Abstract
Selenium (Se) can alleviate the toxicity of antimony (Sb) in plants; however, the associated mechanisms have not been fully clarified. In this study, we hypothesize that Se can affect the subcellular distribution of Sb to regulate Sb toxicity. To test our hypothesis, two nested hydroponic experiments were performed by using paddy rice (Fengmeizhan). The results showed that Sb exerted toxic effects on the growth of paddy rice, and Se caused beneficial effects that were limited to the shoot growth. In general, Se and Sb mutually showed antagonistic effects on their uptake and concentrations in different subcellular fractions. However, in some cases, the stimulation effects of Sb on the Se concentration in chlorophyll (Chl) and cytosol (Cy) fractions or of Se on the Sb concentration in the cell wall fraction (Cw) were also observed in the shoots, which might suggest that Sb detoxification by Se is also related to the migration of both Se and Sb in cells. Selenium and Sb were primarily concentrated in the Cw and Cy, suggesting the important roles of these two fractions in detoxifying Se and Sb. When paddy rice was subjected to increasing Sb concentrations and a fixed Se concentration, most of the Se in the shoots was sequestered in the Cy (59.81–79.51 % of total Se) and more Se was transferred into the inner cell from Cw; however, in the roots, Se was primarily concentrated in the Cw (53.28–72.10 %). When paddy rice was exposed to increasing Se concentrations with a fixed Sb concentration, the Cw in both the shoots and roots might play an important role in binding Se, especially in the roots where up to 78.92 % of the total Se was sequestered in the Cw.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic processes, such as mining and ore extraction, have caused serious antimony (Sb) contamination worldwide, especially in China (Flakova et al. 2012; Fu et al. 2010, 2011; Hiller et al. 2012; Kelepertsis et al. 2006; Mori et al. 1999; Pérez-Sirvent et al. 2012; Serfor-Armah et al. 2006; Shumilin et al. 2001; Steinnes et al. 1997). In Slovakia, mine waste samples that were collected from five abandoned Sb mines contained up to 9,861 mg kg−1 Sb. The local groundwater, surface waters, and mine waters all contained elevated Sb concentrations, reaching up to 9,300 μg L−1 Sb (Hiller et al. 2012). In the northwestern Zamora Province of Spain (Losacio village), the total Sb concentrations in the uppermost soil layer ranged from 14 to 324 mg kg−1 (Álvarez-Ayuso et al. 2012).
Antimony is a nonessential but toxic element for plants, and they can accumulate a large amount of Sb (Tschan et al. 2009). Antimony contamination of soil and water systems has resulted in excess Sb accumulation in plants, especially in crops grown around the contaminated areas. For example, elevated Sb concentrations (109 to 4,029 mg kg−1) were found in ten plant species residing in the vicinity of the XiKuangShan mine (Okkenhaug et al. 2011). Excessive Sb accumulation in plants can cause damage such as oxidative stress (Feng et al. 2009b; Pan et al. 2011), the inhibition of essential element uptake (Feng et al. 2013d; Shtangeeva et al. 2011), photosynthesis inhibition (Pan et al. 2011; Zhang et al. 2010), and the synthesis of soluble proteins (our unpublished data). In addition, Sb is a toxic and carcinogenic element for humans and, therefore, high Sb contents in plants could threaten human health if this element enters the food chain.
Appropriate Se doses are reported to ameliorate Sb toxicity (Feng et al. 2011b; Feng et al. 2013d). An antidotal role for Se in detoxifying many other toxic heavy metals, such as arsenic (Feng et al. 2009a; Malik et al. 2012), mercury (Belzile et al. 2006), lead (He et al. 2004; Mroczek-Zdyrska and Wójcik 2012), aluminum (Cartes et al. 2010), and cadmium (Feng et al. 2013c; Filek et al. 2008, 2009, 2010; Pedrero et al. 2008), has been identified in plants. The detoxification mechanisms of Sb by Se in plants have not been elucidated; however, some mechanisms have been proposed, such as alleviating the oxidative stress generated from Sb, inhibiting Sb uptake through antagonistic effects, and facilitating the uptake of some essential elements that support the maintenance of cell integrity (Feng et al. 2013d). Additional information from studies that explore the detoxification mechanisms of Sb by Se remains insufficient.
Recently, studies have shown that the compartmentalization of Sb in the cytosol and the immobilization of Sb in the cell wall are important tolerance and accumulation mechanisms in fern plants, including Pteris cretica (Feng et al. 2011a), P. cretica ‘Albo-Lineata’, Pteris fauriei, Pteris ensiformis Burm., and Humata tyermanii Moore (Feng et al. 2013a). However, it is not clear as to whether and how common plants can tolerate Sb toxicity, and whether this ability is also associated with the compartmentalization and immobilization of Sb, as seen in fern plants. Furthermore, whether Se supplementation can influence the subcellular distribution of Sb in plants and thus detoxify Sb also remains unknown.
We conducted this study by exposing a paddy rice species (Fengmeizhan) to Sb and Se and investigating the following: (1) the interactions of Sb and Se; (2) the subcellular distribution of Se and Sb; and (3) the effects of Sb and Se on the subcellular distribution of different tissues in paddy rice.
Materials and methods
Experimental design
A two-factor, five-level orthogonal rotation regression design was used in this study, with two nested experiments. The details of the experimental design and the treatment concentrations of Se and Sb are described in a study by Feng et al. (2013d). In brief, there are two sets of treatment concentrations for both Se and Sb in this study. One set is the coded concentrations that have constant values according to the model (i.e., −1.414, −1, 0, 1, and 1.414 for both Se and Sb), and the other is the actual concentration. First, the actual center value of Se and Sb should be selected. A 5 mg L−1 Sb concentration can induce oxidative stress but does not significantly affect the growth of paddy rice, and 1 mg L−1 Se is considered to be marginally toxic (Feng et al. 2013d). We selected 5 and 0.8 mg L−1 as the actual center concentrations of Sb and Se, respectively. The other four actual Sb and Se treatment concentrations were determined according to Eq. (1).
in which, X, X 0, x, and Δj denote the actual treatment concentration, central value, coded value (Supplementary Table 1), and scaling factor (2.829 for Sb and 0.495 for Se). Consequently, the actual treatment concentrations for Sb were 1, 2.171, 5, 7.829, and 9 mg L−1, and for Se they were 0.1, 0.305, 0.8, 1.295, and 1.5 mg L−1.
Experiment I was a single-factor experiment that was designed to investigate the effects of Se on the uptake and subcellular distribution of Sb by fixing the Sb concentration at 5 mg L−1 and simultaneously increasing the Se concentrations from 0.1 to 1.5 mg L−1. The effects of Sb on the uptake and subcellular distribution of Se were also investigated by fixing the Se concentration at 0.8 mg L−1 and simultaneously increasing the Sb concentrations from 1 to 9 mg L−1 (Supplementary Table 1). Experiment II involved 16 experimental runs (runs 1–16, Supplementary Table 2) and was performed to investigate the interactive effects of Se and Sb on uptake and subcellular distribution. Because some treatment concentrations in experiment I were the same as those used in experiment II, experiment I was nested in experiment II by adding the Sb and Se treatment concentrations to experiment II (runs 17–20, Supplementary Table 2), and the two experiments were conducted synchronously. There were four replications for each treatment excluding run 9 of experiment II, which had eight replications to minimize experimental error.
Plant materials and treatments
The seed germination and seedling cultivation of paddy rice (Fengmeizhan) were performed according to Feng et al. (2013d). After approximately 3 weeks, uniform seedlings were transferred into a full-strength Espino nutrient solution to acclimate for 3 weeks. The solution consisted of the following: Ca(NO3)2·4H2O, 0.089 g L−1; MgSO4·2H2O, 0.250 g L−1; (NH4)2SO4, 0.049 g L−1; KH2PO4, 0.034 g L−1; FeCl2, 0.030 g L−1; H3BO2, 2.86 mg L−1; ZnSO4·7H2O, 0.22 mg L−1; MnCl2·2H2O, 1.81 mg L−1; CuSO4·5H2O, 0.08 mg L−1; and H2MoO4·4H2O, 0.02 mg L−1. Three weeks later, the seedlings were subjected to different concentrations of Se (Na2SeO3) and Sb (KSbC4H4O7·1/2H2O) for 2 weeks. The solution was vigorously aerated and replaced twice per week. The pH of the solution was adjusted to 5.5 with diluted HCl or NaOH. The temperature in the greenhouse ranged from 20 °C (night) to 25 °C (day) and there was a relative humidity of 60–70 %. A 16 (day)/8 (night)-h photoperiod with an average photon flux density of 100 μmol m−2 s−1 was supplied.
The fractionation of different tissues
After 2 weeks, the seedlings were harvested, rinsed, and separated into roots and shoots (stems plus leaves). The samples of both the shoots and roots were separated into two parts. One part was used for subcellular fractionation, and the other part was used to determine the concentrations of Se and Sb. Approximately 0.5 g of fresh shoot and root tissue was used to perform the subcellular fractionation based on the method by Feng et al. (2011a) with some modifications. In brief, the weighed tissues were homogenized with a pre-cooled mortar and pestle by using an extracted solution (pH 7.8) of 0.25 mM sucrose, 50 mM Tris-maleate buffer (pH 7.8), 1 mM MgCl2, and 10 mM cysteine. The homogenate was centrifuged at 300×g for 10 min, and the residue was the cell wall (Cw) fraction. The supernatant from the first shoot centrifugation was then centrifuged again at 2,000×g for 10 min and the residue from the second centrifugation step was the chlorophyll (Chl). The supernatants from the second shoot centrifugation and the first root centrifugation were then centrifuged at 10,000×g for 45 min; the residue was considered to be the cytoplasmic organelle (Co) fraction, and the supernatant was considered to be the cytosol (Cy) fraction. The Se and Sb concentrations in different fractions (Cw, Chl, Co, and Cy) were then determined. The remaining seedlings were weighed, oven-dried at 75 °C for 48 h, and pulverized by using a grinder to determine the total Se and Sb.
Element determination
The powdered shoots and roots, in addition to the different subcellular fractions, were digested with concentrated HNO3-HClO4 (Wei et al. 2006), and their Se and Sb concentrations were assayed by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500a, USA) in the Central Laboratory at the Tianjin Academy of Agricultural Sciences of China. The accuracy of the elemental analysis was confirmed by using standard reference materials (bush leaves, GBW07603, GSV-2) from the Centre for Standard Reference of China.
Data analysis
The differences in means among the different treatments in experiment I were compared by using a one-way ANOVA with Duncan’s test at P < 0.05. For experiment II, the interactive effects of Se and Sb on the uptake and subcellular distribution were modeled by fitting a polynomial quadratic equation (Eq. 2) (Al-Attar and Nickless 1988; Tu and Ma 2003) as follows:
in which y is the Se or Sb concentration in different tissues or subcellular fractions; x is the Se or Sb concentration in the solution; b j , b ij , and b jj are the regression coefficients; m is the number of factors; and i and j are the order numbers of the variables.
The terms that were significant at P < 0.05 were included, and only the equations containing the [Se][Sb] term were used to draw three-dimensional surface plots to elucidate the interactive effects of Se and Sb on uptake and subcellular distribution (Supplementary Table 3). In experiment II, the modeling and statistical analyses of the results were completed by using SAS software, and the graphs of three-dimensional surface plots were drawn in SigmaPlot software (SigmaPlot 10.0) according to the resulting equations containing the [Se][Sb] term.
Results
The simple effects of Se and Sb on the biomass
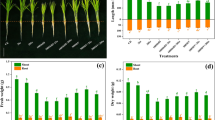
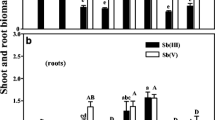
The Sb recovery from the reference material was 91.58 % and the Sb recovery in compartments vs total Sb ranged from 82.01 to 132.12 %. When the Se was imposed at its center value of 0.8 mg L−1, increasing Sb solution concentrations from 1 to 9 mg L−1 significantly inhibited the shoot biomass of paddy rice (F = 65.999, P < 0.001; Fig. 1a, white triangle Sb) and significantly reduced the root biomass to a lesser extent (F = 12.896, P = 0.001; Fig. 1b, white triangle Sb). When Sb was added at its center value of 5 mg L−1, the Se addition appeared to demonstrate beneficial effects on the shoot biomass, especially at a Se treatment concentration of 1.5 mg L−1, at which a significant increase was observed in comparison with a single treatment of 5 mg L−1 Sb (F = 3.660, P = 0.044; Fig. 1a, black circle Se). However, when 5 mg L−1 Sb was present in the solution, the Se addition did not increase but in fact significantly decreased the root biomass (F = 5.958, P = 0.010) as shown in Fig. 1b (black circle Se).
Simple effects of Sb and Se on the biomass of paddy rice and on their contents in the shoots and roots. Each treatment was replicated three times. Each of the curves was drawn based on the related single factor experiment. Symbols and vertical lines in the curves are means and standard error of means. Line white triangle Sb represents that Se was maintained at its central value of 0.8 mg L−1 while increasing the Sb concentration from 1 to 9 mg L−1; line black circle Se represents the experiments in which Sb was maintained at its central of 5 mg L−1 while increasing the Se concentration from 0.1 to 1.5 mg L−1
The simple effects of Se and Sb on the total contents in the shoots and roots
The total Se contents in the shoots and roots of paddy rice significantly increased with the increasing Se solution concentrations when Sb was supplied at its center value of 5 mg L−1 (for shoots, F = 108.845, P < 0.001; for roots, F = 81.525, P < 0.001; and Fig. 1c, d, black circle Se). Increasing Sb solution concentrations appeared to have no obvious effect on the shoot Se content (F = 0.479, P = 0.751) but significantly reduced the root Se content (F = 9.253, P = 0.002) when Se was supplied at 0.8 mg L−1 (Fig. 1c, d, white triangle Sb). Similarly, the total Sb contents in both the shoots and roots of paddy rice were increased by the increased Sb solution concentrations when 0.8 mg L−1 Se was present in the culture solution (for shoots, F = 30.684, P < 0.001; for roots, F = 80.438, P < 0.001; and Fig. 1e, f, white triangle Sb). When Sb was added at 5 mg L−1, Se only exerted negative effects on the shoot Sb uptake at the highest Se concentration of 1.5 mg L−1 (F = 4.356, P = 0.027) but demonstrated negative effects on the roots (F = 18.990, P < 0.001) regardless of the Se treatment concentration (Fig. 1e, f, black circle Se).
The simple effects of Se and Sb on the concentrations in different subcellular fractions
The cytosolic Se concentrations in both the shoots and roots of paddy rice significantly increased with increasing Se concentrations when Sb was added at its center value of 5 mg L−1 (for shoots, F = 35.523, P < 0.001; for roots, F = 18.129, P < 0.001; and Fig. 2a, b, black circle Se). However, when Se was added at its center concentration of 0.8 mg L−1, the increasing Sb solution concentrations increased the Cy Se in the shoots (F = 6.327, P = 0.008) but had a non-significantly negative effect in the roots (F = 2.997, P = 0.072; Fig. 2a, b, white triangle Sb). The Sb concentrations in the Cy fraction from both the shoots and roots of paddy rice were significantly increased with increasing Sb concentrations in the presence of 0.8 mg L−1 Se in the culture solution (for shoots, F = 56.654, P < 0.001; for roots, F = 19.939, P < 0.001; and Fig. 2c, d, white triangle Sb). However, adding Se to the solution containing 5 mg L−1 Sb significantly reduced the Cy Sb concentrations in both the shoots and roots of paddy rice (for shoots, F = 12.817, P = 0.001; for roots, F = 29.618, P < 0.001; and Fig. 2c, d, black circle Se).
Simple effects of Sb and Se on the subcellular distributions of Se and Sb in the cytosol (Cy) and cell wall (Cw) fractions of the shoots and roots of paddy rice. Each treatment was replicated three times. Each of the curves was drawn based on the related single factor experiment. Symbols and vertical lines in the curves are means and standard error of means. For an explanation of the lines, white triangle Sb and black circle Se refer to the caption for Fig. 1
In the Cw fraction of shoots and roots from paddy rice, the Se concentration was significantly increased by elevating the Se concentrations in the solution when Sb was added at its center concentration (for shoots, F = 53.750, P < 0.001; for roots, F = 75.013, P < 0.001; and Fig. 2e, f); Similarly, the Sb concentration in the Cw fraction of shoots and roots from paddy rice was significantly increased by elevating the Sb concentrations in the solution (for shoots, F = 40.716, P < 0.001; for roots, F = 32.949, P < 0.001; and Fig. 2g, h). However, adding increasing Sb concentrations to a solution containing 0.8 mg L−1 Se reduced the Cw Se concentration in both the shoots and roots (for shoots, F = 7.909, P = 0.004; for roots, F = 83.548, P < 0.001; and Fig. 2e, f, white triangle Sb). The Cw Sb concentration in the shoots was significantly (F = 9.200, P = 0.002) increased by increasing the Se concentrations until 1.5 mg L−1 Se was added to a solution containing 5 mg L−1 Sb, and the Cw Sb concentration then returned to the initial level of the single treatment with 5 mg L−1 Sb (Fig. 2g, black circle Se). However, in the roots, adding elevated Se concentrations significantly reduced the Cw Sb concentration (F = 29.110, P < 0.001) when the Sb concentration was imposed at its center value of 5 mg L−1 (Fig. 2h, black circle Se).
The elevated Se solution concentrations significantly increased the Chl Se concentration (F = 27.486, P < 0.001) when Sb was added to the solution at the center concentration (Fig. 3a). Interestingly, the Chl Se concentration was also enhanced by the increased Sb solution concentrations (F = 21.797, P < 0.001) when 0.8 mg L−1 Se was present in the solution. For the Chl Sb concentration, elevated Sb concentrations increased the shoot Chl Sb concentration (F = 22.597, P < 0.001) when Se was imposed at its center concentration, but elevated Se concentrations decreased the shoot Chl Sb concentration when Sb was added to rice at its center concentration (F = 16.657, P < 0.001; Fig. 3b).
Simple effects of Sb and Se on the subcellular distributions of Se and Sb in the chloroplast (Chl) and cytoplasmic organelle (Co) fractions of the shoots and/or roots of paddy rice. Each treatment was replicated three times. Each of the curves was drawn based on the related single factor experiment. Symbols and vertical lines in the curves are means and standard error of means. For an explanation of the lines, white triangle Sb and black circle Se refer to the caption for Fig. 1
In the Co fraction of shoots and roots, elevated Se concentrations significantly increased the Se concentration when 5 mg L−1 Sb was simultaneously present in the culture solution (for shoots, F = 96.782, P < 0.001; for roots, F = 36.647, P < 0.001; and Fig. 3c, d, black circle Se). The addition of elevated Sb concentrations led to non-significant effects on the shoot Co Se concentration (F = 0.103, P = 0.979) but significantly reduced the root Co Se concentration (F = 29.809, P < 0.001) when the Se concentration was at 0.8 mg L−1, except for an Sb concentration of 2.17 mg L−1, in which a significant increase in the root Co Se concentration was observed in comparison with a single treatment of 0.8 mg L−1 Se (Fig. 3c, d, white triangle Sb). The Co Sb concentrations in both the shoots and roots significantly increased with elevated Sb concentrations in the presence of 0.8 mg L−1 Se in the solution (for shoots, F = 18.852, P < 0.001; for roots, F = 18.734, P < 0.001; and Fig. 3e, f, white triangle Sb). However, the elevated Se concentrations mostly have significantly negative effects on both the shoot Co Sb concentration (F = 3.895, P = 0.037) and the root Co Sb concentration (F = 19.630, P < 0.001) (Fig. 3e, f, black circle Se).
The interactive effects of Se and Sb on the concentrations in different subcellular fractions
The interactive effects of Se and Sb on their concentrations in different subcellular fractions are demonstrated in the contour plots shown in Figs. 4 and 5. As shown in Fig. 4a with or without Sb addition, the Se concentration in the Chl fraction initially increased with increasing Se concentrations and then remained constant at high Se concentrations. When the plant was subjected to a specific concentration of Se (for example, 1.5 mg L−1), low Sb concentrations (for example, 2.5 mg L−1) decreased but high Sb concentrations (e.g., 9 mg L−1) increased the Chl Se concentration. Without the presence of Se, the Chl Sb concentration increased with increasing Sb concentrations in the solution. When low Sb concentrations (e.g., <4.5 mg L−1) were added to the solution, the increasing Se concentrations appeared to have limited effects on the Chl Sb concentration; however, upon adding high concentrations of Sb (for example, 9 mg L−1), the addition of elevated Se concentrations always led to negative effects on the Chl Sb concentration (Fig. 4b).
The interactive effects between Sb and Se on the Se concentrations in the shoot and root cytosol fraction (Cy), in the root cell wall fraction (Cw), and in the root cytoplasmic organelle fraction (Co). Each treatment was replicated three times, with the exception of run 9, which was replicated eight times to minimize the experimental error
In the absence of Sb in the culture solution, the shoot Cy Se concentration increased with increasing Se concentrations; however, with high Sb concentrations in the solution (for example, 9 mg L−1), low Se concentrations (e.g., 0.5 mg L−1) increased, but high concentrations of Se (for example, 1.5 mg L−1) began to decrease the shoot Cy Se concentration. When the paddy rice seedlings were subjected to a specific low concentration of Se (for example, 0.305 mg L−1), elevated Sb concentrations increased the shoot Cy Se concentration. However, when high Se concentrations were present in the solution, for example, 1.5 mg L−1, low concentrations of Sb (<5 mg L−1) were shown to increase, but high concentrations of Sb (for example, 9 mg L−1) decreased the shoot Cy Se concentration (Fig. 5a). With or without the low Sb concentrations in the solution (for example, 1 mg L−1), elevated Se concentrations constantly increased the root Cy Se concentration; however, when high Sb concentrations were present in the solution, e.g., 9 mg L−1, low Se concentrations (≤0.8 mg L−1) were found to decrease the Cy Se concentration, but high concentrations of Se (>0.8 mg L−1) began to increase the Cy Se to return to initial concentrations in comparison with a single Sb treatment of 9 mg L−1 (Fig. 5b). With low concentrations of Se in the solution (for example, 0.305 mg L−1), the addition of Sb enhanced the root Cy Se concentration; however, when high concentrations of Se were added (for example, 1.5 mg L−1), the Sb showed a negative effect on the root Cy Se concentration (Fig. 5b). The root Cw Se concentration increased with increasing Se concentrations whenever low or high Sb concentrations were present in the solution. However, Sb supplementation always had a negative effect on the accumulation of Se in the root Cw regardless of the added Se concentrations in the solution (Fig. 5c). With respect to the root Co Se concentration, Se addition always increased the root Co Se concentration with low doses of Sb (for example, ≤5 mg L−1), but it began to decrease with high concentrations of Sb (9 mg L−1) in the solution (Fig. 5d). The addition of Sb exerted different effects on the root Co Se concentration when different doses of Se were added. For example, with low concentrations of Se in the solution (for example, 0.305 mg L−1), increasing Sb concentrations increased the root Co Se concentration. However, when the highest Se concentration was added (e.g., 1.5 mg L−1), the elevated Sb sharply decreased the root Co Se concentration (Fig. 5d).
Distribution percentages of Se and Sb in different subcellular fractions
The results of the changes in Se and Sb distribution percentages in different subcellular fractions are listed in Tables 1 and 2. The majority of Se in both the shoots and roots of paddy rice was concentrated in the Cw and Cy fractions. In the shoots, the percentage of Se in the Cy fraction was much higher than that in the Cw fraction. However, the opposite result was observed in the roots except when ≤0.305 mg L−1 Se and 5 mg L−1 Sb were simultaneously present in the solution, in which most of the Se was accumulated in the root Cy fraction. In the shoots and roots, the Cy and Cw fractions sequestered most of the Sb, except when imposing Se at its center value, in which the percentage of Sb in the Co fraction was also high.
When Se was added at its center value, the distribution percentages of Se in the shoot Chl and Cy fractions increased with increasing Sb concentrations; however, increasing Sb concentrations decreased the percentage of Se in the shoot Cw fraction and slightly decreased the percentage in the shoot Co fraction. With 0.8 mg L−1 Se in the solution, the percentages of Sb in the Cw, Chl, and Co fractions in the shoots were increased by adding Sb in comparison with the lower concentration treatment of 1 mg L−1 Sb plus 0.8 mg L−1 Se despite some fluctuations. However, the percentages of Sb in the Cy fraction were observed to decrease with increasing Sb concentrations.
In the roots of paddy rice, elevated Sb concentrations non-significantly increased the Se percentage in the Co but significantly increased it in the Cy fractions. However, adding Sb significantly decreased the percentage of Se in the Cw fraction when Se was imposed at its center value of 0.8 mg L−1. Sb concentrations lower than 7.829 mg L−1 significantly decreased the Sb percentage in the Co fractions but significantly increased it in the Cy fraction when Se was imposed at its center value; however, when the highest Sb concentration was added to the solution, the Sb percentages were non-significantly increased in the Cw and Co fractions and significantly reduced in the Cy fraction in comparison with a 7.829 mg L−1 Sb treatment plus 0.8 mg L−1 Se.
When Sb was fixed at its center value, the elevated Se concentrations significantly increased the Se percentages in the shoot Cw and Co fractions but markedly decreased the percentage in the shoot Cy fraction. The Se percentage in the shoot Chl fraction initially increased sharply by 0.305 mg L−1 Se and then slowly decreased but still remained higher than the 5 mg L−1 Sb treatment plus 0.1 mg L−1 Se. Regarding the percentage of Sb in the shoots, Se concentrations lower than 0.8 mg L−1 appeared to increase the percentage of Sb in the Cw fraction but decreased it in the Chl, Co, and Cy fractions. However, when an increase to 1.5 mg L−1 Se was added to the solution, the Sb percentages in the Chl and Co showed an increase and were higher than that in the 5 mg L−1 Sb treatment plus 0.8 mg L−1 Se; the percentages of Sb in the Cw and Cy fractions decreased.
In the paddy rice roots, the percentage of Se in the Cw fraction sharply increased with increasing Se concentrations in the solution when Sb was fixed at its center value; however, the percentage of Se in the Cy fraction was largely reduced by increasing the Se concentrations, especially at Se concentrations of ≥0.8 mg L−1. It appeared that 0.8 mg L−1 Se was a key concentration for the Se percentage in the root Co fraction; below this concentration, the percentage of Se was increased, but above this concentration, it was reduced. Se concentrations lower than 0.305 mg L−1 appeared to exert limited effects on the percentages of Sb in the Cw, Co, and Cy fractions; however, when 0.8 mg L−1 Se was added to the solution, there was an increase in the Sb in the Co percentages but a decrease in the Sb percentages in the Cy. When the Se concentrations were higher than 0.8 mg L−1, the Sb percentages in the Cw and Co fractions began to decrease; the Sb percentage in the Cy fraction began to increase.
Discussion
The present study was conducted to investigate the subcellular distribution of Se and Sb and the mechanisms on Se-mediated detoxification of Sb in paddy rice. The highest shoot Sb content was 197.2 mg kg−1 for a treatment with Sb9 + Se0.8 and the highest root Sb content was 1,193.8 mg kg−1 for a treatment with Sb5 + Se0.1. In all treatments, the majority of the Sb was sequestered in the roots of paddy rice. The greater Sb accumulation in the roots than in the shoots suggested that paddy rice showed a limited ability to transfer Sb to shoots from roots. The high Sb accumulation resulted in significant decreases in the biomass of paddy rice, in particular for the shoot biomass (Fig. 1a, b), which suggested the toxicity of Sb to paddy rice. Similar growth inhibition by Sb was also observed in some paddy rice species (Weiyou402 and Fengmeizhan) (Feng et al. 2011b, 2013d), fern plants such as Cyrtomium fortunei, Cyclosorus dentatus, and Microlepia hancei (Feng et al. 2009b), maize (Zea mays) (Pan et al. 2011), and lichen (Xanthoria parietina L. Th. Fr) (Paoli et al. 2013). The toxicity of Sb to plants might be connected to the antioxidative stress generated by Sb exposure (Feng et al. 2009b; Pan et al. 2011), photosynthesis disorders (Zhang et al. 2010; Pan et al. 2011), imbalances in the uptake of essential elements and metabolite synthesis (Shtangeeva et al. 2012), and injury to thylakoids (Paoli et al. 2013).
Paddy rice also showed a relatively strong ability to accumulate Se in its tissues. The highest Se content in the shoots and roots was 155.0 and 2,367.5 mg kg−1 upon Sb5 + Se1.5 treatment, respectively, suggesting a relatively high accumulation of Se in paddy rice. However, most of the accumulated Se was concentrated in the roots of paddy rice, suggesting a weak ability to transfer Se to the shoots from the roots in paddy rice. Low concentrations of Se reportedly benefit plant growth (Ding et al. 2014; Feng et al. 2013b). Stimulated shoot biomass was also observed in this study, especially at a Se concentration of 1.5 mg L−1, although this Se concentration has been reported to be toxic to plants (Ding et al. 2014; Feng et al. 2013c). Nevertheless, Se showed different effects on the root biomass than on the shoot biomass, demonstrating a decreased root biomass when Sb was imposed at the center value of 5 mg L−1 (Fig. 1b). Here, we are not able to explain the exact reasons for the stimulation of shoot biomass at this “toxic” concentration of Se and the decreases in the root biomass after adding Se. It might be closely related to metabolism changes in nutrient elements. Reports have shown that exposure to Se and/or Sb can affect the accumulation of essential elements in plants (Feng et al. 2013c, d); furthermore, it has been well established that deficiencies of nitrogen, phosphorus, potassium, and magnesium can lead to carbohydrate accumulation in leaves and roots and modify the shoot-to-root biomass ratio (Hermans et al. 2006). Similar results were also observed in our previous study, in which the addition of Se to paddy rice that was subjected to Cd also increased the shoot biomass but had negative effects on root development by decreasing the root length, surface area, and proportion of fine roots, and increased the proportion of medium roots (Ding et al. 2014).
It is well documented that the associated mechanisms for the Se-mediated detoxification of heavy metals might be partially connected to the regulation of antioxidative systems and the uptake inhibition of heavy metals (Feng et al. 2013b). In this study, a similar inhibition of uptake was also observed for Sb after adding Se, indicating an antagonistic effect by Se on Sb uptake in paddy rice. Decreases in the Sb content in plant tissue after Se supplementation were also reported by Feng et al. (2011b, 2013d). The decreased root Sb content observed in this study was ascribed to changes in root morphology, i.e., by altering the root morphology to reduce Sb uptake, and this mechanism has been reported in a study by Ding et al. (2014). In this study, Sb also showed an antagonistic effect on Se uptake in the roots of paddy rice when Se was fixed at its center value of 0.8 mg L−1, which was in accordance with the results of our previous study (Feng et al. 2013d).
Information on Se and Sb subcellular distribution is scarce or non-existent. The results of this study showed that the cytosol and cell wall are the primary locations for sequestering Se and Sb, especially when high concentrations of Se and Sb were present in the culture solution (Table 1). It is well known that the cytosol and cell wall play important roles in the toleration of toxicity from heavy metals, such as As (Chen et al. 2005), Cd (Ma et al. 2005; Wu et al. 2005), Zn (Küpper et al. 1999), and Sb (Feng et al. 2011a, 2013a). When Se was at its center value, increasing the Sb concentrations increased the concentrations and percentages of Se in the shoot Cy and Chl fractions (Figs. 2a, 3a, 4a, and 5a, and Table 1) and simultaneously decreased the concentrations and percentages of Se in the shoot Cw fraction (Fig. 2e and Table 1). This result suggests the increased transportation of Se into the protoplasm after exposure to increasing Sb concentrations. Reports have shown that Se can be used to rebuild the damaged chloroplast ultrastructure, reorganize the structure of the thylakoids and stroma, and increase the chloroplast size, fatty acid unsaturation and fluidity of the cell membrane (Filek et al. 2010). Therefore, we speculate that the increased transportation of Se into the protoplasm might be used to counteract the negative effects on thylakoids as described in a study by Paoli et al. (2013) and rebalance photosynthesis. It notable that when Sb was added at its center value, increasing Se concentrations resulted in decreases in the Se percentage in the shoot Cy fraction but led to increases in the shoot Cw fraction (Table 1). It was presumed that paddy rice tends to transfer more Se to the Cw fraction to avoid the toxicity from high Se concentrations to cytoplasmic organelles despite the increasing Se concentrations in the Cy, Cw, Co, and Chl with increasing Se concentrations (Figs. 2a, e, 3a, c, 4a, and 5a). In contrast to the paddy rice shoots, the Cw fraction in the roots might play more important roles in tolerating the toxicity of Se than the Cy fraction on the basis of the generally higher percentages of Se in the Cw fraction than the Cy fraction in the presence of high Se concentrations (Table 1). Increasing Sb concentrations tended to enhance the transportation of the Se in roots into the protoplasm from the cell wall when Se was fixed at its center value, based on the Se percentage changes in the root Cw, Co, and Cy fractions (Table 1), despite their decreased Se concentrations (Figs. 2b, f, 3d, 5b–d). However, when 5 mg L−1 Sb was added, increasing Se concentrations were sequestrated in the Cw fraction, but decreasing Se concentrations were sequestrated in the Co and Cy fractions (Table 1), possibly indicating a key role of the Cw fraction in restricting Se uptake in the roots of paddy rice.
In addition to the direct uptake inhibition of Sb by Se, the Se-mediated detoxification of Sb in paddy rice might also be related to the redistribution of Sb in different subcellular fractions. When Sb was imposed at the center value, the increasing Se concentrations reduced the concentration and percentage of Sb in the shoot Cy and Chl fractions but increased the concentration and percentage of Sb in the shoot Cw fraction (Figs. 2c, 3b, 4b and Table 1), suggesting that Se resulted in the transfer of more Sb to the Cw fraction to reduce the toxicity of Sb to Chl. However, the almost unchanged concentration of Sb in the shoot Co fraction (Fig. 3e, black circle Se) possibly indicated a limited role for Se in the shoot Co Sb concentration despite the reduction in the percentage of Sb in this fraction by Se concentrations as low as 0.8 mg L−1 (Table 1). In the roots, Se could also detoxify Sb toxicity by affecting the Sb concentrations and distribution percentages, which were exhibited as a decreased Sb concentration in the Cy fraction (Fig. 2d), Cw fraction (Fig. 2h), and Co fraction (Fig. 3f); a decreased Sb percentage in the Co fractions (Table 1); and an increased Sb percentage in the Cy fraction (Table 1).
Conclusions
In this study, it is shown that paddy rice can accumulate high concentrations Se and Sb in its tissues, in particular in the roots; however, this plant showed a limited ability to transport Se and Sb to its aerial part from the roots. The addition of Sb significantly reduced the shoot and root biomass, demonstrating toxicity to paddy rice. However, Se exerted beneficial effects on shoot growth but not root growth. Generally, Se and Sb showed negative effects on each other’ uptake and concentration in different subcellular fractions. The cell wall and cytosol sequestrated most of the accumulated Se and Sb in both the shoots and roots of paddy rice, suggesting their important roles in detoxifying their toxicity. Se can reduce the toxicity via regulating the subcellular distribution of Sb, and in some cases, Sb possibly mobilized more Se to counteract Sb toxicity in the chlorophyll. Paddy rice could avoid the toxicity of Se and Sb via regulating concentrations and distribution in different subcellular fractions, in which the cell wall and cytosol fractions might act as “vessels” to sequestrate excess Se and Sb and rebalance concentrations in different subcellular fractions.
References
Al-Attar AF, Nickless G (1988) Response surface methodology for an investigation of the influence of selenium, cadmium and mercury on the growth of Lolium perenne seedlings. Chemosphere 17:1851–1861
Álvarez-Ayuso E, Otones V, Murciego A, García-Sánchez A, Regina IS (2012) Antimony, arsenic and lead distribution in soils and plants of an agricultural area impacted by former mining activities. Sci Total Environ 439:35–43
Belzile N, Wu GJ, Chen YW, Appanna VD (2006) Detoxification of selenite and mercury by reduction and mutual protection in the assimilation of both elements by Pseudomonas fluorescens. Sci Total Environ 367:704–714
Cartes P, Jara AA, Pinilla L, Rosas A, Mora ML (2010) Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann Appl Biol 156:297–307
Chen TB, Yan XL, Liao XY, Xiao XY, Huang ZC, Xie H, Zhai LM (2005) Subcellular distribution and compartmentalization of arsenic in Pteris vittata L. Chinese Sci Bull 50:2843–2849
Ding YZ, Feng RW, Wang RG, Guo JK, Zheng XQ (2014) A dual effect of Se on Cd toxicity: evidence from plant growth, root morphology and responses of the antioxidative systems of paddy rice. Plant Soil 375:289–301
Feng RW, Wang XL, Wei CY, Tu SX (2013a) The accumulation and subcellular distribution of arsenic and antimony in four fern plants. Int J Phytoremediat. doi:10.1080/15226514.2013.773281
Feng RW, Wei CY, Tu SX (2013b) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Feng RW, Wei CY, Tu SX, Ding YZ, Song ZG (2013c) A dual role of Se on Cd toxicity: evidences from the uptake of Cd and some essential elements and the growth responses in paddy rice. Biol Trace Elem Res 151:113–121
Feng RW, Wei CY, Tu SX, Liu ZQ (2013d) Interactive effects of selenium and antimony on the uptake of selenium, antimony and essential elements in paddy-rice. Plant Soil 365:375–386
Feng RW, Wei CY, Tu SX, Sun X (2009a) Interactive effects of selenium and arsenic on their uptake by Pteris vittata L. under hydroponic conditions. Environ Exp Bot 65:363–368
Feng RW, Wei CY, Tu SX, Tang SR, Wu FC (2011a) Simultaneous hyperaccumulation of arsenic and antimony in Cretan brake fern: evidence of plant uptake and subcellular distributions. Microchem J 97:38–43
Feng RW, Wei CY, Tu SX, Wu FC, Yang LS (2009b) Antimony accumulation and antioxidative responses in four fern plants. Plant Soil 317:93–101
Feng RW, Wei CY, Tu SX, Tang SR, Wu FC (2011b) Detoxification of antimony by selenium and their interaction in paddy rice under hydroponic conditions. Microchem J 97:57–61
Filek M, Gzyl-Malcher B, Zembala M, Bednarska E, Laggner P, Kriechbaum M (2010) Effect of selenium on characteristics of rape chloroplasts modified by cadmium. J Plant Physiol 167:28–33
Filek M, Keskinen R, Hartikainen H, Szarejko I, Janiak A, Miszalski Z, Golda A (2008) The protective role of selenium in rape seedlings subjected to cadmium stress. J Plant Physiol 165:833–844
Filek M, Zembala M, Hartikainen H, Miszalski Z, Kornaś A, Wietecka-Posłuszny R, Walas P (2009) Changes in wheat plastid membrane properties induced by cadmium and selenium in presence/absence of 2,4-dichlorophenoxyacetic acid. Plant Cell Tiss Org 96:19–28
Flakova R, Zenisova Z, Sracek O, Krcmar D, Ondrejkova I, Chovan M, Lalinska B, Fendekova M (2012) The behavior of arsenic and antimony at Pezinok mining site, southwestern part of the Slovak Republic. Environ Earth Sci 66:1043–1057
Fu Z, Wu F, Amarasiriwardena D, Mo C, Liu B, Zhu J, Deng Q, Liao H (2010) Antimony, arsenic and mercury in the aquatic environment and fish in a large antimony mining area in Hunan, China. Sci Total Environ 408:3403–3410
Fu Z, Wu F, Mo C, Liu B, Zhu J, Deng Q, Liao H, Zhang Y (2011) Bioaccumulation of antimony, arsenic, and mercury in the vicinities of a large antimony mine, China. Microchem J 97:12–19
He PP, Lv XZ, Wang GY (2004) Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ Int 30:167–172
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11:610–617
Hiller E, Lalinska B, Chovan M, Jurkovic L, Klimko T, Jankular M, Hovoric R, Sottnik P, Flakova R, Zenisova Z, Ondrejkova I (2012) Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Appl Geochem 27:598–614
Kelepertsis A, Alexakis D, Skordas K (2006) Arsenic, antimony and other toxic elements in the drinking water of Eastern Thessaly in Greece and its possible effects on human health. Environ Geol 50:76–84
Küpper H, Zhao FJ, McGrath SP (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119:305–312
Ma JF, Ueno D, Zhao F, McGrath SP (2005) Subcellular localisation of Cd and Zn in the leaves of a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Planta 220:731–736
Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H (2012) Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot 77:242–248
Mori C, Orsini A, Migon C (1999) Impact of arsenic and antimony contamination on benthic invertebrates in a minor Corsican river. Hydrobiologia 392:73–80
Mroczek-Zdyrska M, Wójcik M (2012) The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol Trace Elem Res 147:320–328
Okkenhaug G, Zhu YG, Luo L, Lei M, Li X, Mulder J (2011) Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ Pollut 159:2427–2434
Pan X, Zhang D, Chen X, Bao A, Li L (2011) Antimony accumulation, growth performance, antioxidant defense system and photosynthesis of Zea mays in response to antimony pollution in soil. Water Air Soil Pollut 215:517–523
Paoli L, Fiorini E, Munzi S, Sorbo S, Basile A, Loppi S (2013) Antimony toxicity in the lichen Xanthoria parietina (L.) Th. Fr. Chemosphere 93:2269–2275
Pedrero Z, Madrid Y, Hartikainen H, Camara C (2008) Protective effect of selenium in broccoli (Brassica oleracea) plants subjected to cadmium exposure. J Agric Food Chem 56:266–271
Pérez-Sirvent C, Martínez-Sánchez MJ, Martínez-López S, Bech J, Bolan N (2012) Distribution and bioaccumulation of arsenic and antimony in Dittrichia viscosa growing in mining-affected semiarid soils (in southeast Spain). J Geochem Explor 123:128–135
Serfor-Armah Y, Nyarko BJB, Dampare SB, Adomako D (2006) Levels of arsenic and antimony in water and sediment from Prestea, a gold mining town in Ghana and its environs. Water Air Soil Pollut 175:181–192
Shtangeeva I, Bali R, Harris A (2011) Bioavailability and toxicity of antimony. J Geochem Explor 110:40–45
Shtangeeva I, Steinnes E, Lierhagen S (2012) Uptake of different forms of antimony by wheat and rye seedlings. Environ Sci Pollut Res 19:502–509
Shumilin E, Páez-Osuna F, Green-Ruiz C, Sapozhnikov D, Rodriguez-Meza GD, Godinez-Orta L (2001) Arsenic, antimony, selenium and other trace elements in sediments of the La Paz Lagoon, Peninsula of Baja California, Mexico. Mar Pollut Bull 42:174–178
Steinnes E, Allen RO, Petersen HM, Rambæk JP, Varskog P (1997) Evidence of large scale heavy-metal contamination of natural surface soils in Norway from long-range atmospheric transport. Sci Total Environ 205:255–266
Tschan M, Robinson BH, Nodari M, Schulin R (2009) Antimony uptake by different plant species from nutrient solution, agar and soil. Environ Chem 6:144–152
Tu S, Ma LQ (2003) Interactive effects of pH, arsenic and phosphorus on uptake of As and P and growth of the arsenic hyperaccumulator Pteris vittata L. under hydroponic conditions. Environ Exp Bot 50:243–251
Wei CY, Sun X, Wang C, Wang WY (2006) Factors influencing arsenic accumulation by Pteris vittata: a comparative field study at two sites. Environ Pollut 141:488–493
Wu FB, Dong J, Qian QQ, Zhang GP (2005) Subcellular distribution and chemical form of Cd and Cd-Zn interaction in different barley genotypes. Chemosphere 60:1437–1446
Zhang D, Pan X, Mu G, Wang J (2010) Toxic effects of antimony on photosystem II of Synechocystis sp. as probed by in vivo chlorophyll fluorescence. J Appl Phycol 22:479–488
Acknowledgments
This research was supported by the Central Public Research Institute Basic Fund for Research and Development (2014-szjj-frw-08), the National Science Foundation of China (41103075, 41101306), and the Special Fund for Agro-scientific Research in the Public Interest (201203045).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 14 kb)
Supplementary Table 2
(DOCX 15 kb)
Supplementary Table 3
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Ding, Y., Wang, R., Guo, J. et al. The effect of selenium on the subcellular distribution of antimony to regulate the toxicity of antimony in paddy rice. Environ Sci Pollut Res 22, 5111–5123 (2015). https://doi.org/10.1007/s11356-014-3865-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3865-9