Abstract
The toxicity role of insecticides affecting nontarget vertebrate of wildlife population has become essential subject to focus on. In this vein, the current study aimed to illustrate some biochemical and histopathological alterations induced by two neonicotinoids in Egyptian toads. Forty-five toads were collected and divided equally into three groups (15 toads/group): control group, thiamethoxam group, and acetamiprid group. Both treatment groups received thiamethoxam and acetamiprid (30 and 40 mg/L, respectively) four times within 12 days for induction of sublethal toxicity. Blood and liver tissue samples were collected. Both insecticides cause the same changes, but acetamiprid group exhibited a pronounced significant (P ≥ 0.001) effect than thiamethoxam group on increasing serum lipid profile, ALT, and AST. Moreover, acetamiprid showed a significant (P ≥ 0.001) decrease in hepatic total protein, GSH, and SOD and increase in MDA levels in comparison with thiamethoxam and control groups, respectively. The histopathological hepatic examination showed markable alterations in hepatic architecture in treatment groups that was distinct in acetamiprid group. Finally, our findings illustrate the indirect effect of neonicotinoids on toads and may realize their life-threatening factors.

Graphical Abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphibians are the highlighted examples of the recent biodiversity crisis as they notably decline in population since the late of the twentieth century (Brühl et al. 2013). The survival of amphibian wildlife populations is threatened by habitat loss and fragmentation, infectious diseases, global climate change, invasive species, over-harvesting for human consumption or illegal pet trade, pollution, and pesticide contamination (Lenhardt et al. 2017). Underlying causes of habitat loss, fragmentation, and pollution are the expansion and intensification of agriculture (Hartel et al. 2010).
Amphibians have many characteristics that make them sensitive to environmental changes than other wild species. The biphasic life cycle is an essential aspect of amphibian biology, which makes them reliable models for studying the environmental problems. Moreover, frog skin is highly permeable than that of mammals as the stratum corneum which is the primary barrier to percutaneous absorption in mammals and is much thinner in frogs consists of one or two cell layers (Helmer and Whiteside 2005). Skin absorption is a main route in the uptake process especially in the adult frogs (Katagi 2014). The skin plays an important role in the absorption of water and soluble substance including toxic substance and oxygen; thus, the amphibians can survive through their skin interaction with the surrounded environment and their ability to feat different kinds of habitats and ecological conditions (Hopkins 2007) that makes them act as bioindicator to any environmental condition.
The use of pesticides in agriculture has increased rapidly during the past decades as they are highly effective against pests. The agricultural runoff is the main source for amphibian exposure to pesticides which picked up into adult frogs by passive diffusion via skin and into tadpoles via gills and skin (Katagi 2014). Their effect on amphibian could be lethal or sublethal. Lethal effects are mainly associated with overspray which may let to 100% mortality or with chronic exposure over long period of time. Sublethal effect is mainly described as hindering of growth, delayed metamorphoses, and reproduction problems (Mann et al. 2009), besides the immunosuppression (Smalling et al. 2015).
There are five groups of insecticides: methyl carbamates, chlorinated hydrocarbons, pyrethroids, organophosphorus compounds, and neonicotinoid (Çamlıca et al. 2018). Neonicotinoids have become the most important and effective insecticides on the global market because they have a wide spectrum activity, used in small doses with long-lasting control, safe to many crops, and taken systemically throughout the entire plant, water soluble, and easy applied through many routes: dressing, spraying, and drenching (Uneme 2011).
Neonicotinoids act on nicotinic acetylcholine receptors in the central nervous system of the target organism causing neurotoxicity (Anderson et al. 2015). They are more toxic to invertebrates than mammals, birds, and other higher organisms. Furthermore, neonicotinoids may have sublethal effects on the immune response, development, and growth of amphibians (Gabor et al. 2018; Stuart et al. 2004).
The estimated ecological level of neonicotinoids was found to be in range up to 320mg/L (Morrissey et al. 2015; Robinson et al. 2017). The value of lethal concentration for neonicotinoids for 50% mortality (LC50) in frog species was found to be ranged from 52.6 to 219 mg/L (Feng et al. 2004; Ruiz de Arcaute et al. 2014). Sublethal toxic effects of neonicotinoids on amphibians have been examined in few studies. Although an LC50 for imidacloprid in tadpoles of black-spotted pond frogs (Pelophylax nigromaculatus) was 129–210 mg/L, it caused DNA damage even at lower concentration of 50 mg/L (Beasley 2020).
Lipid peroxidation and oxidative stress are involved in the molecular mechanisms induced by pesticide toxicity that are represented by enzymes as antioxidant defense system including superoxide dismutase (SOD) that scavenges superoxide radicals and glutathione peroxidase (GSH). Malondialdehyde (MDA) is the last result of peroxidized polyunsaturated fatty acids that is indicative for lipid peroxidation of cell membranes. So, changes in antioxidant enzyme activities and MDA contents are good molecular bioindicators to detect the toxic effects in organisms (Büyükgüzel 2009; Winston and Di Giulio 1991; Xing et al. 2012).
The most important organ in detoxification processes of chemicals is the liver (Steinel and Bolnick 2017). Protein, lipid, and carbohydrates are important energy sources used by organisms for detoxification of toxic chemicals; besides other vital functions, they are frequently affected by toxicants (Emre et al. 2013; Yucel and Kayis 2019).
Egyptian toads (Sclerophrys regularis) were selected as a model organism because they are a widespread and native species in Egypt and their habitats might be impacted by agricultural runoff. They are categorized as least concern by International Union of Conservation nature (IUCN red list 2016).
In this vein, the study was designed to validate the intoxication alterations in Egyptian toads with sublethal doses of thiamethoxam and acetamiprid via assessing of the biochemical parameters (protein, lipid, and triglycerides), oxidative stress biomarkers (GSH, SOD, and MDA levels), and liver histopathological changes.
Materials and methods
Animals
All the procedures of the study are conformed according to the ethical and humane principles of Ethics and Animal Experimentation Committee of Suez Canal University (approval no. 2020045).
Forty-five adult male toads were caught with hand net from ponds some distance away from any agricultural sites or pollution sources that could be considered relative unpolluted area. Then, the toads were transported to the laboratory in a covered basket. Their exact ages were not obtainable as these frogs originated from the wild. The average body weight of the toads was 35 ± 10 g. Adult toads were acclimatized in glass tanks (51 × 60 × 33 cm3) for a week under laboratory conditions (12/12-h light/dark cycle and at 26–30 °C) before the experiment starts in the first day of the second week.
To simulate the natural habitat where the toads live, we provided clean tanks with 2 L dechlorinated tap water that settled and exposed for 36 h, sand and mud. Tanks were placed on a slant to provide both aqueous and dry environment (Allran and Karasov 2001). Water was changed twice weekly and administered ad libitum. The food including earth worm and small insects was added every 2 days.
Pesticides and chemicals
Two neonicotinoids pesticides were used in this study. Thiamethoxam (Actara 25%, Syngenta Company) and acetamiprid (Acetamore 20%, Chema Industries) were obtained from a local market. All chemicals used in this study were purchased from Sigma-Aldrich. Kits for measuring SOD, GSH, and MDA were purchased from BioVision Inc., Milpitas, USA.
Experimental design
The toads were divided into three groups in three tanks (15 toads/tank); the first was a control group where toads are exposed only to dechlorinated tap water. The second and the third were thiamethoxam and acetamiprid groups, where toads are exposed to 1/6 of the Egyptian recommended field concentration 30 mg/L and 40 mg/L, respectively, according to Loutfy and Kamel (2018). The experiment lasted for 12 days. Fresh solutions of pesticides were prepared every 4 days. The duration of 4 days falls in the range of biological half-life of both insecticides as described by manufacturers in the enclosed pamphlets. Thiamethoxam (Actara) degradation half-life in water and soil is 11 and 51 days, respectively. Acetamiprid (Acetamore) has 34 days half-life in water and 1–8 days in soil.
Hence, the toads were subjected to sublethal doses of tested pesticides 4 times within 12 days at interval (day 0, day 4, day 8, day 12). At the end of the treatment, blood samples were collected from toads in plain tubes through heart puncture after inhalation anesthesia with tetrahydrofuran. All the toads were euthanized by pithing. Collected blood was incubated for 15 min at 37°C to clot and then centrifuged for 15 min at 3000 rpm until the serum was separated. The separated serum and toads’ livers were kept at −20°C until analysis of biochemical parameters.
Biochemical analyses
Serum total lipid index was measured using the sulfo-phospho-vanillin colorimetric method described by Knight et al. (1972). Serum total cholesterol (chol) and triglycerides (TG) concentrations were determined by enzymatic colorimetric method (PAP) according to Allain et al. (1974) and Bucolo and David (1973), respectively.
The activity of liver enzymes, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured in serum based upon the International Federation of Clinical Chemistry recommendations by Bergmeyer et al. (1986).
Protein contents of the liver tissue homogenate (2.5%) were measured according to the method of Lowry et al. (1951) using bovine serum albumin as standard. To obtain liver homogenate, liver was removed and washed in cooled 0.65% saline, kept on ice, and subsequently blotted on filter paper. One gram of liver was homogenized in 5 mL cold phosphate buffer. The homogenates were centrifuged at 10000 rpm for 10 min at 4 °C, and the supernatants were used for the estimation of total protein and lipid peroxidation.
The activities of the antioxidant enzymes and lipid peroxidation were measured in frozen liver using a spectrophotometer (Shimadzu UV-160). GSH was estimated as described by Sedlak and Lindsay (1968). SOD activity was determined as described by Sun and Zigman (1978). MDA was determined using the method of Buege and Aust (1978).
Histopathological examination
Immediately after scarification of toads of each group, liver pieces were obtained and fixed in 10% formalin. Then the fixed livers were dehydrated in graded alcohol, embedded in paraffin, dissected into sections of 5 micron, and stained with routine hematoxylin and eosin (H&E) stain as described by Bancroft and Gamble (2008).
Statistical analysis
Data was tested for normality using Kolmogorov-Smirnova and Shapiro-Wilk tests, and it was found to follow normal distribution curve. Data was analyzed by using SPSS one-way ANOVA (version 20, USA) to compare between different groups regarding biochemical parameters and oxidative stress biomarkers. Further comparison between different groups was performed by Bonferroni test for multiple comparisons. The obtained results are presented as mean values ± SEM. Significance was designated at probability value of (P ≥ 0.001).
Results
Lipid profile
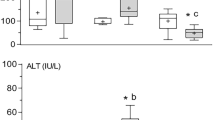
Acetamiprid toads group showed a significant (P ≥ 0.001) elevation in the serum levels of total lipid, cholesterol, and triglyceride when compared with thiamethoxam and control groups. Moreover, both of the pesticides groups revealed significant (P ≥ 0.001) increase in lipid profile than control toad group (Table 1).
Liver function enzymes
Our results reported that acetamiprid intoxication in Egyptian toads led to a significant (P ≥ 0.001) increase in serum level of AST and ALT in comparison with thiamethoxam and control groups (Table 1).
Hepatic total protein
Both of the pesticides toads’ groups showed significant (P ≥ 0.001) decrease in the hepatic levels of total protein as compared with the control group.
Oxidative stress biomarkers
Egyptian toads exposed to acetamiprid showed a significant (P ≥ 0.001) decrease in hepatic GSH and SOD and a significant increase in MDA in comparison with toads exposed to thiamethoxam and control groups (Table 2). In addition, hepatic GSH and SOD decreased, and MDA increased significantly (P ≥ 0.001) in pesticides groups as compared to control group.
Liver histopathology
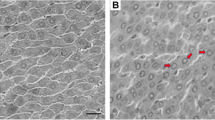
Liver of control group (Fig. 1a) revealed normal hepatic cells and hepatic cords which were arranged in a radiating shape around central veins. The hepatic cells were normal in size and shape with homogenous eosinophilic cytoplasm and centrally located nuclei. The liver of toads treated with thiamethoxam (Fig. 1b–f) showed mild to moderate vacuolation of hepatic cells (Fig. 1c), congestion of hepatic blood vessels, and perivascular edema (Fig. 1e). Hyperplasia of bile ducts and focal lymphocytic infiltrations (Fig. 1b) along with hyperactivity of melanin pigments were also observed (Fig. 1 d and f). The liver of toads treated with acetamiprid (Fig. 2a–f) showed moderate to severe histological alterations. All blood vessels of hepatic areas, central veins, and hepatic sinusoids were congested (Fig. 2c) with occasional perivascular edema and hyperplasia of bile ducts (Fig. 2 b and f). Massive focal lymphocytic infiltrations (Fig. 2d) and hyperreactivity of melanin pigments (Fig. 2e) were also observed. Disarrangement of hepatic cells with severe cytohepatomegally, multifocal degeneration, and focal necrosis were also observed (Fig. 2 a and e). Some hepatic cell revealed mitotic figures and binucleation (Fig. 2f).
Liver sections of Egyptian toads stained with H&E, bar 50, ×40 showing normal tissue architecture of control group (a). Mild to moderate histopathological changes in thiamethoxam-treated group (b–f). Central vein (cv), hyperplasia of bile ducts (black arrows), melanin pigments (red arrows), mitotic figures of nuclei (white arrows), degeneration of hepatocytes and necrosis (white arrow heads), perivascular edema (black arrow heads). Lymphocytic infiltration (asterisk).
Liver sections of Egyptian toads stained with H&E, bar 50, ×40 showing moderate to severe histopathological alteration in acetamiprid-treated group (a–f). Central vein (cv), hyperplasia of bile ducts (black arrows), melanin pigments (red arrows), mitotic figures of nuclei (white arrows), degeneration of hepatocytes and necrosis (white arrow heads), perivascular edema (black arrow heads). Lymphocytic infiltration (asterisk).
Discussion
Wildlife greatly is affected by the increasing use of pesticides especially in the developing countries due to intensification of agriculture and consequently pesticides applications (Schreinemachers and Tipraqsa 2012). The increased use of pesticides is one of the main causes of declining frog populations.
Neonicotinoids could persist in aquatic environments up to 1 year after application; only a small amount (2–28%) of the applied neonicotinoid is taken up by target crops, indicating that (72–98%) of the application may penetrated into the surrounding soils and adjacent water bodies. Thus, nontarget species may accidentally be exposed to harmful concentrations of neonicotinoids through irrigation events or runoff rainfall, overspray, and wastewater drainage (Morrissey et al. 2015; Robinson et al. 2019). Documented half-lives in soils are highly variable. Existence and fate of the neonicotinoids in water depend upon some factors such as light, pH, temperature, and microbial action (Anderson et al. 2015).
There are huge gaps in amphibian ecotoxicological data because of the variety of amphibian species, different life stages, several chemicals, and the changing levels of exposures. In a trial to assess the pesticide exposures of adult northern leopard frogs (L. pipiens) in two wetlands, surrounding grasslands, and adjacent agricultural sites in the USA, >70 individuals were tracked by radiotelemetry, and samples of water, sediment, and specimens from the frogs were tested for pesticide concentrations. The highest environmental pesticide concentrations were in the agricultural sites and then in wetlands, and the lowest concentrations were in the grassland (Beasley 2020).
The steps of pesticide toxicity in nontarget organisms are mainly related with oxidative stress and lipid peroxidation. In the present study, toads were chronically exposed to two neonicotinoid pesticides: thiamethoxam (30 mg/L) and acetamiprid (40 mg/L), and some biomarkers were analyzed including total protein, lipid profile, tissue-related oxidative stress parameters (GSH, SOD), lipid peroxidation markers (MDA), and histopathological changes.
The increased levels of total lipids, cholesterol, and triglycerides in this study could indicate high lipid peroxidation, which is related to glycolipid metabolism disorders, loss of integrity of the cell membrane, and increase energy demand, which cause cell damage, lipid accumulation, and apoptosis (Dornelles and Oliveira 2014). These results agreed with Wilkens et al. (2019) who found an increase in the levels of triglycerides and VLDL in the plasma of bullfrog tadpoles exposed to two herbicides (sulfentrazone and glyphosate). Several studies showed an increase in both total lipids and triglycerides in the liver of amphibians and fish exposed to pesticides, such as El-Banna et al. (2009), Persch et al. (2017), Salbego et al. (2010), and Sounderraj et al. (2011). Emam et al. (2018) also reported a significant increase in serum lipid profile in Japanese quails exposed to imidacloprid.
Liver is the organ of detoxification in the body. Hepatic enzymes, such as AST and ALT, are good indicators for evaluation of hepatotoxicity and liver damage. The increased activity of liver function enzymes in serum occurs due to its release into bloodstream due to cell membrane damage or necrosis which indicates a liver injury in the current study. These results come in accordance with Jalili et al.’s (2019) findings which revealed that pesticide administration causes increase in the liver enzymes in male rat. Emam et al. (2018) also get the same results in Japanese quails. On the other side, thiamethoxam has been proved to cause reduction in liver enzyme activity, increasing bilirubin levels, and hepatic structural changes in rabbit liver (El Okle et al. 2018), but this may be attributed to using of different concentrations.
Disturbance in proteins is one of the early indicators of pesticides toxicity. The decreased protein level in liver tissue could be attributed to less incorporation of amino acids in the translation process which mostly happens in hepatic tissue (Park et al. 2014). Our findings agree with Dornelles and Oliveira (2014) who recorded a decrease in protein level in liver tissue of tadpoles in response to different concentrations of herbicides.
Oxidative stress is a biochemical disturbance that occurs when production of free radicals exceeds the capacity of antioxidant agents, resulting in membrane lipid peroxidation and damage to cellular organelles (Ahmed et al. 2017). Our study demonstrated that GSH and SOD were significantly decreased in intoxicated groups than control. This indicated that toads suffered from serious damage due to reduction in their antioxidant capacity. These results could be attributed to consumption of GSH and SOD to compensate the increased production of free radicals (Kapoor et al. 2010). Furthermore, neonicotinoids could induce accumulation of lipid oxidative metabolites MDA in the liver by inhibiting the activity of antioxidant enzymes system producing tissue damage effects (Wang et al. 2019).
The oxidative damage of neonicotinoids to animal liver has been proven in several animal models. Emam et al. (2018) found a significant decrease in SOD activity and GSH concentration and a significant elevation in MDA in the liver of Japanese quails exposed to imidacloprid. Also, oxidative stress effects were reported in common carp by Xing et al. (2012), in zebrafish by Ge et al. (2015), and in earthworm by Liu et al. (2017).
Liver is the marked organ for evaluation of histopathological changes caused by pollutants, as it contains significant amounts of polyunsaturated fatty acids which are prone to be damaged by free radicals (Ortiz-Ordoñez et al. 2016). The histopathological results of liver revealed normal hepatic architecture in toads of control group. Thiamethoxam causes mild to moderate changes, while acetamiprid made moderate to severe histological alterations. Increased melanin density has been observed in naturally polluted frogs (Fenoglio et al. 2005) indicating its ability to scavenge reactive oxygen (Różanowska et al. 1999) and hence protect pigment cells against the oxidative stress. The vacuolation occurred via retention of fluid inside the hepatocytes which may be due to high levels of lipid peroxidation (Emam et al. 2018; Ortiz-Ordoñez et al. 2016). The cytoplasmic vacuolization of hepatocyte could be an early response to neonicotinoid exposure, such as oxidative stress and lipid disorder (Milić et al. 2018). Significant nuclear accumulation and binucleation occurred in the exposure group, which were the result of accumulation of lipid metabolites (Wang et al. 2014), indicating significant oxidative stress damage in the liver. These results meet the results obtained by Paunescu et al. (2010) from the liver of frog rana (pelophylax ridibunda) treated by Reldan 40EC insecticide.
Conclusion and limitations
The present work focused on the sublethal effect of thiamethoxam and acetamiprid insecticides widely applied to crops in the Egyptian fields which may affect other nontarget organism. Our results revealed that acetamiprid has more serious effect on the Egyptian toads including changes in some biochemical parameters, oxidative stress, and lipid peroxidation responses in liver which put toads into risk of population decline, in addition to histopathological alterations in the hepatic cells. Therefore, Egyptian toads could be used as a mirror for the environmental pollution. The toxicological features of these insecticides must be taken into consideration with respect to vertebrates, raising the probability of wild species that can be affected under field natural exposure conditions. There may be some possible limitations in this study regarding the value of sublethal dose of thiamethoxam and acetamiprid in addition to lack of data about the estimated ecological level of these two insecticides in the Egyptian environment. There may be some possible limitations in this study regarding the value of sublethal dose of thiamethoxam and acetamiprid in addition to lack of data about the estimated ecological level of these two insecticides in the Egyptian environment and presence of gaps in the prior literatures. Further research could be performed on toads to evaluate the toxic effect of various doses and concentrations of those insecticides on growth and immune status.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ahmed EA, Elsayed DH, Kilany OE, El-Beltagy MA (2017) Multivitamins preventive therapy against subclinical endometritis in buffaloes: its correlation to NEFA and oxidative stress. Reprod Biol Endocrinol 17(3):239–245. https://doi.org/10.1016/j.repbio.2017.05.008
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20(4):470–475
Allran JW, Karasov WH (2001) Effects of atrazine on embryos, larvae, and adults of anuran amphibians. Environ Toxicol Chem 20(4):769–775. https://doi.org/10.1897/1551-5028(2001)020<0769:eoaoel>2.0.co;2
Anderson JC, Dubetz C, Palace VP (2015) Neonicotinoids in the Canadian aquatic environment: a literature review on current use products with a focus on fate, exposure, and biological effects. Sci Total Environ 505:409–422. https://doi.org/10.1016/j.scitotenv.2014.09.090
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier Health Sciences, Churchill Livingstone, UK
Beasley VR (2020) Direct and indirect effects of environmental contaminants on amphibians Reference Module in Earth Systems and Environmental Sciences. Elsevier
Bergmeyer H, Horder M, Rej R (1986) International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate: 2-oxoglutarate aminotransferase, EC 2.6.1.1). J Clin Chem Clin Biochem Z Klin Chem Klin Biochem 24:481–495
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: an underestimated cause of global decline? Sci Rep 3:1135
Bucolo G, David H (1973) Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 19(5):476–482
Buege JA, Aust SD (1978) [30] Microsomal lipid peroxidation. In: Fleischer S, Packer L (eds) Methods in Enzymology. Academic Press, pp. 302-310
Büyükgüzel E (2009) Evidence of oxidative and antioxidative responses by Galleria mellonella larvae to malathion. J Econ Entomol 102(1):152–159. https://doi.org/10.1603/029.102.0122
Çamlıca Y, Bediz SC, Çömelekoğlu Ü, Yilmaz ŞN (2018) Toxic effect of acetamiprid on Rana ridibunda sciatic nerve (electrophysiological and histopathological potential). Drug Chem Toxicol 42(3):264–269
Dornelles MF, Oliveira GT (2014) Effect of atrazine, glyphosate and quinclorac on biochemical parameters, lipid peroxidation and survival in bullfrog tadpoles (Lithobates catesbeianus). Arch Environ Contam Toxicol 66(3):415–429. https://doi.org/10.1007/s00244-013-9967-4
El Okle OS, El Euony OI, Khafaga AF, Lebda MA (2018) Thiamethoxam induced hepatotoxicity and pro-carcinogenicity in rabbits via motivation of oxidative stress, inflammation, and anti-apoptotic pathway. Environ Sci Pollut Res Int 25(5):4678–4689. https://doi.org/10.1007/s11356-017-0850-0
El-Banna S, Attia AM, Hafez AA, El-Kazaz SA (2009) Effect of garlic consumption on blood lipid and oxidant/antioxidant parameters in rat males exposed to chlorpyrifos. Slovak J Anim Sci 42(3):111–117
Emam H, Ahmed E, Abdel-Daim M (2018) Antioxidant capacity of omega-3-fatty acids and vitamin E against imidacloprid-induced hepatotoxicity in Japanese quails. Environ Sci Pollut Res Int 25(12):11694–11702. https://doi.org/10.1007/s11356-018-1481-9
Emre I, Kayis T, Coskun M, Dursun O, Cogun HY (2013) Changes in antioxidative enzyme activity, glycogen, lipid, protein, and malondialdehyde content in cadmium-treated Galleria mellonella Larvae. Ann Entomol Soc Am 106(3):371–377. https://doi.org/10.1603/an12137
Feng S, Kong Z, Wang X, Zhao L, Peng P (2004) Acute toxicity and genotoxicity of two novel pesticides on amphibian, Rana N. Hallowell. Chemosphere 56(5):457–463. https://doi.org/10.1016/j.chemosphere.2004.02.010
Fenoglio C, Boncompagni E, Fasola M, Gandini C, Comizzoli S, Milanesi G, Barni S (2005) Effects of environmental pollution on the liver parenchymal cells and Kupffer-melanomacrophagic cells of the frog Rana esculenta. Ecotoxicol Environ Saf 60(3):259–268
Gabor CR, Knutie SA, Roznik EA, Rohr JR (2018) Are the adverse effects of stressors on amphibians mediated by their effects on stress hormones? Oecologia 186(2):393–404. https://doi.org/10.1007/s00442-017-4020-3
Ge W, Yan S, Wang J, Zhu L, Chen A, Wang J (2015) Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J Agric Food Chem 63(6):1856–1862. https://doi.org/10.1021/jf504895h
Hartel T, Schweiger O, Öllerer K, Cogălniceanu D, Arntzen JW (2010) Amphibian distribution in a traditionally managed rural landscape of Eastern Europe: probing the effect of landscape composition. Biol Conserv 143(5):1118–1124. https://doi.org/10.1016/j.biocon.2010.02.006
Helmer PJ, Whiteside DP (2005) Amphibian anatomy and physiology. In: O'Malley B (ed) Clinical anatomy and physiology of exotic species, Elsevier Saunders, London, pp 3-14
Hopkins WA (2007) Amphibians as models for studying environmental change. ILAR J 48(3):270–277
IUCN SSC Amphibian Specialist Group (2016) Sclerophrys regularis, The IUCN Red List of Threatened Species 2016, 2016, e. T54747A107349840.
Jalili C, Farzaei MH, Roshankhah S, Salahshoor MR (2019) Resveratrol attenuates malathion-induced liver damage by reducing oxidative stress. J Lab Physicians 11(3):212–219. https://doi.org/10.4103/JLP.JLP_43_19
Kapoor U, Srivastava MK, Bhardwaj S, Srivastava LP (2010) Effect of imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive its no observed effect level (NOEL). J Toxicol Sci 35:577–581. https://doi.org/10.2131/jts.35.577
Katagi T (2014) Bioconcentration and metabolism of pesticides and industrial chemicals in the frog. J Pestic Sci 39:55–68. https://doi.org/10.1584/jpestics.D13-047
Knight JA, Anderson S, Rawle JM (1972) Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin Chem 18(3):199–202
Lenhardt PP, Brühl CA, Leeb C, Theissinger K (2017) Amphibian population genetics in agricultural landscapes: does viniculture drive the population structuring of the European common frog (Rana temporaria)? PeerJ 5:e3520. https://doi.org/10.7717/peerj.3520
Liu T, Wang X, Xu J, You X, Chen D, Wang F, Li Y (2017) Biochemical and genetic toxicity of dinotefuran on earthworms (Eisenia fetida). Chemosphere 176:156–164. https://doi.org/10.1016/j.chemosphere.2017.02.113
Loutfy NM, Kamel MS (2018) Effects of two neonicotinoids insecticides on some anti-oxidant enzymes and hematological parameters in Egyptian frogs, Bufo regularis. Egypt Acad J Biol Sci F Toxicol Pest Control 10(1):25–36. https://doi.org/10.21608/eajbsf.2018.17017
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157(11):2903–2927. https://doi.org/10.1016/j.envpol.2009.05.015
Milić M, Žunec S, Micek V, Kašuba V, Mikolić A, Lovaković BT, Semren T, Pavičić I, Čermak AMM, Pizent A, Vrdoljak AL, Valencia-Quintana R, Sánchez-Alarcón J, Želježić D (2018) Oxidative stress, cholinesterase activity, and DNA damage in the liver, whole blood, and plasma of Wistar rats following a 28-day exposure to glyphosate. Arh Hig Rada Toksikol 69(2):154–168. https://doi.org/10.2478/aiht-2018-69-3114
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303. https://doi.org/10.1016/j.envint.2014.10.024
Ortiz-Ordoñez E, López-López E, Sedeño-Díaz JE, Uría E, Morales IA, Pérez ME, Shibayama M (2016) Liver histological changes and lipid peroxidation in the amphibian Ambystoma mexicanum induced by sediment elutriates from the Lake Xochimilco. J Environ Sci 46:156–164. https://doi.org/10.1016/j.jes.2015.06.020
Park KT, Yun C-H, Bae C-S, Ahn T (2014) Decreased level of albumin in peripheral blood mononuclear cells of streptozotocin-induced diabetic rats. J Vet Med Sci 76(8):1087–1092. https://doi.org/10.1292/jvms.13-0631
Paunescu A, Ponepal CM, Drghici O, Marinescu AG (2010) Liver histopathologic alterations in the frog Rana (Pelophylax) ridibunda induce by the action of Reldan 40EC insecticide. Analele Univ din Oradea Fasc Biol 17(1):166–169
Persch TSP, Weimer RN, Freitas BS, Oliveira GT (2017) Metabolic parameters and oxidative balance in juvenile Rhamdia quelen exposed to rice paddy herbicides: Roundup®, Primoleo®, and Facet®. Chemosphere 174:98–109. https://doi.org/10.1016/j.chemosphere.2017.01.092
Robinson SA, Richardson SD, Dalton RL, Maisonneuve F, Trudeau VL, Pauli BD, Lee-Jenkins SS (2017) Sublethal effects on wood frogs chronically exposed to environmentally relevant concentrations of two neonicotinoid insecticides. Environ Toxicol Chem 36(4):1101–1109. https://doi.org/10.1002/etc.3739
Robinson S, Richardson S, Dalton R, Maisonneuve F, Bartlett A, de Solla S, Trudeau V, Waltho N (2019) Assessment of sublethal effects of neonicotinoid insecticides on the life-history traits of 2 frog species. Environ Toxicol Chem 38(9):1967–1977
Różanowska M, Sarna T, Land EJ, Truscott TG (1999) Free radical scavenging properties of melanin: interaction of eu-and pheo-melanin models with reducing and oxidising radicals. Free Radic Biol Med 26(5-6):518–525
Ruiz de Arcaute C, Pérez-Iglesias JM, Nikoloff N, Natale GS, Soloneski S, Larramendy ML (2014) Genotoxicity evaluation of the insecticide imidacloprid on circulating blood cells of Montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae) by comet and micronucleus bioassays. Ecol Indic 45:632–639. https://doi.org/10.1016/j.ecolind.2014.05.034
Salbego J, Pretto A, Gioda CR, de Menezes CC, Lazzari R, Radünz Neto J, Baldisserotto B, Loro VL (2010) Herbicide formulation with glyphosate affects growth, acetylcholinesterase activity, and metabolic and hematological parameters in Piava (Leporinus obtusidens). Arch Environ Contam Toxicol 58(3):740–745. https://doi.org/10.1007/s00244-009-9464-y
Schreinemachers P, Tipraqsa P (2012) Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 37(6):616–626. https://doi.org/10.1016/j.foodpol.2012.06.003
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Smalling KL, Reeves R, Muths E, Vandever M, Battaglin WA, Hladik ML, Pierce CL (2015) Pesticide concentrations in frog tissue and wetland habitats in a landscape dominated by agriculture. Sci Total Environ 502:80–90. https://doi.org/10.1016/j.scitotenv.2014.08.114
Sounderraj SL, Sekhar P, Kumar PS, Nancy L (2011) Effect of systemic pesticide phosphamidon on haematological aspects of common frog Rana tigrina. Int J Pharm Bio Sci 2(6):1776–1780
Steinel NC, Bolnick DI (2017) Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Front Immunol 8:827–827. https://doi.org/10.3389/fimmu.2017.00827
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306(5702):1783–1786. https://doi.org/10.1126/science.1103538
Sun M, Zigman S (1978) An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem 90(1):81–89. https://doi.org/10.1016/0003-2697(78)90010-6
Uneme H (2011) Chemistry of clothianidin and related compounds. J Agric Food Chem 59(7):2932–2937. https://doi.org/10.1021/jf1024938
Wang Y, Guo B, Gao Y, Xu P, Zhang Y, Li J, Wang H (2014) Stereoselective degradation and toxic effects of benalaxyl on blood and liver of the Chinese lizard Eremias argus. Pestic Biochem Physiol 108:34–41. https://doi.org/10.1016/j.pestbp.2013.11.004
Wang Y, Zhang Y, Li W, Yang L, Guo B (2019) Distribution, metabolism and hepatotoxicity of neonicotinoids in small farmland lizard and their effects on GH/IGF axis. Sci Total Environ 662:834–841. https://doi.org/10.1016/j.scitotenv.2019.01.277
Wilkens ALL, Valgas AAN, Oliveira GT (2019) Effects of ecologically relevant concentrations of Boral® 500 SC, Glifosato® Biocarb, and a blend of both herbicides on markers of metabolism, stress, and nutritional condition factors in bullfrog tadpoles. Environ Sci Pollut Res Int 26(23):23242–23256. https://doi.org/10.1007/s11356-019-05533-z
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19(2):137–161. https://doi.org/10.1016/0166-445X(91)90033-6
Xing H, Li S, Wang Z, Gao X, Xu S, Wang X (2012) Oxidative stress response and histopathological changes due to atrazine and chlorpyrifos exposure in common carp. Pestic Biochem Physiol 103(1):74–80
Yucel MS, Kayis T (2019) Imidacloprid induced alterations in oxidative stress, biochemical, genotoxic, and immunotoxic biomarkers in non-mammalian model organism Galleria mellonella L.(Lepidoptera: Pyralidae). J Environ Sci Health B 54(1):27–34. https://doi.org/10.1080/03601234.2018.1530545
Acknowledgements
The authors would like to thank Dr. Doaa H. Elsayed, Assistant Professor, Department of Theriogenology, Faculty of Veterinary Medicine, Suez Canal University, for her revision of the manuscript.
Funding
This work was completely supported by personal funding from authors.
Author information
Authors and Affiliations
Contributions
EM: Conceptualization, experimental investigations, funding, and writing of the original draft. NM: Supervision, data interpretation, funding, and editing of the original draft. SA: Funding, statistical analysis, reviewing, and editing of the original draft. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the procedures of the study conformed according to the ethical and humane principles of Ethics and Animal Experimentation Committee of Suez Canal University (approval no. 2020045).
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saad, E.M., Elassy, N.M. & Salah-Eldein, A.M. Effect of induced sublethal intoxication with neonicotinoid insecticides on Egyptian toads (Sclerophrys regularis). Environ Sci Pollut Res 29, 5762–5770 (2022). https://doi.org/10.1007/s11356-021-15976-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15976-y