Abstract
Bixafen (BIX) is a succinate dehydrogenase inhibitor (SDHI)-class fungicide that is used to control crop diseases. However, data on the toxicity of BIX to zebrafish are limited. Here, zebrafish embryos were exposed to 0.1, 0.3, and 0.9 μM BIX. After BIX exposure, zebrafish embryos exhibited cardiac dysplasia and dysfunction, including pericardial edema, reduced heart rate, and drastically decreased erythrocytes in the cardiac area; the severity of these negative effects increased with BIX concentration and the duration of BIX exposure. In addition, the transcription levels of erythropoiesis-related genes decreased significantly in BIX-treated embryos, as compared to untreated control embryos. Similarly, compared with the control, key genes responsible for cardiac development (myh6, nkx2.5, and myh7) also exhibited dysregulated expression patterns in response to BIX treatment, suggesting that BIX might specifically affect cardiac development. Finally, cell apoptosis was induced in embryos after BIX treatment. In combination, our results suggested that exposure to BIX induced cardiac toxicity in zebrafish. These data will be valuable for future evaluations of the environmental risks of BIX.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of fungicide use, fungicide may potentially enter water bodies through surface runoff or leaching, posing threats to aquatic life, including fish (Schriever and Liess 2007; Schriever et al. 2007). Previous studies have reported the toxicity of fungicide to various fish organs; for example, hematotoxicity, cardiac toxicity, hepatotoxicity, neurotoxicity, or immunotoxicity (Li et al. 2019a, b; Bojarski and Witeska 2020; Huang et al. 2020; Jiang et al. 2020; Wang et al. 2021).
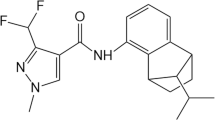
Bixafen (BIX; C18H12Cl2F3N3O), a broad-spectrum succinate dehydrogenase inhibitor (SDHI)-class fungicide, is currently widely used for crop fungal disease control and to improve production yields; BIX inhibits the succinate dehydrogenase activity critical for the mitochondrial respiratory chain in fungi (Berdugo et al. 2012; Lalève et al. 2014; Yamashita and Fraaije 2018). The widespread use of BIX has elicited much concern in recent years due to its potential adverse effects on the environment (Wu et al. 2018; Li et al. 2020). That is, BIX is not readily biodegradable, and thus may be highly persistent and chemically stable in the environment (EFSA 2012); the half-life of BIX is more than 254 days in aerobic soil and 82 days in water (PPD 2019). The maximum residue levels of BIX are 0.01–0.15 mg/kg in plants and 0.02–1.5 mg/kg in livestock (EFSA 2020).
Although BIX is not particularly toxic to mammals (oral LD50 > 5000 mg/kg bw per day), it is highly toxic to aquatic organisms (EFSA 2012). For example, the 96-h lethal concentration 50% (LC50) values of BIX were 0.095 mg/L for rainbow trout (Oncorhynchus mykiss) and 0.105 mg/L for the fathead minnow (Pimephales promelas). In addition, the 48-h effective concentration 50% (EC50) of BIX was 1.2 mg/L for the aquatic invertebrate Daphnia magna, while the 72-h EC50 of BIX was 0.097 mg/L for the alga Pseudokirchneriella subcapitata (EFSA 2012; PPD 2019). Similarly, Wu et al. (2018) reported that treatment with 0.12–1.46 mg/L BIX led to lethal and teratogenic effects (e.g., microcephaly, hypopigmentation, somite segmentation, and narrow fin) in Xenopus tropicalis embryos. Acute exposure to 0.2 and 0.5 μM BIX also induced microcephaly and defects during motor neuron axon development in zebrafish embryos (Brenet et al. 2021). Moreover, we recently demonstrated that BIX exposure (0.1, 0.3, and 0.9 μM) during early embryogenesis (3–48 hpf) caused developmental toxicity in zebrafish embryos (Li et al. 2020). Finally, high concentrations of BIX (≥10 μM) induced reactive oxygen species (ROS) production and genotoxicity in the Jurkat cell line (Graillot et al. 2012). As BIX is a potentially persistent environmental chemical, the adverse effects of BIX on aquatic organisms should be carefully investigated.
Zebrafish have become a premier vertebrate model for investigations of cardiovascular toxicity (Zakaria et al. 2018; Sun and Li 2019; Arman and İşisağ Üçüncü 2020). The first zebrafish organ to form and function during embryonic development is the heart; cardiac contractions begin around 24 hours post fertilization (hpf) (Bakkers 2011; Brown et al. 2016). It has been reported that exposure to the SDHI thifluzamide, boscalid, and penthiopyrad induced pericardial edema and significantly affected heart rate in zebrafish (Yang et al. 2016; Qian et al. 2018, 2019). However, to the best of our knowledge, the effects of BIX on heart development in zebrafish have yet to be investigated.
To address this knowledge gap, we studied the toxic effects of BIX on cardiac development in zebrafish by exposing zebrafish embryos to BIX. The effects of BIX on cardiac phenotype, heart rate, and erythropoiesis were evaluated. We also characterized transcriptional changes in marker genes associated with heart development and cellular apoptosis. Our data provide valuable evidence of the ecological risks posed by BIX to aquatic organisms.
Materials and methods
Zebrafish and BIX treatment
Adult zebrafish strain AB was maintained, embryos were produced, and embryos were exposed to BIX as previously described (Li et al. 2020). Briefly, healthy embryos were harvested, raised to the blastula stage (3 hpf), and then treated with BIX per OECD 212 guidelines (OECD 1998). BIX stock solution (45 g/L) was prepared by dissolving BIX powder (Sigma-Aldrich, USA) in dimethyl sulfoxide (DMSO) (Biosharp, China). Zebrafish embryos were treated with nominal concentrations of BIX (0.1, 0.3, and 0.9 μM) for four days. These BIX concentrations, which may cause malformations but are not fatal, were selected based on our previous study. In that study, we determined the actual BIX concentrations in the exposure solutions (Li et al. 2020). Control groups were exposed to 0.0008% (v/v) DMSO only (no BIX). For each treatment, three replicates were used, each containing 40 mL exposure solution and 70 embryos. After adding the BIX and DMSO, the embryos were maintained at 28.5 °C. All exposure solutions were renewed every 24 h, and there were no changes in BIX concentrations within 24 hours.
Acute toxicity of BIX
The survival, malformation rate, hatching rate, and heart rate of embryos were recorded under a stereomicroscope (Leica, M205FA). Death was assessed via cardiac arrest. The embryos with pericardial edema, tail malformation, and spinal curvature were considered malformations. The emergence of an embryo from an eggshell was considered hatching. The heart rates were counted as the number of beats per 30 seconds.
o-Dianisidine staining
To investigate the effects of BIX on erythrocyte development, and whether the levels of circulating erythrocytes were affected by BIX, we used o-Dianisidine staining to detect red blood cells in the zebrafish embryos at 48, 72, and 96 hpf. The embryos were euthanized in 0.03% MS-222 (Sigma-Aldrich, USA) and then were treated with o-Dianisidine solution (0.6 mg/mL, 0.01 M sodium acetate, 40% (v/v) ethanol, and 0.65% H2O2), which stains hemoglobin, for 15 minutes in the dark. After staining, the embryos were fixed in 4% paraformaldehyde (Sigma-Aldrich, USA) overnight at 4 °C and then rinsed in phosphate buffer saline (PBS). After rinsing, the embryos were examined and mounted for imaging under a stereomicroscope (Leica, M205FA). The percentages of BIX-exposed embryos exhibiting reduced o-Dianisidine signals were calculated using Image-Pro Plus software (Media Cybernetics, Bethesda, MD).
Whole-mount in situ hybridization (WISH)
To determine the potential effects of BIX on cardiac development in zebrafish, we performed WISH using 72-hpf embryos to investigate the spatiotemporal expression patterns of three molecular markers associated with cardiac development: myh6 (previous named amhc, atrial myosin heavy chain), a myocardia marker that promotes the expansion of ventricular myocardial cells (Sarantis et al. 2019); nkx2.5, a cardiac transcription factor that is expressed in zebrafish cardiomyocytes and is required for cardiac development (Colombo et al. 2018); and myh7 (previously named vmhc, ventricle myosin heavy chain), a chamber-specific marker that is expressed in the cardiac ventricle (Jin et al. 2009).
Antisense RNA probes for myh6 (GenBank accession no. NM_198823.1), nkx2.5 (GenBank accession no. NM_131421.2), and myh7 (GenBank accession no. NM_001112733.1) were prepared using DIG RNA Labeling Kits (Roche, Mannheim, Germany), following the manufacturer’s instructions. The specific primers, containing T7 promoter sequences for probe synthesis, are shown in Table S1. WISH was performed as described previously (Thisse and Thisse 2008), with minor modifications. In brief, the embryos were fixed with 4% paraformaldehyde (Sigma-Aldrich, USA) overnight at 4 °C and then were hybridized with the RNA probes detailed above overnight at 60 °C. For each probe, there were at least 15 embryos from the treatment and control groups, and 3–4 biological replicates were performed. After washing the excess probes, the embryos were incubated with anti-DIG antibody alkaline phosphatase (Roche) overnight at 4 °C. After washing the excess antibody, the embryos were stained with the staining solution (Beyotime, Shanghai, China) in the dark at room temperature. After staining, the embryos were observed and imaged under a stereomicroscope (Leica, M205FA).
Acridine orange (AO) staining
We used AO staining to visualize cellular apoptosis. Embryos (72 hpf) from each treatment group were incubated with 5 μg/mL AO solution for 30 minutes at room temperature. The embryos were kept in darkness. After staining, the embryos were rinsed with water three times with gentle agitation and observed and photographed under a fluorescence stereomicroscope (Leica, M205FA).
Differentially expressed gene (DEG) analysis
To characterize differences in the mRNA transcription of certain genes among zebrafish embryos exposed to different concentrations of BIX, we performed transcriptome sequencing. We assessed differences in the expression levels of genes associated with potential molecular mechanisms underlying BIX-induced defects in erythropoiesis (Table S2), as well as genes involved in the apoptotic signaling pathway (Table S3). Sequencing libraries from 30 embryos (24 hpf) from the control, 0.3 μM BIX, and 0.9 μM BIX treatment groups were prepared and sequenced as reported previously (Li et al. 2020). Three independent replicate embryos from each group were sequenced. After removing the low-quality reads (the percentage of bases with quality ≤20 was higher than 50%, or the percentage of N bases was higher than 10%), the clean reads from each group were aligned to the zebrafish reference genome (Ensembl database release 91) using Hisat2 v2.2.1 (Kim et al. 2019). The fragments per kilobase of transcript per million mapped fragment (FPKM) values for all target genes were calculated using the Subread R package v1.6.4 (Liao et al. 2019). We assessed differences in gene expression between the control and BIX-treated embryos using the DESeq2 R package (Love et al. 2014). Genes with adjusted P values <0.05 (adjusted based on the false discovery rate, FDR) and absolute fold change >2 were considered differentially expressed. Heatmaps and volcano plots were generated using TBtools (Chen et al. 2020).
RNA isolation and qRT-PCR
Total RNAs were isolated from 30 embryos (24 hpf) in the control and treatment groups using TRIzol. Total RNA samples (1 μg) were reverse transcribed to synthesize cDNA using the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China), following the manufacturer’s protocols. qRT-PCRs were performed using a SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biotechnology, Hunan, China) on a CFX 96 Touch System (Bio-Rad, USA), following the manufacturer’s protocols. The primers used for qRT-PCR are given in Table S4. Relative mRNA expression levels were normalized to the housekeeping gene gapdh (Li et al. 2020).
Statistical analysis
Differences of survival, malformation rate, hatching rate, and heart rate between the control and treatment groups were evaluated using the Statistic Package for Social Science 25.0 (SPSS, Chicago, IL, USA). Significant differences between the control and all treatment group means were identified using a one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) test. We considered P < 0.05 is statistically significant.
Results
BIX negatively affects cardiac development
Embryos exposed to the lowest tested concentration of BIX (0.1 μM) did not exhibit any significant change in heart morphology at 72 hpf or at 96 hpf (Fig. 1). However, embryos exposed to the higher concentrations of BIX (0.3 μM and 0.9 μM) developed pericardial edema in a dose-dependent manner (Fig. 1c, d, g, h). Embryos exposed to the highest tested concentration of BIX (0.9 μM) also exhibited tail malformation and spinal curvature at 72 hpf and 96 hpf, respectively (Fig. 1d, h).
Bixafen (BIX)-induced cardiac-toxicity phenotypes in zebrafish embryos. a–d Lateral views of representative embryos at 72 hpf. e–h Lateral views of representative embryos at 96 hpf. a, e Control embryos (treated with DMSO, but not exposed to BIX). b, f Embryos treated with 0.1 μM BIX. c, g Embryos treated with 0.3 μM BIX. d, h Embryos treated with 0.9 μM BIX. Scale bars: 250 μm. Red arrows indicate pericardial edema
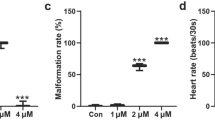
Treatment with 0.9 μM BIX significantly affected the survival of zebrafish embryos at 72 hpf (P < 0.05, Fig. 2a); cardiac malformations at 72 hpf were obvious in the embryos treated with 0.3 and 0.9 μM BIX (Fig. 1). At 72 hpf, malformation rates in groups treated with 0.3 or 0.9 μM BIX were significantly greater than malformation rates in the group treated with 0.1 μM BIX or in the untreated control (P < 0.001, Fig. 2b). In addition, although the hatching rates of the 72-hpf embryos were near 100% in the control, 0.1 μM BIX, and 0.3 μM BIX groups, the hatching rate decreased significantly in the group treated with 0.9 μM BIX: most of these 72-hpf embryos did not hatch (P < 0.001, Fig. 2c). To further evaluate BIX-induced defects in cardiac function, heart rates were compared between the BIX-treated embryos and the control embryos at 48-, 72-, and 96-hpf. There were no significant differences in heart rate between the control and the 0.1 μM BIX treatment group at 48-, 72-, or 96-hpf (Fig. 2d–f). In contrast, heart rate was significantly higher in the 0.3 μM treatment group as compared to the control at 48 hpf (P < 0.01, Fig. 2d). Meanwhile, heart rates in the group treated with 0.9 μM BIX were significantly lower than those in the control group at all three developmental stages (P < 0.05, Fig. 2d–f). At 96 hpf, heart rates in the group treated with 0.3 μM BIX were also significantly lower than those in the control group (P < 0.001, Fig. 2f).
The toxic effects of 0.1, 0.3, and 0.9 μM bixafen (BIX) on zebrafish embryos. a Survival rates, b malformation rates, and c hatching rates of 72-hpf embryos. d–f Embryonic heart rates at d 48 hpf, e 72 hpf, and f96 hpf. Con: control, treated with DMSO only. Values represent mean ± SEM of 3 replicate samples (70 embryos per replicate). ***P < 0.001; **P < 0.01; *P < 0.05
BIX affects erythropoiesis
To determine whether the levels of circulating erythrocytes were affected by BIX, we used o-Dianisidine staining to detect red blood cells in the zebrafish embryos at 48, 72, and 96 hpf (Fig. 3a). We then calculated the percentages of BIX-exposed embryos exhibiting reduced o-Dianisidine signals (Fig. S1). No significant differences in erythrocyte circulation or o-Dianisidine signal intensity between control embryos and those treated with 0.1 μM BIX at 48, 72, and 96 hpf were observed (Fig. 3a). However, far fewer o-Dianisidine-positive cells were observed in the heart areas of embryos treated with 0.3 and 0.9 μM BIX as compared to the control (Fig. 3a; circled in blue), and most of these embryos exhibited reduced o-Dianisidine staining at 48, 72, and 96 hpf (Fig. 3a). In addition, erythrocytes were much more widely distributed in the embryos treated with 0.9 μM BIX as compared to the control embryos at 72 and 96 hpf (Fig. 3a; red arrows). Transcriptome analysis revealed that, of the 12 erythropoiesis-related genes analyzed, three were downregulated in the 0.3-μM BIX treatment group at 24 hpf as compared to the control, and 10 were downregulated in the 0.9-μM BIX treatment group at 24 hpf as compared to the control (Table S2, Fig. S2a, and Fig. 3b). Compared with the control, transcriptional levels of hbbe2 and alas1 at 24 hpf increased significantly after treatment with 0.9 μM BIX (Fig. S2a and Fig. 3b).
Bixafen (BIX) treatment (0.1, 0.3, and 0.9 μM) affects erythropoiesis in zebrafish embryos. a o-Dianisidine staining of erythrocytes in 48-hpf, 72-hpf, and 96-hpf zebrafish embryos. In each image, the area around the heart is outlined in blue; red arrows indicate abnormal erythrocyte distributions. b Heatmap showing the expression patterns of key genes involved in erythropoiesis in 24-hpf zebrafish embryos of 3 replicate samples (30 embryos per replicate). Heatmap colors reflect the log10-transformed FPKM expression levels of functional genes. Con: control group, treated with DMSO only.
BIX altered the expression patterns of cardiac transcription factors
Our WISH results showed that, compared to the control, the expression levels of myocardial marker myh6 decreased substantially in all BIX-treated embryos (Fig. 4a–d). Similarly, the expression levels of cardiac transcription factor nkx2.5 decreased significantly in the embryos treated with 0.1 and 0.3 μM BIX as compared to the control (Fig. 4e–g) and showed ectopic expression patterns in the embryos treated with 0.9 μM BIX (Fig. 4 h). The expression levels of cardiac-specific marker myh7 were similar between the embryos treated with 0.1 μM BIX and control embryos (Fig. 4i–j). However, a pronounced increase in myh7 expression levels was observed in both the 0.3- and the 0.9-μM-BIX treatment groups (Fig. 4 k, l).
Bixafen (BIX) affects the expression patterns of representative cardiac developmental markers in zebrafish embryos. Lateral views of 72-hpf embryos treated with 0.1, 0.3, and 0.9 μM BIX, as well as controls (Con, treated with DMSO only). a–d Expression of the myh6 gene. e–h Expression of the nkx2.5 gene. i–l Expression of the myh7 gene.
BIX exposure induces cell apoptosis and interferes with apoptosis-related gene expression
Using AO staining, we identified apoptotic cells in 72-hpf embryos from all treatment groups (Fig. 5; apoptotic cells are shown as green fluorescent dots). Little fluorescence was observed in the control embryos (Fig. 5a) and those treated with 0.1 μM BIX (Fig. 5b). However, compared with the control, embryos treated with higher concentrations of BIX (0.3 μM and 0.9 μM) exhibited greater levels of fluorescence intensity (i.e., apoptosis) in the cardiac region, indicating cardiac edema (Fig. 5c, d).
Transcriptome analysis of genes associated with the apoptotic signaling pathway indicated that 34 genes were downregulated in embryos treated with 0.3 μM or 0.9 μM BIX as compared to the control (Fig. S2 and Fig. 6a), while 39 genes were upregulated (Fig. S2 and Fig. 6b). The genes involved in the positive (GO:0043065) or negative (GO:0043066) regulation of the apoptotic process were classified following the Gene Ontology database. Of the 34 downregulated genes, 15 positively regulated the apoptotic process and 19 negatively regulated the apoptotic process (Fig. 6a). Of the 39 downregulated genes, 13 positively regulated the apoptotic process and 26 negatively regulated the apoptotic process (Fig. 6b). Consistent with transcriptome sequencing results, the qRT-PCR analyses showed that cdk10, eya3, mtch2, psenen, rb1, and tiprl were significantly downregulated in the BIX-treated embryos at 24 hpf as compared to the control, while bco2l, pawr, pim2, slc2a1a, rela, and scrib were significantly upregulated (Fig. 6c, d).
Exposure to BIX affects the expression patterns of key genes involved in the apoptotic pathway in 24-hpf zebrafish embryos. a, b Heatmaps showing the genes in the apoptotic signaling pathway a downregulated or b upregulated in the embryos treated with BIX (0.1, 0.3, or 0.9 μM) as compared to the control embryos (Con, treated with DMSO only). Heatmap colors reflect the log10-transformed FPKM expression levels of functional genes. c, d qRT-PCR values are presented as the mean ± standard error of the mean (SEM) of 3 replicate samples (30 embryos per replicate). n = 3, *P < 0.05.
Discussion
As an environmental pollutant, the risks of BIX use have received considerable attention (Wu et al. 2018; Li et al. 2020). Here, we evaluated the toxic effects of BIX on cardiac development and associated mechanism in an embryonic/larval zebrafish model. BIX significantly increased mortality rates and pericardial edema in zebrafish embryos. In addition, BIX treatment decreased embryo heart rates and expanded the distribution of circulating erythrocytes in a concentration-dependent manner. This suggested that BIX inhibited normal cardiac development in zebrafish embryos.
Cardiac development, which is an extremely elaborate and sensitive process, can be affected by exposure to toxic chemicals (e.g., persistent organic pollutants, heavy metals, pesticides, or fungicides) during embryonic development (Wu et al. 2013; Li et al. 2019a, b; Bai and Tang 2020; Cunha et al. 2020; Meng et al. 2020b). Cardiac toxicity is often determined based on pericardial edema, which reflects cardiac development, and heart rate, which reflects cardiac function (De et al. 2014; Sarmah and Marrs 2016). Our results showed that exposure to concentrations of BIX greater than or equal to 0.3 μM increased the area of pericardial edema (Fig. 1) and significantly decreased heart rates (Fig. 2d–f) during zebrafish embryogenesis. Previous studies have shown that other SDHI fungicides, such as thifluzamide and penthiopyrad, also significantly increased the area of pericardial edema and decreased heart rates (Yang et al. 2016; Qian et al. 2019). Thus, cardiac development might represent a specific target of SDHI fungicides and therefore might represent a useful endpoint for ecological risk assessments of SDHIs.
Cardiac output is a significant indicator of cardiac function (Vincent 2008). Here, we used erythrocyte staining to indicate cardiac output in zebrafish embryos (Fig. 3a). Changes in cardiac output often lead to disturbances in cardiac contractions by impeding blood flow through the cardiac chambers (Choi and Park 2012). Here, treatment with 0.3 and 0.9 μM BIX led to a severe reduction in the number of erythrocytes in the heart area (Fig. 3a, Fig. S1; circled in blue); treatment with 0.9 μM BIX also led to abnormal erythrocyte distributions in 72-hpf embryos (Fig. 3a; red arrows around the yolk sac region). We hypothesized that, despite the ectopic distribution of erythrocytes, erythropoiesis still proceeded somewhat normally. We also used the changes in gene expression in the zebrafish embryos exposed to BIX to better understand the mechanisms associated with BIX toxicity. Transcriptome sequencing indicated that erythropoiesis-related genes were downregulated in the BIX-exposed embryos at 24 hpf (Fig. 3b), suggesting that the effects of BIX on erythropoiesis could be identified as early as 24 hpf based on differences in the transcription of related genes. In addition, the mRNA levels of erythroid markers hbbe2 and alas1 increased significantly in the 0.9 μM BIX-exposed group at 24 hpf (Fig. 3b), suggesting that the compensatory expression of some erythropoiesis-related genes, such as hbbe2 and alas1, may explain why BIX does not completely inhibit erythropoiesis.
In zebrafish, cardiac development is regulated by a large number of genes and transcription factors (Shu and Chi 2012). The atrial myosin heavy chain (myh6) and ventricular myosin heavy chain (myh7) genes, which are essential for heart muscle differentiation, are specifically expressed in the atrium and the ventricle, respectively (Yelon et al. 1999; Berdougo et al. 2003). In addition, the conserved gene nkx2.5 plays an essential role in cardiomyocyte differentiation, helping to maintain cardiac chamber-specific characteristics (George et al. 2015; Harrington et al. 2017). Our results showed that the expression patterns of myh6, nkx2.5, and myh7 were normal in the cardiac cones of control embryos (Fig. 4a, e, i) and aberrant in the cardiac cones of embryos treated with BIX (Fig. 4). Moreover, the expression of myh7 begins at the 13-somite stage, and myh7 is expressed in both the ventricular myocardium and the somite before the pharyngula period in zebrafish (Yelon et al. 1999; Zeng et al. 2007). From 48 hpf, myh7 is mainly expressed in the ventricle and is crucial for cardiac remodeling (Jin et al. 2009). Recently, we showed that BIX exposure (0.9 μM) caused developmental delays in zebrafish embryos (Li et al. 2020). Therefore, we hypothesized that the strong signal of myh7 in the somites of the embryos exposed to 0.9 μM BIX was caused by developmental delay. The dysregulated expression patterns of these cardiogenesis-associated genes, in conjunction with the cardiac malformations observed in BIX-treated individuals, strongly suggested that BIX exposure may disrupt cardiac morphogenesis. However, the exact molecular mechanisms underlying the toxic effects of BIX on cardiac development require further characterization.
Apoptosis is a strictly regulated cell death process that contributes to various cardiovascular diseases (Haunstetter and Izumo 1998; Kim and Kang 2010; Xia et al. 2016). Here, higher concentrations of BIX (0.3 μM and 0.9 μM) effectively increased the number of AO-positive (apoptotic) cells (Fig. 5c, d), indicating that the development of the cardiovascular system in zebrafish embryos may be partially affected by BIX-induced apoptosis. We used transcriptome sequencing to explore the potential mechanisms associated with BIX-induced apoptosis in zebrafish embryos at 24 hpf. Most of the genes associated with the positive or negative regulation of the apoptotic processes were significantly differentially expressed between the BIX-treated embryos and the control at 24 hpf (Fig. 6), demonstrating that BIX may affect apoptosis in zebrafish embryos by altering apoptosis-associated gene expression levels as early as 24 hpf. Several genes were significantly differentially expressed between the BIX-treated groups and the controls (Fig. 6a, b): anxa1b, tnfrsfa, dab2ipb, bco2l, slc2a1a, and mcl1b. The overexpression of ANXA1, which is a critical mediator of apoptosis, promotes apoptosis in monocytic cells (Solito et al. 2001), while the genes tnfrsfa and dab2ip are involved in the positive regulation of the apoptotic process and are upregulated during apoptosis (Wang et al. 2016; Aliper et al. 2019). BCO2 plays a crucial role in protecting cells from apoptosis (Lobo et al. 2012), and SLC2A1 contributes to the engulfment of the apoptotic body or cell (Morioka et al. 2018). Finally, Mcl1b is an anti-apoptotic protein (Kratz et al. 2006). The significant differences in the expression profiles of these genes between the BIX-treated and control groups (Fig. 6b) imply that BIX-induced apoptosis might be caused by the misexpression of apoptosis-related genes and that apoptosis may be induced by the upregulation of various genes that positively regulate the apoptotic process, such as anxa1b, tnfrsfa, and dab2ip. Subsequently, genes that participate in the negative regulation of apoptosis (e.g., bco2l, slc2a1a, and mcl1b) may be induced to inhibit apoptosis and promote zebrafish survival after BIX exposure. The downregulation of apoptosis-related genes (Fig. 6a) implies that multiple pathways are activated to inhibit BIX-induced apoptosis. However, it is also possible that BIX-induced apoptosis is partially due to the interference of BIX in the mitochondrial respiratory chain. Because mitochondrial-dependent apoptotic signaling is an intrinsic apoptosis pathway, some environmental pollutants (e.g., fungicides, metals, and persistent organic pollutants) preferentially cause apoptosis through the mitochondrial-dependent pathway (Luzio et al. 2013; Jiang et al. 2019; Meng et al. 2020a; Zhang et al. 2020). Thus, the exact molecular mechanisms underlying BIX-induced apoptosis require further investigation.
The effects of BIX on apoptosis in this study were similar to the observed effects of another SDHI fungicide, boscalid (Qian et al. 2018). Emerging research indicates that DNA damage induces apoptosis in multicellular animals (Norbury and Zhivotovsky 2004; Roos and Kaina 2013). Indeed, in vitro experiments showed that BIX exposure induced DNA damage in the Jurkat cell line (Graillot et al. 2012). Therefore, we hypothesized that DNA damage might first be induced by BIX exposure, subsequently leading to apoptosis. In addition, an in vitro study using a novel pyrazole carboxamide, the structure of which was modified based on BIX, showed that exposure led to the induction of apoptosis in the neuronal cells of Helicoverpa zea (Ren et al. 2018). This suggested that, in addition to BIX, compounds with BIX-type structures have the potential to induce apoptosis.
Conclusions
Our results provided solid evidence that BIX exposure causes significant cardiac damage and apoptosis in zebrafish embryos. We also demonstrated that BIX exposure may induce apoptosis in zebrafish embryos by interfering with the expression of representative apoptosis-related regulators. These data highlight the importance of cardiovascular-toxicity evaluations in SDHI risk assessments.
References
Aliper AM, Bozdaganyan ME, Orekhov PS, Zhavoronkov A, Osipov AN (2019) Replicative and radiation-induced aging: a comparison of gene expression profiles. Aging (Albany NY) 11:2378–2387. https://doi.org/10.18632/aging.101921
Arman S, İşisağ Üçüncü S (2020) Cardiac toxicity of acrolein exposure in embryonic zebrafish (Danio rerio). Environ Sci Pollut Res 27(18):22423–22433. https://doi.org/10.1007/s11356-020-08853-7
Bai C, Tang M (2020) Toxicological study of metal and metal oxide nanoparticles in zebrafish. J Appl Toxicol 40:37–63. https://doi.org/10.1002/jat.3910
Bakkers J (2011) Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res 91:279–288. https://doi.org/10.1093/cvr/cvr098
Berdougo E, Coleman H, Lee DH, Stainier DY, Yelon D (2003) Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development 130:6121–6129. https://doi.org/10.1242/dev.00838
Berdugo CA, Steiner U, Dehne HW, Oerke EC (2012) Effect of bixafen on senescence and yield formation of wheat. Pestic Biochem Physiol 104:171–177. https://doi.org/10.1016/j.pestbp.2012.07.010
Bojarski B, Witeska M (2020) Blood biomarkers of herbicide, insecticide, and fungicide toxicity to fish-a review. Environ Sci Pollut Res Int 27:19236–19250. https://doi.org/10.1007/s11356-020-08248-8
Brenet A, Hassan-Abdi R, Soussi-Yanicostas N (2021) Bixafen, a succinate dehydrogenase inhibitor fungicide, causes microcephaly and motor neuron axon defects during development. Chemosphere 265:128781. https://doi.org/10.1016/j.chemosphere.2020.128781
Brown DR, Samsa LA, Qian L, Liu J (2016) Advances in the study of heart development and disease using zebrafish. J Cardiovasc Dev Dis 3:13. https://doi.org/10.3390/jcdd3020013
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202. https://doi.org/10.1016/j.molp.2020.06.009
Choi SW, Park SM (2012) Analysis of left ventricular impedance in comparison with ultrasound images. Artif Organs 36:479–486. https://doi.org/10.1111/j.1525-1594.2011.01381.x
Colombo S, de Sena-Tomás C, George V, Werdich AA, Kapur S, MacRae CA, Targoff KL (2018) Nkx genes establish second heart field cardiomyocyte progenitors at the arterial pole and pattern the venous pole through Isl1 repression. Development 145:dev161497. https://doi.org/10.1242/dev.161497
Cunha V, Vogs C, Le Bihanic F, Dreij K (2020) Mixture effects of oxygenated PAHs and benzo[a]pyrene on cardiovascular development and function in zebrafish embryos. Environ Int 143:105913. https://doi.org/10.1016/j.envint.2020.105913
De LE, Zaccaria GM, Hadhoud M, Rizzo G, Ponzini R, Morbiducci U, Santoro MM (2014) ZebraBeat: a flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci Rep 4:649–652. https://doi.org/10.1038/srep04898
EFSA (2012) Conclusion on the peer review of the pesticide risk assessment of the active substance bixafen. EFSA J 10:2917. https://doi.org/10.2903/j.efsa.2012.2917
EFSA (2020) Review of the existing maximum residue levels for bixafen according to Article 12 of Regulation (EC) No 396/2005. EFSA J 18:5998. https://doi.org/10.2903/j.efsa.2020.5998
George V, Colombo S, Targoff KL (2015) An early requirement for nkx2.5 ensures the first and second heart field ventricular identity and cardiac function into adulthood. Dev Biol 400:10–22. https://doi.org/10.1016/j.ydbio.2014.12.019
Graillot V, Tomasetig F, Cravedi JP, Audebert M (2012) Evidence of the in vitro genotoxicity of methyl-pyrazole pesticides in human cells. Mutat Res 748:8–16. https://doi.org/10.1016/j.mrgentox.2012.05.014
Harrington JK, Sorabella R, Tercek A, Isler JR, Targoff KL (2017) Nkx2.5 is essential to establish normal heart rate variability in the zebrafish embryo. Am J Phys Regul Integr Comp Phys 313:R265–R271. https://doi.org/10.1152/ajpregu.00223.2016
Haunstetter A, Izumo S (1998) Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res 82:1111–1129. https://doi.org/10.1161/01.res.82.11.1111
Huang Y, Chen Z, Meng Y, Wei Y, Xu Z, Ma J, Zhong K, Cao Z, Liao X, Lu H (2020) Famoxadone-cymoxanil induced cardiotoxicity in zebrafish embryos. Ecotoxicol Environ Saf 205:111339. https://doi.org/10.1016/j.ecoenv.2020.111339
Jiang J, Wu S, Lv L, Liu X, Chen L, Zhao X, Wang Q (2019) Mitochondrial dysfunction, apoptosis and transcriptomic alterations induced by four strobilurins in zebrafish (Danio rerio) early life stages. Environ Pollut 253:722–730. https://doi.org/10.1016/j.envpol.2019.07.081
Jiang J, Chen L, Wu S, Lv L, Liu X, Wang Q, Zhao X (2020) Effects of difenoconazole on hepatotoxicity, lipid metabolism and gut microbiota in zebrafish (Danio rerio). Environ Pollut 265:114844. https://doi.org/10.1016/j.envpol.2020.114844
Jin D, Ni TT, Hou J, Rellinger E, Zhong TP (2009) Promoter analysis of ventricular myosin heavy chain (vmhc) in zebrafish embryos. Dev Dyn 238:1760–1767. https://doi.org/10.1002/dvdy.22000
Kim NH, Kang PM (2010) Apoptosis in cardiovascular diseases: mechanism and clinical implications. Korean Circ J 40:299–305. https://doi.org/10.4070/kcj.2010.40.7.299
Kim D, Paggi JM, Park C, Bennett C, Salzberg SL (2019) Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37:907–915. https://doi.org/10.1038/s41587-019-0201-4
Kratz E, Eimon PM, Mukhyala K, Stern H, Zha J, Strasser A, Hart R, Ashkenazi A (2006) Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ 13:1631–1640. https://doi.org/10.1038/sj.cdd.4402016
Lalève A, Gamet S, Walker AS, Debieu D, Toquin V, Fillinger S (2014) Site-directed mutagenesis of the P225, N230 and H272 residues of succinate dehydrogenase subunit B from Botrytis cinerea highlights different roles in enzyme activity and inhibitor binding. Environ Microbiol 16:2253–2266. https://doi.org/10.1111/1462-2920.12282
Li H, Zhao F, Cao F, Teng M, Yang Y, Qiu L (2019a) Mitochondrial dysfunction-based cardiotoxicity and neurotoxicity induced by pyraclostrobin in zebrafish larvae. Environ Pollut 251:203–211. https://doi.org/10.1016/j.envpol.2019.04.122
Li M, Liu X, Feng X (2019b) Cardiovascular toxicity and anxiety-like behavior induced by deltamethrin in zebrafish (Danio rerio) larvae. Chemosphere 219:155–164. https://doi.org/10.1016/j.chemosphere.2018.12.011
Li WH, Yuan MR, Wu YQ, Liu X (2020) Bixafen exposure induces developmental toxicity in zebrafish (Danio rerio) embryos. Environ Res 189:109923. https://doi.org/10.1016/j.envres.2020.109923
Liao Y, Smyth GK, Shi W (2019) The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res 47:e47. https://doi.org/10.1093/nar/gkz114
Lobo GP, Isken A, Hoff S, Babino D, von Lintig J (2012) BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 139:2966–2977. https://doi.org/10.1242/dev.079632
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Luzio A, Monteiro SM, Fontaínhas-Fernandes AA, Pinto-Carnide O, Matos M, Coimbra AM (2013) Copper induced upregulation of apoptosis related genes in zebrafish (Danio rerio) gill. Aquat Toxicol 128-129:183–189. https://doi.org/10.1016/j.aquatox.2012.12.018
Meng S, Chen X, Gyimah E, Xu H, Chen J (2020a) Hepatic oxidative stress, DNA damage and apoptosis in adult zebrafish following sub-chronic exposure to BDE-47 and BDE-153. Environ Toxicol 35:1202–1211. https://doi.org/10.1002/tox.22985
Meng Y, Zhong K, Xiao J, Huang Y, Wei Y, Tang L, Chen SP, Wu J, Ma JZ, Cao ZG, Liao XJ, Lu HQ (2020b) Exposure to pyrimethanil induces developmental toxicity and cardiotoxicity in zebrafish. Chemosphere 255:126889. https://doi.org/10.1016/j.chemosphere.2020.126889
Morioka S, Perry JSA, Raymond MH, Medina CB, Zhu Y, Zhao L, Serbulea V, Onengut-Gumuscu S, Leitinger N, Kucenas S, Rathmell JC, Makowski L, Ravichandran KS (2018) Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563:714–718. https://doi.org/10.1038/s41586-018-0735-5
Norbury CJ, Zhivotovsky B (2004) DNA damage-induced apoptosis. Oncogene 23:2797–2808. https://doi.org/10.1038/sj.onc.1207532
OECD (1998) Guideline for Testing of Chemicals Method 212. Fish, short-term toxicity test on embryo and Sac-fry stages. OECD 212:1–20
PPD (2019) Bixafen. Pesticide properties database. http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1250.htm
Qian L, Cui F, Yang Y, Liu Y, Qi S, Wang C (2018) Mechanisms of developmental toxicity in zebrafish embryos (Danio rerio) induced by boscalid. Sci Total Environ 634:478–487. https://doi.org/10.1016/j.scitotenv.2018.04.012
Qian L, Qi S, Cao F, Zhang J, Li C, Song M, Wang C (2019) Effects of penthiopyrad on the development and behaviour of zebrafish in early-life stages. Chemosphere 214:184–194. https://doi.org/10.1016/j.chemosphere.2018.09.117
Ren Y, Yang N, Yue Y, Jin H, Tao K, Hou T (2018) Investigation of novel pyrazole carboxamides as new apoptosis inducers on neuronal cells in Helicoverpa zea. Bioorg Med Chem 26:2280–2286. https://doi.org/10.1016/j.bmc.2018.03.010
Roos WP, Kaina B (2013) DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett 332:237–248. https://doi.org/10.1016/j.canlet.2012.01.007
Sarantis P, Gaitanaki C, Beis D (2019) Ventricular remodeling of single-chambered myh6-/- adult zebrafish hearts occurs via a hyperplastic response and is accompanied by elastin deposition in the atrium. Cell Tissue Res 378:279–288. https://doi.org/10.1007/s00441-019-03044-4
Sarmah S, Marrs JA (2016) Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. Int J Mol Sci 17:2123. https://doi.org/10.3390/ijms17122123
Schriever CA, Liess M (2007) Mapping ecological risk of agricultural pesticide runoff. Sci Total Environ 384:264–279. https://doi.org/10.1016/j.scitotenv.2007.06.019
Schriever CA, von der Ohe PC, Liess M (2007) Estimating pesticide runoff in small streams. Chemosphere 68:2161–2171. https://doi.org/10.1016/j.chemosphere.2007.01.086
Shu T, Chi NC (2012) Zebrafish models in cardiac development and congenital heart birth defects. Differentiation 84:4–16. https://doi.org/10.1016/j.diff.2012.05.005
Solito E, de Coupade C, Canaider S, Goulding NJ, Perretti M (2001) Transfection of annexin 1 in monocytic cells produces a high degree of spontaneous and stimulated apoptosis associated with caspase-3 activation. Br J Pharmacol 133:217–228. https://doi.org/10.1038/sj.bjp.0704054
Sun G, Li Y (2019) Exposure to DBP induces the toxicity in early development and adverse effects on cardiac development in zebrafish (Danio rerio). Chemosphere 218:76–82. https://doi.org/10.1016/j.chemosphere.2018.11.095
Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3:59–69. https://doi.org/10.1038/nprot.2007.514
Vincent JL (2008) Understanding cardiac output. Crit Care 12:174. https://doi.org/10.1186/cc6975
Wang Q, Liu S, Hu D, Wang Z, Wang L, Wu T, Wu Z, Mohan C, Peng A (2016) Identification of apoptosis and macrophage migration events in paraquat-induced oxidative stress using a zebrafish model. Life Sci 157:116–124. https://doi.org/10.1016/j.lfs.2016.06.009
Wang X, Li X, Wang Y, Qin Y, Yan B, Martyniuk CJ (2021) A comprehensive review of strobilurin fungicide toxicity in aquatic species: emphasis on mode of action from the zebrafish model. Environ Pollut 275:116671. https://doi.org/10.1016/j.envpol.2021.116671
Wu M, Zuo Z, Li B, Huang L, Chen M, Wang C (2013) Effects of low-level hexabromocyclododecane (HBCD) exposure on cardiac development in zebrafish embryos. Ecotoxicology 22:1200–1207. https://doi.org/10.1007/s10646-013-1107-4
Wu S, Lei L, Liu M, Song Y, Lu S, Li D, Shi H, Raley-Susman KM, He D (2018) Single and mixture toxicity of strobilurin and SDHI fungicides to Xenopus tropicalis embryos. Ecotoxicol Environ Saf 153:8–15. https://doi.org/10.1016/j.ecoenv.2018.01.045
Xia P, Liu Y, Cheng Z (2016) Signaling pathways in cardiac myocyte apoptosis. Biomed Res Int 2016:9583268. https://doi.org/10.1155/2016/9583268
Yamashita M, Fraaije B (2018) Non-target site SDHI resistance is present as standing genetic variation in field populations of Zymoseptoria tritici. Pest Manag Sci 74:672–681. https://doi.org/10.1002/ps.4761
Yang Y, Qi S, Wang D, Wang K, Zhu L, Chai T, Wang C (2016) Toxic effects of thifluzamide on zebrafish (Danio rerio). J Hazard Mater 307:127–136. https://doi.org/10.1016/j.jhazmat.2015.12.055
Yelon D, Horne SA, Stainier DY (1999) Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol 214:23–37. https://doi.org/10.1006/dbio.1999.9406
Zakaria ZZ, Benslimane FM, Nasrallah GK (2018) Using zebrafish for investigating the molecular mechanisms of drug-induced cardiotoxicity. Biomed Res Int 2018:1642684. https://doi.org/10.1155/2018/1642684
Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L (2007) Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell 12:391–402. https://doi.org/10.1016/j.devcel.2007.01.011
Zhang J, Zhang C, Du Z, Zhu L, Wang J, Wang J, Li B (2020) Emerging contaminant 1,3,6,8-tetrabromocarbazole induces oxidative damage and apoptosis during the embryonic development of zebrafish (Danio rerio). Sci Total Environ 743:140753. https://doi.org/10.1016/j.scitotenv.2020.140753
Acknowledgments
The research was supported by the Wuhan Branch, Supercomputing Center, Chinese Academy of Sciences, China.
Funding
This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (Grant No. 31801034), the Scientific Research Funds of Huaqiao University (15BS306), and the Postgraduates’ Innovative Fund in Scientific Research of Huaqiao University.
Author information
Authors and Affiliations
Contributions
Mingrui Yuan: data curation, validation, visualization. Wenhua Li: conceptualization, visualization, writing-original draft preparation. Peng Xiao: data curation, writing-reviewing and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experiments in this study were approved by the Animal Care and Welfare Committee of Huaqiao University (Xiamen, China).
Competing interests
The authors declare no competing financial interest.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(RAR 424 kb)
Rights and permissions
About this article
Cite this article
Yuan, M., Li, W. & Xiao, P. Bixafen causes cardiac toxicity in zebrafish (Danio rerio) embryos. Environ Sci Pollut Res 28, 36303–36313 (2021). https://doi.org/10.1007/s11356-021-13238-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13238-5