Abstract
The Chaohu Lake is highly polluted and suffers from severe eutrophication. Nitrate is a key form of nitrogen that can cause water quality degradation. In this study, hydrochemical and dual isotopic approaches were utilized to identify the seasonal variation of nitrate sources in the Chaohu Lake and its inflowing rivers. The average nitrate concentrations ([NO3−]) of the lake and its inflowing rivers were 89.9 and 140.8 μmol L−1, respectively. The isotopic values of δ15N–NO3− and δ18O–NO3− in the lake ranged from − 1.01 to + 16.67‰ and from − 4.39 to + 22.20‰, respectively. The two major rivers had distinct isotopic compositions, with average δ15N–NO3− values of + 17.52 and + 3.51‰, and average δ18O–NO3− values of + 2.71 and + 7.47‰ for the Nanfei River and Hangbu River, respectively. The results show that soil organic ammonium and urban wastewater discharge were the main sources of nitrate in the Chaohu Lake, and nitrate assimilation was an important process affected [NO3−] and isotopic compositions, especially in the western Chaohu Lake. The elevated [NO3−] and δ15N–NO3− values in the western Chaohu Lake indicate the high influence of human activities. Urban wastewater discharge was the primary nitrate source in the Nanfei River and soil organic ammonium was the main source in the Hangbu River. Although nitrate from direct atmospheric deposition was low, its strong flushing effect can substantially improve riverine [NO3−] and nitrate loading from terrestrial ecosystem. The relatively high nitrate fluxes from the Hangbu River indicate that nitrogen loading from agricultural watershed is unneglectable in watershed nitrogen sources reduction strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last few decades, intense human activities, such as chemical fertilizer use and fossil fuel combustion, have greatly exacerbated nitrogen (N) input to terrestrial ecosystems (Galloway et al. 2003). Elevated nitrogen loading coupled with climate change has dramatically enhanced watershed nitrogen export (Baron et al. 2012; Greaver et al. 2016; Yan et al. 2010). Excessive nitrogen loading in receiving waters may pose a remarkable threat to resident organisms and can cause eutrophication and hypoxia (Liu et al. 2015; Mallin et al. 2006; Paerl et al. 2011). Nitrate is one of main existing forms of nitrogen, and is mobile in terrestrial ecosystems and easily transported to aquatic systems. High nitrate concentration ([NO3−]) can even threaten drinking water supplies for human and livestock (Ward et al. 2005). Therefore, it is crucial to identify nitrate sources and to determine their variabilities in improving regional nutrient management and maintaining water quality.
Nitrate in surface waters is often the mixture of different sources, such as atmospheric deposition, nitrate fertilizer, nitrification of soil organic nitrogen, and nitrification of manure and sewage (Kendall 1998; Li et al. 2010; Liu et al. 2013a; Ohte et al. 2010), nitrate from groundwater supply could also be an important nitrate source (Silva et al. 2002). Stable isotope technique is a promising method of identifying nitrate sources in aquatic ecosystems, and dual isotopic methodology of nitrate (δ15N and δ18O) has been widely applied to identify nitrate sources in groundwater, rivers, and lakes (Elisante and Muzuka 2016; Haberhauer et al. 2002; Liu et al. 2013a; Mayer and Wassenaar 2012). Different nitrate sources often have distinctive δ15N and δ18O values (Kendall et al. 2008). Nitrate from atmospheric deposition is characterized by high δ18O–-NO3− values, ranging from + 25.0 to + 80.0‰, and a wide range of δ15N–NO3− from − 8.0 to + 15.0‰ (Kendall 1998). Nitrate derived from nitrate fertilizer is categorized by a narrow range of δ15N–NO3− values (from − 5.0 to +5.0‰) and high δ18O–NO3− value of approximately + 20.0‰ (Chang et al. 2002). However, the isotopic values of nitrate originating from nitrification depend on substrate, with δ15N–NO3− values ranging from − 10.0 to + 25.0‰ and δ18O–NO3− values ranging from − 10.0 to + 10.0‰, respectively (Mariotti et al. 1988; Wassenaar 1995).
Moreover, microbial activities such as assimilation, denitrification, and nitrification, can cause the change of δ15N and δ18O values in nitrate (Liu et al. 2013a; Mayer and Wassenaar 2012). Assimilation by phytoplankton and denitrification by microorganism are the processes of nitrate retention that leave substance enriched in 15N and 18O (Mayer and Wassenaar 2012). The ratio of increased δ15N and δ18O values in the residual nitrate is assumed to be close to 1.0 for assimilation (Granger et al. 2004), and ranging from 1.3 to 2.1 for denitrification (Böttcher et al. 1990; Kendall et al. 2008). Nitrification is a two-step process of ammonium oxidation, during which NH4+ is firstly oxidized to NO2− and then to NO3−. Unlike the coupled isotopic relationship in the processes of assimilation and denitrification, nitrogen and oxygen isotopes are unrelated during nitrification, with one-third of the oxygen atom in NO3− originating from dissolved oxygen and two-thirds from the surrounding water (Kendall et al. 2008).
Nitrate exported from terrestrial landscape is mostly dependent on watershed nitrogen loading; however, climate change, such as precipitation, can also determine nitrate export fluxes (Baron et al. 2012). The influence of precipitation on watershed nitrate export is primarily reflected by direct atmospheric deposition and the effect of flush on terrestrial landscape. Moreover, the alternation of hydrological condition driven by precipitation can also change nitrate export patterns (Greaver et al. 2016). During dry seasons, with low denitrification rate, terrestrial ecosystems exhibit high retention leading to the accumulation of nitrogen. During wet seasons, high amounts of nitrogen could be flushed into aquatic ecosystems owing to the enhanced effect of hydrological conditions (Kaushal et al. 2008).
As a typical shallow lake located within the capital city of Anhui Province, East China, the Chaohu Lake endured unprecedented nutrient loading in recent decades. The accumulation of nutrient in the lake has deteriorated its water quality since the 1980s. However, it is still unclear how nitrate sources vary throughout the year under different hydrological conditions. In this context, a seasonal study of water samples from the Chaohu Lake and its inflowing rivers was conducted. The purposes of this study are (1) to interpret seasonal nitrate sources in the Chaohu Lake, (2) to identify the major nitrate source in the inflowing rivers under different hydrological conditions, and (3) to assess the influence of hydrological variability on the sources and distribution of nitrate in inflowing rivers.
Methods and materials
Study area
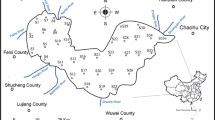
The Chaohu Lake (31°25′ N - 31°43′ N, 117° 17′ E - 117° 52′E), located in the capital city of Anhui Province, is the fifth largest freshwater lakes in China, with a surface water area of 783 km2 (Fig. 1). The watershed experiences a humid temperate climate, and falls in the Asian subtropical monsoon climatic zone. The mean annual temperature, precipitation, and wind speed of the watershed are 15.5 °C, 1000 mm, and 4.1 m s−1, respectively. The Nanfei River and Hangbu River are the two largest inflowing rivers, with watershed area of 1464 and 4246 km2, respectively. The Chaohu Lake was naturally connected to the Changjiang River by the Yuxi River before 1960s, and it was impounded by Chaohu Dam after 1962. The lake can be divided into two parts as the western and eastern lake based on a line crossing Zhongmiao Temple and Mushan Island. The depths of the western and eastern lake range from 2.0 to 3.0 m and from 3.0 to 4.0 m, respectively, with no water column stratification throughout the year.
Sampling and hydrochemical and isotopic analysis
Surface water samples were collected from the Chaohu Lake and its inflowing rivers on September 8, 2015, January 6, 2016, October 21–22, 2016, and June 7, 2017. Each sampling site was positioned by GPS. Four samples were collected from the western lake (CH1, CH2, CH3, and CH6) and three from the eastern lake (CH4, CH5, and CH7) (Fig. 1). Surface water samples were collected from the inflowing rivers of the Nanfei River (NFR) and Hangbu River (HBR). Water samples from Shiwuli River (SWLR), Pai River (PR), and the middle of Nanfei River (NFR-M), and the effluents of sewage treatment plants located near Nanfei River (STP1), Shiwuli River (STP2), and Pai River (STP3) were collected in January 2016. Rainwater was also collected during the sampling trips. All samples were filtered through glass microfiber filter (GF/F Waterman, UK) in situ. For each sample, 300 mL was stored in polyethylene bottles for nitrogen concentration test and 50 mL subsamples were stored in centrifuge tubes for isotopic compositions and ion tests, respectively. All samples were stored at 4 °C in the field and frozen once they were brought to laboratory before further testing. The air temperature and precipitation of the Chaohu Lake watershed during sampling months are listed in Table 1.
[NO3−] of samples were determined in laboratory using Flow Injection Analyzer (FIA-3100, China). Chloride concentrations ([Cl−]) were measured by Dionex-ICS-1500 with an analytical precision of ± 0.01 mg L−1. The oxygen isotope compositions in water were measured by LGR’s Isotopic Water Analyzer (IWA-45EP, Los Gatos Research Inc.), with a precision of ± 0.25‰ for δ18O–H2O. Nitrogen and oxygen compositions were performed using the denitrifier method (Casciotti et al. 2002), by which nitrate was firstly converted into N2O by Pseudomonas aureofaciens, which lack nitrous oxide reductase enzyme to reduce N2O to N2. Then gaseous N2O was tested by isotope ratio mass spectrometer (IRMS, Isoprime100, Isoprime, Cheadle, UK). The isotopic compositions of 15N and 18O were expressed in δ and reported as per mill (‰) relative to the atmospheric N2 and Vienna standard mean ocean water (V-SMOW) for δ15N and δ18O, respectively.
Results
Temporal and spatial variation of water chemistry
The ion concentrations of water samples are listed in Table 2. [NO3−] ranged from 8.64 to 200.96 μmol L−1 (average 89.9 μmol L−1) in the Chaohu Lake. The average [NO3−] of the inflowing rivers was higher, with 168.9 and 92.9 μmol L−1 for NFR and HBR, respectively. In comparison, the [NO3−] of rainwater was much lower than that of surface water, ranging from 23.9 to 40.7 μmol L−1 (average 30.1 μmol L−1). The effluent of sewage treatment plants was found to be with the highest [NO3−], with an average concentration of 561.2 μmol L−1 (ranging from 434.1 to 743.9 μmol L−1).
The [Cl−] of the samples ranged from 242.9 to 1752.8 μmol L−1 (average 801.3 μmol L−1) and from 230.7 to 3187.5 μmol L−1 (average 1321.2 μmol L−1) in the Chaohu Lake and inflowing rivers, respectively. The average [Cl−] of rainwater was lower, with 83.2 μmol L−1 (ranging from 13.9 to 209.8 μmol L−1). The [Cl−] was 1940.1 μmol L−1 (ranging from 1344.6 to 2270.3 μmol L−1) of effluent of sewage treatment plants.
Isotopic compositions of water samples in the Chaohu Lake watershed
The isotopic compositions of samples from the Chaohu Lake watershed are listed in Table 2. The δ15N of nitrate varied from − 1.01 to + 16.67‰ (average + 7.31‰) in the Chaohu Lake. There was a wide range of δ15N–NO3− in the inflowing rivers ranging from − 4.10 to + 22.0‰ with average values of + 17.52 and + 3.51‰ for NFR and HBR, respectively. The δ15N–NO3− values of rainwater were much lower than those in lake and rivers, with an average of + 0.36‰. In comparison, there was a narrow range of δ15N–NO3− in the effluent of sewage treatment plants, ranging from + 12.65 to + 15.71‰ (average + 13.91‰).
The values of δ18O were found to varying from − 4.39 to + 22.20‰ (average + 8.30‰) and from − 4.10 to + 13.69‰ (average + 5.60‰) in the Chaohu Lake and inflowing rivers, respectively. The δ18O values of rainwater were much higher, ranging from + 29.55 to + 68.36‰ (average + 44.87‰). The δ18O–NO3− values ranged from + 4.60 to + 9.27‰ (average + 7.04‰) in sewage treatment plants. In comparison, there was a narrow range of δ18O values in water, ranging from − 8.46 to − 4.06‰ (average − 5.58‰) of water samples from the Chaohu Lake and inflowing rivers. The values of δ18O–H2O were ranging from − 13.44 to − 3.81‰ (average − 7.28‰) and from − 5.40 to − 4.89‰ (average − 5.17‰) in rainwater and effluent of sewage treatment plants, respectively.
Discussion
Hydrochemical compositions and mixing processes
Dissolved ions in surface water are usually the mixture of different sources that can be characterized with distinct hydrochemical signatures. Chloride, unlike nitrate, is conservative and cannot be removed by physical, chemical, and biological processes (Pellerin et al. 2008). Potential Cl− sources in fresh water include atmospheric deposition, hydrogeology, agricultural chemicals, sewage input, industrial wastewater, and other anthropogenic sources. Animal manure and sewage, and urban wastewater discharge are characterized with high [Cl−] and low NO3−/Cl− (Krapac et al. 2002), and agricultural watershed samples typically have a low [Cl−] and high NO3−/Cl− (Liu et al. 2006). Therefore, molar ratio of NO3−/Cl− and [Cl−] can be used as indicators to differentiate the mixing effects of various nitrate sources and provide useful information for identifying nitrate retention processes, such as denitrification and assimilation (Kellman and Hillaire-Marcel 1998; Li et al. 2010; Nestler et al. 2011).
The temporal and spatial variation of samples from the Chaohu Lake can be interpreted by mixing processes of different nitrate sources (Fig. 2). Rainwater was marked by a relatively low [Cl−] and high molar NO3−/Cl− ratio. Samples from the urbanized Nanfei River and the agricultural Hangbu River were characterized by distinct values, and could be regarded as end-members for the Chaohu Lake. During the September 2015 sampling trip, the [Cl−] of the Chaohu Lake exhibited a narrow range, while the NO3−/Cl− was much lower than that of the inflowing rivers. The relatively low NO3−/Cl− can be attributed to in-site nitrate retention processes, such as denitrification and/or assimilation that substantially depleted nitrate. During the January 2016 sampling trip, precipitation was low and the clustered distribution of [Cl−] and NO3−/Cl− suggests that there was no constant mixing process in the Chaohu Lake. The elevated [Cl−] and NO3−/Cl− ratios in the western lake could be inferred that this lake part was subjected to more influence by sewage input than the eastern lake. Moreover, sewage treatment plants were expected to be the main nitrate source for urban rivers during base flow season; however, the higher [Cl−] of urban rives than that of the effluent of sewage treatment plants suggested that there was another potential source that had a marked influence on these rivers. During the October 2016 sampling trip, the [Cl−] of the lake was lower than that observed in the other seasons, which could be attributed to dilution effect by rainwater. The clustered and scattered [Cl−] of the western and eastern lake indicate the occurrence of different mixing processes, respectively. In June 2017, the [Cl−] and NO3−/Cl− were concentrated within the end-members of the Chaohu Lake, excluding CH1. The lower NO3−/Cl− at CH1 could also be attributed to denitrification and/or assimilation.
Differentiating sources of nitrate based on stable isotope composition
Sources of nitrate in the Chaohu Lake
The δ15N and δ18O values of nitrate can be used as a diagnostic tool for discerning major nitrate sources (Kendall 1998). A crossplot of the typical ranges in the δ15N versus δ18O values of various nitrate sources and the nitrogen and oxygen signatures of samples from the Chaohu Lake watershed are presented in Fig. 3. The dual-isotopic composition of NO3− suggests that nitrate in the Chaohu Lake watershed could be the mixture of any pairings of any two end-members or of all four: (1) ammonium fertilizer, (2) soil organic NH4+, (3) urban wastewater discharge, and (4) atmospheric nitrate. Overall, the temporal and spatial variation can be attributed to differences in mixing processes and contributions from these sources.
Crossplot of δ15N–NO3− versus δ18O–NO3− in the Chaohu Lake watershed. The isotopic compositions of various nitrate sources were based on Kendall et al. (2008). The inserted small plot is the regression relationship between δ15N–NO3− and δ18O–NO3− for samples in the Chaohu Lake and the inflowing rivers. WCH, Western Chaohu Lake; ECH, Eastern Chaohu Lake; NFR, Nanfei River; HBR, Hangbu River; PR, Pai River; SWLR, SWLR; STP, Sewage treatment plants
During September 2015, the δ15N–NO3− and δ18O–NO3− crossplot for CH2 to CH5 was within the range of nitrate fertilizer (Fig. 3). However, this does not indicate that the nitrate primarily originated from such a source. As the nitrogen chemical fertilizer applied in the Chaohu Lake watershed are primarily ammonium fertilizer (Wang et al. 2014), such isotopic compositions indicate a mixture of various sources, for example, atmospheric deposition, soil organic ammonium, and urban wastewater discharge. The relatively low values of δ15N-NO3− suggest that soil organic ammonium was the major source of nitrate, especially for sites in the eastern lake. Given the dissolved oxygen δ18O value of + 23.5‰, the values of δ18O–NO3− produced through nitrification should theoretically vary from + 2.66 to + 5.13‰. The relatively high δ18O–NO3− values suggest that either atmospheric deposition was the main nitrate source or other processes changed the δ18O values of the dissolved nitrate (Fig. 4). The rainwater collected from the Chaohu watershed had a lower [Cl−] and a higher NO3−/Cl− ratio (Fig. 2). If the elevated δ18O–NO3− values originated from atmospheric deposition, the hydrochemical character would be marked by low a [Cl−]. However, the high [Cl−] and low NO3−/Cl− ratio indicates that nitrate retention processes such as denitrification and/or uptake by algae, which decrease the [NO3−], had a substantial effect on nitrate isotopic compositions. The relatively high δ15N–NO3− value at CH1 indicates a higher nitrate contribution from urban wastewater discharge. The δ15N–NO3− and δ18O–NO3− values at CH1 were comparable to those of NFR, suggesting that the nitrate at this site could be under the influence of the Nanfei River. Studies have demonstrated that change of δ15N and δ18O in nitrate had positive correlation with 2:1 for denitrification (Mengis et al. 1999; Panno et al. 2006) and 1:1 for assimilation (Granger et al. 2004: Mayer and Wassenaar 2012). According to the kinetic fractionations theory, the isotopic composition of a product would be the same as that of the initial substrate, while the isotopic composition of the substrate would be higher, especially at the end of a reaction (Sulzman 2008). In September 2015, the higher nitrate removal rate resulted in lower [NO3−] and an enriched isotopic composition at CH2 to CH5. The higher [NO3−] at CH1 can be attributed to the nitrate continually exported by the Nanfei River.
Crossplot of δ18O–H2O versus δ18O–NO3− in Chaohu Lake and inflowing rivers. The upper line indicates that oxygen atoms in nitrate produced through nitrification progress are all from dissolved oxygen (δ18O–NO3− = δ18O2 = + 23.5‰), the middle line indicates one-third from dissolved oxygen and two-thirds from surround water (δ18O–NO3− = 1/3 δ18O2 + 2/3 δ18O–H2O) and the lower line indicate all oxygen atom originated from water (δ18O–NO3− = δ18O–H2O). WCH, Western Chaohu Lake; ECH, Eastern Chaohu Lake; NFR, Nanfei River; HBR, Hangbu River; PR, Pai River; SWLR, SWLR; STP, Sewage treatment plants
During January 2016, the hydrochemical compositions were similar throughout the Chaohu Lake, and clustered in the [Cl−] versus NO3−/Cl− crossplot (Fig. 2). The crossplot of δ18O–H2O versus δ18O–NO3− shows that samples from the Chaohu Lake were mainly distributed close to the nitrification equilibrium line (Fig. 4). The air temperature was below 10 °C, and the effect of microbial activities was expected to be low. The isotopic compositions of the samples indicated various nitrate sources. The elevated δ15N–NO3− values in the Chaohu Lake indicated that soil organic nitrogen and urban wastewater discharge were the dominant nitrate sources. Elevated [NO3−] and δ15N–NO3− values were observed in the western lake, indicating a higher contribution from urban wastewater discharge.
In October 2016, precipitation was extremely high in the Chaohu Lake watershed (Table 1). The [Cl−] in the lake was significantly lower than that observed during other sampling trips, suggesting a strong diluting effect by rainwater. The crossplot of δ18O–H2O versus δ18O-NO3− shows that samples from the Chaohu Lake were distributed around the nitrification equilibrium line (Fig. 4), which indicated that nitrate produced from in-site nitrification contributed little to the nitrate, and the nitrate mainly originated from allochthonous input. Considering the low [NO3−] and high δ18O–NO3− value of rainwater, it could infer that direct atmospheric deposition from rainwater had limited contribution to nitrate in the Chaohu Lake. The δ15N–NO3− and δ18O–NO3− of sites CH2 to CH7 show that nitrate from soil organic nitrogen and urban wastewater discharge dominated nitrate input in this season (Fig. 3), and those sources significantly promoted [NO3−] in the Chaohu Lake. The elevated δ15N—NO3− value at CH1 suggested that urban wastewater discharge was the main nitrate source.
During sampling trip of June 2017, the average [NO3−] was comparable to that observed in October 2016, while the average [Cl−] was approximately one time higher. This indicates that nitrate removal processes might occur during in this season. The observed δ15N–NO3− and δ18O–NO3− values were within a relatively narrow range (Fig. 3). In addition, the average value of δ15N–NO3− was + 9.7‰ that was much higher than that observed during the other sampling trips, and the δ18O–NO3− values were slightly higher than the nitrification equilibrium values, indicating less in-site nitrate transfer. During this sampling season, algae were fast growing and assimilating nitrogen resulting in elevated values of δ15N–NO3− and δ18O–NO3− the in surrounding water (Mayer and Wassenaar 2012).The lower [NO3−] and enriched δ15N-NO3− value at CH1 indicated that the assimilation rate was higher than that at the other sampling sites.
Sources of nitrate in rivers
The importance of nitrate sources also varied between different sampling sites in the inflowing rivers. As the linkage between Hefei City and the Chaohu Lake, the Nanfei River was severely polluted and as such, it was a pollutant source for the Chaohu Lake (Yang et al. 2011). During sampling trip of September 2015, the elevated δ15N–NO3− and depleted δ18O–NO3− values at NFR indicated that urban wastewater discharge was the predominant nitrate source. This was consistent with observations of the urban river in the Taihu Lake watershed (Townsend-Small et al. 2007) and the result of a previous study in the Chaohu Lake watershed (Xi et al. 2015). The δ15N–NO3− and δ18O–NO3− values for the agricultural Hangbu River suggested that nitrate originated from soil organic ammonium was the major source.
In January 2016, [NO3−] of NFR, PR, and SWLR were much higher than those measured in the previous sampling trips. Urban wastewater discharge was expected to be the main nitrate source. However, the values of δ15N–NO3− of these rivers were significantly higher than those in effluent of sewage treatment plants (Table 1 and Fig. 3), suggesting that there may be another potential nitrate source that was characterized by high [NO3−] and elevated δ15N. A previous study elucidated that the [NO3−] of groundwater was elevated in winter, and was higher in the northern area of the Chaohu Lake watershed than that in the other regions (Wang et al. 2014). Groundwater in urban area that receives nitrate from overlying soil is enriched in 15N (Chang et al. 2002), and denitrification in groundwater could also result in enriched δ15N in substrate (Liu et al. 2006). Therefore, groundwater could be the main nitrate source for urban rivers during dry season. In comparison, the relatively lower δ15N–NO3− value at HBR suggested that groundwater had a negligible role in riverine nitrate. The isotopic compositions of δ15N–NO3− and δ18O–NO3− in the Hangbu River indicate that soil organic nitrogen was the primary nitrate source.
In October 2016, the [NO3−] of NFR and HBR were similar. However, the δ15N–NO3− value at NFR was much higher than that at HBR, indicating difference in nitrate sources. The δ15N–NO3− and δ18O–NO3− values indicated that the main nitrate sources of the Nanfei River and Hangbu River were urban wastewater discharge, and ammonium fertilizer and/or soil organic nitrogen, respectively. The low [NO3−] and high δ18O–NO3− value of rainwater suggested that direct input from precipitation had little effect on nitrate in rivers. As this was the transition period of rice and winter wheat cultivation, a series of agricultural activities, such as farmland plowing and chemical fertilizer spreading, were performed during the sampling trips. Besides, the improvement of ventilation and substrate content promote nitrification, and the newly produced nitrate could be easily flushed into streams and rivers before infiltration to groundwater or denitrification under intense rainfall (He et al. 2011). This leads to a notable increase of the [NO3−] of the agricultural Hangbu River.
During sampling trip of June 2017, the [NO3−] of NFR was twice as that of HBR. The crossplot of Cl− and NO3−/Cl− suggested that microbial activities had less effect on the removal of nitrate in these two rivers. In addition, the δ18O-NO3− values were slightly higher than the nitrification equilibrium values, indicating lower in-river nitrification. The elevated δ15N–NO3− value of the Nanfei River suggested that the main nitrate source was urban wastewater discharge, while the δ15N–NO3− value of the Hangbu River suggested that ammonium fertilizer and soil organic nitrogen were the main nitrate sources.
There were significant decreasing and increasing trends of δ15N–NO3− and δ18O–NO3− with decreasing [NO3−], respectively (p < 0.01 for δ15N–NO3− and δ18O–NO3−) (Fig. 5a, b). However, the relationships were distinct between different sampling trips. As nitrate was receiving from terrestrial ecosystem, the hydrochemical and isotopic compositions of the nitrate sources varied seasonally. The spatial variation of nitrate sources also differed between the western and eastern Chaohu Lake. The western Chaohu Lake was more influenced by the watershed; therefore, the [NO3−] and δ15N–NO3− values were higher than those of the eastern Chaohu Lake (Table 2). Though denitrification in the sediment and overlying water can cause isotopic fractionations, the range was much smaller than that caused by denitrification in groundwater (Liu et al. 2013b; Sebilo et al. 2003). The positive relationship between [NO3−] and δ15N–NO3− can be attributed to the spatio-temporal variation of nitrate sources.
Influence of precipitation on nitrate export from two watersheds
Climate change caused hydrological cycle by altering precipitation can greatly change the quantity of nitrogen exported from terrestrial ecosystems (Baron et al. 2012). Increased precipitation is a major driving force that results in greater terrestrial landscape nitrogen export to aquatic ecosystems (Greaver et al. 2016). Atmospheric deposition was expected to be a key nitrate source; however, the hydrochemical and isotopic compositions observed in the Chaohu Lake watershed suggested that it plays less of a role in the Chaohu Lake watershed (Table 2). The hydrochemical and isotopic compositions presented significant seasonal variation in the Nanfei River (Fig. 6). The seasonal variation in the δ15N–NO3− and δ18O–NO3− values indicates that nitrate in the Nanfei River originated from various sources. In comparison, less seasonal variation of the [Cl−], δ15N-NO3−, and δ18O-NO3− values was observed in the Hangbu River, indicating a consistent nitrate source. During the dry season in January 2016, elevated [Cl−] and enriched δ15N–NO3− values were observed in both rivers. However, in October 2016 with intensive rainfall, reduced [Cl−] and depleted δ15N-NO3− values were measured. This is consistent with the result of a study on rivers in Austin, USA, which found that groundwater was the main nitrate source during the baseflow season (Silva et al. 2002).
The exported nitrate fluxes from the Nanfei River and the Hangbu River were estimated based on the [NO3−] and riverine discharge (Supplementary Material). In September 2015, the nitrate fluxes from the Nanfei River and Hangbu River were considerable, with 1.07 and 0.70 t N day−1, respectively, and increased to 2.28 and 2.61 t N day−1 in January 2016 (Fig. 7). However, in October 2016, when precipitation was extremely high, nitrate fluxes sharply increased to 10.2 and 25.4 t N day−1 for the Nanfei River and Hangbu River, respectively. The nitrate exported from the Hangbu River was approximately 2.5 times higher than that from the Nanfei River. In June 2017, the nitrate flux from the Hangbu River was 10.93 t N day−1, which was approximately 1.8 times that from the Nanfei River, which was 5.93 t N day−1. Although the seasonal trends in the hydrochemical compositions and isotopic signatures of the Nanfei River and the Hangbu River were similar, the differences in the exported nitrate fluxes indicated that nitrate loadings from these rivers had distinct responses to hydrological condition (Figs. 6 and 7). The highly impervious surface coverage of urbanized watersheds can rapidly cause surface runoff to discharge into rivers (Kaushal et al. 2014), and elevate the riverine [NO3−]. Unlike watershed of Baltimore, USA (Kaushal et al. 2008), the nitrogen fertilizer utilized in the Chaohu Lake watershed is mainly ammonium fertilizer (Wang et al. 2014). Large quantities of ammonium and organic nitrogen could be retained and transformed into nitrate during the dry season and flushed into river during wet season (Greaver et al. 2016). Besides, intense rainfall is associated with a short nutrient resident time in river channel, and it can weaken the denitrification rates of sediment and overlying water (Baron et al. 2012). This indicated that agricultural watershed was more vulnerable to changing hydrological conditions in the Chaohu Lake watershed. The higher fluxes of exported nitrate suggested that nitrate from agricultural areas should also be considered in watershed nitrogen reducing strategies.
Conclusions
Dual stable isotopes in nitrate and the hydrochemical composition of water were used to identify spatial and temporal variations in the nitrate sources in the Chaohu Lake watershed. The results show that the [NO3−] had significant seasonal and spatial variation of the Chaohu Lake and its inflowing rivers, with higher [NO3−] observed in rivers. The δ15N and δ18O values of the nitrate in the Chaohu Lake indicated that it mainly originated from soil organic ammonium and urban wastewater discharge. Combined this with the molar NO3−/Cl− ratio and δ18O–NO3− value suggested that the assimilation of algae played a significant role in nitrate removal of the lake. The higher [NO3−] (> 97.1 μmol L−1) and δ15N–NO3− value (> + 9.8‰) of the Nanfei River indicated that the main source of nitrate was urban wastewater discharge, while soil organic ammonium and ammonium fertilization were the major nitrate source in the Hangbu River during all seasons. The [NO3−] of rainwater was very low (< 41.0 μmol L−1), suggesting that it contributed less nitrate to the rivers and lake. However, the flushing effect of rainwater can substantially promote nitrate export from the Nanfei River and the Hangbu River into the Chaohu Lake.
References
Baron JS, Hall EK, Nolan BT, Finlay JC, Bernhardt ES, Harrison JA, Chan F, Boyer EW (2012) The interactive effects of excess reactive nitrogen and climate change on aquatic ecosystems and water resources of the United States. Biogeochemistry 114:71–92
Böttcher J, Strebel O, Voerkelius S, Schmidt HL (1990) Using isotope fractionation of nitrate-nitrogen and nitrate-oxygen for evaluation of microbial denitrification in a sandy aquifer. J Hydrol 114:413–424
Casciotti KL, Sigman DM, Hastings MG, Bohlke JK, Hilkert A (2002) Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74:4905–4912
Chang CCY, Kendall C, Silva SR, Battaglin WA, Campbell DH (2002) Nitrate stable isotopes: tools for determining nitrate sources among different land uses in the Mississippi River Basin. Can J Fish Aquat Sci 59:1874–1885
Elisante E, Muzuka ANN (2016) Assessment of sources and transformation of nitrate in groundwater on the slopes of Mount Meru, Tanzania. Environ Earth Sci 75:277
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356
Granger J, Sigman DM, Needoba JA, Harrison PJ (2004) Coupled nitrogen and oxygen isotope fractionation of nitrate during assimilation by cultures of marine phytoplankton. Limnol Oceanogr 49:1763–1773
Greaver TL, Clark CM, Compton JE, Vallano D, Talhelm AF, Weaver CP, Band LE, Baron JS, Davidson EA, Tague CL, Felker-Quinn E, Lynch JA, Herrick JD, Liu L, Goodale CL, Novak KJ, Haeuber RA (2016) Key ecological responses to nitrogen are altered by climate change. Nat Clim Chang 6:836–843
Haberhauer G, Gerzabek MH, Krenn A (2002) Nitrate dynamics in an alpine forest site (Mühleggerköpfl) O and N stable isotope analysis in natural water samples. Environ Sci Pollut Res 9:37–41
He B, Kanae S, Oki T, Hirabayashi Y, Yamashiki Y, Takara K (2011) Assessment of global nitrogen pollution in rivers using an integrated biogeochemical modeling framework. Water Res 45:2573–2586
Kaushal SS, Groffman PM, Band LE, Shields CA, Morgan RP, Palmer MA, Belt KT, Swan CM, Findlay SEG, Fisher GT (2008) Interaction between urbanization and climate variability amplifies watershed nitrate export in Maryland. Environ Sci Technol 42:5872–5878
Kaushal SS, McDowell WH, Wollheim WM (2014) Tracking evolution of urban biogeochemical cycles: past, present, and future. Biogeochemistry 121:1–21
Kellman L, Hillaire-Marcel C (1998) Nitrate cycling in streams: using natural abundances of NO3 −-δ15N to measure in-situ denitrification. Biogeochemistry 43:273–292
Kendall C (1998) Tracing nitrogen sources and cycling in catchments. In: McDonnell CKJ (ed) Isotope tracers in catchment hydrology. Elsevier, Amsterdam, pp 519–576
Kendall C, Elliott EM, Wankel SD (2008) Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener RH, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell, Oxford, pp 375–449
Krapac IG, Dey WS, Roy WR, Smyth CA, Storment E, Sargent SL, Steele JD (2002) Impacts of swine manure pits on groundwater quality. Environ Pollut 120:475–492
Li S, Liu C, Li J, Liu X, Chetelat B, Wang B, Wang F (2010) Assessment of the sources of nitrate in the Changjiang River, China using a nitrogen and oxygen isotopic approach. Environ Sci Technol 44:1573–1578
Liu C, Li S, Lang Y, Xiao H (2006) Using δ15N- and δ18O-values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ Sci Technol 40:6928–6933
Liu T, Wang F, Michalski G, Xia X, Liu S (2013a) Using 15N, 17O, and 18O to determine nitrate sources in the Yellow River, China. Environ Sci Technol 47:13412–13421
Liu T, Xia X, Liu S, Mou X, Qiu Y (2013b) Acceleration of denitrification in turbid rivers due to denitrification occurring on suspended sediment in oxic waters. Environ Sci Technol 47:4053–4061
Liu K, Yan W, Lee H, Chao S, Gong G, Yeh T (2015) Impacts of increasing dissolved inorganic nitrogen discharged from Changjiang on primary production and seafloor oxygen demand in the East China Sea from 1970 to 2002. J Mar Syst 141:200–217
Mallin MA, Johnson VL, Ensign SH, MacPherson TA (2006) Factors contributing to hypoxia in rivers, lakes, and streams. Limnol Oceanogr 51:690–701
Mariotti A, Landreau A, Simon B (1988) 15N isotope biogeochemistry and natural denitrification process in groundwater: application to the chalk aquifer of northern France. Geochim Cosmochim Acta 52:1869–1878
Mayer B, Wassenaar LI (2012) Isotopic characterization of nitrate sources and transformations in Lake Winnipeg and its contributing rivers, Manitoba, Canada. J Great Lakes Res 38(Supplement 3):135–146
Mengis M, Schif SL, Harris M, English MC, Aravena R, Elgood RJ, MacLean A (1999) Multiple geochemical and isotopic approaches for assessing ground water NO3 − elimination in a riparian zone. Ground Water 37:448–457
Nestler A, Berglund M, Accoe F, Duta S, Xue D, Boeckx P, Taylor P (2011) Isotopes for improved management of nitrate pollution in aqueous resources: review of surface water field studies. Environ Sci Pollut Res 18:519–533
Ohte N, Tayasu I, Kohzu A, Yoshimizu C, Ki O, Makabe A, Koba K, Yoshida N, Nagata T (2010) Spatial distribution of nitrate sources of rivers in the Lake Biwa watershed, Japan: controlling factors revealed by nitrogen and oxygen isotope values. Water Resour Res 46:W07505
Paerl HW, Xu H, McCarthy MJ, Zhu G, Qin B, Li Y, Gardner WS (2011) Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N and P) management strategy. Water Res 45:1973–1983
Panno SV, Hackley KC, Kelly WR, Hwang HH (2006) Isotopic evidence of nitrate sources and denitrification in the Mississippi River, Illinois. J Environ Qual 35:495–504
Pellerin BA, Wollheim WM, Feng X, Vörösmarty CJ (2008) The application of electrical conductivity as a tracer for hydrograph separation in urban catchments. Hydrol Process 22:1810–1818
Sebilo M, Billen G, Grably M, Mariotti A (2003) Isotopic composition of nitrate-nitrogen as a marker of riparian and benthic denitrification at the scale of the whole Seine River system. Biogeochemistry 63:35–51
Silva SR, Ging PB, Lee RW, Ebbert JC, Tesoriero AJ, Inkpen EL (2002) Forensic applications of nitrogen and oxygen isotopes in tracing nitrate sources in urban environments. Environ Forensic 3:125–130
Sulzman EW (2008) Stable isotope chemistry and measurement: a primer, In: R. Michener and K. Lajtha (eds), Stable isotopes in ecology and environmental science. Blackwell, Oxford,, pp 1–21
Townsend-Small A, McCarthy M, Brandes J, Yang L, Zhang L, Gardner W (2007) Stable isotopic composition of nitrate in Lake Taihu, China, and major inflow rivers. Hydrobiologia 581:135–140
Wang Q, Gu Y, Sun D (2014) Spatial and seasonal variations of nitrate-N concentration in groundwater within Chao Lake watershed. Acta Ecol Sin 34:4372–4379 (In Chinese)
Ward MH, deKok TM, Levallois P, Brender J, Gulis G, Nolan BT, VanDerslice J (2005) Workgroup report: drinking-water nitrate and health—recent findings and research needs. Environ Health Persp 113:1607–1614
Wassenaar LI (1995) Evaluation of the origin and fate of nitrate in the Abbotsford aquifer using the isotopes of 15N and 18O in NO3 −. Appl Geochem 10:391–405
Xi S, Liu G, Zhou C, Wu L, Liu R (2015) Assessment of the sources of nitrate in the Chaohu Lake, China, using a nitrogen and oxygen isotopic approach. Environ Earth Sci 74:1647–1655
Yan W, Mayorga E, Li X, Seitzinger SP, Bouwman AF (2010) Increasing anthropogenic nitrogen inputs and riverine DIN exports from the Changjiang River basin under changing human pressures. Glob Biogeochem Cycles 24
Yang L, Yan W, Ma P, Wang J (2011) Seasonal and diurnal variations in N2O concentrations and fluxes from three eutrophic rivers in Southeast China. J Geogr Sci 21:820–832
Funding
This study was supported by the National Key Research and Development Program of China through grant 2016YFA0601004, and the National Natural Science Foundation of China (41371454).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 53 kb)
Rights and permissions
About this article
Cite this article
Yu, Q., Wang, F., Li, X. et al. Tracking nitrate sources in the Chaohu Lake, China, using the nitrogen and oxygen isotopic approach. Environ Sci Pollut Res 25, 19518–19529 (2018). https://doi.org/10.1007/s11356-018-2178-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2178-9