Abstract
Dye-sensitized solar cells (DSSC) constructed using natural dyes possess irreplaceable advantages in energy applications. The main reasons are its performance, environmentally benign dyes, impressible performance in low light, ecologically friendly energy production, and versatile solar product integration. Though DSSCs using natural dyes as sensitizers have many advantages, they suffer from poor efficiency compared to conventional silicon solar cells. Moreover, the difficulty in converting them to practical devices for the day-to-day energy needs has to be addressed. This review will outline the optimization of conditions to be followed for better efficiency in DSSCs using natural dyes as sensitizers. This review has taken into account the importance of the first step towards the fabrication of DSSC, i.e. the selection process. The selection of plant parts has a noticeable impact on the overall efficiency of the device. Accordingly, a proper study has been done to analyse the plant’s parts that have shown better results in terms of device efficiency. In addition to this, a wide range of techniques and factors such as extraction methods, the solvent used, coating techniques, immersing time, and co-sensitization have been taken into consideration from the studies done over the period of 10 years to examine their influence on the overall performance of the DSSC device. These results have been addressed to stipulate the best suitable condition that will help supplement the efficiency of the device even further. Also, the future perspectives, such as the DSSCs use in wearable devices, incorporating various approaches to enhance the power conversion efficiency of DSSCs using natural dyes, and thermochromism ability for DSSCs have been discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is a well-known fact that the building block necessary for the world’s development is “Energy.” With the increase of modern technology and lifestyle, the need for energy has escalated tremendously. Fossil fuels being the driving force for energy were decreasing rapidly due to overuse along with pollution-causing various environmental hazards. To tackle these problems, renewable energy was introduced. Renewable energy, by definition, is the energy collected from the environment, such as sunlight, wind, water, and others. The main advantage of this energy is that it causes minimal pollution to the environment or other terms known as clean energy and is not perishable.
To harvest the energy, various technologies or devices have been introduced. Among these technologies, solar cells are the one that is discussed in this review. Solar cells are used to convert sunlight or solar energy into electrical energy. A variety of materials have been used for the fabrication of solar cells. So far, silicon solar cells have become commercially popular due to their higher efficiency when compared with other materials. Even so, the manufacturing process of silicon solar cells was not environmentally friendly. Moreover, the investment put into these technologies was also very high (Mashari and Prihanto 2019).
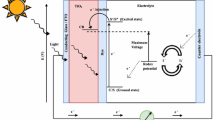
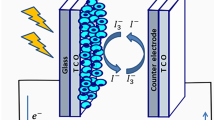
An alternate solution for this problem was found in the form of dye-sensitized solar cells (DSSCs). DSSC is a third-generation solar cell that limits the environmental hazards to the bare minimum with economic benefits. It was first fabricated in 1991 by Professor Gratzel and his co-workers (Peymania 2019) with a working concept similar to plant photosynthesis. The attractive qualities of being cheap, pollution-free, compact, lightweight, and simple have been under the radar for research. DSSC is a thin-film solar cell that is built on a photoelectrochemical process. The general structure of DSSC consists of five major components. The first principal component is the transparent conducting plate. Two conducting and transparent plates in which the semiconductor and dye are deposited act as a current collector. Efficient plates have two main characteristics, i.e., they should have transparency of more than 80% and should have higher charge transfer. This helps in better conductivity and reduction in energy losses. Fluorine-doped tin oxide (FTO, SnO2:F) and indium-doped tin oxide (ITO, In2O3:Sn) plates are most frequently used in DSSC technology. ITO plates have a transparency greater than 80% with sheet resistivity of 18 Ω/cm2, whereas FTO plates have a transparency of nearly 75%, with low sheet resistivity of 8.5 Ω/cm2 (Sharma et al. 2018). Depending on the experiment and the availability of resources, the plates can be chosen. The second component is the working electrode. It is fabricated by depositing a thin layer of semiconducting material such as TiO2, Nb2O5, ZnO, SnO2 (n-type), and NiO (p-type) onto the FTO/ITO glass plate. The commonly used oxide is TiO2 due to its higher bandgap of about 3.2 eV rather than other oxides. The third component is the dye used for sensitization. The dye extracted via proper solvent is vital for DSSC technology as it plays a significant role in the absorption of the incident light. Synthetic dyes and natural plant-based pigments are generally used as sensitizers in DSSCs for improving the flow of Jsc in visible light. The fourth component is the electrolyte which is either organic or inorganic solvents or solid electrolytes. I−/I3− has been proven to be more efficient and is commonly used. But the main drawback in using I−/I3− as an electrolyte was that it tends to corrode the FTO/ITO glass. Due to this reason, solid-state electrolytes were preferred (Wang et al., 2009). The last component consists of the counter electrode, which is used to enhance the reduction reaction of electrolytes.
Revolving around these components, many new optimization methods have been discovered solely to enhance the efficiency of DSSC so as to make it commercially feasible. Studies have indicated that with the increase in charge separation, there has been a considerable decrease in the recombination process between the charges, which stipulates that the recombination was not dependent on the thermodynamics of the charges (Argazzi and Bignozzi 1995). Similarly, other research focuses on linking synthetic dyes onto the semiconductor plate to increase the absorption of light via the electrode, thereby adding to the efficiency and Isc. Experimentally, synthetic dye-sensitized solar cells have reached a maximum efficiency of 11% (Birel et al., 2017).
In an experiment carried out by Jalali et al. (2020), natural pigments from saffron, red onion, mallow, and oregano were used as a dye in the fabrication process of DSSC. With the help of the UV–vis, the presence of anthocyanin or chlorophyll pigment was detected. These pigments are considered essential for the charge carrier generation process when harvesting energy through sunlight. Further, the dye was extracted using a solvent extraction process. The TiO2 paste was coated on the FTO plate using the doctor blade technique. The results depicted the efficiency of the organic dyes being less than 2% and low compared with the standard N719 dye. The reasons for the reduced efficiency were the anchoring group, the pigment stability, and the pigment structure. Likewise, Shanmugam et al. (2014) studied the performance of DSSC fabricated using green grasses. The three types of grasses, Hierochloe odorata (HO), Torulinium odoratum (TO), and Dactyloctenium aegyptium (DA), were used for their light-harvesting properties. The dye extraction was done using absolute ethanol as solvent. Parallelly, a standard cell was also fabricated using cis-bis (isothiocyanato) bis (2,2′-bipyridyl-4,4′-dicarboxylato)-ruthenium(II) commonly known as N3dye, so as to compare the end results of organic and inorganic dyes. Among the organic dyes, the larger efficiency of 0.46% was yielded by HO dye, whereas DSSC fabricated using N3 dye displayed an efficiency of 4.05%. In the same manner, dyes extracted from eight organic dyes using ethyl alcohol and fabricated on an FTO plate have displayed poor performance, with the highest efficiency among them being 0.339%, whereas the DSSC device fabricated using Ru complex dye has resulted in 2.58% efficiency (EL-AGEZ et al. 2014). Likewise, Abdel-Latif et al. (2015) extracted dyes from eleven plant dyes from three different trees, among which the highest efficiency obtained was about 0.40% from zizyphus leaves. These all add to the fact that inorganic or synthetic dyes have proven to have many high results when compared with organic/natural dyes. EL-Ghamri et al. (2014) used two semiconductors for fabrication purposes. The TiO2 and ZnO mesoporous thin films were divided into two sections. The first process required the gradual sintering of TiO2 and ZnO semiconductors, whereas in the second process, continuous sintering was done. The DSSC using natural dyes was fabricated with TiO2 semiconductor, which used gradual sintering intimated good efficiency of 0875%. Apart from this, another DSSC was fabricated using the same process, but the dye used was ruthenium: a synthetic dye. The synthetic dye gave 3.65% output which was way superior to the organic dyes used.
Although synthetic dyes produce better efficiency, they are costly and are toxic to the environment. Researches have been done by using synthetic dyes that can be duplicated using natural dyes from plants in order to overcome the drawbacks caused by synthetic dyes. Natural dyes, when compared with synthetic dyes, can fully fill the dye molecule’s requirement due to their low cost, easy access, and less toxic nature. To make DSSC using natural dyes as a good competitor of DSSC using synthetic dyes.
In this work, many research papers have been gathered to find the optimal conditions to fabricate the DSSC using natural plants, aiming to provide apt ideas suited for fabrication that will enhance the working efficiency of DSSC using plant dyes and make it commercially attractive. This review has focused on conditions in terms of temperature, pH level, dye volume, methods used for extraction, solvents, coating techniques, and semiconductors that can be adjusted to the desired parameter suggested in the studies gathered. There are several other components like open-circuit voltage (Voc), short circuit current (Isc), fill factor (FF), output power (Pout), incident irradiance (Ir), air mass (AM) that need to be monitored while fabricating the optimal DSSC with plant dyes. Likewise, novel ideas are being suggested which in future can be implemented with DSSC technologies. Some of the suggestions have already been used with conventional solar cells but not yet with DSSC technologies using natural plants as a dye. A breakthrough is well needed in the area of DSSC using natural dye to revolutionize the renewable sector.
Framework
Dyes extracted from natural sources have been considered essential due to their eco-friendly nature and portraits as the best example for renewable energy. Natural dyes can be extracted from any part of plants regardless of their family, making them an easily available source. Research has been performed to establish a sturdy, commercial, and budget-friendly green technology for various uses and applications. In the article, regarding the optimal conditions for the fabrication of DSSC, information was gathered using keyword search, specific websites, and other internet sources. In the forthcoming discussion, a detailed analysis has been carried out to find the best favourable conditions for the fabrication of DSSC to accomplish quality performance. The work starts from the selection method, which lays the groundwork for the further process followed by selecting the plant part. Following this, the influence of different conditions on extraction methods, different fabrication methods used with their effect on the overall efficiency, and other dependencies that impact the working of DSSC is also discussed. Figure 1 illustrates the entire flow of the article mentioned earlier. This valuable information was gathered by referring to various journals and conference papers that talked about the different aspects of DSSC technology. Hence, the key additions of the papers can be summarized as below:
-
To encapsulate all the optimal factors responsible for better performance of DSSC technology.
-
To recognize new ways of implementation to attract customers.
-
To categorize new techniques that can be incorporated with DSSC for commercial applications.
-
To analyse all the difficulties that can occur while working with DSSC technology and also methods to eliminate the problems.
-
To capture all the methods and intentions of the process that was carried out to depict ways to minimize the cost and maximize the efficiency of the process.
Methodology
Figure 2 is the pictorial representation of fabricated DSSC and shows it is working. The fundamental component of DSSC includes a counter electrode, working electrode, electrolyte based upon the experiment, and catalyst as discussed in the introduction part. Additional components or materials can be added based on the experiment developments.
Plants
Experiments on DSSC fabrication using synthetic dyes have shown better efficiency over the years. However, the cost of dye used, its toxicity, and the environment cannot be ignored. To avoid this, plant extract dyes were introduced. It was common knowledge that plants perform photosynthesis. In doing so, the main requirement was to absorb sunlight that falls on the plant surface. Keeping this in mind, the idea of DSSC fabrication using natural dyes came to light. Each of the plants has pigments that can absorb the different spectrum of light. All around the world, one can find different variations of fauna. These two qualities make them eco-friendly as well as affordable. In addition to this, it provides a classic combination of renewable and sustainable energy.
Influence of selection of plants
As plants are the main component in the fabrication of DSSC, their selection plays a vital role in the overall process. Plants can be divided into different categories based on their type and family. It was important to know what types of plants are being used, whether it is a shrub, a tree, or a herb and which family it belongs to. It should be kept in mind that not all plants which are available can be used for the fabrication purpose, as the dye extracted from these plants should have properties of absorption for a particular spectrum, bonding with the TiO2 semiconductor as well as the presence of pigments which are responsible for absorption (Syafinar et al. 2015). Selection can also be based on the availability of the plant. It was not wise to use exotic and rare plants or were already in use for some other technology as it would cause more load or consumption of the resource. It was observed that more number of shrub plants were used, followed by trees and herbs, respectively. This could be due to the fact that shrubs were easy to grow and were handy.
Apart from this, the pigments present in the plants were of prime importance as they were responsible for absorption, stability, anchoring with other materials, and cell degradation that affected the overall performance of the DSSC. The absorption spectrum of a pigment was decided by its molecular structure. The plant contains four major pigments, namely chlorophyll, carotenoids, anthocyanins (flavonoids), and betalains. However, listed pigments can only absorb certain wavelengths that may or may not be sufficient for better performance, meaning some were more efficient than the others (Garcia-Salinas et al. 2019). Chlorophyll contained chlorine which was responsible for the absorption of the yellow and blue wavelength of light. Due to the abundant presence of chlorophyll, the plants get their green colour. The existence of chlorophyll pigment in plants has only one primary purpose, i.e., to detain the light falling on the plant surface for the photosynthesis process (Pote et al. 2019).
It was observed that when pigments such as chlorophyll, anthocyanin, and beta-carotene were extracted from the spinach plant and compared, chlorophyll was found to have a higher efficiency than the other pigments (Cari et al. 2014). Other experiments done with green algae (Lim et al. 2015), mallow (Torchani et al. 2015), Cymbopogon schoenanthus, and Ixora coccinea (Al-Alwani et al. 2019), red amaranth (Ramanarayana et al. 2017), Amaranthus caudatus (Godibo et al. 2015), Ziziphus jujube (Taya et al. 2015), papaya plant (Rizali et al. 2019), and spinach (Ammar et al. 2019) have proven that the performance and the absorption of light increase with the presence of chlorophyll when compared with other pigments present in the dyes. Though chlorophyll has proven to be superior to other pigments in terms of its efficiency, less degradation factor (Torchani et al. 2015), and better absorption, xanthophylls have shown better results in terms of stability (Lim et al. 2015). Xanthophylls were one of the significant parts of carotenoids and are accountable for the yellow colour in plant leaves. Other than chlorophyll, anthocyanins and betalains have proved to be good contenders in increasing the performance and stability of the cell. The occurrence of both anthocyanins and betalains in the same plant can never happen. Anthocyanins were obtained from higher tissues of plants and were responsible for the variation of colours in leaves, stems, flowers, fruits, and roots. At the same time, betalains were red or yellow-coloured pigments extracted from the plant’s tyrosine. These were also responsible for the red colour found in beets. One similarity found in both these pigments was that they were water soluble.
Many experiments performed contained pigments of anthocyanins, and betalains have established a decent increase in efficiency. When Calogero et al. (2012) compared anthocyanins and betalain pigments present in plant extracts, it appeared that the dye-containing betalain pigment performed better and also remained stable for about 20 h. In an experiment, Garcia-Salinas et al. (2019) also showed the better performance of betalain pigment over anthocyanins by comparing the performance of bougainvillaea, beetroot, and eggplant plants as sensitizers. One main factor that surfaced during the research was the negative effect of the presence of sugar or sucrose in anthocyanins (Hemalatha et al. 2012) and betalains (Martinez et al. 2011) with respect to their performances. However, at the same time, it had a positive influence on the efficiency of the dye-containing carotenoid pigment. Carotenoid was a yellow, red, or orange colour pigment that acts as an accessory during the photosynthesis process, i.e., it harvests the light and prevents any energy dissipation. This revealed that not all compounds go well with all the pigments. Other than the main pigments, delphinidin pigment was observed to have a better anchoring/bonding capacity with the TiO2 semiconductor when compared with other plants, which resulted in performance in terms of efficiency reaching up to 0.6% (Hamadanian et al. 2014).
Hence, it was safe to say that the stepping stone for fabricating an efficient DSSC relies on the selection of plants based on the type, family, and pigments present in it, and one cannot simply select any plants that they want; there has to be a proper thinking process that needs to be followed.
The segregation of pigments in the plant was widely distributed, meaning some parts of plants had more pigments responsible for better absorption and higher efficiency than the others when used as a dye sensitizer. Different parts of plants were allocated with different duties. Not all parts contained pigments that can be used to harvest light. Among all parts such as leaves, flowers, fruits, roots, stem, and barks, leaves were more frequently used, followed by fruits and flowers. The main reason for these selections was related to the fact that the green colour indicates the presence of chlorophyll which was the main pigment for absorbing the sunlight. Thereby, using the leaves can lead to the fabrication of better DSSC. However, it does not mean that using other parts will not accomplish better performance. It was established that dye extracted from the young flesh of fruit had higher efficiency when compared with pickled flesh and seeds (Jaafar and Safri 2017). Experiments have shown that stems of the Cladode plant have indicated higher efficiency of about 0.740% (Ganta et al., 2017). This eradicates the widespread belief that leaves are the only part that shows better performance.
Moreover, in an experiment done by Zhou et al. (2011), it was clearly stated that out of 20 different plants that were used, a variety of plant parts such as fruits, leaves, and flower extract taken from mangosteen pericarp fruits showed higher efficiency of 1.17% out of all the other extracts. This was possible because of the presence of rutin pigment in the fruit extract. Hence, it was noted that not all plants provide the same result. The plant that comes in the algae category (Saedi et al. 2020) and cactus (Ali and Nayan 2010) has shown a promising DSSC performance with extracts obtained from their fruits and leaves, respectively. The highest efficiency observed was 8.22% that used the leaves of Peltophorum petrocarpum and Acalypha amentacea plants (Sanjay et al. 2018a, b). Therefore, it was advisable to check the presence of major pigments responsible for better performance in the parts of plants being used before going ahead with the process.
Another point to be kept in mind was the terms pigment and dye. Although both contribute towards the colour of a substance, there was a notable difference between the two. Pigments that were found in plants were not directly used for DSSC fabrication. They were first extracted from the plants using extraction methods and solvents. The later results were said to be a dye that can be used for further processes. A dye was typically unstable in UV light; on the other hand, the pigment was usually stable and was also responsible for the absorption of light of a particular wavelength. Size-wise, a dye was small in comparison to a pigment making it more soluble in liquids. Though both the terms seem to represent the same meaning, the actual definition and purpose of these terms were not the same. One should be careful enough to understand and state both the terms in an appropriate manner.
Criteria for dye extraction from plants
Influence of solvent used
After the selection process of plants and their parts, the extraction process comes next in line. The plant dye should be extracted efficiently to obtain the maximum amount of pigment present in the plant. There is a number of solvents, such as ethanol, methanol, acetone, water, acetate, that are used for extraction purposes. The main solvent that has proven to be efficient compared with other solvents was alcohol, i.e., ethanol and methanol (Alwani et al. 2017a, b), among which ethanol has become more popular. Al-Alwani et al. (2019) stated that when solvents were compared with each other based on their solubility with dye, performance, and absorption spectrum, in both times, ethanol was proven to be the better solvent for extraction purposes. Similarly, Taya et al. (2014) compared different solvents for the extraction purpose in which dye extracted from ethanol showed better efficiency than others. Likewise, when a comparison was made between hydrochloric acid (HCL) and ethanol acting as a solvent, it was noticed that ethanol provided better efficiency than the acidic solvent (Godibo et al., 2015). Although ethanol as a solvent has shown remarkable performance, solvents such as water and acetone have also proven their worth. The extract’s efficiency increased even more, when a combination of acetone and water was used as a solvent for extraction (Garcia-Salinas et al. 2019). According to Hosseinpanahi et al. (2017), the optimal condition for extraction of anthocyanin pigment from the plant was using ethanol as a solvent. Similarly, another experiment on Peltophorum pterocarpum and Acalypha amentacea plants dye compared the efficiency of water and ethanol extracted dyes by cold maceration technique. It revealed that the efficiency of A. amantacea and P. pterocarpum plant, when extracted using water, was 4.43% and 5.76%, respectively, while the efficiency increased when ethanol was used as a solvent, i.e., 5.08% and 7.17%, respectively (Sanjay et al. 2018a, b). This indicated that ethanol was best suited for dye extraction purposes.
Apart from ethanol or alcohol, the second most commonly used solvent was water. It was noted that water had shown promising results when used as a solvent. Deionized water used to extract Calotropis gigantean leaves has exhibited higher efficiency (Sharma et al., 2015). Finally, it can be concluded that the solvent used also plays a role in determining the overall performance of DSSC.
Influence of temperature
It has been noted that temperature has quite an impact on the extraction as well as the dye absorption process. Studies have revealed that the absorption of dye onto the thin film increases with the increase of temperature (Park et al., 2013). Experiments have been carried out using a temperature range of 25–35 °C for extraction; the extraction process and extraction carried out under 35 °C temperature has also shown favourable performance (Faiz et al. 2013). However, Al-Alwani et al. (2017a, b), Al-Alwani et al. (2019), and Al-Alwani et al. (2020) mentioned in their research that the optimal temperature best suited for dye extraction ranges from 70 to 80 °C.
Influence of combination/mixture of dyes
Mixing of dyes together has resulted in higher efficiency when compared with individual dyes. Lim et al. (2015) expressed that when two dyes with pigments, i.e., chlorophyll and xanthophylls from the same plant, were mixed, they yielded higher efficiency of about 0.085% than the individual dyes. Similarly, Martinez et al. (2011) also compared the performance of betaxanthin and betacyanin dye mixture and individual dyes. It was found that the mixture of dye resulted in higher efficiency of 0.48% when compared to individual dyes. Other experiments utilizing the mixture dyes from plants such as maqui and blackberry (Leyrer et al. 2018), Bougainvillaea glabra and Bougainvillaea spectabilis (Martinez et al. 2011), amaranthus and carica papaya (Pote et al. 2019), pomegranate and mulberry (cladode and aloe vera), and purple cabbage and wormwood (Chang et al. 2010 & 2013) had shown better efficiency when compared with their individual dyes.
It was interesting to note that using a specific calculated ratio of the mixture of dye together had shown higher efficiency. Zolkepli et al. (2015) experimented using Tradescantia spathacea and Ixora coccinea dye extract by mixing the two dyes in a ratio of 1:4 (Tradescantia/Ixora), which showed higher efficiency than the individual dyes. Another experiment done using Ixora sp. flower and Canarium odontophyllum fruit in a ratio of 1:1 yielded an efficiency of 1.13% (Kumara et al., 2013a, b). Likewise, an experiment was done on raspberries, hibiscus, chlorophyll dye extract, which were mixed in a ratio of 1:1:1, which resulted in higher efficiency of 3.04%, while the efficiency of individual dyes was less when compared (Alhamed et al. 2012).
Another way of mixing was by using a specific percentage combination of natural-coloured dyes. An experiment on a combination of green and red natural dyes was done, and it was observed that the optimal combination of 20% green and 80% red exhibited better performance of DSSC, resulting in 0.847% of efficiency (Kabir et al. 2019). In another experiment conducted by Kabir et al. (2020), an efficiency of 1.572% was established using a combination of 40% red and 60% yellow-coloured dye.
Synthetic dye has already proven to perform better than natural dyes. Keeping this in mind, the natural dyes were mixed with synthetic dyes to boost the cell’s efficiency. Pote et al. (2019) used an appropriate amount, i.e., 0.5 mg of Dn-F015, mixed into the dye extract of spinach and found that the efficiency enhanced 0.25% compared with the individual dye. Further, it was seen that the efficiency and current density also boomed when ruthenium dye was a mixed flame tree and pawpaw dye extract. It was observed that the fill factor (FF) of natural dye proved better than the synthetic dye (Kimpa et al. 2012). In an experiment done by Sanjay et al. (2018a, b), it was observed that when Peltophorum pterocarpum and Acalypha amentacea plant dyes were extracted using ethanol solvent and mixed together, it resulted in higher efficiency of 8.22% when compared with their individual dyes.
It was observed that in the UV–visible light wavelength, the dye mixture has a higher potential to transform the light into electricity, i.e., the dye mixture has a better ability for photon-to-electron conversion. This quality led to the enhanced absorption range of the dye mixture. The mixture contains different dyes that vary in absorption wavelength due to their dissimilar composition, thus causing a wide absorption wavelength. Consequently, the performance of DSSC using dye mixture has better results when correlated to DSSC using individual dyes.
Influence of methods used for extraction
Before the extraction process begins, the plant must be thoroughly washed using distilled water to avoid any impurities, after which the plant was either dried at room temperature or at any suitable temperature. The plant was crushed or squeezed, or cut into small pieces, after which it was poured in a porcelain mortar with a pestle, and a suitable solvent was added in the mortar. This can be kept for a variation of time, i.e., from 15 min to 24 h. Most of the experiments follow this procedure with a slight variation here and there.
There has been an experiment done using the cold extraction method in which the crude dye obtained using the Soxhlet extraction process was kept inside the refrigerator at 4 °C until its next use. The efficiency obtained using this extraction process was 0.0712% which seemed very less (Eop et al. 2019). In the same way, Ismail et al. (2018) also used the cold extraction method and found efficiency of 0.56%. Another method used for extraction was with an ultrasonic cleaner in which the solvent added plant was kept for 15 min at a frequency of 37 Hz in Degas mode for extraction of chlorophyll pigment. This method shows a better efficiency of 0.75% (Syafinar et al., 2015). Taya et al. (2016), in their research, suggested that the efficiency of DSSC can be varied by extracting the leaves in grinded and un-grinded form. In the experiment, results were compared to find which method of extraction yields promising output. Three plant leaves that were used were safflower, Medicago sativa, and Rosmarinus officinalis. These were extracted using ethyl alcohol. Among them, the outcome of Medicago sativa was higher with 0.115% when extracted after grinding. But the method which has resulted in much higher efficiency of 1.50% was the maceration method (Ridwan et al. 2018).
The criteria for the extraction method might depend on the preference of the researcher. The basic knowledge that a researcher should have when selecting a method includes the cost, productiveness, availability, biodegradability, and popularity. While the intent was to extract natural dye because of its low toxicity, the method chosen should also obey the same objective, i.e., the chemical used should have a low degrading effect on the environment yet should be functional enough to extract the maximum amount of dye from the plant part.
Influence of concentration/volume
Considering volume/concentration, i.e., at what proportion the compound should be added to get better performance, has a great overall impact. Muryani et al. (2019) experimented with variations in dye and solvent concentration. It was noted that the dye/solvent concentration of 5 g/100 ml showed better efficiency of 0.066%. Similarly, another experiment conducted by Madnasri et al. (2019) talks about different dye volumes. It was observed that an optimal volume of 0.05 ml resulted in good efficiency of 0.099%. Hosseinpanahi et al. (2019) stated that a solvent concentration of 25.02% will increase the efficiency of DSSC. Moreover, it was discovered that with the optimum concentration of 90 mM, the performance of DSSC and the dye loading capacity displayed improvement (Arifin et al. 2017).
According to these studies, the key points that indicate the relation between dye concentration/volume and DSSC performance were as follows:
-
The absorption ability of the dye was directly linked to the concentration of the dye used. The more the leaves used in the solution, the more will be the presence of the dye molecules in the solution, thereby aiding to higher absorbance of the photon from the visible light.
-
The concentration of dye will influence the dye loading capacity. The presence of a higher quantity of leaves in the dye solution will increase the amount of dye molecules absorbed in the pores of the TiO2 layer.
-
Simply put, the more the concentration/volume of dye, the better will be for the efficiency of the device. However, it was noted that after a certain amount of conc./volume, although the dye loading capacity increased, the efficiency of the device started to deplete. This occurred due to the phenomena of dye precipitation onto the TiO2 electrode. These precipitated molecules cause the dye molecule to become inactive, thus blocking the electron injection process in the DSSC device. As a consequence, the efficiency of the device declines.
Hence, it can be said that the concentration and volume of dye solution somewhat contribute to the overall performance of DSSC.
Fabrication methods used
Coating technique
There are multiple ways for coating the electrode with semiconductor paste. In literature, the coating techniques used for experiments were doctor blade, spin coating, screen printing, slip casting, squeeze printing, brush painting, EPD method, and dip coating. The doctor blade method has been the most popular among the others due to its simple application. But one drawback was that the layer thickness applied to the electrode depends upon its application. So, one must be gentle and careful while applying the paste and avoid multiple applications; otherwise, the layer formed will not be thin. Alhamed et al. (2012) performed the experiment using the sol–gel dip-coating method with different combinations of dyes. It was found that the output efficiency obtained was much higher. A similar experiment had been carried out using the dip-coating method, which resulted in an efficiency of 0.48% (Martinez et al., 2011). Another method used was an electrophoretic deposition, in which the FTO plates were considered as an anode, and a steel mesh was taken as a cathode plate. They were kept 2 m apart from each other. Each deposition cycle lasted for 15 s at an STD temperature of 25 °C. Using this technique has resulted in an efficiency of 0.6% (Hamadanian et al., 2014).
Although the doctor blade method has been popular due to its simplicity, but spin coating method has also gained interest for its flexibility. The major factor that contributes to the higher performance of DSSC was the paste thickness. It was observed that the thin layer leads to lesser resistivity in the electrode, which made the electron transfer easy (Ali and Nayan 2010). Aiding to this, the spin coating allows variation of layer by adjusting the revolutions per minute (rpm) at which it has to run. It was noted that a higher efficiency of 1.95% was obtained using spin coating at a speed of 500 rpm for 5 s that obtained 25-μm thin layers on the electrode (Chang et al. 2013). Similarly, a better efficiency was obtained while using spin coating at a speed of 3000 rpm with the involvement of other factors such as dye volume (Madnasri et al., 2019). In another experiment, Emran et al. (2018) mentioned the merit of applying 4 layers of coating onto the electrode. It implied that due to more depth of the paste, more dye was absorbed onto the plate leading to good efficiency of 0.115% when compared to 2 layers of semiconductor paste. Contradictory to this finding, another researcher, Ferreira et al. (2018) stated that the optimal condition to obtain better efficiency was by applying 2 layers of semiconductor paste onto the electrode that resulted in 0.37% of efficiency. In addition, another experiment revealed an efficiency of 0.43% when the 2-layer coating was used (Jaafar et al., 2017). Hence, it was safe to assume that the optimal condition for achieving a better performance of DSSC was by applying 2 layers of TiO2 paste using the spin coating method.
Immersion time
Once the electrode has been coated successfully with the appropriate technique, the electrode should be dipped in the dye extract so that the dye can anchor onto the surface of the semiconductor-coated electrode. The proposed time for the cell to be placed inside the dye extract solution was implied to be 12 h. It has been stated that if the DSSC was placed inside the dye for more than 12 h, its efficiency decreased (Diantoro et al. 2019). Other experiment results show that the better efficiency of 0.091% and 0.526% were obtained when the electrode was dipped for about 6 h (Anggrainai et al. 2017) and 24 h respectively (Pote et al. 2019). Although it was mentioned that more than 12 h might result in lower efficiency, but it turns out that the efficiency increased when immersed for about 24 h. So to be on the safe side, the optimal immersing time can be assumed to be in a range of 6 to 24 h.
Pre-treated semiconductor paste
TiO2 is considered being better when compared with other semiconductor powders for reasons such as low toxicity, photo corrosion resistance, low cost, insoluble in water, and stability. Moreover, it has good mechanical and structural properties, which make it apt for other applications. Pre-treatment of semiconductor powder has proven to be beneficial in obtaining good overall performance of DSSC. Boyo et al. (2015) discovered that when the temperature was varied from 100 to 500 °C, after the application of TiO2 paste onto the surface of conducting plates, the number of open pores present in the TiO2 paste decreased, which resulted in better absorption of dye leading to the higher efficiency of 0.52%. Similarly, better efficiency of 0.56% was observed when TiO2 paste was subjected to heating (Ismail et al. 2018). So it can be summarized that pre-heating of TiO2 paste before or after it was applied to the electrode has some positive role in the betterment of DSSC performance. Not only pre-heated but pre-dye treated TiO2 powder has also acted as an asset to the high yielding of DSSC. A comparison was made between pure TiO2 paste and pre-dye treated TiO2 paste. The pre-dye treated TiO2 was made by directly adding the extracted dye of Lawsonia inermis seed into the TiO2 paste.
It was established that the DSSC fabricated using pre-dye treated TiO2 showed higher efficiency of 1.47% than the pure TiO2 fabricated DSSC (Anggraini et al. 2020). Apart from the pre-treatment method, the concentration of semiconductors has also played a vital role in the fabrication of well-functioned DSSC. In an experiment carried by Ammar et al. (2017), a comparison was made using individual dyes and TiO2 mixed dyes. The different concentrations of 4%, 6%, 8%, and 10% of TiO2 were mixed with extracted dyes. It was observed that among the individual and TiO2-treated dyes, dye containing 10% concentration of TiO2 showed higher efficiency of about 2.239% when compared with others.
Apart from using TiO2 semiconductor as a paste for its obvious advantages, other semiconductors such as ZrO2 can also be used for the same purpose. Using ZrO2 side by side with TiO2 has yield higher results. An experiment revealed that fabrication done using the layer-by-layer paste of TiO2-ZrO2 gave an efficiency of 3.13%, which was way higher when compared the results with individual TiO2 and ZrO2-fabricated DSSC (Pawar et al. 2019).
The TiO2 nano-powder synthesized using the sol–gel technique has also shown promising results. An experiment on beetroot and henna was conducted with titanium butaoxide (TBT) as a precursor and a mixture of potassium iodide (KI), acetonitrile, and iodine as an electrolyte. It was established that beetroot had better efficiency of 1.3% when compared with the henna plant (Sathyajothi et al. 2017).
Working electrode plate
The plates used in fabrication have much value because the conductivity of the cell will somewhat depend on the properties of the material used. The two plates commonly used in DSSC were FTO (fluorine-doped tin oxide) and ITO (indium-doped tin oxide) plates. FTO has been given more importance in most of the experiments conducted due to its lower sheet resistivity. However, it does not mean that using ITO plates will reduce the overall performance. An experiment done with Syzygium cumini seeds extract using ITO plates showed higher efficiency of 2.0% (Hambali et al. 2015). The FTO plates, though have less sheet resistivity but also have less transparency when compared to ITO, have established a greater performance level of 3.04% (Alhamed et al. 2012), 3.13% (Pawar et al. 20,190, 3.4% (Sharma et al. 2015), and 8.22% (Sanjay et al. 2018a, b). It can be concluded that FTO proves to be the optimal plates that can be used during DSSC fabrication.
Co-sensitization
The other method for improving the working of DSSC using natural dye was by exploring the effect of the co-sensitization method. The dyes were chosen based on their varying absorption of light so as to achieve a broad absorption spectrum. An experiment was performed using Ixora sp. and Canarium odontophyllum plant parts. The dye extract retrieved from the plants was used for double-layered co-sensitization. The dye extracted from C. odontophyllum was first coated on TiO2 electrode via dip coating, then removed using the de-sorption solution. After which, the second dye extract from the Ixora sp. flower was allowed to get absorbed onto the same electrode. A compression was made between the individual dye, mixed dye, and co-sensitized dye, among which co-sensitized dye performed higher efficiency of 1.55% (Kumara et al. 2013a, b). After observing the result, it can be considered that co-sensitization has a positive effect on the overall functioning of DSSC.
Other dependency
pH dependency and co-pigmentation
The presence of pH had a remarkable influence on the absorption power of the dye used for DSSC. In his experiment, Park et al. (2013) affirm that with the increase of acid concentration in the dye, the absorption level of the dye also increased. This stems from the fact that the isoelectric point of the TiO2 dye absorption falls near the acidic pH band (around 3.5 pH), hence, promoting more dye absorption processes. Likewise, in another experiment, it was established that the optical absorption of the extracted dye boosted with the addition of acid in the extraction solvent used (Hemmatzadeh et al., 2013). According to Isah et al. (2014), the ideal pH value of 3 was helpful for intensifying the dye absorption property, which resulted in 0.98% efficiency. Usage of acids such as HCL has shown a higher impact on the overall performance giving efficiency of 1.43% (Lim et al. 2015) and has also been used for hydrolyses of extracted dyes (Alaba et al. 2012). In an experiment conducted by Kumara et al. (2013a, b), it has been proven that using HCL acid helps in better dye absorption and is efficient in light absorption.
Another interesting result was found when two acids, malic acid and ascorbic acid, were mixed with the anthocyanin pigment extracted from the fruit of the mangosteen pericarp plant. This process was said to be known as the co-pigmentation method. It was discovered that using acid increases the efficiency, stabilises the pigment, and helps with the dye’s colour retention. The combination of anthocyanin with malic acid showed better performance (Munawaroh et al. 2016). Apart from using acid in extracted dyes so as to obtain better performance, treatment of electrode plates with acid also proved positive for enhancing the overall efficiency of DSSC. It might be safe to say that with lower pH ranging from 2 to 3 value, the capability of DSSC will always have a positive impact.
Extraction time
The time needed for extracting the pigment from the plant, more commonly known as dye extraction time, can vary from experiment to experiment. An experiment suggested the prime time for extraction of dye from the plant was 24 h, resulting in improved efficiency of 0.162%, which was higher when compared with other samples subjected to different extraction times. A proportional relation was found between the dye extraction time and the concentration of dye which recommended that the concentration of dye increased with the extraction time. Due to the increased concentration, the dye loading capacity when the anode was immersed/dipped in the dye for dye molecules to attach themselves to the TiO2 surface also improved. This considerably accelerated photon absorption that supplemented the efficiency. But suppose the dye extraction time exceeds the prescribed time. In that case, it will negatively impact the efficiency as the concentration of dye will reach the highest point, causing the molecules to recombine at the TiO2 surface, thereby obstructing the electron transmission, which leads to blemishing of DSSC performance (Gu et al. 2018). An alternative result was found when saffron petals were extracted for 15 min which led to 0.66% efficiency (Hosseinpanahiet al. 2017). It might be due to other factors such as a lower pH value of 2 combined with an optimal solvent-acid concentration that has led to such higher results.
Additives
Additives were used for enhancing some of the properties, which might help in better working of DSSC. An experiment conducted on mangosteen pericarp plants used CADA (chenodeoxycholic acid) as an additive. It was used to increase the anchoring power of the dye in order to rush the electron transfer, thereby helping the performance of DSSC. The presence of CADA not only helped in the performance aspects, but also reduced the dye aggregation. An amount of 2 mM was used to achieve a better overall efficiency (Ismail et al. 2018). Similarly, Saedi et al. (2020) added graphene quantum dot (GQD) to the extracted dye from Gracilaria and Ulva algae and compared the individual dyes to the GQD mixed dyes. The result showed that Gracilaria dye mixed with GQD performed better with an efficiency of 0.94%, which was far better when compared with other samples.
Another additive, such as TiCl4, has also been found to help boost up efficiency. It was used onto the FTO/ITO plates and has proven to increase the efficiency up to 1.572%, which was 1.24 times more when compared with another sample (Kabir et al. 2020). Likewise, three acids, HCL, H3PO4, and HNO3, were pre-treated on the FTO plate’s surface and post-treated after the application of TiO2 on the working electrode surface. This was done to check the performance of the acids in both pre-treated and post-treated conditions. The results stated that H3PO4 acid proved better with 0.40% efficiency during pre-treatment, whereas post-treatment HNO3 was immersed with 0.81% efficiency. The extract used was from purple carrot roots (Radwan et al. 2016). In an experiment, copper oxide nanoparticles were used as a thin film that had higher benefits on the working of DSSC. It exhibited a good electrocatalytic process with the iodide ions. In addition, cupric nitrate of amount 5 g was mixed with the dye, which demonstrated a good impact on the overall performance, resulting in 3.4% efficiency (Sharma et al. 2015).
Storage temperature
After the extraction of dye from plants, it was crucial to store the dye at an appropriate temperature so as to avoid any dye degradation as it will directly affect the functioning of DSSC. The optimal temperature which was recorded to store the mixture of dyes was − 20 °C (Zolkepli et al. 2015) and for individual dye was 4 °C (Yusoff et al., 2014).
Stability
The stability of a device has been defined by its ability to produce constant output under all internal as well as external conditions. There are many factors that can lead to instability in the DSSC device. Prabavathy et al. (2017) reported that the main reason for the instability of the device was mostly the interaction of the dye with the photo catalytic activity of semiconductor materials used in the photo electrodes. The PCA of the semiconductor (TiO2) allows it to generate free electrons that, when it comes in contact with any moisture, release free radicals. When these free radicals come in contact with organic dyes, they start degrading them. The phenomena of dye degradation cause photo instability in DSSC. In another experiment, the author Suhaimi et al. (2014) concluded that the solvent used for the extraction purposes exerts an influence on the stability of DSSC. Two solvents, ethanol and water, used for extraction intimated their importance in terms of stability and efficiency of the dyes. After storing the dyes extracted for 24 h from these two solvents, it was observed that the dyes extracted from ethanol solvent proved to have better stability and efficiency. The dye solution with ethanol remained stable even after 24 h of storing, suggesting that solvents also help maintain the dye’s stability.
Apart from these factors, there were other conditions that had a direct impact on the plant pigment stability. The natural dyes used for sensitization mainly contain pigments such as xanthophylls, anthocyanin, betalain, and chlorophyll. Suppose the pigment does not show stability under environmental conditions. In that case, it will cause dye degradation when in contact with sunlight. In an experiment done with freshwater green algae, xanthophyll exhibited better stability when exposed to light over a period of 24 h. On the other hand, chlorophyll underwent higher dye degradation under visible light and temperature. However, continued exposure to light and high temperature caused a high degree of degradation in both the pigments (Lim et al., 2015). Garcia-Salinas et al. (2019) compared the stability between anthocyanins and betalain dyes by gathering an ample literature review and found that anthocyanin pigment proved to be more stable than betalain pigment. The pH suitable for betalain pigment ranges from 5 to 6, whereas anthocyanin exhibited better stability under acidic dye concentration. However, preserving both the pigments can be done for at least 6 months if dry bracts were used.
Summary of the natural dyes and achieved efficiencies
In the above sections, a detailed investigation of various conditions for the development of DSSC solar cells was given. In this section, a summary of plant names from which natural dyes were extracted has been given, along with the lab-scale development of DSSC properties. In Table 1, plant names and the key points were summarized. In Table 2, the detailed properties of DSSCs using various dyes are shown.
Plants that were used for the fabrication have been listed in the above tabular column. It summarizes all the highlights that were needed to get an overview of all the studies to gather from different sources. The table captures all the information related to plants used, such as their name, family, type, pigments present, and parts used for the extraction. Likewise, the plates as well the coating technique used were also listed. The table also shows the efficiency achieved in each research which has been arranged in ascending order of the efficiency. Similarly, other points such as solvent used for extraction and also the key points in the fabrication have also been listed. Overall, the table depicts all the major features which were spotted in the experiments during the DSSC fabrication process.
From the comprehensive literature study carried previously, some of the important information about the plant type and percent share was captured. In Fig. 3, the percentage of what type of plant has been more used for the DSSC study purpose has been represented using a pie chart. It can be considered useful: to know what part of plants has been used more so that research can be expanded to the plant types that have not yet been used or opted more. It was observed that more experiments were performed using shrub plant type followed by tree and herbs.
Figure 4 graph drafts of the total efficiency that each plant has exhibited in various experiments over the past years. It can be well observed that for a maximum number of the experiments conducted, leaves have shown better performance in most cases compared with other parts. Although other plant parts have also resulted in higher efficiency, experiments done using other plant parts were fewer.
Future perspective
Keeping in mind the various advantages and disadvantages of DSSC, many trials were performed by adding various components in the hope of increasing the electrical and process efficiency. With careful fabrication and thoughtful technique, DSSC can be incorporated in various fields using unique methods. Figure 5 is a pictorial representation of some fields where DSSC can be useful. The same was also discussed in the upcoming sections.
Performance enhancers
The third generation dye-sensitized solar cells have been experimented on for some time now. During which, many new ideas related to the dyes, working electrodes, and electrolytes have been surfaced to improve the device’s overall performance. Additives are one of the techniques used to enhance the DSSC device’s output by increasing the device’s Voc and current density. Additives or performance enhancers slow down the recombination process, thereby aiding the current density and making the device stable. Due to their promising properties, many experiments are carried out to find cost-effective additives that can bond with the DSSC components to develop better outcomes. In their study, Yang et al. (2014) showcased the advantages of using nanoparticle additives such as TiO2, Co3O4, and NiO with agarose polymer electrolytes where Co3O4 and NiO were classified as magnetic nanoparticles. The result showed that Co3O4-added agarose polymer provided better performance with 2.11% efficiency and stability of 400 h. Apart from this, triphenyl phosphate (TPP) was used as an alternative for a 4-tertbutylpyridine (TBP) additive due to its cost-effective nature. TPP was used as an additive in DSSC fabricated using N719 dye and TPA dye. The efficiency of these cells seems to improve significantly with the presence of TPP (conc. 1 M). N719-sensitized DSSC gave an efficiency of 7.04%, whereas TPA-sensitized DSSC showed 2.73% efficiency. In both cases, the current density has proven to increases due to the presence of TPP, which slows the rate of electron recombination, causing current density to increase (Afrooz and Dehghani 2015). Similarly, a set of alkaline iodide electrolytes was prepared in two batches, first without any additives and second with additives. The sample with additives was observed to be responsible for enhancing the current density, open-circuit voltage, and efficiency of the fabricated DSSC. The additives used for this experiment were Pr4NI, MPII, and 4TBP. The KI electrolyte within cooperated additive showed higher efficiency of 5.26%, with a current density of 11.35 mA cm−2 from any other samples (Bandara et al. 2017).
Additives such as benzimidazole and 2-ethylimidazole have helped the performance of the DSSC process. Due to its interaction with TiO2, benzimidazole and 2-ethylimidazole were considered to replace the TBP additive in the electrolyte. The cells were fabricated with a different molar ratio of the co-absorbers and found that the current density started decreasing after a certain amount of co-absorbers were added. At an optimal molar ratio of 9.5/0.5, the best efficiency of 7.93% was obtained with a higher Voc value of 0.817 V (Sun et al., 2017). Besides this, Toor et al. (2016) studied the property of CADA to be used as additives and found that CADA can be identified as a low-cost, effective additive to be used for DSSC devices. N719-sensitized DSSC was studied for its performance with and without additives. DSSC with additive provided an efficiency of 5.70% with a current density of 15.36 mA/cm2.
Researchers have been exploring new compounds or materials that can come under a cost-effective additive that can be suitable with synthetic dyes. These searches can be expanded to DSSC using natural dyes so as to make the device easily available, cheap, and environment friendly.
Nanoparticles
The field of nanotechnology has been popular for all the right reasons. The fact that in spite of being compact in size, nanoparticles have contributed abundantly towards enhancing the properties of the devices in which it was used has captured the attention of all the researchers. It was indeed a well-established idea of using nanoparticles in the fabrication of DSSC by replacing the components such as a conventional counter electrode or photoanode.
Besides being used for its obvious benefits as a counter electrode, Pt cannot be a good choice when fabricating a cost-effective DSSC. Alternative for this was using penternary chalcogenides Cu2CoSn(SeS)4 and Cu2ZnSn(SeS) as counter electrodes. Counter electrodes were prepared using the hot injection method. No further changes were made while fabricating, i.e., FTO plate was coated with TiO2 paste using a doctor blade, and photoanode was synthesized using N-719 dye. The efficiency of DSSC fabricated using Cu2CoS resulted in the promising result of efficiency 6.47% Voc of 0.61v and Isc of 6.14 mAcm−2 respectively, which was far better when compared with Cu2ZnSn (SeS)4 and Pt as counter electrodes (Ozel et al. 2016). In an experiment led by Guo et al. (2013), different amounts of Ag nano were mixed with TiO2 and were sensitized with ruthenizer 535-bisTBA (N-719) dye to form photoanode. It was found that 15% Ag when used with TiO2 as a photoanode was best suited for fabrication as it resulted in higher Isc and Voc value, i.e., 10.19 mA cm2, 698 mV, respectively and yielded an efficiency of 5.33%. The presence of AG also helped in increasing the e- lifetime and decreasing the dark current.
The idea of extracting nanoparticles from natural plants has been fantasized by many due to its salient features, such as low cost and readily available nature. Daniel et al. (2013) performed an experiment that showcased silver extraction from henna dye further reduced to silver nanoparticles used as photo synthesizers. The effect of temp, pH, and size on silver nanoparticles was studied. It was concluded that the size of the nanoparticles increased until it reached a temperature of 60 °C, exceeding which the size decreased. The optimal zeta potential was concluded to be − 28.3 mV. The silver nanoparticles were stable for a pH value of 10. The size optimal size was concluded to be 50 nm. It was also found that the absorbance of the dye increased with an increased amount of leaf concentration. Overall, it was concluded that sliver nanoparticles extracted from the henna plant had improved the photocurrent of DSSC. A similar experiment was done using the dye extract from the flower of Kigelia africana. The dye was sensitized with Na-doped TiO2 nanorods and was used as photoanodes for fabrication purposes. It was found that the DSSC using NA-doped TiO2 exhibits higher efficiency of range 1.09 to 1.56%, whereas the non-doped DSSC resulted in 0.87% efficiency (Shalini et al. 2018).
Experiments done by combinations of different nanoparticles have yielded better performances when compared with the conventional DSSC fabrications. In an experiment done by Muduli et al. (2012), TiO2 nanoparticles were mixed with gold nanoparticles using a hydrothermal process; the result obtained showed an increase in open voltage and short circuit current with an efficiency of 6%, which was way better when compared with DSSC fabricated with only TiO2 nanoparticles. It might be due to the fact that the interface of loaded TiO2 was less when compared to unloaded TiO2 when the recombination of e− and I3− occurs. For DSSC using plant dyes, Sanjay et al. (2018a, b) conducted an experiment that stated ZnO nanoparticles as a close alternative for TiO2 nanoparticles for semiconductor paste. The efficiency obtained was 6.07% from P. pterocarpum plant extracted dye.
A novel idea of nanoparticles, i.e., dandelion-like TiO2 nanoparticles, was designed using a hydrothermal process. Large numbers of nanowires were made using the nucleation-growth-assembly mechanism. Two sets of DSSC were fabricated, one with a monolayer and the other with a double layer. The comparison was made between monolayer DSSC of thickness 31 μm and double-layer DSSC with underlayer and overlayer mechanism, where the underlayer was made of nanoparticles and the overlayer was a combination of nano and dandelion-like spheres. It was observed that monolayer DSSC showed an efficiency of 7.4%, whereas the double layer produced a higher efficiency of 8.3%. This novel design can be considered preferable for improving the functionality of DSSC (Gharavi and Mohammadi 2015).
Similarly, Gurulakshmi et al. (2019) indicated a potential alternative for Pt counter electrode. A combination of SSrGO reduced from graphene oxide, and SWCNH were dispersed into dimethylformamide, after which it was coated onto FTO to form a counter electrode. A single cell of Pt free DSSC obtained had an efficiency of 8.27%, and the combined module showed an efficiency of 5.18%. This DSSC was tested by running an electric motor of 15 mW, and it was found that the motor ran surprisingly well in indoor and outdoor light conditions. The implication of using Pt free DSSC has a better future scope for easy fabrication purposes.
Furthermore, the experiment was performed by altering the surface properties of the photoanodes so as to trap maximum light. Pu et al. (2012) suggested a similar idea. The experiment used a KrF excimer laser to roughen the surface of the photoanode fabricated by using TiO2 nanoparticles. It was observed that the otherwise transparent surface of TiO2 became more opaque, which indicated the entrapping of light by the photoanode. This resulted in an increase in the conversion efficiency from 4.49 to 5.59%. Additionally, a notable increase was observed with respect to Voc and Isc values. Thus, it can be summarized that the use of nanoparticles has been useful for the performance of DSSC and can be researched further to obtain organic nanoparticles derived from plants which can pave the way for cheap, easily accessible, and eco-friendly DSSC devices.
Recent advances
Laser drilling
Even after the known benefits of using DSSC, making it commercially available was hard due to higher competition from Si-based solar cells. To mitigate this problem, research has been directed towards flexible electrode-based DSSC, which might have a chance to attract the commercial sectors as the device which can be used in decorative as well as domestic purposes. Ti substrates have been frequently used for this purpose due to their capability to withstand numerous sintering processes and higher conductivity. As flexible electrode-based DSSC will be subjected to a numberless amount of folding, it was important to opt for a process that can help the film stick onto the Ti substrate firmly.
According to research done by Liu et al. (2011), microhole arrays (MHA) done onto the surface of Ti substrate using laser drilling had proven to provide better adhesiveness of NTA onto the surface of Ti substrate, proving to be a good example for flexible electrode-based DSSC. The procedure showed that the Ti foil was washed properly using an ultrasonic bath before drilling MHA using Nd:YAG laser system. The fabrication was performed using two types of counter electrodes, i.e., Pt-coated indium tin oxide-polyethene naphthalate (Pt/ITO/PEN) and platinized fluorine-doped tin oxide (Pt/FTO) glass. The DSSC fabricated using TNA grown on Ti substrate with Pt/FTO counter electrode resulted in 3.45% efficiency, whereas the flexible DSSC fabricated using Pt/ITO/PEN produced 2.67% of efficiency. Figure 6 indicates the SEM images of photoanode and MHA along with the cavity of 5-µm depth and 22-µm diameter caused by laser drilling. Hence, the MHA technique using laser drilling can be seen as a resourceful option when going for flexible DSSC fabrication.
SEM images of photoanode and MHA with laser drilling (Liu et al. 2011)
DSSC as wearable devices
Many studies have been done on implementing DSSC into our day-to-day devices and using it in powering the sensors. Though it has not been fully established, DSSC can be used for commercial use if a proper mechanism is put into action. An experiment was done using eight different dyes of tropical plants, which was studied for their light absorption properties so as to be used in smart window applications. The presence of phytoconstituents indicated a positive result. It had useful properties that were well suited for the manufacturing of smart windows. Among the eight plant dyes, dyes of M. indica L. and P. macrophylla showed better performance in visible light due to the presence of flavonoid and anthraquinone pigments. They showed better performance for the transmittance of light but had low absorption properties. With the help of the solution growth technique, the dyes were made to grow onto the glass surface, which was then tested for visible wavelength using UV/VIS spectroscopy. The result showed that from the range of 90 nm and 1100-nm wavelength, the dyes showed good transmittance and low absorption at 400 nm. Between M. indica L. and P. macrophylla dye extract, M. indica L. dye exhibit better results for a wide range of wavelength, whereas dye extracted from P. macrophylla was better for the lower range where a controlled temperature is needed for long duration (Abodunrin et al. 2015).
As portrayed in Fig. 7, another interesting experiment was done by Yun et al. (2014) where they tried to sew the flexible DSSC onto the cloths. This experiment used paper, cotton, and silk for this purpose, and the dye used was N719 dye. Unlike the conventional fabrication method, here, no transparent conductive oxide (TCO) or glass substrate was used. The process included sewing of textile electrodes onto the surface of cloths via the loom weaving technique. This experiment yielded high energy conversion efficiency of about 5.8% when silk fabric was used. It can be said that this method has a wide range of future applications due to the wide variety of fabrics available and has a higher advantage in expanding DSSC into the commercial as well as domestic sectors.
Picture of sewing woven electrode onto silk gauze along with SEM images of Hanji, cotton, and silk gauze surface (Yun et al. 2014)
As an upcoming technology, it has a whole lot of capability. The fact that this has been used to replace TCO substrate with less expensive components and impart good results makes it more attractive. Cha et al. (2012) experimented with the DSSC fabrication, which was TCO free. The idea was taken from the traditional Korean door structure. In their new design, they incorporated stainless steel mesh on one side of the glass paper along with the photoelectrode filled with Ru-complex dye, whereas on the other side, Pt was deposited to form the cell’s core. The electrolyte was injected into the pores of the glass paper, which protected the device from electrical short during the bending of the device. The structure benefited by avoiding TCO substrate because of its frangibility towards strain that affects its conductivity. In the Korean door inspired structure, the components were substituted on a glass paper that was considered highly flexible, thereby providing better strength to the design. This design performed well when layered with two coats of TiO2 paste, which gave an efficiency of 2.5%, with Voc and Isc reaching 0.7 V and 4.1 mAcm−2, respectively. The experiment paves the way for flexible DSSC to replace conventional DSSC in terms of performance, low cost, and flexibility. The same has been depicted below (Fig. 8), revealing the glass paper-based DSSC under different conditions with the I-V characteristics measured at 1 sun.
Glass paper-based DSSC under different conditions along with I-V and energy conversion efficiency graphs (Cha et al., 2012)
Thermochromism
The ability of a material to change its colour with respect to variation in temperature was termed thermochromism. The colour can be reversed. There was much research yet to be done on using thermochromism in DSSC fabrication. Thermochromic materials were helpful for devices that can either block or allow the near-infrared radiations for their use.
An experiment conducted by Riapanitra et al. (2019) highlighted the property of F–VO2/Nb–TiO2 as a multifunctional coating film. The paper focuses on the use of fluorine-doped vanadium dioxide and niobium-doped TiO2 as a coating layer that can be used in the fabrication of DSSC. Further tests were made by doping it with fluorine and physically mixing it with NTO to study the new properties that were obtained by it. The F–VO2/NTO was synthesized using a solvothermal/hydrothermal process and later coated onto the glass surface using the doctor blade technique. A comparison was made between F–VO2/NTO and VO2/NTO, which revealed that VO2 doped with F and mixed with NTO yielded better multifunctionality than the other. The presence of fluorine proved to project better thermochromic properties in VO2 as fluorine decreases the critical temperature of VO2 and also changes the bandgap, thereby allowing transmittance of visible wavelength. As VO2 has already gained popularity as a thermochromic material due to its reversible metal to semiconductor transition at a temperature of 68 °C (Riapanitra et al. 2020), a similar experiment was done where fluorine chlorine was used for doping VO2 (M) using the hydrothermal method. Although chlorine demonstrated good results when used for smart windows, it was not promising when compared with fluorine-doped VO2 (M). There must be better research that was needed to be done on the process of integration of thermochromic materials with DSSC fabrication to help provide better optical properties.
Conclusion
Although DSSC using natural dyes has promising benefits to offer, due to its low efficiency compared to the conventional Si solar cells, it was not able to captivate the solar market. By recognizing this fact, a comprehensive literature review was conducted in this paper to understand a broader view of the DSSC and to identify the key hotspots in the dye selection and fabrication process. In this review, optimum conditions such as suitable temperature, immersing time, coating techniques, optimum concentration/volume, dye mixture, additives, extraction time, co-sensitization, pre-treatment of semiconductor paste, and solvent influence have been discussed and their impact on the overall efficiency of DSSC. With this review, we provided a catalogue of all the optimum conditions that were considered to play a vital role in the fabrication process to obtain a DSSC with a higher amount of efficiency so as to be used in practical applications. This being the case, the DSSC using natural dyes can emerge as an industrial technology if mentioned conditions are kept in mind while fabricating.
Furthermore, the future scope of DSSC with natural dyes has also been explained with a multidisciplinary focus. We observed that there were many environmental factors that were affecting the working of the solar cells in general. Like, due to vehicles, industries, the dust present in the air tends to settle down over the solar modules, causing performance problems and slowly damaging the equipment. Many solutions have been implemented to aid these damages in the performance of solar devices caused by such impurities, though researchers tossed an idea of DSSC fabricated using photochromic spiropyran derivatives SIBT as photosensitizers (Ma et al. 2015). The advantage of using such sensitizers was to control the transmission from sunlight by reacting to the different intensities of light all along the day. This resulted in proper management of energy as well as higher efficiency. Similarly, we came across many solutions that were used to rectify the problem of dust deposition, such as anti-glare, self-cleaning coating, and energy-efficient coating used for thin-film applications (Anderson et al. 2016). Further research can be extended to incorporate such prevention methods for DSSC devices so as to make them more efficient. Synthetic DSSC has already been integrated with new technologies using thermochromism, laser drilling, and wearable devices. But their practical applications in commercialized sectors are still not up to the standards. Hence, the research on the practical performance of these devices can be considered. For instance, the laser-drilled DSSC that can help penetrate the light could be the best suit for agricultural application; we are currently working on this with the in-house designed experimental testbeds. Here, the practical performance testing also includes the various environmental conditions such as dust deposition, relative humidity, shadow conditions, among others. At the same time, one other work includes the ornamental plant selection for the DSSC.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Abdel-Latif MS, Abuiriban MB, El-Agez TM, Taya SA (2015) Dye-sensitized solar cells using dyes extracted from flowers, leaves, parks, and roots of three trees. Int J Renew Energy Res 5:294–298
Abodunrin T, Uhuegbu C, Olugbuyiro J (2015) Phytochemical analysis of leaf-extracts from eight tropical trees: prospects for environmentally-friendly dye compounds for smart windows. Int J Sci Eng Res 6:682–698
Afrooz M, Dehghani H (2015) Effects of triphenyl phosphate as inexpensive additive on the photovoltaic performance of dye-sensitized nanocrystalline TiO2 solar cells. RSC Adv 5:50483–50493
Alaba AK (2012) Utilization of natural Morinda lucida as photosensitizers for dyesensitized solar cell. Arch Appl Sci Res 4:419–425
Al-Alwani MAM, Al-Mashaan ABSA, Abdullah MF (2019) Performance of the dye-sensitized solar cells fabricated using natural dyes from Ixora coccinea flowers and Cymbopogon schoenanthus leaves as sensitizers. Int J Energy Res 43:1–11
Al-Alwani MAM, Hassimi AH, Al-Shorgani NKN, Al-Mashaan ABSA (2020) Natural dye extracted from Areca catechu fruits as a new sensitiser for dye-sensitised solar cell fabrication: optimisation using D-optimal design. Mater Chem Phys 240:1–9
Al-Alwani MAM, Ludin NA, Mohamad AB, Kadhum AAH, Sopian K (2017a) Extraction, preparation and application of pigments from Cordyline fruticosa and Hylocereus polyrhizus as sensitizers for dye-sensitized solar cells. Spectrochim Acta Part A Mol Biomol Spectrosc 179:23–31
Al-Alwani MAM, Mohamad AB, Kadhum AAH, Ludin NA, Safie NE, Razali MZ, Ismail M, Sopian K (2017b) Natural dye extracted from Pandannus amaryllifolius leaves as sensitizer in fabrication of dye-sensitized solar cells. Int J Electrochem Sci 12:747–761
Alami AH, Aokal K, Zhang D, Tawalbeh M, Alhammadi A, Taieb A (2018) Assessment of Calotropis natural dye extracts on the efficiency of dye-sensitized solar cells. Agron Res 16:1569–1579
Alhamed M, Issa AS, Doubal AW (2012) Studying of natural dyes properties as photo-sensitizer for dye sensitized solar cells (DSSC). JED 16:1370–1383
Ali RAM, Nayan N (2010) Fabrication and analysis of dye-sensitized solar cell using natural dye extracted from dragon fruit. Int J Integrated Engineering 2:55–62
Ammar AM, Mohamed HSH, Yousef MMK, Abdel-Hafez GM, Hassanien AS, Khalil ASG (2019) Dye-sensitized solar cells (DSSCs) based on extracted natural dyes. J Nanomater 2019:1–10. https://doi.org/10.1155/2019/1867271
Ananth S, Vivek P, Arumanayagam T, Murugakoothan P (2014) Natural dye extract of Lawsonia inermis seed as photo sensitizer for titanium dioxide based dye sensitized solar cells. Spectrochim Acta Part A Mol Biomol Spectrosc 128:420–426
Anderson AL, Chen S, Romero L, Top I, Binions R (2016) Thin films for advanced glazing applications. Buildings 6:1–34
Anggraini R, Nurosyid F, Kusumaningsih T (2020) Dye sensitized solar cell (DSSC) with immersion time variation of working electrode on the dye of C4 plant chlorophyll of corn leaves (Zea mays L.). AIP Conf. Proc 2217(030084):1–5. https://doi.org/10.1063/5.0000600
Argazzi R, Bignozzi CA (1995) Long-lived photoinduced charge separation across nanocrystalline TiO2 interfaces. J Am Chem SOC 117:11815–11816
Arifin Z, Soeparman S, Widhiyanuriyawan D, Suyitno S (2017) Performance enhancement of dye-sensitized solar cells using a natural sensitizer. International Journal of Photoenergy 2017:1–5. https://doi.org/10.1155/2017/2704864
Bandara TMWJ, Fernando HDNS, Furlani M, Albinsson I, Ratnasekera JL, DeSilva LA, Dissanayake MAKL, Mellander B-E (2017) Combined effect of alkaline cations and organic additives for iodide ion conducting gel polymer electrolytes to enhance efficiency in dye sensitized solar cells. Electrochim Acta 252:208–214
Batniji A, Abdel-Latif MS, El-Agez TM, Taya SA, Ghamri H (2016) Dyes extracted from Trigonella seeds as photosensitizers for dye-sensitized solar cells. J TheorAppl Phys 10:265–270
Birel O, Nadeem S, Duman H (2017) Porphyrin-based dye-sensitized solar cells (DSSCs): a review. Jfluoresc 27:1075–1085
Boyo A, Boyo H, Kesinro O (2015) Annealing Effect on efficiency of Aspilia africana flowers dye sensitized solar cells. Int J Sustain Green Energy 4:137–140
Calogero G, Marco GD, Cazzanti S, Caramori S, Argazzi R, Carlo AD, Bignozzi CA (2010) Efficient dye-sensitized solar cells using red turnip and purple wild Sicilian prickly pear fruits. Int J Mol Sci 11:254–267
Calogero G, Yum JH, Sinopoli A, Marco GD, Gratzel M, Nazeeruddin MK (2012) Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Solar Energy 86:1563–1575
Cari C, Khairuddin A, Septiawan TY, Suciatmoko PM, Kurniawan D, Supriyanto A (2014) The preparation of natural dye for dye-densitized solar cell (DSSC). AIP Conference Proceedings 2018:020106. https://doi.org/10.1063/1.5054510
Cha SI, Kim Y, Hwang KH, Shin YJ, Seo SH, Lee DY (2012) Dye-sensitized solar cells on glass paper: TCO-free highly bendable dye-sensitized solar cells inspired by the traditional Korean door structure. Energy Environ Sci 5:6071–6075
Chang H, Kao MJ, Chen TL, Chen CH, Cho KC, Lai XR (2013) Characterization of natural dye extracted from wormwood and purple cabbage for dye-sensitized solar cells. International Journal of Photo energy 2013:1–8. https://doi.org/10.1155/2013/159502
Chang H, Lo YJ (2010) Pomegranate leaves and mulberry fruit as natural sensitizers for dye-sensitized solar cells. Sol Energy 84:1833–1837
Daniel SCGK, Mahalakshmi N, Sandhiya J, Nehru K, Sivakumar M (2013) Rapid synthesis of Ag nanoparticles using Henna extract for the fabrication of photoabsorption enhanced dye sensitized solar cell (PE-DSSC). Advanced Materials Research 678:349–360
Diantoro M, Maftuha D, Suprayogi T, Iqbal MR, Solehudin, Mufti N, Taufiq A, Hidayat A, Suryana R, Hidayat R (2019) Performance of Pterocarpus indicus Willd leaf extract as natural dye TiO2-dye/ITO DSSC. Mater Today Proc 17:1268–1276
El-Agez TM, Taya SA, Elrefi KS, Abdel-Latif MS (2014) Dye-sensitized solar cells using some organic dyes as photosensitizers. Opt Appl 44(2):345–351
EL-Ghamri HS, El-Agez TM, Taya SA, Abdel-Latif MS, Batniji AY (2014) Dye-sensitized solar cells with natural dyes extracted from plant seeds. Mater Sci Poland 32(4):547–554
Emran MA, Amin A, Hossain MF (2018) Fabrication and performance test of dye-sensized solar cell using natural dye extracted from Basella alba seeds. In Proceeding of 10th International Conference on Electrical and Computer Engineering, Dhaka, Bangladesh, 20 December-22 December, 2018; pp. 365–368.
Eop TS, Azhari AW, Halin DSC (2019) Natural dyes extracted from leaves, fruits, and roots of Piper Betel, Adonidia Merrillii and Morinda Citrifolia as photosynthesizers for dye sensitized solar cells. IOP Conf Ser Mater Sci Eng. 572:1–5
Faiz MR, Fahmi A (2016) Performance of pandan leaf pigment for dye sensitized solar cell (Dssc): effect of dye extraction temperature. Int J Electric Electron Eng 14:1–5
Ferreira BC, Sampaio DM, Babu RS, de Barros ALF (2018) Influence of nanostructured TiO2 film thickness in dye-sensitized solar cells using naturally extracted dye from Thunbergia erecta flowers as a photosensitizer. Opt Mater 86:239–246
Ganta D, Jara J, Villanueva R (2017) Dye-sensitized solar cells using aloe vera and cladode of cactus extracts as natural sensitizers. Chem Phys Lett 679:97–101
García-Salinas MJ, Ariza MJ et al (2019) Optimizing a simple natural dye production method for dye-sensitized solar cells: examples for betalain (bougainvillea and beetroot extracts) and anthocyanin dyes. Appl Sci 9:2515
Gharavi PSM, Mohammadi MR (2015) The improvement of light scattering of dye-sensitized solar cells aided by a new dandelion-like TiO2 nanostructures. Sol Energy Mater Sol Cells 137:113–123
Gomez-Orti NM, Vazquez-Maldonado IA, Perez-Espadas AR, Mena-Rejon GJ, Azamar-Barrios JA, Oskam G (2010) Dye-sensitized solar cells with natural dyes extracted from achiote seeds. Solar Energy Mater Solar Cells 94:40–44
Godibo DJ, Anshebo ST, Anshebo TY (2015) Dye sensitized solar cells using natural pigments from five plants and quasi-solid state electrolyte. J Braz Chem Soc 26:92–101
Gomesh N, Syafinar R, Irwanto M, Irwan YM, Fareq M, Hashim U, Mariun N (2015) Characteristics of dye-sensitized solar cells using dye from pitaya fruit. Appl Mech Mater 793:450–454
Gu P, Yang D, Zhu X, Sun H, Li J (2018) Fabrication and characterization of dye-sensitized solar cells based on nature plants. Chem Phys Lett 693:16–22
Guo K, Li M, Fang X, Liu X, Sebo B, Zhu Y, Hu Z, Zhao X (2013) Preparation and enhanced properties of dye-sensitized solar cells by surface plasmon resonance of Ag nanoparticles in nanocomposite photoanode. J Power Sources 230:155–160
Gurulakshmi M, Meenakshamma A, Susmitha K, Charanadhar N, Srikanth VVSS, Babu SN, Subbaiah YPV, Venkateswarlu K, Raghavender M (2019) A transparent and Pt-free all-carbon nanocomposite counter electrode catalyst for efficient dye sensitized solar cells. Sol Energy 193:568–575
Hamadanian M, Safaei-Ghomi J, Hosseinpour M, Masoomi R, Jabbari V (2014) Uses of new natural dye photosensitizers in fabrication of high potential dye-sensitized solar cells (DSSCs). Mater Sci Semicond Process 27:733–739
Hambali NAMA, Roshidah N, Hashim MN, Isa SSM (2015) Dye-sensitized solar cell using Syzygium cumini fruit as natural dye utilizing titanium dioxide. Appl Mech Mater 754–755:1177–1181
Hemalatha KV, Karthick SN, Raj CJ, Hong N-Y, Kim S-K, Kim H-J (2012) Performance of Kerria japonica and Rosa chinensis flower dyes as sensitizers for dye-sensitized solar cells. Spectrochim Acta Part A Mol Biomol Spectrosc 96:305–309
Hemmatzadeh R, Mohammadi A (2013) Improving optical absorptivity of natural dyes for fabrication of efficient dye-sensitized solar cells. J Theor Appl Phys 7:1–7
Hosseinpanahi K, Fard MHA, Feizy J, Golzarian MR (2017) Dye-sensitized solar cell using saffron petal extract as a novel natural sensitizer. J Sol Energy Eng 139:1–5
Isah KU, Ahmadu U, Idris A, Kimpa MI, Uno UE, Ndamitso MM, Alu N (2014) Betalain pigments as natural photosensitizers for dye-sensitized solar cells: the effect of dye pH on the photoelectric parameters. Mater Renew Sustain Energy 3:1–5
Ismail M, Ludin NA, Hamid NH, Ibrahim MA, Sopian K (2018) The effect of chenodeoxycholic acid (CDCA) in mangosteen (Garcinia mangostana) pericarps sensitizer for dyesensitized solar cell (DSSC). J Phys: Conf. https://doi.org/10.1088/1742-6596/1083/1/012018
Jaafar H, Safri SN (2017) Study of Eleiodoxa conferta (Kelubi) fruit as dye-sensitized solar cell (DSSC) for energy conversion efficiency. Mater Sci Forum 888:353–356
Jalali T, Arkian P, Golshan M, Jalali M, Osfouri S (2020) Performance evaluation of natural native dyes as photosensitizer in dye-sensitized solar cells. Opt Mater 110:1–9
Kabir F, Bhuiyan MMH, Manir MS, Rahaman MS, Khan MA, Ikegami T (2019) Development of dye-sensitized solar cell based on combination of natural dyes extracted from Malabar spinach and red spinach. Results in Physics 14:1–7
Kabir F, Bhuiyan MMH, Hossain MR, Bashar H, Rahaman MS, Manir MS, Khan RA, Ikegami T (2020) Effect of combination of natural dyes and post-TiCl4 treatment in improving the photovoltaic performance of dye-sensitized solar cells. Comptes Rendus Chimie 1:1–2
Kimpa MI, Momoh M, Isah KU, Yahya HN, Ndamitso MM (2012) Photoelectric characterization of dye sensitized solar cells using natural dye from pawpaw leaf and flame tree flower as sensitizers. Mater Sci Appl 3:281–286
Kumara NTRN, Ekanayake P, Lim A, Iskandar M, Ming LC (2013a) Study of the enhancement of cell performance of dye sensitized solar cells sensitized with Nephelium lappaceum (F: Sapindaceae). J SolEnergy Eng 135:1–5
Kumara NTRN, Ekanayake P, Lim A, Liew LYC, Iskandar M, Ming LC, Senadeera GKR (2013b) Layered co-sensitization for enhancement of conversion efficiency of natural dye sensitized solar cells. J Alloy Compd 581:186–191
Kushwaha R, Srivastava P, Bahadur L (2013) Natural pigments from plants used as sensitizers for TiO2 based dye-sensitized solar cells. J Energy 2013:1–8. https://doi.org/10.1155/2013/654953
Leyrer J, Rubilar M, Morales E, Pavez B, Leal E, Hunter R (2018) Factor optimization in the manufacturing process of dyesensitized solar cells based on naturally extracted dye from a maqui and blackberry mixture (Aristotelia chilensis and Rubus glaucus). J Elec Materi 47:6136–6143
Lim A, Kumara NTRN, Tan AL, Mirza AH, Chandrakanthi RLN, Petra MI, Ming LC, Senadeera GKR, Ekanayake P (2015) Potential natural sensitizers extracted from the skin of Canarium odontophyllum fruits for dye-sensitized solar cells. Spectrochim Acta Part A Mol Biomol Spectrosc 138:596–602
Lim A, Manaf NH, Tennakoon K, Chandrakanthi RLN, Lim LBL, Bandara JMRS, Ekanayake P (2015) Higher performance of DSSC with dyes from Cladophora sp. as mixed cosensitizer through synergistic effect. J Biophys 2015:1–8. https://doi.org/10.1155/2015/510467
Liu Y, Xu H, Wang H, Zhao W, Liang C, Zhong M, Shen H (2011) Flexible TiO2 nanotube-based dye-sensitized solar cells using laser-drilled microhole array electrodes. Appl Phys A 102:127–130
Ma S, Ting H, Ma Y, Zheng L, Zhang M, Xiao L, Chen Z (2015) Smart photovoltaics based on dyesensitized solar cells using photochromic spiropyran derivatives as photosensitizers. AIP Adv 5:1–5
Madnasri S, Wulandari RDA, Hadi S, Yulianti I, Edi SS, Prastiyanto D (2019) Natural dye of Musa acuminata bracts as light absorbing sensitizer for dye-sensitized solar cell. Mater Today Proc 13:246–251
Martínez ARH, Vargas S, Estevez M, Rodríguez R (2011) Dye-sensitized solar cells from extracted bracts bougainvillea betalain pigments. Int J Mol Sci 12:5565–5576
Martinez ARH, Estevez M, Vargas S, Quintanilla F, Rodriguez R (2011) New dye-sensitized solar cells obtained from extracted bracts of Bougainvillea glabra and spectabilis betalain pigments by different purification processes. Int J Mol Sci 12:5565–5576
Martínez ARH, Estévez M, Vargas S, Rodríguez R (2013) Stabilized conversion efficiency and dye-sensitized solar cells from Beta vulgaris pigment. Int J Mol Sci 14:4081–4093
Mashari RM, Prihanto D (2019) Implementation of Gyrinops versteegii Gaharu leaves as a dye sensitized solar cell. FESPE 1:10–13
Muduli S, Game O, Dhas V, Vijayamohanan K, Bogle KA, Valanoor N, Ogale SB (2012) TiO2–Au plasmonic nanocomposite for enhanced dye-sensitized solar cell (DSSC) performance. Sol Energy 86:1428–1434
Munawaroh H, Fadillah G, Saputri LNMZ, Hanif QA, Hidayat R, Wahyuningsih S (2016) The co-pigmentation of anthocyanin isolated from mangosteen pericarp (Garcinia Mangostana L.) as natural dye for dye-sensitized solar cells (DSSC). IOP Conf Ser Mater Sci Eng 107:1–6
Muryani BY, Sarifah N, Kusumawardani DR, Nurosyid F (2019) Effect concentration of dye solution binahong leaves to the efficiency of dye-sensitized solar cell (DSSC). AIP Conf Proceed 2202:020122. https://doi.org/10.1063/1.5141735
Najm AS, Ludin NA, Abdullah MF, Almessiere MA, Ahmed NM, Al-Alwani MAM (2020) Areca catechu extracted natural new sensitizer for dye-sensitized solar cell: performance evaluation. J Mater Sci: Mater Electron 31:3564–3575
Özel F, Sarılmaz A, İstanbullu B, Aljabour A, Kuş M, Sönmezoğlu S (2016) Penternary chalcogenides nanocrystals as catalytic materials for efficient counter electrodes in dye-synthesized solar cells. Sci Rep 6:29207
Pamain A, Pogrebnaya T, Kingondu CK (2014) Natural dyes for solar cell application: UV-visible spectra and outdoor photovoltaic performance. Res J Eng Appl Sci 3:332–336
Park KH, Kim TY, Park JY, Jin EM, Yim SH, Choi DY, Lee JW (2013) Adsorption characteristics of gardenia yellow as natural photo sensitizer for dye-sensitized solar cells. Dyes Pigm 96:595–601
Pawar KS, Baviskar PK, Inamuddin, Nadaf AB, Gawali SS, Pathan HM (2019) Layer-by-layer deposition of TiO2–ZrO2 electrode sensitized with Pandan leaves: natural dye-sensitized solar cell. Materials for Renewable and Sustainable Energy 8:1–9
Peymannia M, Gharanjig K, Arabi AM (2019) New engineered and environmentally friendly dye-sensitized solar cells: efficient extraction of dyes from Cytisus, Alcearosea, and Roselle. Int J Energy Res. 44:1–16
Pote FI, Supriyanto A, Nurosyid F, Diyanahesa NE, Ramelan AH (2019) Performance of DSSC transparent based on hybrid DnF015 with spinach chlorophyll dye. AIP Conference Proceedings 2202:020012. https://doi.org/10.1063/1.5141625
Pote FI, Supriyanto A, Nurosyid F, Kurniawan D (2019) Performance of natural chlorophyll Amaranthus and Carica Papaya dye on TiO2 coating in the making of dye sensitized solar cell (DSSC) through the spin coating method. J Phys Theor Appl 3:69–76
Prabavathy N, Shalini S, Balasundaraprabhu R, Velauthapillai D, Prasanna S, Muthukumarasamy N (2017) Enhancement in the photostability of natural dyes for dye-sensitized solar cell (DSSC) applications: a review. Int J Energy Res. 41:1–25
Pu MY, Chen JZ (2012) Improved performance of dye-sensitized solar cells with laser-textured nanoporous TiO2 photoanodes. Mater Lett 66:162–164
Ramanarayanan R, Nijisha P, Niveditha CV, Sindhu S (2017) Natural dyes from red amaranth leaves as light-harvesting pigments for dye-sensitized solar cells. Materials Research Bulletin 90:156–161
Radwan IM, Taya SA, El-Agez TM, Abdel-Latif MS, Ghamri H (2016) Improvement of the performance of purple carrot sensitized solar cells by acidic treatment of FTO glass substrate and TiO2 film. Acta Phys Pol, A 130(3):795–799
Riapanitra A, Asakura Y, Yin S (2019) Improved thermochromic and photocatalytic activities of F-VO2/Nb–TiO2 multifunctional coating films. Tungsten 1:306–317
Riapanitra A, Asakura Y, Yin S (2020) One-step hydrothermal synthesis and thermochromic properties of chlorine-dope d VO2(M) for smart window application. Funct Mater Lett 13:1–8
Ridwan MA, Noor E, Rusli MS, Akhiruddin A (2018) Fabrication of dye-sensitized solar cell using chlorophylls pigment from sargassum. IOP Conf. Ser.: Earth Environ. Sci. 144:1–7
Rizali D, Suryanto H, Sukarni S (2019) The effect of chlorophyll concentration from papaya leaves on the performance of dye-sensitized solar cell. Journal of Mechanical Engineering Science and Technology 3:59–69
Rosana NTM, Amarnath DJ, Kumar PS, Joseph KLV (2019) Potential of plant-based photosensitizers in dye-sensitized solar cell applications. Environ Prog Sustainable Energy 39:1–5
Saedi A, Moradi AM, Kimiagar S, Panahi HA (2020) Efficiency enhancement of dye-sensitized solar cells based on Gracilaria/Ulva using graphene quantum dot. Int J Environ Res 14:393–402
Sanjay P, Deepa K, Madhavan J, Senthil S (2018a) Optical, spectral and photovoltaic characterization of natural dyes extracted from leaves of Peltophorum pterocarpum and Acalypha amentacea used as sensitizers for ZnO based dye sensitized solar cells. Opt Mater 83:192–199
Sanjay P, Deepa K, Madhavan J, Senthil S (2018b) Performance of TiO2 based dye-sensitized solar cells fabricated with dye extracted from leaves of Peltophorum pterocarpum and Acalypha amentacea as sensitizer. Mater Lett 219:158–162
Sathyajothi S, Jayavel R, Dhanemozhi AC (2017). The fabrication of natural dye sensitized solar cell (Dssc) based on TiO2 Using henna and beetroot dye extracts materials today. Proceedings, 4:668–676
Shalini S, Prabavathy N, Balasundaraprabhu R, Kumar TS, Velauthapilla D, Balraju P, Prasanna S (2018) Studies on DSSC encompassing flower shaped assembly of Na-doped TiO2 nanorods sensitized with extract from petals of Kigelia Africana. Optik - Int J for Light and Electron Optics 155:334–343
Sharma JK, Akhtar MS, Ameen S, Srivastava P, Singh G (2015) Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J Alloy Compd 632:321–325
Sharma K, Sharma V, Sharma SS (2018) Dye-sensitized solar cells: fundamentals and current status. Nanoscale Res Lett 13:381
Shanmugam V, Manoharan S, Anandan S, Murugan R (2014) Green grasses as light harvesters in dye sensitized solar cells. Spectrochim Acta Part A Molecul Biomolecul Spectroscopy 135:1386–1452
Suhaimi S, Shahimin MM (2014) Effect of varied extracting solvent on stability and reliability of DSSCs using natural dyes as photosensitizer. IEEE Student Conference on Research and Development 2014:1–6
Sun X, Li Y, Mao H, Dou J, Wei M (2017) Toward s a high open-circuit voltage by co-additives in electrolyte for high-efficiency dye-sensitized solar cells. J Power Sources 359:142–146
Syafinar R, Gomesh N, Irwanto M, Fareq M, Irwan YM (2015) Chlorophyll pigments as nature based dye for dye-sensitized solar cell (DSSC). Energy Procedia 79:896–902
Taya SA, El-Agez TM, Elrefi KS, Abdel-Latif MS (2015) Dye-sensitized solar cells based on dyes extracted from dried plant leaves. Turk J Phys 39:24–30
Taya SA, El-Agez TM, Abdel-Lati MS, El-Ghamri HS, Batniji AY, El-Sheikh IR (2014) Fabrication of dye-sensitized solar cells using dried plant leaves. Int J Renew Energy Res 4:384–388
Taya SA, El-Agez TM, Abdel-Latif MS, Ghamri H, Batniji A, Tabaza WA (2016) Dyes extracted from safflower, Medicago sativa, and Ros marinus oficinalis as photosensitizers for dye-sensitized solar cells. Journal of Nano- and Electronic Physics 8:01026
Toor RA, Sayyad MH, Nasr N, Sajjad S, Shah SAA, Manzoor T (2016) Efficiency enhancement of dye sensitized solar cells with a low cost co-adsorbant in N719 dye. Int J Sustain Energy Environ Res 5:46–50
Torchani A, Saadaoui S, Gharbi R, Fathallah M (2015) Sensitized solar cells based on natural dyes. Curr Appl Phys 15:307–312
Ung MC, Mansa RF, Sipaut CS, Dayou J (2015) Fabrication and analysis of photoelectrochemical properties of dye sensitized solar cell using local Borneon natural dye extracts. Advanced Materials Research 1107:536–541
Wang M, Anghel AM, Marsan B, Ha NC, Pootrakulchote N, Zakeeruddin SM, Gratzel M (2009) CoS supersedes Pt as efficient electrocatalyst for triiodide reduction in dye-sensitized solar cells. J Am Chem SOC 131:15976–15977
Yang Y, Cui J, Yi P, Zheng X, Guo X, Wang W (2014) Effects of nanoparticle additives on the properties of agarose polymer electrolytes. J Power Sources 248:988–993
Yun MJ, Cha SI, Seo SH, Lee DY (2014) Highly flexible dye-sensitized solar cells produced by sewing textile electrodes on cloth. Sci Rep 4:5322
Yusoff A, Kumara NTRN, Lim A, Ekanayake P, Tennakoon KU (2014) Impacts of temperature on the stability of tropical plant pigments as sensitizers for dye sensitized solar cells. J Biophys 2014:1–8. https://doi.org/10.1155/2014/739514
Zhou H, Wu L, Gao Y, Ma T (2011) Dye-sensitized solar cells using 20 natural dyes as sensitizers. J Photochem Photobiol, A 219:188–194
Zolkepli Z, Lim A, Ekanayake P, Tennakoon K (2015) Efficiency enhancement of cocktail dye of Ixora coccinea and Tradescantia spathacea in DSSC. J Biophys. https://doi.org/10.1155/2015/582091
Author information
Authors and Affiliations
Contributions
All authors have read and agreed to the published version of the manuscript. Conceptualization: NMK, NA; Methodology: RB, NMK; Formal analysis and investigation: RB, PDN, NMK; Writing—original draft preparation: RB, PDN; Writing—review and editing: NMK, MSPS, NA; Resources: NMK; NA Supervision: NA.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baby, R., Nixon, P., Kumar, N. et al. A comprehensive review of dye-sensitized solar cell optimal fabrication conditions, natural dye selection, and application-based future perspectives. Environ Sci Pollut Res 29, 371–404 (2022). https://doi.org/10.1007/s11356-021-16976-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16976-8