Abstract
Nitrate (NO3−) loss and enrichment in water bodies caused by fertilization are a major environmental problem in agricultural areas. However, the quantitative contribution of different NO3− sources, especially chemical fertilizers (CF) and soil organic nitrogen (SON), to NO3− runoff loss remains unclear. In this study, a systematic investigation of NO3− runoff and its sources was conducted in a subtropical agricultural watershed located in Yujiang County, Jiangxi Province, China. A semi-monthly sampling was performed at the inlet and outlet from March 2018 to February 2019. Hydrochemical and dual NO3− isotope (15 N and 18O) approaches were combined to estimate the NO3− runoff loss and quantify the contribution of different sources with a Bayesian isotope mixing model. Source apportionment by Stable Isotope Analysis in R (SIAR) suggested that NO3− in runoff was mainly derived from nitrification of ammonium (NH4+) mineralized from SON (37–52%) and manure/sewage (M&S) (25–47%), while the contribution of CF was relatively small (14–25%). The contribution of various sources showed seasonal variations, with a greater contribution of CF in the wet growing season (March to August). Compared with the inlet which contributed 37–40% to runoff NO3−, SON contributed more at the outlet (49–52%). Denitrification in the runoff was small and appeared to be confined to the dry season (September to February), with an estimated NO3− loss of 2.73 kg N ha−1. The net NO3− runoff loss of the watershed was 34.5 kg N ha−1 yr−1, accounting for 15% of the annual fertilization rate (229 kg N ha−1 yr−1). Besides M&S (22%), fertilization and remineralization of SON (CF + SON) were the main sources for the NO3− runoff loss (78%), suggesting accelerated nitrification of NH4+ from CF (24%) and SON mineralization (54%). Our study indicates that NO3− runoff loss in subtropical agricultural watersheds is dominated by nonpoint source pollution from fertilization. SON played a more important role than CF. Besides, the contribution of sewage should not be neglected. Our data suggest that a combination of more rational fertilizer N application (CF), better management of SON, and better treatment of domestic sewage could alleviate NO3− pollution in subtropical China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing nitrate (NO3−) contamination of surface waters is a pervasive environmental problem worldwide (Erisman et al. 2013; Ju et al. 2009; Nikolenko et al. 2017; Verchot et al. 1997; Zhang et al. 2014, 2018a), threatening to drinking water quality and causing soil acidification and eutrophication in downstream watercourses (Dong et al. 2021; Lee et al. 2008; Parra Suarez et al. 2019; Zhang et al. 2018b, 2021). Understanding NO3− sources is a prerequisite for finding effective measures to prevent N pollution of surface waters (Wu et al. 2020a; Zhang et al. 2021).
Nitrate in surface waters originates from various sources: atmospheric precipitation (AP), manure/sewage (M&S), chemical fertilizers (CF), and soil organic nitrogen (SON). The source of NO3− pollution could be identified by several methods, such as principal component analysis (Song et al. 2006), end-member mixing analysis (Bernal et al. 2006; Wang et al. 2019), decision tree model method (Xue et al. 2015), gray water footprint (Serio et al. 2018), 15 N labelling method (Di and Cameron 2002), and stable isotope methods (Xue et al. 2009; Zhang et al. 2018b). Among them, dual isotope signatures (δ15Nand δ18O) of NO3− have been proven to be an effective approach to apportion NO3− in surface waters to these sources (Kendall 1998; Kendall et al. 2007; Nestler et al. 2011; Silva et al. 2002). However, the approach is limited by uncertainties with regard to overlapping source signatures when different NO3− sources mix (Li et al. 2019; Panno et al. 2008). For example, NO3− derived from AP and CF generally has low, overlapping δ15N-NO3− values (− 6 to + 6‰ and − 13 to + 13‰, respectively), while the δ15N of NO3− derived from M&S has higher values (+ 4 to + 25‰) (Liu et al. 2018). Moreover, the fractionation of isotopes during N cycling processes, such as mineralization, nitrification, and denitrification, can change the original isotopic composition of the NO3− sources (Kendall et al. 2007). Notwithstanding, the use of dual isotope signatures of NO3− combined with water chemistry and land use analysis has been proven suitable to apportion sources with relatively good accuracy and to identify NO3− transformations (Li et al. 2019; Matiatos 2016; Xia et al. 2017). Particularly, Bayesian mixing models, such as Stable Isotope Analysis in R (SIAR), have successfully estimated the proportional contributions of various sources to NO3− runoff by estimating the relative contribution of more than three potential sources using both δ15N and δ18O of NO3− (Ding et al. 2014; Li et al. 2019; Liu et al. 2018; Matiatos 2016; Xue et al. 2012; Yue et al. 2014). Bayesian models reduce some of the uncertainties of linear mixing models by considering the ranges of NO3− distribution instead of fixed values for sources (Parnell et al. 2010; Yue et al. 2015; Zhang et al. 2018b).
Globally, the estimated nitrogen (N) loss by NO3− leaching is 26 Tg N yr−1 (Lin et al. 2001), 65% (17 Tg N yr−1) of which is from agricultural land (Smil et al. 1999), where N fertilizers are applied excessively to guarantee high yields and profits (Ma et al. 2012; Parra Suarez et al. 2019; Perego et al. 2012; Ruidisch et al. 2013; Yang et al. 2014). Usually, chemical fertilizer (CF) is regarded to be the main source of NO3− runoff loss from agroecosystems (Cameron et al. 2013; Padilla et al. 2018; Parra Suarez et al. 2019; Zhang et al. 2018a). Ammoniacal N fertilization directly promotes NO3− leaching by accelerating nitrification of ammonia to NO3− (Sieling and Kage 2006; Wang et al. 2018). Results from a global statistical model and an integrated data analysis suggested that around 20% of the applied fertilizer N is leached in the form of NO3− worldwide (Lin et al. 2001; Zhou and Butterbach-Bahl 2014). A 15 N labelling study with cow urine in New Zealand reported that 16% of the added N was lost by NO3− leaching (Di et al. 2002). By contrast, other studies have demonstrated that a substantial portion of the leached NO3− comes from the mineralization of soil organic nitrogen (SON) or from the remineralization of fertilizer N which has accumulated in the soil organic matter (Addiscott 1996; Di and Cameron 2002; Mengis et al. 2001; Sebilo et al. 2013). It has been shown that the remineralization rates of accumulated SON can be high (Mengis et al. 2001) and long-lasting; Sebilo et al. (2013) found labelled NO3− being released from a cropped soil 28 years after application. In general, N fertilization accelerates N cycling with increased nitrification and NO3− leaching (Aber et al. 1989; McNulty et al. 2005). Given the limited understanding of the effect of fertilization on NO3− loss versus NO3− retention and remineralization, the quantitative contribution of CF and SON to NO3− loss remains unclear, especially in agroecosystems with intensive agriculture and heavy fertilization.
To meet the increasing demand for food, crops, and fiber, agriculture in humid subtropical China has been intensified over the past decades (Zhang et al. 2013). Arable soils in humid subtropical China cover about 446,890 km2, which accounts for 37% of China’s total arable land (Zhao 2002). Concomitant with extensive N fertilization and high atmospheric N deposition (31.7 kg ha−1) (Cui et al. 2012), NO3− export has increased significantly over the past 35 years, resulting in severe NO3− pollution in surface water (Li et al. 2019; Sun et al. 2008). Moreover, due to excessive fertilization, NO3− may accumulate in the soil, a phenomenon so far was only known from arid and semi-arid regions (Jia et al. 2018; Scanlon et al. 2008; Walvoord et al. 2003; Zhou et al. 2016). NO3− accumulation in deep horizons of a regolith was recently reported for uplands and orchards in humid subtropical China (Wu et al. 2019, 2020b; Yang et al. 2020a, 2020b). Deep soil NO3− accumulation acidifies subsoil and contaminates groundwater with NO3−. To find methods to curb NO3− pollution in humid, subtropical agroecosystems, it is important to quantify NO3− runoff loss and its long-term sources. Particularly, the question of how much of the NO3− comes from recent fertilization and how much from N accumulated over time (i.e., CF vs. SON) deserves attention for designing land management strategies that effectively reduce NO3− runoff and protect water resources.
The present study investigated the spatiotemporal variability of NO3− sources and transformations over one hydrological year in the Red Soil Critical Zone Observatory (RSCZO). The observatory with intensively planted mode and substantial N fertilization represents a typical agricultural watershed in humid subtropical China in which rain-fed uplands in hill slope and irrigated paddy fields in valley are tightly interspersed (Wang et al. 2019; Wu et al. 2020a). Completely, different hydrological processes of these two ecosystems result in large differences in NO3− sources and processes and make it difficult to accurately distinguish them at the whole watershed scale. Using SIAR model combined with water chemistry, the objectives were (1) to estimate the NO3− runoff loss in a subtropical agricultural watershed; (2) to identify the main sources of NO3− in the runoff, and (3) to quantitatively differentiate the contribution of CF and SON to the NO3− runoff loss.
Materials and methods
Study area
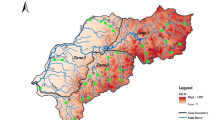
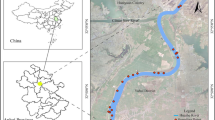
This study was carried out in the RSCZO, located in Yujiang County, Jiangxi Province, China, which is part of the Ecological Experimental Station of Red Soil, Chinese Academy of Sciences (28°15′ N, 116°55′ E) (Fig. 1). The area has a humid subtropical monsoon climate with a mean annual temperature of 17.8 °C and a mean annual rainfall of 1,795 mm (Gao et al. 2016). There is a significant seasonal change in precipitation, with over 50% of the precipitation occurring between April and June. The dominant soils in RSCZO are red soils derived from Quaternary red clay or weathered Cretaceous sandstone (Acrisols and Ferrasols; IUSS Working Group WRB 2015). The physical and chemical properties of the surface soils (0–15 cm) of RSCZO are summarized in Table 1.
The RSCZO is an agricultural watershed without human settlements where agricultural fields account for 93.3% of the area. The main land uses are upland fields (48.7%), paddy fields (24.8%), orchards (19.8%), and others land uses (6.7%) (Fig. 1). Fertilizers are usually applied to the main crops in April and July (Table 2). Based on the fertilization rates and areas of different land uses, the average annual fertilization rate in RSCZO was 229 kg N ha−1 yr−1. The chemical compositions of the applied fertilizers are shown in Table 3.
Apart from precipitation, the runoff in the RSCZO receives water from the upper Luxi River, a tributary of the Xinjiang River. The river water flows from the Luxi River into the RSCZO through the Baita channel, passing several villages and agricultural fields further upstream. The channel provides irrigation water to the agricultural fields, while it does not receive drainage water from the agricultural fields. Therefore, the channel only partly participates in the hydrological cycle of the agricultural fields. During the sampling period, the annual discharges at inlet and outlet were 2506 and 3686 mm, respectively. There is significant seasonal fluctuation, with higher runoff in the wet growing season (March to August) accounting for 62% and 80% of the total flow at the inlet and outlet (Fig. 2).
Sampling and analyses
From March 2018 to February 2019, to trace the main sources of NO3− in the runoff, rainwater was collected by an automatic rainwater collector (PSC-III, Qingdao PR Instrument Co., China) equipped with a high-density polyethylene (HDPE) bucket on the roof of an 8-m building in RSCZO; surface water samples were collected from the inlet and outlet of the watershed (Fig. 1). Only the wet deposition was collected under the control of a wetness detector. After each rainfall event, the rainwater stored in the bucket was transferred into a 1.5 L HDPE bottle manually. Runoff sample collecting was conducted in the stream central at inlet 2 and outlet manually twice a month (usually in the middle and end of the month). During the seasonal drought in the dry seasons (September to February), no surface water samples could be collected at the inlet. In total, 79 rainwater samples and 43 surface water samples were collected. All samples were filtered through 0.45-µm Millipore membrane filters and stored in a refrigerator at 4 °C before analysis. The sampling containers were pre-cleaned with acid (2 M HCl), rinsed with deionized water several times, and dried before use.
Precipitation was monitored and recorded by an auto-meteorological experimental sub-station (MAWS301, Vaisala Corporation, Finland) installed at the Ecological Experimental Station of Red Soil, 4 km away from RSCZO. The streamflow was monitored by V-notches with pressure gauges installed at the inlet and outlet (HOBO, Onset Computer Corporation, USA).
NH4+, NO3−, and total N concentrations were determined using a continuous flow analyzer (San + + System, Skalar Analytical B.V., Netherlands). The Cl− concentration was determined by ion chromatography (ICS-1100, Dionex Corporation, USA). The analysis of 15 N and 18O isotopic abundance in dissolved NO3− at natural abundance was carried out following “Azide method” (McIlvin and Altabet 2005; Ti et al. 2018; Wu et al. 2020a). In brief, the method is based on the chemical conversion of NO3− to nitrous oxide (N2O). The NO3− is reduced to NO2− on a cadmium column and then quantitatively converted to N2O with hydrazoic acid. The 15 N-N2O and 18O-N2O of the produced N2O were analyzed using an isotope ratio mass spectrometer (MAT 253plus, Thermo Fisher Scientific., Germany). 15 N-NO3− and 18O-NO3− values were calibrated against certified reference materials (USGS32 and USGS34) which received the same pre-treatment as the samples. The analytical precision was 0.3‰ for 15 N-NO3− and 0.5‰ for 18O-NO3−. One standard sample for each ten samples and three parallel samples (technical replicates) for each sample were set to ensure the accuracy of the chemical composition measurement. The values for 15 N and 18O are expressed in delta (δ) notation relative to N2 (air) and Vienna Standard Mean Ocean Water (V-SMOW) (Kendall 1998):
where \({R}_{sample}\) and \({R}_{standard}\) are the 15 N/14 N and 18O/16O ratios for sample and standard, respectively.
SIAR mixing model
To estimate the relative contribution of potential NO3− sources to NO3− in the runoff, the Bayesian mixing model provided in the R package SIAR (stable isotope analysis in R) was used, following the Bayesian model framework (Chen et al. 2009; Xue et al. 2012):
where \({X}_{ij}\) is the isotope value j of sample i, with j = 1 and 2, representing δ15N and δ18O, respectively, and i = 1, 2, 3, …, N; k is the potential NO3− source, k = 1, 2, 3, and 4, representing AP, M&S, CF, and SON, respectively, in this study; \({P}_{k}\) is the proportion of source k, which needs to be estimated by the SIAR model; \({S}_{jk}\) is the isotope value j of source k, which is normally distributed with mean \({\mu }_{jk}\) and standard deviation (SD) \({\omega }_{jk}^{2}\); \({C}_{jk}\) is the fractionation factor of isotope j for source k and is normally distributed with mean \({\uplambda }_{jk}\) and SD \({\uptau }_{jk}^{2}\); and \({\varepsilon }_{ik}\) is the residual error of the additional unquantified variation between individual samples and is normally distributed with mean 0 and SD σjk (Parnell et al. 2010).
In the present study, the mean values and standard deviations of NO3− isotopic signatures in AP were measured in rainwaters samples taken throughout the sampling year (Table 4). The values of CF and SON were obtained from a previous study in the same watershed (Wu et al. 2020a). The isotopic values of M&S were adopted from a study conducted in another agricultural watershed (Table 4) (Zhang et al. 2018a).
Statistical analysis and calculations
The extent of denitrification was estimated using the Rayleigh equation, which describes the evolution of the isotopic values of residual NO3− undergoing denitrification in a closed system, which can be expressed as (Mariotti et al. 1981)
where \({\updelta }_{r}\) is the δ15N values of residual NO3−; \({\updelta }_{0}\) is the δ15N values of initial NO3−; ε is the enrichment factor for denitrification; and \({{NO}_{3}^{-}}_{r}\) and \({{NO}_{3}^{-}}_{0}\) are the NO3− concentration of residual NO3− and initial NO3−, respectively. In this study, ε was set as − 15.9%, which is the widely cited value determined by Böttcher et al. (1990) for an agricultural watershed. The lowest observed δ15N-NO3− value (5.26‰) was set as the initial δ15N for NO3−o (Chen et al. 2009).
The runoff NO3− flux was calculated based on the NO3− concentrations in runoff and runoff discharge as follows (Li et al. 2019):
where \(F\) is the annual runoff NO3− flux (kg ha−1 yr−1); \({C}_{N-Runoff}\) is the NO3− concentrations of runoff samples (mg N L−1); \({Q}_{m-runoff}\) is the runoff discharge of each half month (m3); \(S\) is the area of RSCZO (ha), and 1000 is to convert the unit of the result into kg ha−1; usually, the samples collected in the middle of the month represent the first half of the month; the samples collected at the end of the month represent the second half of the month.
The NO3− runoff loss was calculated as follows:
where \(L\) is the NO3− runoff loss (kg ha−1 yr−1); \({L}_{i}\) is the NO3− runoff loss from potential NO3− source i (kg ha−1 yr−1), with i = 1, 2, 3, and 4, representing AP, M&S, CF, and SON, respectively; \({F}_{i-outlet}\) and \({F}_{i-inlet}\) are the annual runoff NO3− flux from source i at the outlet and inlet (kg ha−1 yr−1); \({F}_{outlet}\) and \({F}_{inlet}\) are the annual runoff NO3− flux at the outlet and inlet (kg ha−1 yr−1); and \({P}_{i-outlet}\) and \({P}_{i-inlet}\) are the proportions of source i at the outlet and inlet (%), which are estimated by the SIAR model.
The N deposition was calculated based on the N concentrations and rainfall of each rain event as follows:
where \({D}_{N}\) is the annual wet N deposition (kg ha−1 yr−1); \({C}_{N-Rainwater}\) is the N concentration of each rain event (mg N L−1); \(R\) is the rainfall of each rain even (mm); and 100 is to convert the unit of the result into kg ha−1.
All surface water samples were divided into four groups to investigate the temporal variation of the data and the difference between inlet and outlet: inlet in the dry season, inlet in the wet season, outlet in the dry season, and outlet in the wet season. One-way analysis of variance (ANOVA) was performed to test the difference in the measured variables (NO3−-N concentrations, 15 N-NO3−, and 18O-NO3−) between wet and dry seasons as well as between inlet and outlet. All statistical analyses were conducted by SPSS (version 22.0, IBM Corporation, USA). All figures were made with Origin (Version 2018, OriginLab Corporation, USA).
Results
Wet N deposition
Annual atmospheric wet inorganic N deposition contributed 22.4 kg N ha−1 yr−1, which amounted to 10% of the average fertilization rate in RSCZO. It consisted of 51% NH4+-N and 49% NO3−-N (11.5 and 10.9 kg N ha−1 yr−1, respectively).
Nitrate concentration and flux in runoff
The NO3− concentration at the inlet ranged from 0.24 to 1.07 mg N L−1, with a mean of 0.53 mg N L−1. The concentrations were relatively stable in time, with a low coefficient of variation (39%) (Fig. 3). No significant difference in mean NO3− concentrations was found between the dry season and wet season at the inlet (0.63 vs 0.45 mg N L−1, respectively) (P > 0.05) (Fig. S1).
At the outlet, the NO3− concentration varied from 0.62 to 4.17 mg N L−1, with a mean of 1.71 mg N L−1 (Fig. 3). The NO3− concentrations were consistently higher at the outlet than at the inlet, resulting in significantly higher mean NO3− concentrations at the outlet in both dry and wet seasons (P < 0.001) (Fig. S1). There was a significant seasonal difference in the mean NO3− concentrations at the outlet, with a significantly higher mean value in the dry season than in the wet season (2.11 vs 1.30 mg N L−1) (P < 0.01) (Fig. S1). During the wet season, the NO3− concentrations at the outlet were relatively low except for the first half of March (< 1.5 mg N L−1). Concentrations increased transiently after fertilization in May and July. During the dry season, the NO3− concentration increased to values > 1.5 mg N L−1 from October onwards.
Based on the monthly runoff discharge and NO3− concentrations (Eq. (7)), the monthly NO3− runoff flux for RSCZO was calculated. The annual NO3− fluxes at inlet and outlet were 11.8 and 46.3 kg ha−1 yr−1, respectively (Fig. S2). The monthly NO3− fluxes were significantly higher in the wet season than in the dry season, despite the lower NO3− concentrations in the wet season (P < 0.01) (Fig. S2). The monthly NO3− input from the inlet was lower than the output from the outlet during the entire sampling period, reflecting that RSCZO is a net NO3− source. The annual NO3− runoff loss generated by RSCZO was 34.5 kg N ha−1 yr—1, with 76% of it occurring in the wet season.
Nitrate isotopic composition in runoff
The δ15N-NO3− values ranged from + 4.79 to + 17.80‰ (mean = + 9.98‰) and from + 2.08 to + 10.98‰ (mean = + 7.78‰) at the inlet and outlet, respectively (Fig. 4a). The temporal dynamics of δ15N-NO3− values were almost identical between inlet and outlet. However, during most of the sampling period, the δ15N-NO3− values at the outlet were lower than at the inlet (Fig. 4a), albeit the difference between mean δ15N-NO3− values at the inlet and outlet was only significant in the wet season (P < 0.01) (Fig. S3a).
The δ18O-NO3− values varied from –5.13 to + 4.62‰ at the inlet, with a mean of + 0.69‰ (Fig. 4b). A 74% of the observations were smaller than 2.00‰. By contrast, 83% of δ18O-NO3− values at the outlet were larger than 2.00‰, varying from + 1.31 to + 8.97‰ (mean = + 3.66‰) (Fig. 4b). The δ18O-NO3− values at the outlet were higher than those at the inlet during the entire sampling period (Fig. 4b). Again, a significant difference between inlet and outlet was only found in the wet season (P < 0.001) (Fig. S3b). Temporally, a significant seasonal difference was found at the inlet, with higher δ18O-NO3− values in the dry season (p < 0.05) (Fig. S3b).
Source apportionment of nitrate based on dual isotopes and SIAR
Based on the analysis of NO3− dual isotopes, most samples fell within the source signatures of M&S, SON, and NH4+ in fertilizer or precipitation, indicating that these sources contribute significantly to the NO3− in the runoff (Fig. 5). None of the signatures matched the source signature of NO3− in fertilizer or precipitation.
Relationship between δ15N-NO3− and δ18O-NO3− in runoff. The isotopic composition of various potential sources was modified after Kendall et al. (2007) and Silva et al. (2002); the “NO3− in precipitation,” “NO3− fertilizer,” “NH4+ in fertilizer or precipitation,” “soil N,” and “manure and sewage” represent that the δ15N and δ18O of NO3− which was derived from this
According to the SIAR mixing model, the proportional contribution of the four potential NO3− sources, SON, AP, M&S, and CF, varied independently of season (Fig. 6). SON, M&S, and CF were the dominant NO3− sources, while the contribution of AP was negligible. At the inlet, the order of contribution in the wet season and dry season was as follows: M&S (40–47%) > CF (15–19%) > SON (37–40%) > AP (1%) (Fig. 6a, b). At the outlet, the contribution of CF increased by 12% in both seasons relative to the inlet, while the contribution of M&S decreased by 22% and 7% in the wet season and dry season, respectively. SON (49–52%) was the dominant NO3− source at the outlet, followed by M&S (25–33%), and CF (14–25%), while the contribution of AP was negligible (1%) (Fig. 6c, d). CF contributed more in the wet season (25%) than in the dry season (14%) at the outlet (Fig. 6).
Proportional contribution of four potential NO3− sources in runoff at the inlet in the wet season (a), inlet in the dry season (b), outlet in the wet season (c), and outlet in the dry season (d) estimated using SIAR. SON, AP, M&S, and CF represent N in soil organic matter, NO3− in atmospheric precipitation, manure/sewage, and chemical fertilizers, respectively
Nitrate runoff loss from different nitrate sources
By subtracting NO3− flux at the inlet from that at the outlet (Eqs. (8)–(11)), the NO3− runoff loss from different NO3− sources was calculated (Fig. 7). In the entire sampling year, SON (18.53 kg N ha−1 yr−1), CF (8.36 kg N ha−1 yr−1), and M&S (7.43 kg N ha−1 yr−1) were the most important sources of NO3− runoff loss (Fig. 7). The contribution of CF was larger in the wet season (28%) than in the dry season (12%) (Fig. 7).
Discussion
Biogeochemical transformation of runoff nitrate
Nitrate derived from nitrification
The concentration of NO3− is affected by biogeochemical processes, such as nitrification and denitrification, which are both important drivers of the N cycle (Aravena and Mayer 2010; Kendall 2007). It has been demonstrated that NO3− derived from nitrification is depleted in 18O and has δ18O-NO3− values ranging from − 10 to + 10‰ (Kendall 2007). In the present study, samples fell within this range, indicating that nitrification is the dominating process of NO3− production in RSCZO (Fig. 5). As shown by the SIAR results, SON, M&S, and CF were the main NO3− sources (Fig. 6). Fertilizers applied in RSCZO were mainly urea and compound fertilizer (Table 2). Thus, the NO3− in the runoff of RSCZO was mainly derived from nitrification of mineralized SON, NH4+ from sewage, and urea-containing fertilizers.
Nitrate removal by denitrification
Denitrification in the water environment and locally anoxic soil pockets is the main process of removing NO3−, thus lowering the concentration of NO3− in the runoff (Aravena and Mayer 2010; Rivett et al. 2008). Unlike NO3−, chloride (Cl−) is conservative and not subject to physical, chemical, and biological processes (Liu et al. 2006). Therefore, the molar NO3−/Cl− ratio can be used as an indicator for the extent of denitrification in the watershed (Kellman and Hillaire-Marcel 1998; Li et al. 2010; Liu et al. 2006). Denitrification removes NO3− from the water environment, which results in an enrichment of heavy nitrate isotopes in residual NO3− concomitant with a decrease of NO3−/Cl− ratios (Kendall 2007; Otero et al. 2009; Yue et al. 2014). We found a significant negative correlation between δ15N-NO3− and the NO3−/Cl− as well as ln (NO3−) in the dry season (P < 0.05), suggesting that there was denitrification occurring in the dry season (Fig. S4). The RSCZO has a subtropical monsoonal climate. Based on data obtained from the Yujiang weather station (1981–2010), the monthly mean temperature in the dry season ranges from 5.4 to 24.7 °C, which suggests that there can be substantial denitrification activity even in the cooler dry season (Chen et al. 2009; Li et al. 2019). Compared with the dry season, there was no significant correlation between δ15N-NO3− and NO3−/Cl− or ln (NO3−) in the wet season (P > 0.05), indicating that denitrification was small in the wet season (Fig. S4). This is in agreement with other studies in the same area which found weak or insignificant denitrification during the wet season (Wang et al. 2019; Wu et al. 2020a). One explanation for this unexpected finding is that the residence time of NO3− might be too short for significant denitrification during the wet season. Intense rainfalls cause large runoff episodes (Fig. 2) during which NO3− may bypass anoxic zones in the watershed (Baron et al. 2013; He et al. 2011; Yu et al. 2018, 2019). Another factor that could account for the absence of denitrification in the wet season is that the denitrification signal might be masked by the dilution effect or intense exchange and mixing of water bodies with denitrified NO3− in the wet season (Liu et al. 2018; Xia et al. 2017).
During the dry season when the runoff discharge was relatively low, the amount of denitrified NO3− was calculated based on the Rayleigh equation (Eq. (6)), suggesting that 19% of the NO3− had been removed from the runoff by denitrification. Based on the denitrification ratio and the NO3− flux, the NO3− removal by denitrification in the dry season was estimated to be 2.73 kg N ha−1 in the RSCZO.
Sources of runoff nitrate at the inlet and outlet
SON, M&S, and CF were the dominant NO3− sources, indicating that nonpoint source pollution from agriculture and point source pollution by domestic sewage dominated the NO3− runoff (Figs. 5–6). The runoff at inlet of RSCZO comes from the Baita channel, which flows through several villages with dense human populations and agricultural fields with heavy fertilization. Due to the large distribution of agricultural fields in the adjacent area, the nitrification of urea-containing fertilizers and mineralized SON led to abundant NO3− production and the high contributions of SON and CF to NO3− in runoff (Zhang et al. 2014, 2021). However, the runoff in the Baita channel only partly participates in the hydrological cycle in the adjacent agricultural fields. Therefore, before flowing into RSCZO, the effect of agricultural activities was limited. In the nearby rural area, the domestic sewage from living quarters and livestock farms is discharged directly into the channel, which could explain the prominent role of M&S for nitrate at the inlet (Han et al. 2015; Zhang et al. 2018b). As an important inorganic N source of the watershed, the atmospheric inorganic N deposition was up to 10% of the average fertilization rate in the RSCZO. However, compared with the above sources, the contribution of AP to runoff NO3− was minor (Fig. 6). The main reason might be that most NO3− in AP has already been altered by microbial nitrification before entering the runoff (Buda and Dewalle 2009; Lee et al. 2008; Matiatos 2016; Vrzel et al. 2016).
The relative importance of NO3− sources varied with season, with CF having a larger contribution in the wet season (25%) than the dry season (14%) (Fig. 6). This shows that the fertilization contributes to NO3− as it is usually applied in the wet season (Table 2). In the study area, 70% of rainfall occurred in the wet season (Wang et al. 2019), obviously leaching NO3− derived from nitrification of urea-containing fertilizers. Besides, intensive rainfall conditions may flush soil particulates that contain NO3− and urea-containing fertilizers into the runoff, particularly after soil tillage (Berhe and Torn 2017; Li et al. 2019). It has been demonstrated that mineralization of SON could contribute a lot to NO3− leaching and high NO3− concentrations in runoff especially in the dry season when crops are harvested (Addiscott 1996; Di and Cameron 2002; Poch-Massegú et al. 2014; Schlegel et al. 2005; Thomsen et al. 2010). The unusually high NO3− concentration in March in the runoff may thus be the result of SON mineralization during the previous dry season and the relatively low runoff discharge in March (Fig. 3).
Nitrate runoff loss in the RSCZO
In the RSCZO, there was a net NO3− runoff loss of 34.5 kg N ha−1 yr−1, accounting for 16% of the local annual N fertilization (Fig. 7). This can be mainly explained by the N fertilization in RSCZO. Fertilization directly increases NO3− production by promoting the nitrification of urea-containing fertilizers (Cameron et al. 2013; Zhao and Xing 2009). Meanwhile, as discussed above, SON mineralization may also result in abundant NO3− production and leaching. Over the last three decades, N fertilizer consumption in Chinese has increased greatly, but not all can be utilized by crops (Ju et al. 2007; Guo et al. 2010). As a result, excess fertilizer N which is not leached is incorporated into the soil organic matter (Di et al. 2002; Ju et al. 2007; Sebilo et al. 2013). Meanwhile, there is a large amount of fertilizer N returning to the soil through straw mulching (Li and Li 2017), which may be released in subsequent years. It has been proposed that cultivation in subsequent years enhances the mineralization of accumulated SON and further increases the leaching of NO3− derived from nitrification of mineralized SON (Francis et al. 1995; Sebilo et al. 2013). Fertilization can result in NO3− loss by accelerating NO3− loss derived from the nitrification of chemical fertilizers and mineralized SON. The contribution of SON may thus be added to CF (CF + SON) and be used to assess the overall effect of fertilization on NO3− loss. Taking this approach, 78% of the NO3− runoff loss was caused by fertilization (26.9 N ha−1 yr−1), 78% of which occurred in the wet season (Fig. 7). By labelling the fertilizer with 15 N, it has been proven that most of the NO3− leaching as affected by fertilization is not from CF; rather, it comes from the mineralization of SON (Di and Cameron 2002; Gurmesa et al. 2016; Sebilo et al. 2013). Interestingly, in the present study, the contribution of SON (54%) was higher than that of CF (24%) (Fig. 7). Though the mineralization and immobilization processes will mask the effect of CF by changing the isotopic fingerprint of NO3− from CF (Mengis et al. 2001), the contribution rate twice as high as that of CF still indicated a dominant role of SON in NO3− runoff loss. Thus, to effectively control the NO3− runoff loss in the agricultural area, NO3− loss derived from long-term accumulated SON should be taken into account.
As a result of the agronomic intensification and economic growth over the last 20 years in China, more than 3.0 × 1010 m3 of sewage derived from domestic and industrial sources is discharged into rivers and/or used for irrigation of agricultural fields with low-level or no treatment (Chen et al. 2005). Sewage effluent has been shown to be a major source of NO3− pollution in the seriously polluted rivers of China (Liu et al. 2013; Zhang et al. 2014). In this study, 22% of the NO3− runoff loss was derived from M&S (7.43 kg N ha−1 yr−1), indicating that M&S is also an important source of NO3− runoff loss in RSCZO (Fig. 7). Though there is no human settlement in the RSCZO, some local farmers (5 families) live across the road from the northern boundary of the RSCZO. The sewage effluent discharged from these families might attribute to the NO3− runoff loss from M&S. Therefore, in addition to developing rational fertilization schemes and improving N fertilizer use efficiency, better wastewater treatment systems and technologies are needed to control the NO3− pollution in agricultural areas.
Limitations and implications of this research
The present study aimed to assess the contribution of fertilizer N to NO3− runoff loss and denitrification loss in an agricultural model watershed in subtropical China, based on concentrations and isotopic signatures of NO3− in inlet and outlet. However, this approach has noteworthy uncertainties:
-
1. The method of isotope analysis fails to distinguish nitrate from nitrite, and the possible presence of nitrite in runoff samples will bias the obtained isotopic compositions (Xue et al. 2009; Zhang et al. 2018b). In the present study, the nitrite concentrations in the runoff at RSCZO are relatively low (0.02 ± 0.02 mg N L−1) (unpublished data) compared with NO3−, which results in a negligible bias.
-
2. Although the SIAR model effectively quantifies the proportional contributions of different sources, uncertainties exist when using only two isotopic signals (δ15N-NO3− and δ18O-NO3−) to trace four potential sources. The δ15N-NO3− and δ18O-NO3− values of the four potential NO3− sources have relatively wide ranges and show overlap. Small variations in isotopic composition of NO3− could lead to large changes in source apportionment obtained by SIAR (Xue et al. 2009, 2012). N transformations, including nitrification and denitrification, result in isotopic fractionation, and the quantitative basis is lacking in analyzing the degree of fractionation (Zhang et al. 2018b). As a result, the change of the compositions of original δ15N-NO3− and δ18O-NO3− caused by isotopic fractionation will aggrandize the uncertainty in the SIAR analysis. It has been demonstrated that denitrification affects the isotopic signature of nitrate depending on the sources and may thus distort the results of the source apportionment (Zhang et al. 2018b). In the present study, the results indicated that denitrification was weak in the runoff during the dry season with only 2.73 kg N ha−1 NO3− removed by denitrification. This partly justifies to not take account for isotopic fractionation by denitrification in the SIAR modeling as it would largely increase the uncertainty in the SIAR analysis. Besides denitrification, the “mineralization–immobilization turnover” will change the isotopic fingerprint of NO3−−from CF and hence affect the distinction of the contribution from CF and SON, partly masking the effect of CF (Deutsch et al. 2006b; Mengis et al. 2001; Nestler et al. 2011). However, the dual isotope method can still trace NO3− sources if the NO3− is rapidly transported through the soils (Mengis et al. 2001). A recent study conducted in the RSCZO showed that the residence time of waters in RSCZO is relatively in the rain period when N input occurs (Wang et al. 2019). The unassimilated NO3− produced from CF leached rapidly along with the heavy rainfall. Moreover, the choice of sampling in autumn and winter supports the quality of our results as microbial processes are slow due to lower temperatures which also decreases the effect of microbial processes on the isotopic composition of NO3− (Deutsch et al. 2006a; Nestler et al. 2011).
-
3. As discussed in the section “Nitrate runoff loss in the RSCZO,” fertilization increases NO3− production directly by promoting nitrification of urea-containing fertilizers and indirectly by enhancing the mineralization of SON. Therefore, both the contributions of CF and SON are assumed to be driven by fertilization. Since not all the SON is derived from fertilizer N, this assumption may lead to an overestimation of the contribution of fertilization to NO3− runoff loss. However, in agricultural fields with long-term fertilization, all SON will eventually be derived from fertilizer N applied during the crop growing season (Addiscott 1996; Di and Cameron 2002). It has been shown that remineralization of fertilizer N accumulated in the SON may gradually contribute to NO3− leaching more than a quarter century after fertilization (Sebio et al. 2013). Therefore, most of the contribution of SON may be attributed to previous fertilization, and the overestimation should be negligible.
-
4. The estimation of denitrification losses was based on the assumption that the watershed is a relatively closed system, while certain processes, such as the exchange of N in groundwater in the river aquifer during the monsoon season, were ignored. In fact, surface water and groundwater are both important pathways of NO3− loss. In this study, only surface water was taken into consideration, rendering an underestimation of NO3− pollution. Therefore, to allow for a more accurate and systematic evaluation of NO3− pollution in agricultural watersheds, the source identification of NO3− in groundwater recharge should be analyzed in future work.
Despite the above limitations and uncertainties, the results obtained from this study have multiple impacts:
-
1. This study provided a simple and effective method to trace the sources of NO3− runoff and loss in subtropical agricultural watersheds with mixed land uses. By comparing the differences in fluxes and sources of NO3− between input and output of the watershed, the NO3− runoff loss and its main sources could be effectively quantified at the whole watershed scale.
-
2. This study is important for environmental managers and policymakers when proposing more targeted environmental management strategies in humid subtropical agricultural regions. Using SIAR model, this study quantitatively differentiated the contribution of SON and CF to NO3− runoff loss in a typical subtropical agricultural watershed with long-term intensive fertilization. The results showed that SON plays a dominant role in NO3− runoff loss, reflecting the accumulation of reactive N in agricultural soils by decadal over-fertilization and reinforcing the significances of it as a source of NO3− pollution of surface waters and groundwaters. The study also showed that, besides fertilization, sewage is an important source for NO3− runoff loss. Thus, adequate control of nonpoint sources from agriculture and point sources from domestic sewage should work together to effectively reduce the NO3− runoff loss in subtropical agricultural regions. Adopting advanced techniques to improve the N use efficiency of chemical fertilizers, reducing fertilizer N accumulation in soil organic matter, setting a stricter effluent standard, and upgrading domestic sewage treatment are the essential measures in controlling NO3− loss in subtropical China.
Conclusion
The main sources of the NO3− in the runoff from RSCZO were in the order of SON > M&S > CF. There was obvious seasonal variation in the contribution of various sources, with more contribution of CF in the wet season. Denitrification in the runoff was small and only observed in the dry season. The RSCZO is a net source for NO3−. Nonpoint source pollution from fertilization (SON + CF) was identified as the main source of NO3− runoff loss, with SON playing a more important role than CF in the NO3− runoff loss. Sewage was identified as another important source. To alleviate NO3− pollution from subtropical agriculture, rational fertilization schemes, better management of SON, and improved wastewater treatment should be advocated.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM (1989) Nitrogen saturation in northern forest ecosystems. Bioscience 39:378–386
Addiscott TM (1996) Fertilizers and nitrate leaching. Issues Environ Sci Technol 5:1–26
Aravena R, Mayer B (2010) Isotopes and processes in the nitrogen and sulfur cycles. In: Aelion CM, Höhener P, Hunkeler D, Aravena R (eds) Environmental isotopes in biodegradation and bioremediation. Taylor & Fransis Group, Boca Raton, pp 203–246
Baron JS, Hall EK, Nolan BT, Finlay JC, Bernhardt ES, Harrison JA, Chan F, Boyer EW (2013) The interactive effects of excess reactive nitrogen and climate change on aquatic ecosystems and water resources of the United States. Biogeochemistry 114:71–92
Berhe AA, Torn MS (2017) Erosional redistribution of topsoil controls soil nitrogen dynamics. Biogeochemistry 132:1–18
Bernal S, Butturini A, Sabate F (2006) Inferring nitrate sources through end member mixing analysis in an intermittent Mediterranean stream. Biogeochemistry 81:269–289
Böttcher J, Strebel O, Voerkelius S, Schmidt HL (1990) Using isotope fractionation of nitrate nitrogen and nitrate oxygen for evaluation of denitrification in a sandy aquifer. J Hydrol (amst) 114:413–424
Buda AR, Dewalle DR (2009) Dynamics of stream nitrate sources and flow pathways during stormflows on urban, forest and agricultural watersheds in central Pennsylvania, USA. Hydrol Processes 23:3292–3305
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann App Biol 162:145–173
Chen F, Jia G, Chen J (2009) Nitrate sources and watershed denitrification inferred from nitrate dual isotopes in the Beijiang River, south China. Biogeochemistry 94:163–174
Chen JY, Tang CY, Sakura Y, Yu JJ, Fukushima Y (2005) Nitrate pollution from agriculture in different hydrogeological zones of the regional groundwater flow system in the North China Plain. Hydrogeol J 13:481–492
Cui Cui J, Zhou J, Peng Y, He YQ, Chan A (2012) Atmospheric inorganic nitrogen in wet deposition to a red soil farmland in Southeast China, 2005–2009. Plant Soil 359:387–395
Deutsch B, Mewes M, Liskow I, Voss M (2006a) Quantification of diffuse nitrate inputs into a small river system using stable isotopes of oxygen and nitrogen in nitrate. Org Geochem 37:1333–1342
Deutsch B, Kahle P, Voss M (2006b) Assessing the source of nitrate pollution in water using stable N and O isotopes. Agron Sustainable Dev 26:263–267Di HJ, Cameron KC (2002) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cycl Agroecosys 46:237–256
Di HJ, Cameron KC (2002) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cycl Agroecosys 46:237–256
Di HJ, Cameron KC, Silva RG, Russell JM, Barnett JW (2002) A lysimeter study of the fate of 15N-labelled nitrogen in cow urine with or without farm dairy effluent in a grazed dairy pasture soil under flood irrigation. New Zeal J Agr Res 45:235–244
Ding J, Xi B, Gao R, He L, Liu H, Dai X, Yu Y (2014) Identifying diffused nitrate sources in a stream in an agricultural field using a dual isotopic approach. Sci Total Environ 484:10–18
Dong Y, Yang JL, Zhao XR, Yang SH, Zhang GL (2021) Contribution of different proton sources to the acidification of red soil with maize cropping in subtropical China. Geoderma 392:114995
Erisman JW, Galloway JN, Seitzinger S, Bleeker A, Dise NB, Petrescu AR (2013) Consequences of human modification of the global nitrogen cycle. Philos Trans R Soc B Biol Sci 368:1621
Francis GS, Haynes RJ, Williams PH (1995) Effect of the timing of ploughing-in temporary leguminous pastures and two winter cover crops on nitrogen mineralization, nitrate leaching and spring wheat growth. J Agr Sci 24:1–9
Gao L, Lv YJ, Wang DD, Muhammad T, Biswas A, Peng XH (2016) Soil water storage prediction at high space–time resolution along an agricultural hillslope. Agr Water Manage 165:122–130
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KW, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010
Gurmesa GA, Lu X, Gundersen P, Mao Q, Zhou K, Fang Y, Mo J (2016) High retention of 15N-labeled nitrogen deposition in a nitrogen saturated old-growth tropical forest. Global Change Biol 22:3608–3620
Han Z, Dan Z, Shi G, Shen L, Xu W, Xie Y (2015) Characteristics and management of domestic waste in a rural area of the Tibetan Plateau. J Air Waste Manage 65:1365–1375
He B, Kanae S, Oki T, Hirabayashi Y, Takara K (2011) Assessment of global nitrogen pollution in rivers using an integrated biogeochemical modeling framework. Water Res 45:2573–2586
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, Update 2015, International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. Food and Agriculture Organization of the United Nations, Rome, Italy.
Jia X, Zhu Y, Huang L, Wei X, Fang Y, Wu L, Binley A, Shao M (2018) Mineral N stock and nitrate accumulation in the 50 to 200 m profile on the loess plateau. Sci Total Environ 633:999–1006
Ju XT, Kou CL, Christie P, Dou ZX, Zhang FS (2007) Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Enviro Pollut 145:497–506
Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc Natl Acad Sci USA 106:3041–3046
Kellman L, Hillaire-Marcel C (1998) Nitrate cycling in streams: using natural abundances of NO3–δ15N to measure in-situ denitrification. Biogeochemistry 43:273–292
Kendall C (1998) Tracing nitrogen sources and cycling in catchments. In: Kendall C, McDonnell JJ (eds) Isotope Tracers in Catchment Hydrology. Elsevier BV, Amsterdam, pp 531–547
Kendall C, Elliott EM, Wankel SD (2007) Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener RH, Lajtha K (eds) Stable Isotopes in Ecology and Environmental Science, 2nd edn. Blackwell, Oxford, pp 375–449
Lee KS, Bong YS, Lee D, Kim Y, Kim K (2008) Tracing the sources of nitrate in the Han River watershed in Korea, using delta15N-NO3- and delta18O-NO3- values. Sci Total Environ 395:117–124
Li X, Li S (2017) Temporal and spatial distribution characteristics of crop straw nutrient resources and returning to agricultural fields in China. Trans Chin Soc Agr Eng 33:1–19 (In Chinese)
Li SL, Liu CQ, Li J, Liu XL, Chetelat B, Wang BL, Wang FS (2010) Assessment of the sources of nitrate in the Changjiang River, China using a nitrogen and oxygen isotopic approach. Environ Sci Technol 44:1573–1578
Li C, Li SL, Yue FJ, Liu J, Zhong J, Yan ZF, Zhang RC, Wang ZJ, Xu S (2019) Identification of sources and transformations of nitrate in the Xijiang River using nitrate isotopes and Bayesian model. Sci Total Environ 646:801–810
Lin BL, Sakoda A, Shibasaki R, Suzuki M (2001) A modelling approach to global nitrate leaching caused by anthropogenic fertilisation. Water Res 35:1961–1968
Liu CQ, Li SL, Lang YC, Xiao HY (2006) Using delta15N- and delta18O-values to identify nitrate sources in karst ground water, Guiyang, southwest China. Environ Sci Technol 40:6928
Liu T, Wang F, Michalski G, Xia XH, Liu SD (2013) Using (15)n, (17)o, and (18)o to determine nitrate sources in the Yellow River, China. Environ Sci Technol 47:13412–13421
Liu S, Wu F, Feng W, Guo W, Song F, Wang H, Wang Y, He Z, Giesy JP, Zhu P, Tang Z (2018) Using dual isotopes and a Bayesian isotope mixing model to evaluate sources of nitrate of Tai Lake, China. Environ Sci Pollut Res Int 25:32631–32639
Ma L, Velthof GL, Wang FH, Qin W, Zhang WF, Liu Z, Zhang Y, Wei J, Lesschen JP, Ma WQ, Oenema O, Zhang FS (2012) Nitrogen and phosphorus use efficiencies and losses in the food chain in China at regional scales in 1980 and 2005. Sci Total Environ 434:51–61
Mariotti A, Germon J, Hubert P, Kaiser P, Letolle R, Tardieux A, Tardieux P (1981) Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes. Plant Soil 62:413–430
Matiatos I (2016) Nitrate source identification in groundwater of multiple land-use areas by combining isotopes and multivariate statistical analysis: a case study of Asopos basin (Central Greece). Sci Total Environ 541:802–814
McIlvin MR, Altabet MA (2005) Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal Chem 77:5589–5595
McNulty SG, Boggs J, Aber JD, Rustad L, Magill A (2005) Red spruce ecosystem level changes following 14 years of chronic N fertilization. For Ecol Manag 219:279–291
Mengis M, Walther U, Bernasconi SM, Wehrli B (2001) Limitations of using delta 18o for the source identification of nitrate in agricultural soils. Environ Sci Technol 35:1840–1844
Nestler A, Berglund M, Accoe F, Duta S, Xue DM, Boeckx P, Taylor P (2011) Isotopes for improved management of nitrate pollution in aqueous resources: review of surface water field studies. Environ Sci Pollut Res 18:519–533
Nikolenko O, Jurado A, Borges AV, Knller K, Brouyre S (2017) Isotopic composition of nitrogen species in groundwater under agricultural areas: a review. Sci Total Environ 621:1415–1432
Otero N, Torrentó C, Soler A, Menció A, Maspla J (2009) Monitoring groundwater nitrate attenuation in a regional system coupling hydrogeology with multi-isotopic methods: the case of Plana de Vic (Osona, Spain). Agr Ecosyst Environ 133:103–113
Padilla FM, Gallardo M, Manzano-Agugliaro F (2018) Global trends in nitrate leaching research in the 1960–2017 period. Sci Total Environ 643:400–413
Panno SV, Kelly WR, Hackley KC, Hwang HH, Martinsek AT (2008) Sources and fate of nitrate in the Illinois River Basin, Illinois. J Hydrol 359:174–188
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PloS one 5:e9672
Parra Suarez S, Peiffer S, Gebauer G (2019) Origin and fate of nitrate runoff in an agricultural catchment: Haean, South Korea - comparison of two extremely different monsoon seasons. Sci Total Environ 648:66–79
Perego A, Basile A, Bonfante A, De Mascellis R, Terribile F, Brenna S, Acutis M (2012) Nitrate leaching under maize cropping systems in Po Valley (Italy). Agric Ecosyst Environ 147:57–65
Poch-Massegú R, Jiménez-Martínez J, Wallis KJ, de Cartagena FR, Candela L (2014) Irrigation return flow and nitrate leaching under different crops and irrigation methods in Western Mediterranean weather conditions. Agri Water Manag 134:1–13
Rivett MO, Buss SR, Morgan P, Smith JWN, Bemment CD (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res 42:4215–4232
Ruidisch M, Bartsch S, Kettering J, Huwe B, Frei S (2013) The effect of fertilizer best management practices on nitrate leaching in a plastic mulched ridge cultivation system. Agric Ecosyst Environ 169:21–32
Scanlon BR, Reedy RC, Bronson KF (2008) Impacts of land use change on nitrogen cycling archived in semiarid unsaturated zone nitrate profiles, Southern High Plains. Texas Environ Sci Technol 42:7566–7572
Schlegel AJ, Grant CA, Havlin JL (2005) Challenging approaches to N fertilizer recommendations in continuous cropping systems in the great plains. Agron J 97:391–398
Sebilo M, Mayer B, Nicolardot B, Pinay G, Mariotti A (2013) Long-term fate of nitrate fertilizer in agricultural soils. Proc Natl Acad Sci USA 110:18185–18189
Serio F, Miglietta PP, Lamastra L, Ficocelli S, Intini F, De Leo F, De Donno A (2018) Groundwater nitrate contamination and agricultural land use: a grey water footprint perspective in Southern Apulia Region (Italy). Sci Total Environ 645:1425–1431
Sieling K, Kage H (2006) N balance as an indicator of N leaching in an oilseed rape – winter wheat – winter barley rotation. Agr Ecosyst Environ 115:261–269
Silva SR, Ging PB, Lee RW, Ebbert JC, Tesoriero AJ, Inkpen EL (2002) Forensic applications of nitrogen and oxygen isotopes in tracing nitrate sources in urban environments. Environ Forensics 3:125–130
Smil V (1999) Nitrogen in crop production: an account of global flows. Global Biogeochem Cy 13:647–662
Song Y, Xie S, Zhang Y, Zeng L, Salmon LG, Zheng M (2006) Source apportionment of PM2. 5 in Beijing using principal component analysis/absolute principal component scores and UNMIX. Sci Total Environ 372:278–286
Sun B, Chen DL, Li Y, Wang, XX (2008) Nitrogen leaching in an upland cropping system on an acid soil in subtropical China: lysimeter measurements and simulation. Nutr Cycl Agroecosys 81:291–303
Thomsen IK, Lægdsmand M, Olesen JE (2010) Crop growth and nitrogen turnover under increased temperatures and low autumn and winter light intensity. Agric Ecosyst Environ 139:187–194
Ti C, Wang X, Yan X (2018) Determining delta(15)N-NO3- values in soil, water, and air samples by chemical methods. Environ Monit Assess 190:341
Verchot LV, Franklin EC, Gilliam JW (1997) Nitrogen Cycling in Piedmont Vegetated Filter Zones: I. Surface Soil Processes J Environ Qual 26:327–336
Vrzel J, Vuković-Gačić B, Kolarević S, Gačić Z, Kračun-Kolarević M, Kostić J, Aborgiba M, Farnleitner A, Reischer G, Linke R (2016) Determination of the sources of nitrate and the microbiological sources of pollution in the Sava River Basin. Sci Total Environ 573:1460–1471
Walvoord MA, Phillips FM, Stonestrom DA, Evans RD, Hartsough PC, Newman BD, Striegl RG (2003) A reservoir of nitrate beneath desert soils. Science 302:1021–1024
Wang X, Zou C, Gao X, Guan X, Zhang Y, Shi X, Chen X (2018) Nitrate leaching from open-field and greenhouse vegetable systems in China: a meta-analysis. Environ Sci Pollut Res Int 25:31007–31016
Wang Y, Gao L, Peng X (2019) Hydrologic separation and their contributions to N loss in an agricultural catchment in hilly red soil region. Sci China Earth Sci 62:1–14
Wu H, Song X, Zhao X, Peng X, Zhou H, Hallett PD, Hodson M, Zhang G (2019) Accumulation of nitrate and dissolved organic nitrogen at depth in a red soil critical zone. Geoderma 337:1175–1185
Wu H, Dong Y, Gao L, Song X, Liu F, Peng X, Zhang G (2020) Identifying nitrate sources in surface water, regolith and groundwater in a subtropical red soil critical zone by using dual nitrate isotopes. Catena 198:104994
Wu H, Song X, Liu F, Zhao X, Zhang G (2020) Regolith property controls on nitrate accumulation in a typical vadose zone in subtropical China. Catena 192:104589
Xia Y, Li Y, Zhang X, Yan X (2017) Nitrate source apportionment using a combined dual isotope, chemical and bacterial property, and Bayesian model approach in river systems. J Geophys Res-Biogeo 122:2–14
Xue DM, Botte J, De Baets B, Accoe F, Nestler A, Taylor P, Vam Cleemput O, Berglund M, Boeckx P (2009) Present limitations and future prospects of stable isotope methods for nitrate source identification in surface-and groundwater. Water Res 43:1159–1170
Xue DM, De Baets B, Van Cleemput O, Hennessy C, Berglund M, Boeckx P (2012) Use of a Bayesian isotope mixing model to estimate proportional contributions of multiple nitrate sources in surface water. Environ Pollut 161:43–49
Xue DM, Pang F, Meng F, Wang Z, Wu W (2015) Decision-tree-model identification of nitrate pollution activities in groundwater: a combination of a dual isotope approach and chemical ions. J Contam Hydrol 180:25–33
Yang XL, Lu YL, Tong YA, Yin XF (2014) A 5-year lysimeter monitoring of nitrate leaching from wheat–maize rotation system: comparison between optimum N fertilization and conventional farmer N fertilization. Agr Ecosyst Environ 199:34–42
Yang SH, Wu HY, Dong Y, Zhao X, Song X, Yang L, Hallett PD, Zhang GL (2020a) Deep nitrate accumulation in a highly weathered subtropical critical zone depends on the regolith structure and planting year. Environ Sci Technol 54:13739–13747
Yang SH, Wu HY, Song XD, Dong Y, Zhao XR, Cao Q, Yang JL, Zhang GL (2020) Variation of deep nitrate in a typical red soil critical zone: effects of land use and slope position. Agr Ecosyst Environ 297:106966
Yu Q, Wang F, Li X, Yan W, Li Y, Lv S (2018) Tracking nitrate sources in the Chaohu Lake, China, using the nitrogen and oxygen isotopic approach. Environ Sci Pollut R 25:19518–19529
Yu LF, Mulder J, Zhu J, Zhang KS, Wang ZW, Dörsch P (2019) Denitrification as a major regional nitrogen sink in subtropical forest catchments: evidence from multi-site dual nitrate isotopes. Global Change Biol 25:1765–1778
Yue FJ, Liu CQ, Li SL, Zhao ZQ, Liu XL, Ding H, Liu BJ, Zhong J (2014) Analysis of δ15N and δ18O to identify nitrate sources and transformations in Songhua River, Northeast China. J Hydrol 519:329–339
Yue FJ, Li SL, Hu J (2015) The contribution of nitrate sources in Liao Rivers, China, based on isotopic fractionation and bayesian mixing model. Procedia Earth & Planetary Science 13:16–20
Zhang J, Zhu T, Meng T, Zhang Y, Yang J, Yang W, Müller C, Cai Z (2013) Agricultural land use affects nitrate production and conservation in humid subtropical soils in China. Soil Biol Biochem 62:107–114
Zhang Y, Li F, Zhang Q, Li J, Liu Q (2014) Tracing nitrate pollution sources and transformation in surface- and ground-waters using environmental isotopes. Sci Total Environ 490:213–222
Zhang Y, Shi P, Li F, Wei A, Song J, Ma J (2018a) Quantification of nitrate sources and fates in rivers in an irrigated agricultural area using environmental isotopes and a Bayesian isotope mixing model. Chemosphere 208:493–501
Zhang Y, Shi P, Song J, Li Q (2018b) Application of nitrogen and oxygen isotopes for source and fate identification of nitrate pollution in surface water: a review. Appl Sci 9:18
Zhang S, Zhang G, Wang D, Liu Q, Xu M (2021) Investigation into runoff nitrogen loss variations due to different crop residue retention modes and nitrogen fertilizer rates in rice-wheat cropping systems. Agri Water Manage 247:106729
Zhao QG (2002) Material cycling and regulation in red soils of China. Science Press, Beijing (in Chinese)
Zhao X, Xing GX (2009) Variation in the relationship between nitrification and acidification of subtropical soils as affected by the addition of urea or ammonium sulfate. Soil Biol Biochem 41:2584–2587
Zhou M, Butterbach-Bahl K (2014) Assessment of nitrate leaching loss on a yield-scaled basis from maize and wheat cropping systems. Plant Soil 374:977–991
Zhou J, Gu B, Schlesinger WH, Ju X (2016) Significant accumulation of nitrate in Chinese semi-humid croplands. Sci Rep 6:25088
Funding
This study was financially supported by the National Natural Science Foundation of China (No. 41571130051; 41877010; 41771251). Yue Dong acknowledges the support of scholarship provided by the University of Chinese Academy of Sciences (UCAS).
Author information
Authors and Affiliations
Contributions
YD conducted the experiments, analyzed the data, and wrote the main manuscript; JLY and GLZ designed the experiments and revised the manuscript; XRZ and SHY analyzed the data and prepared the first draft of the manuscript; JM and PD revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, Y., Yang, JL., Zhao, XR. et al. Nitrate runoff loss and source apportionment in a typical subtropical agricultural watershed. Environ Sci Pollut Res 29, 20186–20199 (2022). https://doi.org/10.1007/s11356-021-16935-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16935-3