Abstract
This review highlights and summarizes the impact of different fabrication processes on the efficiency of dye-sensitized solar cells (DSSCs). Energy conversion efficiency of cell depends upon semiconductor, sensitizer, electrolyte, and counter electrode. Efficiency of DSSCs can be enhanced by properly selecting the optimum significance of various parameters of fabrications process. Major challenges of these solar cells are non-vegetal, noxious, extreme sensitizers. Application of natural dyes in this field plays a significant role. An optimized CdSe-TiO2 photoanode showed a power conversion efficiency (PCE) of 13.29% and short circuit current density of 15.30 mA cm−2 for the DSSC. Power conversion efficiency of 3.26% was achieved by using TTO electrode for DSSC device that is ascribed to the improved electrical and optical properties due to doping with Ta element. Absorbance of betalain was shown in the visible range of 530–535 nm for betanin while 450–559 nm for anthocyanin pigment. The natural dyes are economical, readily available, and environmentally friendly. This compilation would be beneficial for researchers working on dye solar cell.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
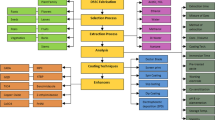
All over the world, non-renewable energy based power plants such as thermal power plants, nuclear power plants, diesel and gas turbine power plants, etc. are used to generate electricity. These fossil fuels pollute the environment due to carbon emissions (Polo et al. 2004). On the other hand, the application of renewable energy sources results in clean surroundings (Awasthi et al. 2020) and a healthy environment across the universe (Sharma et al. 2015). There are various renewable energy sources such as wind energy, solar energy, and hydro energy (Fukurozaki et al. 2013). Solar energy is the more convenient renewable energy source due to simple principles and easy construction of conversion devices. Photovoltaic is a device that converts solar energy into electricity (Kumar et al. 2015). There are three generations of solar cells: (i) crystalline solar cell, (ii) thin-film solar cell, and (iii) dye-sensitized solar cell (Ludin et al. 2014). A dye-sensitized solar cell (DSSC) is particularly interested in research and industries due to the novel and emerging solar cell generation. These solar cells have different parts, photosensitizer, photoelectrode, liquid electrolyte, and counter electrode, as shown in Fig. 1.
Photosensitizer converts incident solar radiation into electric voltage. Photoelectrode is made up of a nanocrystalline semiconductor that absorbs solar radiation. During illumination, electrons move from sensitizer to the working electrode (semiconductor). Liquid electrolyte consists of a redox couple that regenerates the dye. Some researchers have been examined the factors that influence DSSC performance, resulting in good alternatives. The porphyrin-dyed ZnPorph-L-H2Porph(ZnP-H2P) and metal-free organic dye solar cell were used to make co-sensitized DSSC (Sharma et al. 2014). Co-sensitized (mixed) dye solar cells C (Zn{Porph}-L-H2{Porph}/DC) and D (Zn{Porph}-L-H2{Porph}) were obtained by immersing the working electrode in separate solutions of sensitizers in varying sequences. Power conversion efficiency of C and D was observed as 6.16% and 4.80%, respectively. Three different types of dye solar cells were prepared by treating titanium dioxide electrodes in three different ways, untreated, post-treated, and pre- and post-treated, through titanium tetrachloride (TiCl4) solution. Pre- and post-treated working electrodes showed the best results and gave 5.1% conversion efficiency. This novel working electrode raises the thickness of TiO2, thus increasing the current density of the solar cell. Therefore, the thickness of TiO2 raises cell performance (Sedghi and Miankushki 2013). Nitrogen-doped TiO2 (NSND-TiO2) nanoparticle was prepared using modified flame spray pyrolysis (FSP) equipment. NSND-TiO2 was formed by spraying ammonia water above the flame in which TiO2 particles were made. Addition of nitrogen elements to TiO2 showed a decreased energy band gap to 2.90eV and recorded 6.03% power conversion efficiency (Huo et al. 2014). Naturally occurring dyes available in various kinds of roots, leaves, fruits, and flowers show a viable environmentally friendly alternative and can be employed in DSSCs. Various natural dyes named Anethum graveolens, parsley, arugula, Spinacia oleracea, and green algae to fabricate DSSCs were treated using two different methods, i.e., before and after drying raw materials. It has been found that some DSSCs using after drying method showed better performance and others revealed better results using before drying method. The Spinacia oleracea extract gave the highest efficiency after the drying method was recorded as 0.29% (Taya et al. 2013). Benzonitrile-based electrolytes have low vapor pressure, thus causing more extended stability to the solar cells using N179 dye (Latini et al. 2014). Five aldimine derivatives were prepared by condensing the suitable amine with salicylaldehyde (m1–m4) and 4-aminobenzoic acid with 2-thiophene carboxaldehyde (m5). Highest efficiency of 0.575% was obtained with 2-(2-hydroxybenzylideneamino) benzoic acid-based dye solar cell (Batniji et al. 2014).
The objectives of this review are (i) to describe different fabrication methodologies of dye-sensitized solar cell while analyzing the impact of various technical procedures on the photovoltaic performance of DSSC; (ii) to discuss various ideal features of performance parameters for DSSCs; (iii) to discuss the detailed description of various natural sensitizers for the healthy environment; and (iv) to discuss performance evaluation between natural and synthetic DSSC. This study will be fruitful for the researcher to understand the construction and functioning of natural DSSC. Also, it provides knowledge in the field of natural dyes in the environment.
Performance parameters of DSSCs
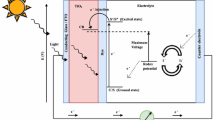
Open-circuit voltage, short circuit current, fill factor, maximum voltage, and maximum current are the various distinct performance parameters of dye solar cell. All the parameters of DSSC discussed are as follows (Fig. 2):
Performance parameters of DSSC (Sharma et al. 2020)
Open-circuit voltage
Open-circuit voltage is the difference in potential between the two terminals in the cell under light illumination when the circuit is open. It is influenced by the fermi level (semiconductor) and the level of dark current. It is calculated when the current through the DSSC is zero (open circuit). Open-circuit voltage is dependent on the cell temperature. At the open terminals of DSSC, the voltage is mentioned as open-circuit voltage. The following is the expression for open-circuit voltage (Takagi et al. 2013):
Short circuit current
Short circuit current density (Isc) is the photocurrent per unit area (mA/cm2) when an illuminated DSSC is short-circuited. It relies on various factors such as light absorption, light intensity, regeneration of the oxidized dye, and injection efficiency. It is strongly associated with incident photon conversion efficiency and theoretical values of the current density can be determined from the IPCE spectrum. At the short circuit terminals of DSSC, the current is mentioned as short circuit current. When the cell temperature rises, short circuit current also rises (Takagi et al. 2013). The expression for short circuit current is as follows (Lee et al. 2005):
Fill factor
Fill factor measures the ideality of the DSSC and is defined as the ratio of the maximum power output per unit area to the product of open-circuit voltage and current density. Several factors can affect the fill factor, such as a high internal resistance value that will offer a low fill factor and a reduced overall efficiency. It is expressed as follows (Zhou et al. 2007):
Efficiency
Overall solar energy to the electrical conversion efficiency of a dye solar cell is defined as the maximum value of the cell output divided by the incident light power. It is estimated by measuring photocurrent density at short circuit (Jsc), the open-circuit photovoltage (Voc) at open-circuit terminals, and intensity of the incident light and fill factor of the cell (FF), as shown in Eq. 4. Since it is reliant on all the three factors under standard conditions (Mbonyiryivuze et al. 2015), it is of great significance to optimize each one of them for high overall efficiency (Nan et al. 2017):
Fabrication elements
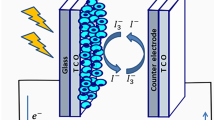
Structure of DSSC is shown in Fig. 3, displaying all components of the cell. Structure of DSSC is varied from the first and second generations of the solar cell. DSSC consists of specially four parts: (i) working electrode (WE); (ii) sensitizer; (iii) electrolyte; (iv) counter electrode (Gratzel 2003). Optimization of all the components is of immense meaning to enhance the overall efficiency of the cell. Working substrate is made up of semiconductor material deposited on the transparent conductive oxide. In general, metal oxides with wide band gaps are used as materials for working electrodes. Titanium dioxide, zinc oxide, and tin oxide have been extensively used in dye-sensitized solar cells (Hagfeldt et al. 2010). Semiconductor metal oxides nanoparticles form nanoporous and semi-transparent working electrodes. To sensitize working electrode, sensitizer is used that enhances semiconductor conductivity. On the surface of the working electrode, a layer of dye molecules is liable for electron harvest. Ruthenium-based dyes such as N719 (known as black dye) (Nazeeruddin et al. 1999) and N3 have been extensively employed as synthetic dyes for DSSCs (Nazeeruddin et al. 1993). N719 or N3 based DSSCs have been shown the highest efficiency of the cell. Electrolyte is filled between the working and counter electrode to complete the flow of electrons. Iodide/triiodide (I−/I3−) redox couple has been widely preferred because of its appropriate redox potential (Daeneke et al. 2011). Other redox couples such as Br−/Br3− (Wang et al. 2005), SCN−/(SCN)3−, and SeCN−/(SeCN)3− (Oskam et al. 2001) have been striking due to their redox potentials closer to the ground state of the dyes, which provide a higher output voltage. Counter electrode is used to catalyze the regeneration of I−. Platinum is mostly used as a counter electrode due to its better performance. Efficient regeneration of iodide enhances the diffusion rate of iodide and thus increases the reduction rate of the oxidized dye.
Constructional parts of a dye-sensitized solar cell (Rawal et al. 2015)
Working substrate
Working electrode (anode) consists of a mesoporous extensive bandgap semiconductor layer. Titanium dioxide is the most common material used as a semiconductor for DSSC. Nanoparticle layer of titanium dioxide is sintered on the working electrode to enhance electronic conduction. Mesoporous layer is deposited on a transparent conducting oxide (TCO). The fluorine-doped tin oxide (FTO) and indium-doped tin oxide are mainly transparent conducting oxide. Plastic foils (Richhariya and Kumar 2016) and metal sheets (Heo et al. 2009) are also used as materials for the working substrate of DSSC (Jun et al. 2007). Merits and failures of different substrates are shown in Table 1.
DSSC efficiency can be increased by using suitable functionalized semiconducting metal oxides having quantum dots, organic conjugated polymers, etc. Photoanodes are fabricated using semiconductor oxides having wide bandgap. Such metal oxides can absorb almost all the incident sunlight and have stability against photo-corrosion (Kumar et al. 2020). An optimized CdSe-TiO2 photoanode showed a power conversion efficiency (PCE) of 13.29% and short circuit current density of 15.30 mA cm−2 for the DSSC, as shown in Fig. 4 (Bhattacharya and Datta 2020).
J-V curve for CdSe-TiO2-based DSSC (Bhattacharya and Datta 2020)
A facile spray pyrolysis method was used to deposit a thin film of Ta-doped SnO2 (TTO). Resistance stability of sheet resistance was relatively better up to 400°C representing the appropriateness of TTO electrode to provide as conducting photoanode electrode in DSSC. XRD study showed the crystallinity, phase purity, and texture properties of the thin film (Fig. 5). Power conversion efficiency of 3.26% was achieved using the TTO electrode for DSSC device ascribed to the improved electrical and optical properties due to doping with Ta element (Ramarajan et al. 2020).
XRD pattern of the TTO thin film (Ramarajan et al. 2020)
Materials of working substrate
Titanium dioxide is the best semiconductor for DSSC due to its suitable properties that match the solar cell requirement. Conversion efficiency of 2.61% was achieved by employing two-step hydrothermal method for synthesizing TiO2 materials. Paste was prepared by grinding 6.0g of TiO2 with ethanol and water, and then mixed with terpineol and ethyl cellulose after sonication (40). Photoanode with 23μm thickness was prepared using shear-exfoliated graphene, causing less electron recombination and giving cell efficiency of 8.9%. Nanofiber-based photoanode causes a higher electron diffusion coefficient (Lo and Leung 2019). Two grams of titania powder was placed in a beaker and 6mL citric acid of 0.1M, 0.2mL polyethylene glycol, 0.2mL titanium (IV) isopropoxide, and 0.1mL non-ionic surfactant Triton X-100 were added to prepare the solution. Table 2 reveals that dyes extracted using acetone acquired every parameter of DSSC superior to the other four solvents. Therefore, that experiment verified the need for solvent effect and showed that the solvent having less polarity, being as acetone, was more able to extract an effective fraction of red amaranth dye than the other four solvents (Uddin et al. 2015).
TiO2 working substrate was synthesized using a hydrothermal gel method employing polyvinylpyrrolidone (PVP) by adding 100mL of deionized water in 10mL of titanium tetrabutanolate under vigorous agitation at room temperature. Colloid was mixed with 0.8g of polyethylene glycol, 1ml of OP emulsifier, and stoichiometric PVP (0.0270, 0.0405, 0.0540, 0.0810, 0.108, and 0.135) and subsequently concentrated at 80°C. The resultant titanium dioxide nanocrystallites were calculated as TiO2-1 wt%, TiO2-1.5 wt%, TiO2-2 wt%, TiO2-3 wt%, TiO2-4 wt%, and TiO2-5 wt%, respectively. The TiO2-0 wt% was considered zero PVP. Compared with dense titanium dioxide working substrate, the power conversion efficiency was reported as 9.86%, as shown in Table 3 (Hu et al. 2014).
TiO2 colloidal dispersions synthesize hydrothermally at 200°C by using titanium isopropoxide gels and acetic acid under non-ionic surfactant. Particle size varying from 15 to 20nm showed the cell’s average efficiency as 5.2% (Muniz et al. 2011). TiO2-based working electrode was fabricated using silver coating photo-deposition with distinct light durations. In a solution of 5×10−4 mol/L AgNO3, TiO2 working electrode was dipped in distinct durations of 5, 10, 15, and 30 min, and ultraviolet radiations irradiated the solutions. This novel fabrication enhanced the conversion efficiency of the DSSC 6.86% in deposition time of 10 min with fill factor of 69.4% (Table 4). IPCE spectra of dye solar cell using TiO2 and TiO2-Ag-coated electrode are shown in Fig. 6. There is higher IPCE in the case of the silver-coated electrode (Peng et al. 2013). Anatase TiO2 was synthesized using a hydrothermal process in two steps using amine ligands and achieved a conversion efficiency of 2.61% for the smallest nanoparticles (Phonkhokkong et al. 2016).
IPCE spectra of dye solar cell using TiO2 and TiO2-Ag-coated electrode (Peng et al. 2013)
Sensitizer
A layer of the sensitizer is adsorbed onto the surface of the semiconductor by chemical bonding. The primary role of the sensitizer is to absorb the incident light, infuse the excited electron into the semiconductor, and turn it into regenerated by the redox couple in the electrolyte. Dye (sensitizer) is the heart of dye-sensitized solar cells, making the DSSC different from other solar cells. Metal complex sensitizers, metal-free organic sensitizers, and natural sensitizers are the most common varieties of sensitizers in DSSC. Natural sensitizers are environmentally friendly and easily extractable using a simple extraction process (Calogero et al. 2012). Two new organic dyes were DRA-BDC and DTB-BDC, with electron acceptors as rhodanine-3-acetic acid/thiobarbituric acid while electron donor as N,N-butyldicarbazole, which have been used as dye for DSSC. DRA-BDC and DTB-BDC revealed higher absorption peaks at 440 and 370 nm, respectively, in UV-visible spectra of dyes (Fig. 7). Novel sensitizer has a larger energy gap between its LUMO and conduction band of TiO2, leading to increased current density (2.46mA/cm2), thus contributing to the enhancement of the PCE (1.16%) (Moustafa et al. 2021).
Absorption spectra of organic dyes (Moustafa et al. 2021)
Two novel compounds derived from the phenyltetrazole system, 5-(4-decyloxyphenyl)tetrazole (LTz-4) and N,N-diethyl-4-{((2′-nitro-4′-tetrazoyl)phenyl)diazenyl}aniline (SD - 6), were used as co-adsorbents for DSSC. Highest open-circuit voltage (0.71V) and PCE (9.20%) were observed in the case of dye HD-14 (ruthenium complex) with co-adsorbent DCA (Fig. 8). High value of photocurrent density can be due to the co-adsorbent, favoring the capture of photons and encouraging the introduction of electrons into semiconductors (Silva et al. 2020).
J-V characteristics for HD-14 (ruthenium complex) with co-adsorbent DCA (Silva et al. 2020)
Natural dye was extracted from the daisy flowers family (Leucanthemum vulgare), namely yellow daisy, purple daisy, and wine daisy, containing luteolin flavonoid used to fabricate DSSCs. The yellow daisy-, wine daisy-, and purple daisy-based DSSC revealed PCE of 0.6%, 0.4%, and 0.8%, respectively (Fig. 9) (Ferreira et al. 2020).
J-V curves for dyes: a purple; b yellow; c wine (Ferreira et al. 2020)
Materials of sensitizer
There are various methods used for the fabrication of dye for DSSC using different natural dyes. Extracts from Dianthus barbatus, Lepidium sativum, and Raphanus raphanistrum were employed as dyes in titanium dioxide nano-powder-based dye solar cell. Highest efficiency and fill factor of 0.15% and 0.48 were observed from Dianthus barbatus (Researcher et al. 2016). One gram of rosella and blue pea flower was taken in 100mL of two different solvents at various temperatures (25, 50, 50, 70, and 100°C). The observed conversion efficiency of DSSCs using extracts of rosella, blue pea, and mixed dye extracted at 100°C was 0.37%, 0.05%, and 0.15%, respectively (Table 5) (Hamadanian et al. 2014).
Anthocyanin was extracted from maqui berry by drying 0.5g of maqui berries at 45°C and dipped in 5mL of distilled water. At 750 and 1500mg of anthocyanin/L, the extracted concentration of maqui was examined and power conversion efficiency was obtained as 0.14 % and 0.19%, respectively (Table 6) (Ozuomba et al. 2013).
Natural dyes from Reseda luteola, Berberis integerrima, Panica granatum, Pleniflora, Consolida orientalis, Reseda gredensis, Clematis orientalis, Adonis flammea, Salvia sclarea, and Consolida ajacis plants were extracted using Soxhlet extractor. Identifying suitable natural dyes for improving VOC without resulting dye degradation may result in additional improvement of cell performance. The maximum energy conversion of DSSC using Reseda luteola extract is 0.22%. (Table 7) (Phinjaturus et al. 2016).
Two dye solar cells were fabricated using anthocyanin extracted from Hibiscus sabdariffa and plain (un-dyed) cells, which reported an energy conversion efficiency of 0.58% for dyed cells while 0.03% for un-dyed solar cell. Husk, cob, and silk of purple corn were extracted in acetone, ethanol, and DI waters. Maximum conversion efficiency of 1.06% was obtained from husk extracted from acetone, as given in Table 8 (Ananth et al. 2015).
TiO2-based DSSC was sensitized using natural dye formed by Pterocarpus marsupium stem bark. One hundred milliliters of distilled water was added in 250g of Pterocarpus marsupium stem bark (cut pieces) for a duration of 14 h, and filtered out. In this concentrated solution, distilled water of 250mL and isopropanol of 15mL were added. Initial pH value was recorded as 8.75. Nitric acid was added drop wise to raise the pH value up to 2, and the solution was vigorously stirred. After that, the titanium isopropoxide solution of 5mL was mixed drop wise until white precipitation was formed. Solution was then heated up to 80°C for 2–3 h. At room temperature, the solution was going to foraging for 1 h. The resultant white precipitate was left to dry at 150°C for 15–20 h to form fine particles of pure titanium dioxide. Table 9 displays the conversion efficiency of DSSC using pre-dyed titanium nanoparticles as 0.49% (Jia et al. 2018).
Novel X type D-(π-A)2 organic dyes JX1 and JX2 were developed and co-sensitized with porphyrin dyes JP1 and JP3 for dye solar cells. JX1-based DSSC showed photoconversion efficiency (PCE) of 3.67%, while JX2-based DSSC showed PCE of 4.63%. Co-sensitized dye solar cell (JP3+JX2) showed the highest efficiency of 8.08% (Zeng et al. 2010).
Electrolyte
Electrolyte regenerates the sensitizer of dye solar cells. Redox couple present in the electrolyte is an essential feature of the electrolyte for better cell performance. The function of electrolytes is to transfer the electron between two electrodes. Active transport of electrons within the dye solar cell is essential, and many different redox systems have been explored. Iodide triiodide is the most common liquid electrolyte. However, severe problems such as electrolyte volatility can lead to long-term stability concerns because of complications in sealing the device. Transform of the volatile electrolyte into a nonvolatile ionic liquid (Choi et al. 2008) has been recognized to be victorious (Gorlov and Kloo 2008), and high efficiencies and excellent stabilities have been reported (Tian et al. 2000). Numerous other iodine-free redox mediators have been experienced. Both complexes (Wang et al. 2010) and organic redox couples have been verified to be potential alternatives (Ayalew and Ayele 2016). Electrolyte configuration can be further changed to enhance the performance of the cell. DSSC has been fabricated using gel polymer electrolytes (GPEs) with polyacrylonitrile (PAN)-based polymer with variant number of tetrabutylammonium iodide (TBAI) salt and iodine. The S3 electrolyte-based DSSC showed the highest PCE of 3.45%, with Voc of 582 mV and Jsc of 12.9 mA cm−2 (Fig. 10). High PCE of DSSC (S3 electrolyte) over a visible wavelength range shows superior photon harvesting efficiency (Chowdhury et al. 2020).
J-V characteristics of DSSCs using different electrolytes (Chowdhury et al. 2020)
Gel polymer electrolyte (GPE) has been prepared using different lithium iodide concentrations (LiI). Solution was contained PEO and PVA in same quantity, ethylene carbonate (EC), tetrabutylammonium iodide (TBAI), dimethyl sulfoxide (DMSO), and iodine crystals (I2). DSSC fabricated from GPE with 1.34 wt% LiI gave the highest efficiency of 6.26%, as shown in Fig. 11 (Teo et al. 2018).
J-V characteristics of DSSCs using PVA-PEO-based GPEs consisting of different ratios of TBAI and LiI salts (Teo et al. 2018)
Materials of electrolyte
Usually, iodide triiodide redox couple is generally used in the fabrication of dye solar cells. Evaporation and long-term stability are the main issues in front of liquid electrolytes. Gel polymer electrolytes were synthesized using distinguished weights (0%, 2%, 4%, 5%, 6%, and 8%) of acetamide in poly(ethylene oxide) (PEO) with LiI/I2. Gel electrolyte was formed by mixing 0.53g of polyethylene oxide with acetonitrile and propylene carbonate (20:1) stirred for 2h continuously. After that, 0.2g of LiI, 0.04g of I2, and different wt% of acetamide (0%, 2%, 4%, 5%, 6%, and 8%) were added and dropping was continued for 2 h; homogeneous sol was evaporated at 80°C to get gel polymer electrolyte. The highest cell efficiency 9.01% was observed with 5%wt of acetamide, as discussed in Table 10 (Pavithra et al. 2015).
Single-step methodology was employed for preparing counter electrodes using copper sulfide nanoparticle that was treated with two distinct electrolytes, Co(II)/(III) bipyridine and Fe(II)/(III) ferrocene-based liquid electrolyte. Ferrocene electrolyte was prepared by mixing 0.1M of ferrocene and 0.05M of ferrocenium tetrafluoroborate in propylene carbonate. The sol was kept under the nitrogen atmosphere, thus avoiding degradation of ferrocenium ion in contact with air. A high limiting current (11.8mAcm−2) was observed by the ferrocene/ferrocenium redox couple (Congiu et al. 2016). Ionic liquid electrolyte enhances the thermal durability of DSSC with an energy conversion efficiency of 2.06% and a fill factor of 0.49 (Ito and Takahashi 2012). An electrolyte was developed by adding 0.172g of arrowroot powder in 20 mL of double-distilled water (D2) and stirred for 30 min at 70°C. On the other hand, potassium iodide was taken in D2. Finally, two solutions were mixed drop-by-drop and stirred continuously to produce biopolymer electrolyte, and 0.63% efficiency observed in this was observed in Table 11 (Singh et al. 2014).
Different electrolytes, liquid, solid-state, and quasi-solid-state electrolytes used in DSSC and concluded quasi-solid-state electrolytes, were more suitable for providing the best performance of the cell. Table 12 shows the performance parameters of different electrolytes (Wu et al. 2008). Photoconversion efficiency of 2.06% was achieved using heteroleptic copper(I) dyes and homoleptic copper(I)/(II) redox shuttles. It shows the potential for all copper-based dye solar cells (Karpacheva et al. 2018).
Counter electrode
The function of the counter electrode in dye solar cells is to regenerate the electrolyte. Counter electrode consists of a conducting layer on a glass (TCO) or a plastic substrate. Catalytic material is used for resulting fast chemical reactions in the DSSC, thus reducing transition time. A layer of platinum is commonly coated on the substrate for the effective regeneration of the redox couple. High catalytic activity towards the iodide/triiodide redox reaction (Pettersson et al. 2007) is the most significant advantage of using platinum as a counter electrode. In electrolytes, platinum is also chemically stable (Kay and Gratzel 1996). Various catalytic materials have been tested in dye solar cells, such as carbon (Saito et al. 2002), platinum (Bay et al. 2006), and graphene (Kay and Gratzel 1996). Increasing an extremely efficient double-featured electrode material is imperative to the identity charged integrated electronics that unite the energy conversion unit of the dye-sensitized solar cell (DSC) and the energy storage unit of the supercapacitor. Nitrogen-doped carbon sphere (NCS) was incorporated to encourage electrode activity. A power energy conversion efficiency of 8.64% was recorded, much higher than the conventional solid carbon sphere (SCS), as shown in Fig. 12 (Wang et al. 2020).
J-V characteristics of nitrogen-doped carbon sphere-based DSSC (Wang et al. 2020)
A structured configuration (n-MWCNT-TiO2/N3/MWCNT) was used for DSSC. In this configuration, expensive Pt was replaced by economical MWCNT that ensures less costly DSSC. Absorbance spectrum of dye is shown in Fig. 13. Presence of MWCNT in n-MWCNT-TiO2 nanocomposite encourages the introduction of electron initiated because of dye to conduction band MWCNT-TiO2 nanocomposite semiconductor (Younas et al. 2019a).
UV-Vis spectra of N3 dye in n-MWCNT-TiO2/N3, TiO2/N3, and EtOH films (Younas et al. 2019b)
Pristine perfect and defective graphene nanosheets (GNSs) were formed and applied as substrates for catalytic activity against the reduction reaction of T2 to T–. It was observed that pristine GNSs could accomplish high reactivity to T2/T– through an exothermic adsorption reaction (Tontapha et al. 2019). Admirable electrocatalytic properties and economics are major necessities for the appropriate counter electrodes of dye-sensitized solar cells. DSSCs with the optimal PEDOT-Ni2P-3 electrode and PEDOT-CO2P-3 electrode displayed the power conversion efficiency of 7.14% and 6.85%, respectively (Table 13) (Di et al. 2019).
DSSC using tungsten oxide/titanium oxide nanocomposites (nWO3-TiO2) with three dissimilar WO3-TiO2 (1%WO3-TiO2/N719/MWCNT) displayed an efficiency improvement of about 40% as corresponding to the conventional TiO2/dye/Pt solar cell as shown in Fig. 14 (Younas et al. 2019a).
J-V curves for nWO3-TiO2-based DSSC (Younas et al. 2019b)
Due to its remarkably low price, polyaniline (PANI) is a striking option to platinum counter electrode for dye-sensitized solar cell. Though, deposition of PANI film up to fluorine-doped tin oxide glass surface is too complicated. DSSC using Gr/PANI and Pt counter electrode displayed photoconversion efficiency of 3.58% and 3.97%, respectively (Fig. 15) (Shahid et al. 2019).
J-V curves of Gr/PANI, Gr, FTO/PANI, and Pt counter electrode-based DSSCs (Shahid et al. 2019)
Materials of counter electrode
Counter electrode can be fabricated by using different fabrication methods. One-step hydrothermal method was used to prepare nickel sulfide hollow spheres as a counter electrode using 0.1g nickel sulfide (NiS) and 0.025 g polyethylene glycol powder in 1mL absolute ethanol solution stirred in an agate mortar to make a paste. NiS counter electrode-based DSSC showed power conversion efficiency of 6.90% (Chen et al. 2009). Novel platinum-free counter electrode named poly-3-methylthiophene (P3MT) was fabricated using an electrochemical deposition method. At room temperature, the P3MT electro-deposition was taken out in acetonitrile solution containing 0.1 M 3-methylthiophene (3MT) and 0.1M tetrabutylammonium tetrafluoroborate. An efficiency of 2.76% with a fill factor of 0.50 was reported for P3MT counter electrode-based DSSC (Yang et al. 2014). Carbon was generated in the presence of argon from the graphitization of glucose at high temperatures. Counter electrode was prepared using 1g of carbon and 0.12g of PVP solution in a mortar pestle and the mixture was carried on pot mill for 2 days for homogenous slurry. Conversion efficiency was observed as 3.63% for carbon cathode (Table 14) (Torabi et al. 2014).
H2-reduced carbon counter electrode showed the best performance of DSSC as compared to the conventional cell. Counter electrode was prepared using H2 reduction technique. In this process, carbon was heated in a mixture of H2-Ar gas at 450°C for half an hour. The highest power conversion efficiency was found 7.7%, with a fill factor of 0.69% and an open-circuit voltage of 0.65V, as shown in Table 15 (Kumar and Bharg 2015).
Hummers methodology was used to synthesize graphene oxide (GO) and obtained solar-reduced graphene oxide (SRGO) by reducing GO under focused sunlight. Before spraying substrate, 1mg of GO (or SRGO) was scattered in 1mL of isopropanol and then the solution was ultrasonicated for half an hour. SRGO-DSSC has shown better results (Table 16) (Takada et al. 2015).
4. Natural vs synthetic dye
Recent development in natural photosensitizer
Natural dye solar cells have tremendous advantages over synthetic dyes-based solar cells. Environmental friendly, economic, and facile synthesis chooses fabrication of dye solar cells using natural dyes. Performance of dye solar cell can be understood by evaluating open-circuit voltage (Voc), short circuit current density (Jsc), fill factor (FF), and efficiency of the cell (η). Various photoelectrochemical parameters of dye solar cell with different natural sensitizers are given in Table 17.
Natural resources like Crocus sativus (Saffron), Allium cepa L (red onion), Malva sylvestris (Mallow), and oregano (Origanum vulgare) were used to prepare natural dye for DSSC. The efficiency of 0.54% was observed by red onion L. Existence of carbonyl and hydroxyl groups enables these dyes to bind to semiconductor layer (Jalali et al. 2020). Betalain and anthocyanin pigments were extracted from prickly pear and mulberry, respectively. UV-Vis spectroscopy (Fig. 16) shows absorbance in the visible range of 530–535 nm for betanin while 450–559 nm for anthocyanin pigment (Obi et al. 2020).
Absorbance spectra of different mix concentrations of anthocyanin (a) and betalain (b) (Obi et al. 2020)
The effect of different organic solvents, temperatures, and pH levels for the extraction of dye from Areca catechu was studied. The most favorable state of dye extraction was recorded at 80°C, pH 10, and ethanol as extraction solvent, as shown in Fig. 17 (Al-Alwania et al. 2020).
Effect of a solvent, b temperature, and c pH on the absorbance of the dye (Al-Alwania et al. 2020)
Natural and synthetic dyes
The most important field of the DSSC compared to the other types of solar cells is applying the dye. It is well determined that the energy gap size of the semiconductors examines the absorption frequency of light in the solar cells. A vital purpose for using the dyes in the DSSC is to explore the absorption spectra on the visible light because the visible light has about 96% energy of the sunlight (Phonkhokkong et al. 2016). Absorption spectra of dye solar cells are determined by grouping the photoelectrode’s nanoparticles, e.g., titanium dioxide and sensitizer, where dyes can assist dye solar cell in exploring their absorption spectra. Synthetic dyes synthesized from a complex methodology. On the other hand, natural dyes are extracted from natural resources which results in economic feasibility. Natural and synthetic dyes are evaluated based on economics, environmental aspects, the methodology used, performance, stability, and absorbance, as given in Table 18.
Performance evaluation between natural and synthetic
Natural and synthetic dyes are compared based on performance and fabrication parameters, as shown in Table 19.
Conclusion and recommendations
This article summarizes various factors affecting performance of the DSSC with critical review. The various fabrication parameters (semiconductor, sensitizer, electrolyte, and counter electrode) affect the performance of the DSSC.
The following concluding remarks are drawn based on the above review:
-
There are various fabrication methods employed for DSSC. The use of titanium dioxide showed the best results in semiconductor material, while mangosteen used as natural dye gave the highest power conversion efficiency. Also, the quasi-solid-state electrolyte used in DSSC was more suitable for DSSC performance. Conversion efficiency of 7.5% was achieved by carbon as a counter electrode.
-
Shear-exfoliated graphene-based photoanode with 23μm thickness gave conversion efficiency of 8.9%, resulting in lesser electron recombination.
-
Extracts of Dianthus barbatus gave an efficiency of 0.05%, with a fill factor of 0.48 showing progress in dye solar cells using natural dyes.
-
Porphyrin dyes co-sensitized with new X organic dyes showed excellent enhancement in conversion efficiency (8.08%) compared to individual dye (4.63%).
-
Dye-sensitized solar cells with copper(I) dyes and copper(I)/(II) redox shuttles achieved an efficiency of 2.06%, showing the potential of all copper-based DSSCs.
-
An optimized CdSe-TiO2 photoanode showed a power conversion efficiency (PCE) of 13.29% and short circuit current density of 15.30 mA cm−2 for the DSSC.
-
TTO electrode-based DSSC gave power conversion efficiency of 3.26% ascribed to the improved electrical and optical properties due to doping with Ta element.
-
Absorbance of betalain was shown in the visible range of 530–535 nm for betanin while 450–559 nm for anthocyanin pigment.
Significant issues in the progress of dye solar cells are less efficient and have poor stability. The following are the various issues regarding the efficiency and stability of dye solar cell:
-
Viscous nature of electrolyte used in DSSC.
-
Weak performance of sensitizers in the near to infra-red region (NIR) of solar spectrum.
-
Fast degradation of sensitizer resulting in less life of DSSC.
-
The following recommendations can improve the efficiency and stability of dye solar cell:
-
Structure of sensitizer—enhancement in dye structure to provide high efficiency in NIR region of the solar spectrum.
-
Viscosity of electrolyte—electron mobility can be improved by a less viscous electrolyte that enhances the stability of the DSSC.
-
Morphology of working substrate—dark current can be decreased by the proper morphology of the working substrate.
-
Expensive platinum-based counter electrode can be replaced by numerous materials such as conducting polymers, carbonaceous materials, sulfides, and oxides.
-
Flexible working electrode is very useful for curved surfaces. The lesser weight and low cost promote the application of flexible electrodes rather than transparent conducting oxide glasses.
Availability of data and materials
Here, critical analysis has been done with reference to earlier research work. This is a kind of comprehensive review. Hence, there is no data used.
Abbreviations
- DSSC :
-
dye-sensitized solar cell
- FF :
-
fill factor
- FSP :
-
flame spray pyrolysis
- I sc :
-
short circuit photocurrent (mA)
- P max :
-
maximum value power (mW)
- J sc :
-
photocurrent current density (mA/cm2)
- PCE :
-
power conversion efficiency (%)
- PVP :
-
polyvinylpyrrolidone
- V oc :
-
open-circuit voltage of DSSC (V)
- V m :
-
maximum value of voltage corresponds to maximum power (mV or V)
- η :
-
efficiency (%)
References
Adel R, Abdallah T, Moustafa YM, Al Sabagh AM, Talaat H (2015) Effect of polymer electrolyte on the performance of natural dye sensitized solar cells. SuperlatticMicrostruc 86:62–67. https://doi.org/10.1016/j.spmi.2015.07.024
Al-Alwania MAM, Hassimi AH, Al-Shorgani NKN, Al-Mashaan ABSA (2020) Natural dye extracted from Areca catechu fruits as a new sensitizer for dye-sensitised solar cell fabrication: optimisation using D-Optimal design. Mater Chem Phys 240:122204. https://doi.org/10.1016/j.matchemphys.2019.122204
Ananth S, Vivek P, Solaiyammal T, Murugakoothan P (2015) Pre dye treated titanium dioxide nano particles sensitized by natural dye extracts of Pterocarpus marsupium for dye sensitized solar cells. Optik 126:1027–1031. https://doi.org/10.1016/j.ijleo.2015.02.066
Awasthi A, Shukla AK, Shukla KN, Porwal D, Richhariya G (2020) Review on sun tracking technology in solar PV system. Energy Rep 6:392–405. https://doi.org/10.1016/j.egyr.2020.02.004
Ayalew WA, Ayele DW (2016) Dye sensitized solar cells using natural dye as light-harvesting materials extracted from Acanthus sennii chiovenda flower and Euphorbia cotinifolia leaf. J Sci: Adv Mater Device 1:488–494. https://doi.org/10.1016/j.jsamd.2016.10.003
Batniji AY, Morjan R, Abdel-Latif MS (2014) Aldimine derivatives as photosensitizers for dye-sensitized solar cells. Turk J Phys 38:86–90
Bay L, West K, JensenW B, Jacobsen T (2006) Electrochemical reaction rates in a dye-sensitised solar cell the iodide/tri-iodide redox system. Sol Energy Mater Sol Cells 90(3):341–351. https://doi.org/10.1016/j.solmat.2005.04.040
Bhattacharya A, Datta J (2020) Wide-low energy coupled semiconductor layers of TiO2- CdX boosting the performance of DSSC. Sol Energy 208:674–687. https://doi.org/10.1016/j.solener.2020.08.024
Calogero G, Yum JH, Sinopoli A, Marco GD, Gratzel M, Nazeeruddin MK (2012) Anthocyanins and betalains as light harvesting pigments for dye sensitized solar cells. Sol Energy 86:1563–1575. https://doi.org/10.1016/j.solener.2012.02.018
Campbell WM, Jolley KW, Wagner P, Wagner K, Walsh PJ, Gordon KC (2007) Highly efficient porphyrin sensitizers for dye sensitized solar cells. J Phys Chem C 111:11760–11762. https://doi.org/10.1021/jp0750598
Cerda B, Sivakumar R, Paulraj M (2016) Natural dyes as sensitizers to increase the efficiency in sensitized solar cells. J Phys Conf Ser 720:1–5. https://doi.org/10.1088/1742-6596/720/1/012030
Chen J, Li K, Luo YA (2009) A flexible carbon counter electrode for dye-sensitized solar cells. Carbon 47:2704–2708. https://doi.org/10.1016/j.carbon.2009.05.028
Chien CY, Hsu BD (2013) Optimization of the dye sensitized solar cell with anthocyanin as photosensitizer. Sol Energy 98:203–211. https://doi.org/10.1016/j.solener.2013.09.035
Chien CY, Hsu BD (2014) Performance enhancement of dye-sensitized solar cells based on anthocyanin by carbohydrates. Sol Energy 108:403–411. https://doi.org/10.1016/j.solener.2013.09.035
Choi H, Baik C, Kang SO, Ko J, Kang MS, Nazeeruddin MK, Gratzel M (2008) Highly efficient and thermally stable organic sensitizers for solvent-free dye-sensitized solar cells. Chem, Int Ed 47(2):327–330. https://doi.org/10.1002/ange.200703852
Chowdhury FI, Buraidah MH, Arof AK, Mellander BE, Noor IM (2020) Impact of tetrabutylammonium, iodide and triiodide ions conductivity in polyacrylonitrile based electrolyte on DSSC performance. Sol Energy 196:379–388. https://doi.org/10.1016/j.solener.2019.12.033
Congiu M, Neto ON, Marco DML, Dini D, Graeff CFO (2016) Cu2−xS films as counter-electrodes for dye solar cells with ferrocene-based liquid electrolytes. Thin Solid Films 61:22–28. https://doi.org/10.1016/j.tsf.2016.05.033
Dadkhah M, Niasari MS, Mir N (2014) Synthesis and characterization of TiO2 nanoparticles by using new shape controllers and its application in dye sensitized solar cells. J Ind Eng Chem 20:4039–4044. https://doi.org/10.1016/j.jiec.2014.01.003
Daeneke T, Kwon T, Holmes AB (2011) High-efficiency dye-sensitized solar cells with ferrocene-based electrolytes. Nat Chem 3:211–215. https://doi.org/10.1038/nchem.966
Di Y, Jia S, Li N, Hao C, Zhang H, Hu S, Liu H (2019) Electrocatalytic films of PEDOT incorporating transition metal phosphides as efficient counter electrodes for dye sensitized solar cells. Sol Energy 189:8–14. https://doi.org/10.1016/j.solener.2019.07.039
Esteban ACMS, Enriquez EP (2013) Graphene anthocyanin mixture as photo sensitizer for dye sensitized solar cell. Sol Energy 98:392–399. https://doi.org/10.1016/j.solener.2013.09.036
Ferreira FC, Babu RS, Barros ALF, Raja S, Conceicao LRB, Mattoso LHC (2020) Photoelectric performance evaluation of DSSCs using the dye extracted from different color petals of Leucanthemum vulgare flowers as novel sensitizers. Spectrochim Acta Part A: Mol Biomol Spectrosc 233:118198. https://doi.org/10.1016/j.saa.2020.118198
Fukurozaki SH, Zilles R, Sauer IL (2013) Energy payback time and CO2 emissions of 1.2 kWp photovoltaic roof top system in Brazil. Int J Smart Grid Clean Energy 2:164–169. https://doi.org/10.12720/sgce.2.2.164-169
Godibo DJ, Anshebo ST, Anshebo TY (2015) Dye sensitized solar cells using natural pigments from five plants and quasisolid state electrolyte. J Braz Chem Soc 26:92–101. https://doi.org/10.5935/0103-5053.20140218
Gorlov M, Kloo L (2008) Ionic liquid electrolytes for dye-sensitized solar cells. Dalton Trans 20:2655–2666. https://doi.org/10.1039/B716419J
Gratzel M (2003) Review: dye-sensitized solar cells. J PhotochemPhotobiol C: Photochem Rev 4:145–153. https://doi.org/10.1016/S1389-5567(03)00026-1
Hagfeldt A, Boschoo G, Sun L (2010) Dye sensitized solar cells. Chem Rev 110:6595–6663. https://doi.org/10.1021/cr900356p
Hamadanian M, Ghomi JS, Hosseinpour M, Masoomi R, Jabbari V (2014) Uses of new natural dye photo sensitizers in fabrication of high potential dye-sensitized solar cells (DSSCs). Mater Sci Semicond Process 27:733–739. https://doi.org/10.1016/j.mssp.2014.08.017
Han HG, Weerasinghe HC, Kim KM, Kim JS (2015) Ultrafast Fabrication of flexible dye sensitized solar cells by ultrasonic spray coating technology. Mater Sci Eng 5:1–9. https://doi.org/10.1038/srep14645
Hao S, Wu J, Huang Y, Lin J (2005) Natural dyes as photo sensitizers for dye sensitized solar cell. Sol Energy 80:209–214. https://doi.org/10.1016/j.solener.2005.05.009
Hashmi G, Miettunen K, Peltola T, Halme J (2011) Review of materials and manufacturing options for large area flexible dye solar cells. Renew Sust Energ Rev 15:3717–3732. https://doi.org/10.1016/j.rser.2011.06.004
Heo JH, Jung KY, Kwak DJ (2009) Fabrication of titanium doped indium oxide films for dye-sensitized solar cell application using reactive RF magnetron sputter method. IEEE Trans Plasma Sci 37:1586–1592. https://doi.org/10.1109/TPS.2009.2023477
Hu B, Tang Q, He B, Lin L, Chen H (2014) Mesoporous TiO2 anodes for efficient dye-sensitized solar cells: an efficiency of 9.86% under one sun illumination. Power Sourc 267:445–451. https://doi.org/10.1016/j.jpowsour.2014.05.119
Hug H, Bader M, Mair P, Glatzel T (2013) Biophotovoltaic natural pigments in dye sensitized solar cells. Appl Energy 115:216–225. https://doi.org/10.1016/j.apenergy.2013.10.055
Huo J, Hu Y, Jiang H, Hou X (2014) Continuous flame synthesis of near surface nitrogen doped TiO2 for dye sensitized solar cells. J Chem Eng 258:163–170. https://doi.org/10.1016/j.cej.2014.07.026
Ito S and Takahashi K (2012). Fabrication of monolithic dye sensitized solar cell using ionic liquid electrolyte. Int J Photoenergy 6. https://doi.org/10.1155/2012/915352
Jalali T, Arkian P, Golshan M, Jalali M, Osfouri S (2020) Performance evaluation of natural native dyes as photosensitizer in dye-sensitized solar cells. Opt Mater 110:110441. https://doi.org/10.1016/j.optmat.2020.110441
Jia HL, Chen YC, Ji L, Lin XL, Guana MY, Yang Y (2018) Cosensitization of porphyrin dyes with new X type organic dyes for e-cient dye-sensitized solar cells. Dyes Pigments 163:589–593. https://doi.org/10.1016/j.dyepig.2018.12.048
Jun Y, Kim J, Kang MG (2007) A study of stainless steel based dye-sensitized solar cells and modules. Sol Energy Mater Sol Cells 91:779–784. https://doi.org/10.1016/j.solmat.2007.01.007
Karpacheva M, Malzner FJ, Wobill C, Buttner A, Constable EC, Housecroft, Constable CE (2018) Dye-sensitized solar cells with copper(I) dyes and copper(I)/(II) redox shuttles. Dyes Pigments 156:410–416. https://doi.org/10.1016/j.dyepig.2018.04.033
Kavan L, Liska P, Zakeeruddin SM, Michael G (2016) Low temperature fabrication of highly efficient, optically transparent (FTO-free) graphene cathode for co-mediated dye-sensitized solar cells with acetonitrile free electrolyte solution. Electrochim Acta 195:34–42. https://doi.org/10.1016/j.electacta.2016.02.097
Kay A, Gratzel M (1996) Low cost photovoltaic modules based on dye sensitized nanocrystalline titanium dioxide and carbon powder. Sol Energy Mater Sol Cells 44:99–117. https://doi.org/10.1016/0927-0248(96)00063-3
Kumar R, Bharg P (2015) Fabrication of a counter electrode using glucose as carbon material for dye sensitized solar cells. Mater Sci Semicond Process 40:331–336. https://doi.org/10.1016/j.mssp.2015.06.009
Kumar A., Richhariya G., Sharma A.2015. Solar photovoltaic technology and its sustainability. Energy Sustain through Green Energy, edited by Atul Sharma, Sanjay Kumar Kar. Chapter 1. 3-25. Springer. ISBN: 978-81-322-2336-8. https://doi.org/10.1007/978-81-322-2337-5_1
Kumar DK, Kriz J, Bennett N, Chen B, Upadhayaya H, Reddy KR, Sadhu V (2020) Functionalized metal oxide nanoparticles for efficient dye-sensitized solar cells (DSSCs): a review. Mater Sci Energy Technol 3:472–481. https://doi.org/10.1016/j.mset.2020.03.003
Kumara NTRN, Ekanayake P, Lim A (2013) A layered co-sensitization for enhancement of conversion efficiency of natural dye sensitized solar cells. J Alloys Compd 581:186–191. https://doi.org/10.1016/j.jallcom.2013.07.039
Kumara NTRN, Lim A, Lim CM, Petra MI, Ekanayake P (2017) Recent progress and utilization of natural pigments in dye sensitized solar cells: a review. Renew Sust Energ Rev 78:301–317. https://doi.org/10.1016/j.rser.2017.04.075
Latif MSA, Abuiriban MB, El-Agez TM, Taya SA (2015) Dye sensitized solar cells using dyes extracted from flowers, leaves, parks, and roots of three trees. Int J Renew Energy Res 5:294–298
Latini A, Aldibaja FK, Cavallo C, Gozzi D (2014) Benzonitrile based electrolytes for best operation of dye sensitized solar cells. J Power Sources 269:308–316. https://doi.org/10.1016/j.jpowsour.2014.06.154
Law M, Greene LE, Johnson JC, Saykally R, Yang P (2005) Nano wire dye sensitized solar cell. Nat Mater 4:455–459. https://doi.org/10.1038/nmat1387
Lee WJ, Lee DY, Song JS, Min BK (2005) Effect of process parameters on the efficiency of dye sensitized solar cells. Met Mater Int 11:465–471. https://doi.org/10.1007/bf03027496
Lim A, Kumara NTRN, Tan AL, Mirza AH, Chandrakanthi RLN, Petra MI (2015) Potential natural sensitizers extracted from the skin of Canarium odontophyllum fruits for dye-sensitized solar cells. Spectroc Acta Part A: Mol BiomolSpectros 13:596–602. https://doi.org/10.1016/j.saa.2014.11.102
Lo KSK, Leung WWF (2019) Dye-sensitized solar cells with shear-exfoliated graphene. Sol Energy 180:16–24. https://doi.org/10.1016/j.solener.2018.12.077
Ludin NA, Al-Alwani Mahmoud AM, Mohamad AB, Kadhum AAH, Sopian K, Abdul Karim NS (2014) Review on the development of natural dye photo sensitizer for dye sensitized solar cells. Renew Sust Energ Rev 31:386–396. https://doi.org/10.1016/j.rser.2013.12.001
Mali SS, Betty CA, Bhosale PN, Patil PS (2012) Eosin-Y and N3-dye sensitized solar cells (DSSCs) based on novel nanocoral TiO2: a comparative study. Electrochim Acta 59:113–120. https://doi.org/10.1016/j.electacta.2011.10.043
Maurya IC, Neetu, Gupta AK, Srivastava P, Bahadur L (2016) Callindrahaematocephata C and Peltophorum pterocarpum flowers as natural sensitizers for TiO2 thin film based dye-sensitized solar cells. Opt Mater 60:270–276. https://doi.org/10.1016/j.optmat.2016.07.041
Mbonyiryivuze A, Omollo I, Ngom BD, Mwakikunga, Dhlamin SM, Park E, Maaza M (2015) Natural dye sensitizer for gratzel cells: Sepia melanin. Phys Mater Chem 3:1–6. https://doi.org/10.12691/pmc-3-1-1
Mehmood U, Rahman S, Harrabi K, Hussein IA, Reddy BVS (2014). Review article: recent advances in dye sensitized solar cells. Adv Mater Sci Eng. 1-13. https://doi.org/10.1155/2014/974782
Mehmood U, Al-Ahmed A, Al-Sulaiman FA, Malik MI, Shehzad F, Khan AH (2017) E-ect of temperature on the photovoltaic performance and stability of solid state dye sensitized solar cells: a review. Renew Sust Energ Rev 79:946–959. https://doi.org/10.1016/j.rser.2017.05.114
Moustafa S, Abusaif A, Fathy M, Abu-Saied MA, Elhenawy AA, Kashyout AB, Mohamed R, Yousry S, Ammara A (2021) New carbazole-based organic dyes with different acceptors for dye-sensitized solar cells: Synthesis, characterization, DSSC fabrications and density functional theory studies. J Mol Struct 1225:129297. https://doi.org/10.1016/j.molstruc.2020.129297
Muniz EC, Goes MS, Silv JJ, Varela JA, Joanni E (2011) Synthesis and characterization of mesoporous TiO2 nanostructured films prepared by a modified sol–gel method for application in dye solar cells. Ceram Int 37:1017–1024. https://doi.org/10.1016/j.ceramint.2010.11.014
Nagavolu C, Susmitha K, Raghavender M, Giribabu L, Mills CA, Silva SRP (2016) Pt-free spray coated reduced graphene oxide counter electrodes for dye sensitized solar cells. Sol Energy 137:143–147. https://doi.org/10.1016/j.solener.2016.08.002
Nan H, Shen HP, Wang G, Xie SD, Yang GJ, Lin H (2017) Studies on the optical and photoelectric properties of anthocyanin and chlorophyll as natural co-sensitizers in dye sensitized solar cell. Opt Mater 73(2017):172–178. https://doi.org/10.1016/j.optmat.2017.07.036
Nandakumar VG, Suresh S, Sreekala CO, Sudheer SK, Pillai MVP (2017) Hemigraphiscolorata as a natural dye for solar energy conversion. Mater Today: Proc 4:4358–4365. https://doi.org/10.1016/j.matpr.2017.04.006
Narayan MR (2012) Review: dye sensitized solar cells based on natural photosensitizers. Renew Sust Energ Rev 16:208–215. https://doi.org/10.1016/j.rser.2011.07.148
Nazeeruddin MK, Kay A, Rodicio I (1993) Conversion of light to electricity by cis-X2bis(2,2'-bipyridyl-4,4’-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X =Cl-, Br-,I-,CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J Am Chem Soc 115:6382–6390. https://doi.org/10.1021/ja00067a063
Nazeeruddin MK, Zakeeruddin SM, Baker HR (1999) Acid-base equilibria of (2,2‘-Bipyridyl-4,4‘-dicarboxylic acid)ruthenium(II) complexes and the effect of protonation on charge transfer sensitization of nanocrystalline titania. Inorg.Chem. 38:6298–6305. https://doi.org/10.1021/ic990916a
Obi K, Frolova L, Fuierer P (2020) Preparation and performance of prickly pear (Opuntia phaeacantha) and mulberry (Morus rubra) dye-sensitized solar cells. Sol Energy 208:312–320. https://doi.org/10.1016/j.solener.2020.08.006
Oskam G, Bergeron BV, Meyer GJ (2001) Pseudohalogens for dye sensitized TiO2 photoelectrochemical cells. J Phys Chem B 105:6867–6873. https://doi.org/10.1021/jp004411d
Ozuomba JO, Okoli LU, Ekpunobi AJ (2013) The performance and stability of anthocyanin local dye as a photosensitizer for DSSCs. Adv Appl Sci Res 4:60–69 ISSN: 0976-8610
Park KH, Kim TY, Park JY, Jin EM, Yim SH, Choi DY, Lee JW (2012) Adsorption characteristics of gardenia yellow as natural photo sensitizer for dye sensitized solar cells. Dyes Pigments 96:595–601. https://doi.org/10.1016/j.dyepig.2012.10.005
Pavithra N, Asiri AM, Anandan SF (2015) Fabrication of dye sensitized solar cell using gel polymer electrolytes consisting poly(ethylene oxide)-acetamide composite. J Power Sources 286:346–353. https://doi.org/10.1016/j.jpowsour.2015.03.160
Peng W, Zeng Y, Gong H, Leng YQ, Yan YH, Hu W (2013) Silver coated TiO2 electrodes for high performance dye-sensitized solar cells. Solid State Electron 89:116–119. https://doi.org/10.1016/j.sse.2013.07.011
Pettersson H, Gruszecki T, Bernhard R, Haggman L, Gorlov M, Boschloo G, Edvinsson T, Kloo L, Hagfeldt A (2007) The monolithic multicell: a tool for testing material components in dye-sensitized solar cells. Prog Photovolt 15(2):113–121. https://doi.org/10.1002/pip.713
Phinjaturus K, Maiaugree W, Suriharn B, Pimanpaeng S, Amornkitbamrung V, Swatsitang E (2016) Dye sensitized solar cells based on purple corn sensitizers. Appl Surf Sci 380:101–107. https://doi.org/10.1016/j.apsusc.2016.02.050
Phonkhokkong T, Thongtem T, Thongtem S, Phuruangrat A, Promnopas W (2016) Synthesis and characterization of TiO2 nanopowders for fabrication of dye sensitized solar cells. Digest J Nanomater Biostruct 1:81–90 ISSN: 1842-3582
Polo AS, Itokazu MK, Iha NYM (2004) Metal complex sensitizers in dye-sensitized solar cells. Coord Chem Rev 248:1343–1361. https://doi.org/10.1016/j.ccr.2004.04.013
Ramarajan R, Purushothamreddy N, Dileep RK, Kovendhan M, Veerappan G, Thangaraju K, Joseph DP (2020) Large-area spray deposited Ta-doped SnO2 thin film electrode for DSSC application. Sol Energy 211:547–559. https://doi.org/10.1016/j.solener.2020.09.042
Rawal N, Vaishaly AG, Sharma H, Mathew B (2015) Dye sensitized solar cells: the emerging technology. Energy Power Eng Sci 2:46–52. https://doi.org/10.12966/epes.05.03.2015
Researcher J.L.E, Hunter R, Rubilar M, Pavez B, Morales E, Torres S (2016) Development of dye sensitized solar cells based on naturally extracted dye from the maqui berry (Aristotelia chilensis). Opt Mater 60:411–417. https://doi.org/10.1016/j.optmat.2016.08.021
Richhariya G, Kumar A (2016) A Review on performance affected parameters for dye sensitized solar cell. Energy Secur Sustain, CRC Press, Boca Raton, pp 93–107 Print ISBN: 978-1-4987-5443-9, eBook ISBN: 978-1-4987-5444-6
Ruhane TA, Islam MT, Rahaman MS, Bhuiyan MMH, Islam JMM, Newaz MK, Khan KA, Khan MA (2017) Photo current enhancement of natural dye sensitized solar cell by optimizing dye extraction and its loading period. Optik 149:174–183. https://doi.org/10.1016/j.ijleo.2017.09.024
Saito Y, Kitamura T, Wada Y, Yanagida S (2002) Application of poly (3,4-ethylenedioxythiophene) to counter electrode in dye-sensitized solar cells. Chem Lett 10:1060–1061. https://doi.org/10.1021/jp303958r
Sathyajothi S, Jayavel R, Dhanemozhi AC (2017) The fabrication of natural dye sensitized solar cell (DSSC) based on TiO2 using henna and beetroot dye extracts. Mater Today: Pro 4:668–676. https://doi.org/10.1016/j.matpr.2017.01.071
Sedghi A, Miankushki HN (2013) Influence of TiCl4 treatment on structure and performance of dye sensitized solar cells. Jpn J Appl Phys 52:1–5. https://doi.org/10.7567/JJAP.52.075002
Shahid MU, Mohamed NM, Muhsan AS, Bashiri R, Shamsudin AE, Zaine SNA (2019) Few-layer grapheme supported polyaniline (PANI) film as a transparent counter electrode for dye-sensitized solar cells. Diamond Relat Mater 94:242–251. https://doi.org/10.1016/j.diamond.2019.03.009
Sharma GD, Zervaki GE, Angaridis PA, Vatikioti A, Gupta KSV, Gayathri T, Nagarjuna P (2014) Stepwise co-sensitization as a useful tool for enhancement of power conversion efficiency of dye sensitized solar cells: the case of an unsymmetrical porphyrin dyad and a metal free organic dye. Org Electron 15:1324–1337. https://doi.org/10.1016/j.orgel.2014.03.033
Sharma A, Srivastava J, Kumar A (2015) A comprehensive overview of renewable energy status in India. Environ Sustain 2015:91–105. https://doi.org/10.1007/978-81-322-2056-5_5
Sharma K, Sharma V, Sharma SS (2020) Dye sensitized solar cells: fundamentals and current status. Nanoscale Res Lett 13:381. https://doi.org/10.1186/s11671-018-2760-6
Silva MDSDP, Diogenes ICN, Carvalho IMMD, Zanoni KPS, Amaral RCM, Iha NY (2016) Novel heteroleptic ruthenium complexes for dye sensitized solar cells. J Photochemphtobiol A:Chem 314:75–80. https://doi.org/10.1016/j.jphotochem.2015.08.012
Silva LD, Sanchez M, Freeman HS (2020) New tetrazole based dyes as efficient co-sensitizers for DSSCs: Structure-properties relationship. Org Electron 87:105964. https://doi.org/10.1016/j.orgel.2020.105964
Singh R, Bhattacharya B, Rhee HW, Singh PK (2014) New biodegradable polymer electrolyte for dye sensitized solar cell. Int J Electrochem Sci 9:2620–2630 ISSN 1452-3981
Susanti D, Nafi M, Purwaningsih H, Fajarin R, Kusuma GE (2014) The preparation of dye sensitized solar cell (DSSC) from TiO2 and tamarillo extract. Proc Chem 9:3–10. https://doi.org/10.1016/j.proche.2014.05.002
Takada H, Obana Y, Sasaki R, Kuribayashi M, Kanno M, Zhu C (2015) Improved durability of dye sensitized solar cell with H2 reduced carbon counter electrode. J Power Sources 274:1276–1282. https://doi.org/10.1016/j.jpowsour.2014.10.148
Takagi K, Magaino S, Saito H, Aoki T, Aoki D (2013) Measurements and evaluation of dye-sensitized solar cell performance. J PhotochemPhotobiol C: Photochem Rev 14:1–12. https://doi.org/10.1016/j.jphotochemrev.2012.08.003
Taya SA, El-Agez TM, El-Ghamri HS, Latif MSA (2013) Dye sensitized solar cells using fresh and dried natural dyes. Int J Mater Sci Appl 2:37–42. https://doi.org/10.11648/j.ijmsa.20130202.11
Teo LP, Tiong TS, Buraidah MH, Arof AK (2018) Effect of lithium iodide on the performance of dye sensitized solar cells (DSSC) using poly(ethylene oxide) (PEO)/poly (vinyl alcohol) (PVA) based gel polymer electrolytes. Opt Mater 85:531–537. https://doi.org/10.1016/j.optmat.2018.09.026
Tian H, Jiang X, Yu Z, Kloo L, Hagfeldt A, Sun L, Angew. (2000) Efficient organic dye sensitized solar cells based on an iodine free electrolyte. Chem Int Ed 49(40):7328–7331. https://doi.org/10.1002/anie.201003740
Tontapha S, Aroon WS, Promgool T, Kanokmedhakul S, Maiaugree W, Swatsitang E, Homrahad V, Amornkitbumrung V (2019) Electrocatalytic activity of disulfide/thiolate with graphene nanosheets as an efficient counter electrode for DSSCs: a DFT study. Mater Today Commun 22:100742. https://doi.org/10.1016/j.mtcomm.2019.100742
Torabi N, Behjat A, Jafari F (2014) Dye sensitized solar cells based on porous conjugated polymer counter electrodes. Thin Solid Films 573:112–116. https://doi.org/10.1016/j.tsf.2014.11.034
Uddin J, Islam JMM, Karim E, Khan SMM, Akhter S (2015) Preparation and characterization of dye sensitized solar cell using natural dye extract from Red Amaranth (Amaranthus sp.) as sensitizer. Int J Thin Film Sci Tech 4:41–146. https://doi.org/10.12785/ijtfst/040212
Wang ZS, Sayama K, Sugihara H (2005) Efficient eosin y dye-sensitized solar cell containing Br-/Br3-electrolyte. J Phys Chem B 109:22449–22455. https://doi.org/10.1021/jp053260h
Wang M, Chamberland N, Breau L, Moser JE, Baker HR (2010) An organic redox electrolyte to rival triiodide/iodide in dye-sensitized solar cells. Nat Chem 2(5):385–389. 32. https://doi.org/10.1038/nchem.610
Wang H, Gao J, Zhu J, Ma JY, Zhou H, Xiao J, Wu M (2020) Design bifunctional nitrogen doped flexible carbon sphere electrode for dye-sensitized solar cell and supercapacitor. Electrochim Acta 334:135–582. https://doi.org/10.1016/j.electacta.2019.135582
Weerasinghe HC, Huang F, Cheng YB (2013) Fabrication of flexible dye sensitized solar cells on plastic substrates. Nano Energy 2:174–189. https://doi.org/10.1016/j.nanoen.2012.10.004
Wu J, Lan Z, Hao S, Li P, Lin J, Huang M, Fang L, Huan Y (2008) Progress on the electrolytes for dye-sensitized solar cells. Pure Appl Chem 80:2241–2258. https://doi.org/10.1351/pac200880112241)
Yang X, Luo J, Zhou L, Yang B, Zuo X, Li G (2014) A novel Pt-free counter electrode for dye-sensitized solar cells: nickel sulfide hollow spheres. Chem Mater Let 136:241–244. https://doi.org/10.1016/j.matlet.2014.08.053
Younas M, Gondal MA, Dastageer MA, Harrabi K (2019a) E-cient and cost-e-ective dye-sensitized solar cells using MWCNT-TiO2 nanocomposite as photoanode and MWCNT as Pt-free counter electrode. Sol Energy 188:1178–1188. https://doi.org/10.1016/j.solener.2019.07.009
Younas M, Gondal MA, Dastageer MA, Baig U (2019b) Fabrication of cost effective and efficient dye sensitized solar cells with WO3-TiO2 nanocomposites as photoanode and MWCNT as Pt-free counter electrode. Ceram Int 45:936–947. https://doi.org/10.1016/j.ceramint.2018.09.269
Yugis AR, Mansa RF, Sipaut CS (2015) Review on metallic and plastic flexible dye sensitized solar cell. Mater Sci Eng 78:1–7. https://doi.org/10.1088/1757-899X/78/1/012003
Yune JH, Karatchevtseva I, Triania G, Wagner K, Officer DJ (2013) A study of TiO2 binder-free paste prepared for low temperature dye-sensitized solar cells. Mater Res 28:488–496. https://doi.org/10.1557/jmr.2012.354
Zalas M, Gierczyk B, Bossi A, Mussini PR, Klein M, Pankiewicz R (2017) The influence of anchoring group position in ruthenium dye molecule on performance of dye-sensitized solar cells. Dyes Pigments 150:335–346. https://doi.org/10.1016/j.dyepig.2017.12.029
Zeng W, Cao Y, Bai Y, Wang Y, Shi Y, Zhang M, Wang F, Pan C, Wang P (2010) Efficient dye sensitized solar cells with an organic photosensitizer featuring orderly conjugated ethylene dioxythiophene and dithienosiloleblocks. Chem Mater 22:1915–1925. https://doi.org/10.1021/cm9036988
Zhou W, Yang W, Fang Z (2007) A novel model for photovoltaic array performance prediction. Appl Energy 84:1187–1198. https://doi.org/10.1016/j.apenergy.2007.04.006
Acknowledgements
The authors are highly thankful to the Centre for Energy and Environment, Delhi Technological University, and Energy Centre, Maulana Azad National Institute of Technology, Bhopal, for providing the basic facility for this review compiling.
Author information
Authors and Affiliations
Contributions
Geetam Richhariya: methodology, writing (original draft), and investigation. Bhim Charan Meikap: conceptualization and supervision. Anil Kumar: conceptualization, writing (review and editing), visualization, and supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•Different fabrication methodologies of dye-sensitized solar cells are described.
• Ideal features of fabrication parameters of DSSCs are explained.
• Various natural sensitizers for a healthy environment are discussed.
• Performance evaluation between natural and synthetic dye is described.
Rights and permissions
About this article
Cite this article
Richhariya, G., Meikap, B.C. & Kumar, A. Review on fabrication methodologies and its impacts on performance of dye-sensitized solar cells. Environ Sci Pollut Res 29, 15233–15251 (2022). https://doi.org/10.1007/s11356-021-18049-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18049-2