Abstract
In order to assess the toxicity of heavy metals on the early development of Meretrix meretrix, the effects of mercury (Hg), cadmium (Cd) and lead (Pb) on embryogenesis, survival, growth and metamorphosis of larvae were investigated. The EC50 for embryogenesis was 5.4 μg l−1 for Hg, 1014 μg l−1 for Cd and 297 μg l−1 for Pb, respectively. The 96 h LC50 for D-shaped larvae was 14.0 μg l−1 for Hg, 68 μg l−1 for Cd and 353 μg l−1 for Pb, respectively. Growth was significantly retarded at 18.5 μg l−1 (0.1 μM) for Hg, 104 μg l−1 (1 μM) for Cd and 197 μg l−1 (1 μM) for Pb, respectively. The EC50 for metamorphosis, similar to 48 h LC50, was higher than 96 h LC50. Our results indicate that the early development of M. meretrix is highly sensitive to heavy metals and can be used as a test organism for ecotoxicology bioassays in temperate and subtropical regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large amounts of pollutants have been released into marine and estuarine environments over the last few decades. Among these pollutants, heavy metals have long been recognized as major pollutants of the marine environment, constituting a hazard to marine organisms (Depledge et al. 1994). In marine environments, bivalves are common, highly visible, and ecologically and commercially important on a global scale as food and nonfood resources. Because of their benthic and sedentary mode of life, they are easily exposed to environmental pollution (heavy metals, persistent organic pollutants, etc.) and bioaccumulate these pollutants. Therefore, bivalves are usually used as models in the field of environmental toxicology (Rittschof and McClellan-Green 2005).
In fact, the early developmental stages are the most sensitive in the life cycle of bivalves. Heavy metal concentrations causing lethal toxicity in embryos and larvae are much lower than those which are lethal to adults (Connor 1972; Beiras and His 1994). It has been shown that early developmental stages are highly sensitive to toxicants such as heavy metals (His et al. 1999), pesticides (Wessel et al. 2007) and antifouling paints (Bellas 2006). The toxicity of heavy metals such as copper to bivalves is well known since they have been used as antifouling agents for centuries (Laughlin et al. 1988; Lapota et al. 1993). Mercury (Hg) has also been shown to be highly toxic to Argopecten irradians juveniles (Nelson et al. 1977), Crassostrea gigas (Beiras and His 1994) and Mytilus galloprovincialis embryos and larvae (Beiras and His 1995). Similar toxicities have also been found for lead (Pb) and cadmium (Cd) in Ruditapes decussatus and M. galloprovincialis embryos, however, these metals are less toxic than Hg (Beiras and Albentosa 2004).
Because they are easy to collect and culture, very sensitive to pollutants and provide rapid responses, both embryos and larvae of invertebrates (bivalves, echinoderms, crustaceans, etc.) are usually used to test the toxicity of pollutants (Beiras and His 1994; Warnau et al. 1996; Reish and Gerlinger 1997; Itow et al. 1998; His et al. 1999). Besides sea urchins, oysters of the genus Crassostrea and mussels of the genus Mytilus have been proposed as sentinel organisms for marine ecotoxicological tests to assess seawater quality (His et al. 1997). However, most of these studies have concerned the genus Crassostrea and Mytilus. To our knowledge, there is no report on heavy metal toxicity on embryogenesis and larval development of widespread Asian bivalves such as R. philippinarum and M. meretrix.
The clam M. meretrix, which is widely distributed along the coastal and estuarine areas of East Asia, is an important commercial marine bivalve in China (Wang et al. 1993). It mainly inhabits sandy or muddy substrates in the estuarine lower intertidal and shallow subtidal areas where pollution is more severe than in the sea areas (He et al. 1997). Marine environments in China have deteriorated significantly and pose a great threat to marine resources including M. meretrix. Heavy metals are one of the many reasons why M. meretrix numbers have decreased, as Pb, Cd and Hg have been found in high concentrations in marine environments (Wang and Wang 2007; Meng et al. 2008).
Therefore, it is necessary to find out how these heavy metals affect the entire early development of M. meretrix. Moreover, in contrast to the majority of bioassays which usually use embryos or larval stages, the sensitivity of the entire early life stages of bivalves to heavy metals has been less studied. This data will help us to establish dose-response relationships for each metal tested, and give biological criteria for the implementation of marine water quality standards to protect these organisms.
Materials and methods
Brood stock and larvae collection
Adults of M. meretrix were collected from Wenzhou (Zhejiang province) and reared at ambient temperature (28 ± 1°C) in aquaria with filtered seawater. To induce spawning, the adults were taken out of the seawater and placed in the shade for 5–6 h, then transferred to tanks and stimulated using flowing seawater. Gametes were released after 2 or 3 h, following fertilization. The zygotes then developed into the larval stage. The larval stages of this clam include trochophore, D-shaped larvae (the larval shell looks like a “capital D”) and pediveliger, as illustrated in Fig. 1.
The zygote developed to the D-shaped phase 24 h after fertilization. The D-shaped larvae were collected using a 50 μm mesh and cultured in 2 l tanks at an initial density of 4–6 ind. ml−1. The larvae in all treatments were cultured at 28 ± 1°C and fed with Isochrysis spp. at a concentration of 1–10 × 104 cells ml−1 three times a day. The culture medium was renewed with fresh seawater daily. Temperature (28 ± 1°C), salinity (20‰) and pH (7.8) were controlled throughout heavy metal exposure and air was supplied via gentle aeration.
Metal solutions and analysis
Experiments were performed using the following analytical grade salts: HgCl2, CdCl2 2.5 H2O and Pb(NO3)2. Stock solutions were prepared in deionized water, at concentrations (1 M) high enough to prevent weighing errors and salinity change. Toxicity tests included five metal concentrations: 0.01, 0.1, 1, 10 and 100 μM (0.1, 1, 10, 100 and 1000 μM for Cd embryogenesis experiment).
Concentrations of metals (Pb, Cd and Hg) in the experimental solutions were measured using an atomic absorption spectrophotometer (AAS, Thermo SOLAAR, USA). Lead and Cd were measured according to the method of “The specification for marine monitoring” (GB17378 4-2007), China) using a graphite furnace AAS. Mercury was analyzed by cold vapor AAS, according to Weltz and Schubert-Jacobs (1991). The detection limits for Pb, Cd and Hg were 0.03, 0.01 and 0.001 μg l−1, respectively. All analyses were carried out thrice.
Embryotoxicity experiment
General methods followed those of Beiras and His (1994, 1995). For the embryotoxicity assays, 2-cell embryos (~15 min after fertilization) were exposed to different metal concentrations for 24 h at 28 ± 1°C in beakers containing 100 ml sea water (3 replicates per treatment). This incubation time allowed the embryos to develop to the D-shell stage. The number of embryos in each beaker was calculated before adding the heavy metals. The density of the embryos was about 6–10 ind. ml−1 which had no effect on embryo development.
After 24 h, the larvae were fixed with formalin, and the number of normal D-shaped larvae per beaker, assessed using microscopy, was recorded. The endpoint measured was the percentage of normal D-shaped larvae, excluding embryos and abnormally developed larvae. Larvae were considered abnormal when they were an irregular shape, had a convex hinge, and/or protruding mantle (His et al. 1997). More than 150 embryos per treatment were observed to determine embryogenesis success. The median effective concentration (EC50) was defined as the metal concentration that resulted in 50% abnormal development.
Growth and survival experiments
The D-shaped larvae (2–3 ind. ml−1) were reared at 28 ± 1°C in 2 l plastic (polyethylene) vessels, and were continuously exposed to different metals during larval development (3 replicates per treatment). Larvae were fed as indicated above. Seawater change and larval sampling were carried out daily. The mean length of the larvae (30 individuals per treatment) was recorded with a graduated eyepiece. The EC50 for growth was defined as the metal concentration that resulted in 50% reduction in growth.
The numbers of dead and live larvae were noted and live larvae were transferred to fresh food suspensions daily. The LC50 (median lethal concentration) was defined as the metal concentration that resulted in 50% mortality.
Metamorphosis experiments
This experiment was carried out when pediveligers were competent to metamorphose, as indicated by a larval size >180 μm and a high percentage (>80%) of eyed larvae. The number of pediveligers was calculated first, and then pediveligers (4–6 ind. ml−1) were placed in 10 ml petri dishes filled with seawater containing different metal concentrations (3 replicates per treatment). The number of postlarvae was recorded 48 h later using microscopy. The EC50 was defined as the metal concentration that resulted in a 50% reduction in larval settlement.
Statistical analysis
Values are presented as mean ± SD. One-way analysis of variance (one-way ANOVA) was performed on all data using SPSS 13.0 statistical software, and P < 0.05 was accepted as significant. Percentage data were transformed (arcsine of the square root) before ANOVA, and presented in figures as non-transformed percentages.
EC50 calculations were normalized to the control mean percentage of larval abnormality using Abbot’s formula (Emmens 1948), P = (Pe−Pc/100−Pc) × 100 where Pc and Pe are control and experimental percentage response, respectively. The EC50 values and their 95% confidence intervals (CI) in this experiment were calculated by the probit method (Newman 1995) using SPSS 13.0 statistical software.
Results
Chemical analyses
The results of the measured concentrations in this study are shown in Table 1. In the present study, PbCl2 precipitated in the seawater solutions containing added Pb at concentrations >10 μM and the measured dissolved Pb was significantly lower than the nominal concentration. For other solutions, the concentrations in the vessels ranged from 90 to 102% of the nominal concentrations. Therefore, nominal concentrations were used for presentation and calculation of toxicity parameters.
Embryo toxicity
When M. meretrix embryos were exposed for 24 h to increasing Hg, Cd and Pb concentrations, the percentage of normal larvae was significantly decreased (P < 0.05). Thus, increasing concentrations of metals inhibited embryo development in a dose-dependent manner. In the control and in lower concentrations of heavy metals, the embryos reached the D-shaped larval stage. Higher concentrations of heavy metals completely blocked embryo development.
At 18.5 μg l−1 (0.1 μM) of Hg, embryo development was arrested at the gastrula or blastula stage, whilst at 187 μg l−1 (1 μM) Hg only morulae were found. Following exposure to Cd, D-shaped larvae were usually found at 1,046 μg l−1 (10 μM), whereas differentiation was inhibited at the morula stage at 10,167 μg l−1 (100 μM). Similarly, for Pb at 197 μg l−1 (1 μM), embryos developed to the D-shaped larval stage, while for Pb at 1,016 μg l−1 usually gastrulae or blastula were found. As shown in Table 2, the lowest Hg concentration to significantly affect larval hatching was 18.5 μg l−1 (0.1 μM), for Cd this concentration was 104 μg l−1 (1 μM) and was 197 μg l−1 (1 μM) for Pb. Mercury showed the highest toxicity and completely inhibited larval hatching at 18.5 μg l−1 (0.1 μM), while Cd 1,046 μg l−1 (10 μM) severely blocked incubation, and Pb 1,016 μg l−1 completely inhibited larval incubation.
The EC50 values and their 95% confidence intervals are shown in Table 3. Hg was ~55 times more toxic to M. meretrix embryos than Pb, and ~188 times more toxic than Cd. Therefore, Hg was the most toxic of the three metals tested to the embryos. The heavy metals were consistently ranked in the following order from highest to lowest toxicity: Hg > Pb > Cd.
Larval growth
When the larvae were exposed to heavy metals for about 24 h, dead larvae which presented with extruded velum and granulated tissues appeared in the more toxic treatments. A sublethal effect, injury to the velum and swimming inhibition were also observed, occurring at lower concentrations than those causing lethal effects.
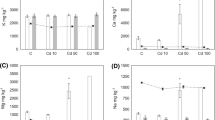
The increases in larval shell length for the different metal concentrations are shown in Fig. 2. Concentration-dependent growth inhibition was also found for all the metals tested. A reduction in growth rate was evident in the larvae following exposure to most concentrations from 24 h. The larvae exposed to 187 μg l−1 (1 μM) Hg grew little or died after 24 h and only grew about 20 μm when exposed to 18.5 μg l−1 (0.1 μM) Hg. Larval growth was limited at a Cd concentration of 1,046 μg l−1 (10 μM) and was significantly retarded at 104 μg l−1 (1 μM) (P < 0.01), whereas larval growth was about 10 μm at 1,016 μg l−1 Pb and was significantly inhibited at 197 μg l−1 (1 μM) Pb (P < 0.05).
Effects of mercury, cadmium and lead on larval growth of M. meretrix. Values shown are the mean ± SD (n = 30). a The larvae exposed to 10 μM Hg and 100 μM Hg died after 24 h, so the growth curves ceased at 24 h. b The larvae exposed to 10 μM and 100 μM died after 72 h, so the growth curves ceased at 72 h. c The larvae exposed to 100 μM Cd died after 24 h and the growth curve ceased at 24 h. Similarly, the growth curve of 10 μM Cd ceased at 48 h
At the end of this experiment (96 h), the lowest concentration of each metal had little influence on growth. Relatively higher concentrations of these heavy metals, 18.5 μg l−1 (0.1 μM) Hg, 104 μg l−1 (1 μM) Cd and 197 μg l−1 (1 μM) Pb, significantly inhibited growth (P < 0.05). A significant reduction in larval growth of 13.8% at 18.5 μg l−1 Hg was noted, and 104 μg l−1 Cd caused a reduction of 60.4% in mean larval growth. Larval growth was also inhibited by 57.2% at 197 μg l−1 Pb. The EC50 values and their 95% confidence intervals are shown in Table 3. Among the three metals, Hg inhibited larval growth more than Pb and Cd.
Larval survival
Heavy metals not only had severe effects on M. meretrix larval growth, but also had adverse affects on larval survival. The effects of Hg, Cd and Pb on the survival rate of M. meretrix are shown in Table 4. Concentration-dependent survival inhibition was evident. In this experiment, when exposed for 24 h, larvae could survive in 187 μg l−1 (1 μM) Hg, 1,046 μg l−1 (10 μM) Cd and 7,158 μg l−1 Pb, respectively, while no larvae survived in 18.5 μg l−1 (0.1 μM) Hg, 1,046 μg l−1 (10 μM) Cd and 1,016 μg l−1 Pb when exposed for 96 h. However, 1.89 μg l−1 (0.01 μM) Hg, 104 μg l−1 (1 μM) Cd and 197 μg l−1 (1 μM) Pb had no significant effect on larval survival for 96 h. The LC50 values and their 95% confidence intervals are shown in Table 3. According to the 96 h LC50 values, Hg was ~4.9 times more toxic to M. meretrix larvae than Cd, and ~25 times more toxic than Pb. Thus, Hg was most toxic to M. meretrix larvae.
Metamorphosis
The percentages of metamorphosed individuals from competent pediveligers exposed to different heavy metal concentrations are listed in Table 5. In this experiment, Pb was the least toxic for metamorphosis, even 1,016 μg l−1 and 7,158 μg l−1 Pb reduced the metamorphosis rate only by about 10%. 104 μg l−1 (1 μM) Cd reduced larval attachment by 44.5%, while 187 μg l−1 (1 μM) Hg inhibited metamorphosis by 41.8%. 20.4 μg l−1 (0.1 μM) Pb, 11.0 μg l−1 (0.1 μM) Cd and 18.5 (0.1 μM) μg l−1 Hg had no adverse effect on larval settlement; on the other hand, enhanced average metamorphosis was also observed at 1.95 μg l−1 (0.01 μM) Pb and 18.5 μg l−1 (0.1 μM) Hg (not statistically significant). Table 3 lists the EC50 values and their 95% confidence intervals. It can be seen that Hg was most toxic and Pb was least toxic to metamorphosing larvae.
Discussion
Embryogenesis
Based on the EC50 values in our study, Hg was the most toxic to M. meretrix embryos, followed in decreasing order of toxicity, by Pb and Cd. This agrees with toxicity data for trace metals on the embryonic development of other marine invertebrates (Dinnel et al. 1989; Ramachandran et al. 1997; Fernández and Beiras 2001). The EC50 value obtained in the present study for Hg inhibition of embryogenesis of 5.4 μg l−1, compares with 4.6 μg l−1 for Meretrix lusoria (Chin and Chen 1993), 4.2 μg l−1 (48 h EC50) for M. galloprovincialis, 5.1 μg l−1 for R. decussatus (Beiras and Albentosa 2004), 5.6 μg l−1 for C. virginica (Calabrese et al. 1973), 6.7 μg l−1 (Martin et al. 1981) and 13 μg l−1 (Beiras and His 1994) for C. gigas, respectively. For Cd, the EC50 value for embryogenesis was 1,014 μg l−1 in the present study, compared to a value of 424 μg l−1 for M. galloprovincialis and 1,925 μg l−1 for R. decussates (Beiras and Albentosa 2004). Similarly, Calabrese et al. (1973) reported an EC50 for C. virginica of 3,800 μg l−1 and Nadella et al. (2009) showed a 48 h EC50 value of 502 μg l−1 for Mytilus trossolus. For Pb, an EC50 for inhibition of embryogenesis of 296 μg/l was obtained in the present study, whereas the EC50 for C. virginica was 2,450 μg l−1 (Calabrese et al. 1973), 221 μg l−1 for M. galloprovincialis and 156–312 μg l−1 for R. decussatus (Beiras and Albentosa 2004).
To compare EC50 values among experiments, the exposure period should be taken into account, since the duration of dosing is a major factor influencing toxicity. The EC50 values found in the present study for embryos were comparable with data from the literature for oyster species, most of these studies referred to exposure times of 24–48 h (Beiras and His 1994). As illustrated in our results, the EC50 values of the heavy metals for M. meretrix embryos were similar to those of the above bivalves, which indicated that the sensitivity of M. meretrix embryos were comparable to those of Mytilus and Crassostrea. In conclusion, the embryos of M. meretrix are sufficiently sensitive for use in seawater assessments.
Larval survival
For M. meretrix larvae, the 48 h LC50 for Hg, Cd and Pb were 109.3 μg l−1, 237 μg l−1 and >7.1 mg l−1, respectively. The 48 h LC50 for Pb could not be determined as it would exceed the solubility of Pb in seawater. On the other hand, the 96 h LC50 was more sensitive and the deviation of the LC50 value was much less at 48 h. Therefore, we suggest that the 96 h LC50 may be more stable and reliable than the 48 h LC50, and we propose that this value better reflects toxicity to the entire life of larvae in toxicity tests.
From the above results, it is evident that M. meretrix embryos were more sensitive to metal pollutants than larvae. The findings from this study were consistent with those in C. virginica (Calabrese et al. 1977) and C. gigas (Beiras and His 1994). Wong et al. (1993) also found that tolerance to heavy metals increased with age in crustacean larvae. In these studies, exposure periods for embryos and larvae were comparable (a little longer for larvae), so the comparison between them was reasonable. Larvae are more resistant to pollutants possibly because the larval shell protects them when exposed to pollutants for a short time.
In conclusion, embryo testing is faster and more sensitive than larvae testing. In addition, there is no interference from variables such as larval condition and the presence of algal food, thus M. meretrix embryos are more suitable and reliable than larvae in acute toxicity tests.
Growth
From D-shaped larvae to spat, M. meretrix only need about five days to complete this stage of development at 26°C. Thus, this experiment lasted only 96 h, during which the larvae were at the pelagic stage. Moreover, because the shell length and height of M. meretrix showed a stable correlation, y = 0.8576x + 40.544 μm, R 2 = 0.9954, this suggested that the cultured larvae were developing normally and shell length could be used as an appropriate indicator of growth (Tang et al. 2006).
Besides embryogenesis and larval mortality, the larval growth test is also used as an endpoint in bioassays. In fact, toxicants first affect the behavior (swimming) and physiological response (growth), and then cause mortality. Although it is difficult to perform, this type of test is more sensitive and useful for a realistic assessment of the impact of a potential pollutant in the wild (Geffard et al. 2002).
Experimental evidence shown in this study indicates that larval growth is reduced at low metal concentrations. 18.5 μg l−1 (0.1 μM) Hg, 104 μg l−1 (1 μM) Cd and 197 μg l−1 (1 μM) Pb inhibited larval growth after 96 h exposure. As shown in Table 3, the sensitivity of growth tests is similar or a little higher than mortality tests. These results are similar to those in other studies. Watling (1982) found that metal concentrations causing a 50% reduction in growth were consistently lower than both embryo and larval LC50 for oyster species. Beiras and His (1994) showed that C. gigas larval growth rate decreased by a factor of 0.4 at a nominal concentration of 4 μg l−1 Hg, and by a factor of 0.7 at 8 μg l−1 Hg, whilst effects on acute mortality were not apparent until 32 μg l−1. Geffard et al. (2002) also demonstrated that C. gigas larval growth testing was more sensitive to pollutant sediments than the embryo test. However, our experimental time was much shorter than the time stated in that study, therefore it was relatively easy to carry out.
Although the larval growth test was sometimes more sensitive, it was easily influenced by many factors such as larval condition, algal food and other environmental factors. Thus, we think this test may be used as an alternative.
Our results suggest that heavy metals of relatively low concentrations (realistic concentrations in some areas) may significantly reduce larval growth. A retardation of growth, which prolongs the pelagic life of bivalves, will have some ecological implications on the recruitment of the bivalves (Calabrese et al. 1977).
Metamorphosis
In contrast to D-shaped larvae, pediveligers showed significant resistance to metals when the exposure time was only 48 h, thus the EC50 values for metamorphosis were relatively high. The EC50 values for metamorphosis were at least several times higher than those for embryogenesis. In this experiment, dissolvable lead in high concentrations was much lower than the nominal concentration and the Pb concentrations were somewhat not reasonable, thus the EC50 values for Pb were not so believable and were somewhat high. The sensitivity of metamorphosis was comparable to that of 48 h mortality.
Enhancement of biological response at low concentrations of the toxicants (hormesis) was also detected in our study. 18.5 μg l−1 (0.1 μM) Hg and 1.95 μg l−1 (0.01 μM) Pb both promoted settlement. Therefore, lower concentrations of metals may be positive inductors triggering settlement. This effect might be a sublethal response by the larvae, resulting in swimming cessation and attachment. In other studies, 1 μg l−1 Hg also stimulated the settlement of C. gigas larvae (Beiras and His 1994). Bellas et al. (2004) showed that the number of attached ascidian larvae was significantly greater at 32 μg l−1 for Hg and 8,192 μg l−1 for Cd than in controls.
In marine environments, metal ions may combine with inorganic ions or organic matter to form complexes, therefore the actual concentrations of the metal ions would be lower than nominal concentrations. Thus, it should be noted that toxicity was probably underestimated in these experiments, taking into account the loss of heavy metals in the vessels. In addition, the concentration span between treatments was high which may have caused inaccuracies in the EC50 values.
The concentrations of heavy metals used here were unrealistically high in order to thoroughly understand the toxicity. Nevertheless, the possibility still remains in some areas, particularly near the seashore, that the present rate of pollution could have led to such levels of heavy metals. In estuarine areas, even these heavy metals are in low concentrations, but they may act synergistically and significantly influence the larvae. Furthermore, because adult bivalves rarely migrate, recruitment in the population depends on the transport of larvae from other areas. In addition, several years are required for growth, maturation, and reproduction in M. meretrix. Therefore, some disturbance in the larvae may have a long-term impact on the population.
Conclusion
The sensitivity of early development of M. meretrix to heavy metals was investigated in laboratory experiments using the following endpoints: embryogenesis, larval survival, growth and metamorphosis. Most of these endpoints were easy to quantify, but not all of them are suitable for use in routine testing. Embryogenesis is, very easy, sensitive and can be used as an endpoint in toxicity tests. However, it is inappropriate to use larval growth or metamorphosis as an endpoint to assess the toxicity of heavy metals in this species, as larval growth was affected by many factors (larval condition, algal food, etc.) and metamorphosis was not so sensitive.
This data will help to determine the heavy metal level which may adversely affect the wild population, and the suitability of this species for the toxicity testing of chemicals and environmental samples. This study may provide knowledge to assist in developing marine water quality guidelines in Asian regions.
References
Beiras R, Albentosa M (2004) Inhibition of embryo development of the commercial bivalves Ruditapes decussates and Mytilus galloprovincialis by trace metals; implications for the implementation of seawater quality criteria. Aquaculture 230:205–213. doi:10.1016/S0044-8486(03)00432-0
Beiras R, His E (1994) Effects of dissolved mercury on embryogenesis, survival, growth and metamorphosis of Crassostrea gigas oyster larvae. Mar Ecol Prog Ser 113:95–103. doi:10.3354/meps113095
Beiras R, His E (1995) Effects of dissolved mercury on embryogenesis, survival and growth of Mytilus galloprovincialis mussel larvae. Mar Ecol Prog Ser 126:185–189. doi:10.3354/meps126185
Bellas J (2006) Comparative toxicity of alternative antifouling biocides on embryos and larvae of marine invertebrates. Sci Total Environ 367:573–585. doi:10.1016/j.scitotenv.2006.01.028
Bellas J, Beiras R, Vazquez E (2004) Sublethal effects of trace metals (Cd, Cr, Cu, Hg) on embryogenesis and larval settlement of the Ascidian Ciona intestinalis. Arch Environ Contam Toxicol 46:61–66. doi:10.1007/s00244-003-0238-7
Calabrese A, Collier RS, Nelson DA, MacInnes JR (1973) The toxicity of heavy metals to the embryos of the American oyster Crassostrea virginica. Mar Biol 18:162–166.
Calabrese A, Mac Innes JR, Nelson DA, Millar JE (1977) Survival and growth of bivalve larvae under heavy metal stress. Mar Biol (Berl) 41:179–184. doi:10.1007/BF00394024
Chin TS, Chen HC (1993) Toxic effects of mercury on the hard clam, Meretrix lusoria, in various salinities. Comp Biochem Physiol 105(Part C):501–507. doi:10.1016/0742-8413(93)90092-Y
Connor PM (1972) Acute toxicity of heavy metals to some marine larvae. Mar Pollut Bull 3(12):190–192. doi:10.1016/0025-326X(72)90268-8
Depledge MH, Weeks JM, Bjerregaard PB (1994) Heavy metals. In: Calow P (ed) Handbook of ecotoxicology II. Blackwell Scientific Publications, Cambridge, pp 79–105
Dinnel PA, Link JM, Stober QJ, Letourneau MW, Roberts WE (1989) Comparative sensitivity of sea urchin bioassays to metals and pesticides. Arch Environ Contam Toxicol 18:748–755. doi:10.1007/BF01225012
Emmens CW (1948) Principles of biological assay. Chapman and Hall, London
Fernández N, Beiras R (2001) Combined toxicity of dissolved mercury with copper, lead and cadmium on embryogenesis and early larval growth of the Paracentrotus lividus sea urchin. Ecotoxicology 10:263–271. doi:10.1023/A:1016703116830
GB17378 4 (2007) The specification for marine monitoring, part 4: seawater analysis. Standards Press of China, Beijing, pp 15–17
Geffard O, Budizinski H, His E (2002) The effects of elutriates from PAH and heavy metal polluted sediments on Crassostrea gigas (Thunberg) embryogenesis larval growth and bio-accumulation by the larval of pollutants from sedimentary origin. Ecotoxicology 11:403–416. doi:10.1023/A:1021024415695
He CB, Xu SJ, Zhang C (1997) Study on the growth and ecological characteristics of Meretrix meretrix cultivated on tidal flat. Chin J Fish Sci 16:17–20 in Chinese with English abstract
His E, Seaman MNL, Beiras R (1997) A simplification the bivalve embryogenesis and larval development bioassay method for water quality assessment. Water Res 31:351–355. doi:10.1016/S0043-1354(96)00244-8
His E, Beiras R, Seaman MNL (1999) The assessment of marine pollution-bioassays with bivalve embryos and larvae. In: Southward A, Tyler P, Young C (eds) Advances in Marine Biology, vol 37. Academic Press, London, pp 1–178
Itow T, Loveland RE, Botton ML (1998) Developmental abnormalities in Horseshoe crab embryos caused by exposure to heavy metals. Arch Environ Contam Toxicol 35:33–40. doi:10.1007/s002449900345
Lapota D, Rosenberger DE, Platter-Rieger MF, Seligman PF (1993) Growth and survival of Mytilus edulis larvae exposed to low levels of dibutyltin and tributyltin. Mar Biol (Berl) 115:413–419. doi:10.1007/BF00349840
Laughlin RB, Gustafson R, Pendoley P (1988) Chronic embryo-larval toxicity of tributyltin (TBT) to the hard shell clam Mercenaria rnercenana. Mar Ecol Prog Ser 48:29–36. doi:10.3354/meps048029
Martin M, Osborn KE, Billig P, Glickstein N (1981) Toxicities of ten metals to Crassostrea gigas and Mytilus edulis embryos and Cancer magister larvae. Mar Pollut Bull 12:305–308. doi:10.1016/0025-326X(81)90081-3
Meng W, Qin YW, Zheng BH, Zhang L (2008) Heavy metal pollution in Tianjin Bohai Bay, China. J Environ Sci (China) 20:814–819. doi:10.1016/S1001-0742(08)62131-2
Nadella SR, Fitzpatrick JL, Franklin N, Bucking C, Smith S, Wood CM (2009) Toxicity of dissolved Cu, Zn, Ni and Cd to developing embryos of the blue mussel (Mytilus trossolus) and the protective effect of dissolved organic carbon. Comp Biochem Physiol 105(Part C):340–348
Nelson DA, Calabrese A, Maclnnes JR (1977) Mercury stress on juvenile Bay scallops, Argopecten irradians, under various salinity-temperature regimes. Mar Biol (Berl) 43:293–297. doi:10.1007/BF00396923
Newman MC (1995) Quantitative methods in aquatic toxicology. CRC Press, Boca Raton, p 426
Ramachandran S, Patel TR, Colbo MH (1997) Effect of cadmium on three Malaysian tropical estuarine invertebrate larvae. Ecotoxicol Environ Saf 36:183–188. doi:10.1006/eesa.1996.1508
Reish DJ, Gerlinger TV (1997) A review of the toxicological studies with polychaetous annelids. Bull Mar Sci 60:584–607
Rittschof D, McClellan-Green P (2005) Molluscs as multidisciplinary models in environment toxicology. Mar Pollut Bull 50:369–373. doi:10.1016/j.marpolbul.2005.02.008
Tang BJ, Liu BZ, Wang GD, Zhang T, Xiang JH (2006) Effects of various algal diets and starvation on larval growth and survival of Meretrix meretrix. Aquaculture 254:526–533. doi:10.1016/j.aquaculture.2005.11.012
Wang CY, Wang XL (2007) Spatial distribution of dissolved Pb, Hg, Cd, Cu and As in the Bohai Sea. J Environ Sci (China) 19:1061–1066. doi:10.1016/S1001-0742(07)60173-9
Wang RC, Wang ZP, Zhang JZ (1993) Marine Shellfish Aquaculture. Qingdao Ocean University Press, Qingdao, China, pp 322–324 (in Chinese)
Warnau M, Iaccarino M, De Biase A, Temara A (1996) Spermiotoxicity and embryotoxicity of heavy metals in the echinoid Paracentrotus lividus. Environ Toxicol Chem 15:1931–1936. doi:10.1897/1551-5028(1996)015<1931:SAEOHM>2.3.CO;2
Watling HR (1982) Comparative study of the effects of zinc, cadmium and copper on the larval growth of three oyster species. Bull Environ Contam Toxicol 28:195–201. doi:10.1007/BF01608575
Weltz B, Schubert-Jacobs M (1991) Evaluation of a flow injection system and optimization of parameters for hydride generation atomic absortion spectrometry. Atom Spectrosc 12:91–103
Wessel N, Rousseau S, Caisey X, Quiniou F, Akcha F (2007) Investigating the relationship between embryotoxic and genotoxic effects of benzo[a]pyrene, 17-α-ethinylestradiol and endosulfan on Crassostrea gigas embryos. Aquat Toxicol 85:133–142. doi:10.1016/j.aquatox.2007.08.007
Wong CK, Chu KH, Tang KW, Tam TW, Wong LJ (1993) Effects of chromium, copper and nickel on survival and feeding behaviour of Metapenaeus ensis larvae and postlarvae (Decapoda: Penaeidae). Mar Environ Res 36:63–78. doi:10.1016/0141-1136(93)90082-B
Acknowledgment
The authors thank for the help of staff in Qingjiang hatchery, Zhejiang Mariculturre Research Institute. This research was supported by the National Natural Science Foundation of China (No.40821004/3067991), the National Key Foundational Research Project of China (No.2007CB407305), the National Marine Public Welfare Research Project (No.200805069) and the National Agricultural Public Welfare Research Project (No.nyhyzx07-047).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Liu, B., Yang, H. et al. Toxicity of lead, cadmium and mercury on embryogenesis, survival, growth and metamorphosis of Meretrix meretrix larvae. Ecotoxicology 18, 829–837 (2009). https://doi.org/10.1007/s10646-009-0326-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0326-1