-5
Dobrzański Z, Bykowski P, Iwaniuk Z, Usydus Z, Górecka H, Trziszk T (2002). Evaluation of the chemical composition of fish oil: a by-product from fish processing plants. Bull Sea Fish Inst 1(155):39–46. https://www.mir.gdynia.pl/pliki/osrodek/biuletyn/biulet1-02.pdf#page=37
EFSA (2010). Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA Journal 8(10):1874. https://doi.org/10.2903/j.efsa.2010.1874
European Commission (1997) Draft commission regulation setting limits for certain contaminants in food stuffs. DocIII/5125/95/REV.3.
FAO-WHO (2010). Fats and fatty acids in human nutrition: report of an expert consultation, FAO food and nutrition paper #91, FAO, WHO: Geneva, Switzerland.
FAO/WHO (2011). Joint FAO/WHO food standards programme codex committee on contaminants in foods, fifth session.
Fierens C, Corthout J (2007) Omega-3 fatty acid preparations-a comparative study. J Pharm Belg 62(4):115–119
Gamez-Meza N, Higuera-Ciapara L, Calderon De La Barca AM, Vazquez-Moreno L, Noriega-Rodriguez J, Angulo-Guerrero O (1999) Seasonal variation in the fatty acid composition and quality of sardine oil from Sardinops sagax caeruleus of the Gulf of California. Lipids 34:639–642
Garcia-Hernandez VM, Gallar M, Sanchez-Soriano J, Micol V, Roche E, Garcia-Garcia E (2013). Effect of omega-3 dietary supplements with different oxidation levels in the lipidic profile of women: a randomized controlled trial. Int J Food Sci Nutr 64:993e1000. https://doi.org/10.3109/09637486.2013.812619
García-Moreno PJ, Morales-Medina R, Pérez-Gálvez R, Bandarra NM, Guadix A, Guadix EM (2014) Optimisation of oil extraction from sardine (Sardina pilchardus) by hydraulic pressing. Int J Food Sci Techn 49(10):2167–2175. https://doi.org/10.1111/ijfs.12527
Givens DI, Gibbs RA (2008) Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them: symposium on ‘how can the n-3 content of the diet be improved?’ Proceedings of the Nutrition Society 67(3):273–280. https://doi.org/10.1017/S0029665108007167
GOED (2012). GOED Voluntary Monograph (v. 4). Omega-3 EPA, omega-3 DHA, omega-3 EPA & DHA. Salt Lake City, UT: Global Organization for EPA and DHA Omega-3, available at: http://www.goedomega3.com.
Gomez-Caminero A, Howe PD, Hughes M, Kenyon E, Lewis DR, Moore M, ... Ng J (2001). Arsenic and arsenic compounds. World Health Organization.
Güzelsoy NA, İzgi B (2015) Optimization of analytical parameters for determination of (As, Hg, Cd and Pb) in fish oil supplements. J Food Feed Sci Techn 15:19–26
GVR (2021). Grand View Research, Omega 3 supplements market size and share report 2020–2020, Report ID: GVR-1–68038–818–3, 210 pages. https://www.grandviewresearch.com/industry-analysis/omega-3-supplement-market
Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ (2005) Long-chain conversion of [13C] linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res 46:269–280. https://doi.org/10.1194/jlr.M400225-JLR200
Ichihara K, Shibahara A, Yamamoto K, Nakayama T (1996) An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 31:535–539
Ikem A, Egiebor NO (2005) Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America). J Food Comp Analy 18(8):771–787. https://doi.org/10.1016/j.jfca.2004.11.002
Institute of Medicine (2003). Dietary reference intakes: applications in dietary planning. Subcommittee on Interpretation and Uses of Dietary Reference Intakes and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Institute of Medicine of the National Academies, The National Academies Press, Washington, DC, p. 248.
Jackowski SA, Alvi AZ, Mirajkar A, Imani Z, Gamalevych Y, Shaikh NA, Jackowski G (2015) Oxidation levels of North American over-the-counter n-3 (omega-3) supplements and the influence of supplement formulation and delivery form on evaluating oxidative safety. J Nutr Sci 4:e30. https://doi.org/10.1017/jns.2015.21
Kargın E, Erdem C (1992) Bakır-çinko etkileşiminde Tilapia nilotica’nın karaciğer, solungaç ve kas dokularındaki metal birikimi. Doğa Tr J Zool 16:343–348
Kolanowski W (2010) Omega-3 LC PUFA contents and oxidative stability of encapsulated fish oil dietary supplements. Int J Food Prop 13:498–511. https://doi.org/10.1080/10942910802652222
Kosker AR, Özogul F, Ayas D, Durmus M, Ucar Y, Regenstein JM, Özogul Y (2019) Tetrodotoxin levels of three pufferfish species (Lagocephalus sp.) caught in the North-Eastern Mediterranean Sea. Chemosphere 219:95–99. https://doi.org/10.1016/j.chemosphere.2018.12.010
Kremmyda LS, Tvrzicka E, Stankova B, Zak A (2011). Fatty acids as biocompounds: their role in human metabolism, health and disease-a review. Part 2: Fatty acid physiological roles and applications in human health and disease. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc, 155(3). https://doi.org/10.5507/bp.2011.052
Kris-Etherton PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757. https://doi.org/10.1161/01.CIR.0000038493.65177.94
Kumari P, Chowdhury A, Maiti SK (2018) Assessment of heavy metal in the water, sediment, and two edible fish species of Jamshedpur Urban Agglomeration, India with special emphasis on human health risk. Hum Ecol Risk Assess 24(6):1477–1500. https://doi.org/10.1080/10807039.2017.1415131
Kürklü NS, Başıbüyük HH, Altun HK (2020). Assessment of heavy metal levels and fatty acid compositions of some krill oil capsules marketed in Turkey. Int J Agric Envirn Food Sci 4(4):418–424. https://doi.org/10.31015/jaefs.2020.4.4
Lazzarin N, Vaquero E, Exacoustos C, Bertonotti E, Romanini ME, Arduini D (2009) Low-dose aspirin and omega-3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertil Steril 92:296–300. https://doi.org/10.1016/j.fertnstert
Lee JB, Kim MK, Kim BK, Kim JY, Lee KG (2016) Analysis of polychlorinated biphenyls (PCBs), heavy metals and omega-3 fatty acids in commercially available Korean functional fish oil supplements. Int J Food Sci Techn 51(10):2217–2224. https://doi.org/10.1111/ijfs.13198
Mendil D, Uluözlü ÖD, Tüzen M, Soylak M (2009) Investigation of the levels of some element in edible oil samples produced in Turkey by atomic absorption spectrometry. J Hazard Mater 165(1–3):724–728. https://doi.org/10.1016/j.jhazmat.2008.10.046
Mol S (2011) Levels of selected trace metals in canned tuna fish produced in Turkey. J Food Comp Analy 24(1):66–69. https://doi.org/10.1016/j.jfca.2010.04.009
Naz A, Chowdhury A, Chandra R, Mishra BK (2020) Potential human health hazard due to bioavailable heavy metal exposure via consumption of plants with ethnobotanical usage at the largest chromite mine of India. Environ Geochem Health 42:4213–4231. https://doi.org/10.1007/s10653-020-00603-5
Neff LM, Culiner J, Cunningham-Rundles S, Seidman C, Meehan D, Maturi J, Wittkowski KM, Levine B, Breslow JL (2011) Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J Nutr 141:207–213. https://doi.org/10.3945/jn.110.130021
Nogueira M, Kessuane M, Lobo Ladd A, Lobo Ladd F, Cogliati B, Castro I (2016). Effect of long-term ingestion of weakly oxidised flaxseed oil on biomarkers of oxidative stress in LDL-receptor knockout mice. Br J Nutr 1e12. https://doi.org/10.1017/S0007114516001513
Opperman M, Benade AS, Marais DW (2011) Analysis of omega-3 fatty acid content of South African fish oil supplements: cardiovascular topics. Cardiovasc J Afr 22(6):324–329. https://doi.org/10.5830/CVJA-2010-080
Özyurt G, Polat A, Ozkütük S (2005) Seasonal changes in the fatty acids of gilthead sea bream (Sparus aurata) and white sea bream (Diplodus sargus) captured in Iskenderun Bay, Eastern Mediterranean coast of Turkey. Eur Food Res Technol 220:120–124. https://doi.org/10.1007/s00217-004-1060-9
Özyurt G, Şimşek A, Etyemez M, Polat A (2013) Fatty acid composition and oxidative stability of fish oil products in Turkish retail market. J Aquatic Food Prod Techn 22(3):322–329. https://doi.org/10.1080/10498850.2011.644882
Packaged Facts (2012) Global market for EPA/DHA Omega-3 products. Maryland, Md.: Packaged Facts, p. 53.
Ritter JC, Budge SM, Jovica F (2013). Quality analysis of commercial fish oil preparations. J Sci Food Agric 93:1935e1939. https://doi.org/10.1002/jsfa.5994
Rosenfeld ME, Palinski W, Yla-Herttuala S, Butler S, Witztum JL (1990) Distribution of oxidation specific lipid-protein adducts and apolipoprotein b in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis 10(3):336–349. https://doi.org/10.1161/01.ATV.10.3.336
Saiki MK, Castleberry DT, May TW, Martin BA, Bullard FN (1995) Copper, cadmium, and zinc concentrations in aquatic food chains from the upper Sacramento River (California) and selected tributaries. Arch Environ Contam Toxicol 29(4):484–491
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137(1–2):65–83. https://doi.org/10.1016/S0378-4274(02)00381-8
Schormüller J (1969). Handbuch der Lebensmittelchemie (Band III/2). Triesrische Lebensmittel Eier, Fleisch, Fisch, Buttermich, 1584, Springer Verlag, Berlin/Heidelberg, Germany/New York, NY p. 1561–1578.
Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361. https://doi.org/10.1038/nri2294
Shibata N, Nagai R, Uchida K, Horiuchi S, Yamada S, Hirano A, … Kobayashi M (2001) Morphological evidence for lipid peroxidation and protein glycoxidation in spinal cords from sporadic amyotrophic lateral sclerosis patients. Brain Res 917(1):97-104https://doi.org/10.1016/S0006-8993(01)02926-2
Shukla VKS, Perkins EG (1998) Rancidity in encapsulated health-food oils. Inform 9:955–961
Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B (2011) Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 93:402–412. https://doi.org/10.3945/ajcn.110.005611
Soldo B, Šimat V, Vlahović J, Skroza D, Ljubenkov I, GeneralićMekinić I (2019) High quality oil extracted from sardine by-products as an alternative to whole sardines: production and refining. Europ J Lipid Sci Techn 121(7):1800513. https://doi.org/10.1002/ejlt.201800513
Steffens W (1997) Effects of variation in essential fatty acids in fish feeds on nutritive value of freshwater fish for humans. Aquaculture 151(1–4):97–119
Swanson D, Block R, Mousa SA (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3(1):1–7. https://doi.org/10.3945/an.111.000893
Tatarczyk T, Engl J, Ciardi C, Laimer M, Kaser S, Salzmann K, …Ebenbichler CF (2007) Analysis of long-chain ω-3 fatty acid content in fish-oil supplements .Wien Klin Wochenschr 119(13)417-422https://doi.org/10.1007/s00508-007-0820-5
Türkmen M, Türkmen A, Tepe Y, Ateş A, Gökkuş K (2008) Determination of metal contaminations in sea foods from Marmara, Aegean and Mediterranean seas: twelve fish species. Food Chem 108(2):794–800. https://doi.org/10.1016/j.foodchem.2007.11.025
Turner R, McLean CH, Silvers KM (2006) Are the health benefits of fish oils limited by products of oxidation? Nutr Res Rev 19(1):53–62. https://doi.org/10.1079/NRR2006117
Usydus Z, Szlinder-Richert J, Polak-Juszczak L, Malesa-Ciećwierz M, Dobrzański Z (2009) Study on the raw fish oil purification from PCDD/F and dl-PCB-industrial tests. Chemosphere 74(11):1495–1501. https://doi.org/10.1016/j.chemosphere.2008.11.039
Walter MF, Jacob RF, Bjork RE, Jeffers B, Buch J, Mizuno Y, Mason RP (2008). Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease: the prevent study. J Am Coll Cardiol 51:1196e1202. https://doi.org/10.1016/j.jacc.2007.11.051
WHO (2011). World Health Organization Technical Report Series, No. 959. Evaluation of certain contaminants in food. 72nd report of the Joint FAO/WHO Expert Committee on Food Additives
Yi YJ, Zhang SH (2012) Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ Sci Pollut Res 19(9):3989–3996. https://doi.org/10.1007/s11356-012-0840-1
Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y (1996) Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci 93(7):2696–2701. https://doi.org/10.1073/pnas.93.7.2696
Yu X, Khan S, Khan A, Tang Y, Nunes LM, Yan J, ... & Li G. (2020). Methyl mercury concentrations in seafood collected from Zhoushan Islands, Zhejiang, China, and their potential health risk for the fishing community: capsule: methyl mercury in seafood causes potential health risk. Environ Int 137, 105420.
Zhong H, Yin H (2015) Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol 4:193–199. https://doi.org/10.1016/j.redox.2014.12.011
Zhou W, Park S, Liu G, Miller DP, Wang LI, Pothier L, … Christiani DC (2005) Dietary iron, zinc, and calcium and the risk of lung cancer. Epidemiology 16:772-779https://doi.org/10.1097/01.ede.0000181311.11585.59
Zhu F, Fan W, Wang X, Qu L, Yao S (2011) Health risk assessment of eight heavy metals in nine varieties of edible vegetable oils consumed in China. Food Chem Toxic 49(12):3081–3085. https://doi.org/10.1016/j.fct.2011.09.019
Zohra BS, Habib A (2016) Assessment of heavy metal contamination levels and toxicity in sediments and fishes from the Mediterranean Sea (southern coast of Sfax, Tunisia). Environ Sci Pollut Res 23(14):13954–13963. https://doi.org/10.1007/s11356-016-6534-3
Funding
This work was supported by project number FBA-2019–11995 of the Research Foundation of Cukurova University. The authors thank the Research Foundation of Cukurova University for its financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization: Gülsün Ozyurt and Dilan Ekmen. Methodology: Dilan Ekmen, Mustafa Durmuş, and Yilmaz Ucar. Software (statistical analyses): Dilan Ekmen. Writing—original draft preparation: Gülsün Ozyurt, Mustafa Durmuş, and Yilmaz Ucar. Writing—review and editing—and supervision: Gülsün Ozyurt. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Fish oil supplements were purchased from a commercial market in Adana, Turkey. Therefore, ethical approval was not required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Oxidative qualities and fatty acid and heavy metal contents of fish oil supplements were assessed.
• While all the capsule groups confirmed the label information, one-third of the syrup groups had less than the specified content.
• The chemical quality of syrup form needs to be improved, and their reliability in terms of content should be increased.
• Considering the heavy metals, it seems important to follow up the fish oil products more strictly.
Rights and permissions
About this article
Cite this article
Ozyurt, G., Ekmen, D., Durmuş, M. et al. Assessment of the safety of dietary fish oil supplements in terms of content and quality. Environ Sci Pollut Res 29, 25006–25019 (2022). https://doi.org/10.1007/s11356-021-17581-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17581-5