Abstract
Isopyrazam (IPZ) is a broad spectrum succinate dehydrogenase inhibitor fungicide. Little is known about its potential ecological risks of aquatic organisms recently. The present study examined the embryonic development effects of zebrafish exposed to IPZ under static condition using a fish embryo toxicity test. The lowest observed effect concentration of IPZ was 0.025 mg/L in 4-day exposure. Developmental abnormalities, including edema, small head deformity, body deformation and decreased pigmentation, and mortality were observed in zebrafish embryos of 0.05 mg/L and higher concentrations, which shown concentration dependency. The heart rate of zebrafish was disrupted by IPZ. Moreover, enzyme and gene experiments shown that IPZ exposure caused oxidative stress of zebrafish. Furthermore, it induced a decrease of succinate dehydrogenase (SDH) enzyme activity and gene transcription level in zebrafish larvae. It can be speculated that IPZ may have a lethal effect on zebrafish, which is accompanied by decreased SDH activity, oxidative stress, and abnormality. These results provide toxicological data about the IPZ on aquatic non-target organisms, which could be useful for further understanding potential environmental risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural chemicals have been shown to play an important role in social development. However, numerous studies illustrate that these compounds enter the environment, especially aquatic systems, along with multi-component chemical “cocktails,” and could pose different threats to non-target organisms (Mnif et al. 2011; Avetta et al. 2014; Bunzel et al. 2015; Fernández et al. 2015).
Isopyrazam (IPZ), 3-(difluoromethyl)-1-methyl-N-[1,2,3,4-tetrahydro-9-(1-methylethyl)-1,4-methano-naphthalen-5-yl]-1H-pyrazole-4-carboxamide, is a broad spectrum fungicide which inhibits mitochondrial respiration by binding to succinate dehydrogenase (SDH, so-called complex II in the mitochondrial respiration chain) in the inner mitochondrial membrane of cells. SDH is a fundamental component of the tricarboxylic cycle, within the mitochondrial electron transport chain (Keon et al. 1991). Succinate dehydrogenase inhibitor (SDHI), as stated by the Fungicide Resistance Action Committee, includes eight chemical classes recently and is a group of fungicides which act against spore germination, germination tube growth, mycelial growth, and sporulation of fungal growth (FRAC 2017; Avenot and Michailides 2010; Scalliet et al. 2012; Abad-Fuentes et al. 2015). IPZ is one of the SDHIs with pyridinyl-ethyl-benzamides and belongs to the ortho-substituted phenyl amides class of fungicides (Fig. 1), which have been widely used in agriculture against diseases affecting bananas, peppers, tomatoes, and other vegetable crops. Previous study reported that IPZ has the potential to play an important role in the management of gray mold with median effective concentration (EC50) values of 0.07 and 0.68 mg/L for the inhibition of Botrytis cinerea spore germination and mycelial growth, respectively (Song et al. 2016). Another report showed that IPZ has protective and curative activity against Podosphaera xanthii, with EC50 values of 0.04 and 0.05 mg/L, respectively, and exhibited a long duration of efficacy against cucumber powdery mildew at 60 mg/L (He et al. 2017).
Several SDHIs have been detected in the environment (Tanabe et al. 2001; Tsuda et al. 2009; Smalling et al. 2013; Gulkowska et al. 2014). For example, boscalid, a SDHI, has been detected in surface water in the USA (Reilly et al. 2012), in headwater streams in Denmark (Rasmussen et al. 2013), and in a coastal lagoon in Spain (Moreno-González et al. 2013). Previous works have demonstrated that IPZ could be long-term maintained in aquatic ecosystems, with degradation half-life (DT50) values in the water-sediment systems maintained in the dark of greater than 1 year, and was fairly to very slightly degradable in soils under aerobic laboratory conditions with the DT50 values in the range of 40–976 days (Hand and Oliver 2010; APVMA 2018). IPZ only moderately degradation fast in a photolysis study in buffered pure water (DT50 > 60 days), although it was susceptible to both direct and indirect photodegradation and metabolism by algae and macrophytes (DT50 values of 11 and 37 days) (Hand and Oliver 2010; Hand and Moreland 2014). Moreover, existing studies have shown that this kind of chemicals can be hazardous to non-target organisms. For example, fluopyram, another SDHI, could cause increased incidence of thyroid and hepatic tumor formation in mammals (Tinwell et al. 2014; Rouquie et al. 2014). Thifluzamide, a SDHI, displayed toxicity toward embryos, larvae, and adult zebrafish, and the 96-h median lethal concentration (LC50) values were 3.08, 3.52, and 4.19 mg/L, respectively (Yang et al. 2016a, b). Therefore, the potential threat of pesticides has attracted much attention, and SDHIs may be of concern due to possible long-term effects on aquatic organisms (Reilly et al. 2012).
The zebrafish embryo model has been extensively used in developmental toxicity researches, and many pioneering studies suggest that in many cases, there are very similar toxicological effects in zebrafish embryos and humans (Hill et al. 2005; Selderslaghs et al. 2012; Strähle et al. 2012; Driessen et al. 2015; Stengel et al. 2018). The mortality, edema, hatching rate, heart rate, and so on are usefully parameters in the embryo toxicity test (Hermsen et al. 2011; Noyes et al. 2016; Wang et al. 2017). Oxidative stress has the potential to become an important subject in chemicals induced aquatic toxicity, and many toxicity effects induced by pesticide may be directly involved in this process (Di Giulio et al. 1989; Lushchak 2011; Blahová et al. 2013). Andrade et al. (2016) showed that carbendazim, a benzimidazole fungicide, elicited several developmental anomalies and GST increased in zebrafish embryos with 96 h-LC50 of 1.75 mg/L. Thifluzamide was reported to cause oxidative damage in zebrafish embryos (Yang et al. 2016a). The heart is the first functional organ developed in zebrafish, heart development is an extremely sensitive process, and heart rate has become an important toxicology endpoint in embryonic development testing (Glickman and Yelon 2002; Sarmah and Marrs 2016). Previous study showed that difenoconazole could inhibit the heart rate of zebrafish embryos and cause significant pericardial edema at 2.0 mg/L (Mu et al. 2016). Fong et al. (2016) tested benzophenone-2 exposure to zebrafish embryos and reported that the enlarged yolk was due to the impaired lipid metabolism and poor blood circulation was due to the decreased heart rate.
IPZ is classified as “Likely to be Carcinogenic to Humans” based on increased incidence of uterine endometrial adenocarcinomas and liver hepatocellular adenomas in female rats and increased incidence of thyroid follicular cell adenomas and/or carcinomas in male rats (EPA 2017). The stable induction of tissue specific miRNAs (miR200a, 200b, and 429) by liver non-genotoxic carcinogens may serve as early predictors (biomarkers) of hepatocarcinogenic potential. Plummer et al. (2018) reported that a statistically significant dose-dependent increase in miRNAs 200a, 220b, and 429 in both male and female rats under IPZ exposure. Another study also found that IPZ and other six pesticides showed treatment-related increases in endometrial adenocarcinomas for rats combined chronic toxicity and carcinogenicity studies (Yoshida et al. 2015). Besides, Wu et al. (2018) reported that IPZ induced lethal and malformations including microcephaly, hypopigmentation, somite segmentation and narrow fin of Xenopus tropicalis embryos, with the LC50 and the median teratogenic concentration of 2.87 and 0.31 mg/L, respectively. IPZ was practically non-toxic to birds and mammals on an acute basis, while was very highly acutely toxic to fish with a 96-h LC50 of 25.8 μg ac/L for the Cyprinus carpio, and 21-day EC50 of 53 μg ac/L for the Daphnia magna, but the toxicology knowledge of IPZ to zebrafish and underlying mechanisms is limited (APVMA 2018). Against this background, the present study examined the lethal effect, hatching rate, malformation, heart rate, oxidative stress, and SDH activity in zebrafish embryos for evaluation of the developmental effects of IPZ on early-life zebrafish.
Materials and methods
Chemicals
The IPZ (purity ≥ 99%) was provided by Dr. Chengrong Ding from the Zhejiang University of Technology (Hangzhou, China). Stock solution of IPZ was prepared in DMSO (purity ≥ 99.9%, Aladdin, China) and stored at 4 °C in a refrigerator. The remaining chemicals or solvents used in this study were at least of analytical grade or relevant biochemical grade except for special instructions. Exposure solutions were prepared by diluting the stock solution in zebrafish culture medium to achieve working solutions with a final DMSO concentration of 0.1% (v/v) in all DMSO control and treatment groups.
Zebrafish maintenance
Adult zebrafish (AB strain) were maintained following the standard procedures (27.0 ± 1 °C, 12:12-h light:dark cycle) and fed twice daily with live brine shrimp as previously described (Tu et al. 2013). Zebrafish embryos were obtained by natural breeding of spawning groups at a sex ratio approximately of 1:1 in a glass tank, with spawning occurring after the light was turned on the next morning. After spawn, zebrafish embryos were collected and cleaned three times with embryo medium. The fertilized and normal embryos were inspected and staged for the exposure experiment after being checked under an inverted microscope (Leica, Germany) (Westerfield 1995).
Embryo exposure and microscope observations
According to the procedure specified by OECD TG 236 (OECD 2013), the normal fertilized embryos, 6 h post-fertilization (hpf), were randomly distributed in 24-well microplates and exposed to various concentrations of IPZ (0, 0.025, 0.05, 0.1, 0.15, 0.2, 0.3, 0.4, and 0.5 mg/L) with 20 embryos per dose group at 27.0 ± 0.5 °C. The exposure solution in each well was renewed daily. Embryos were cultivated for 4 days post-fertilization (dpf) and observed under an inverted microscope to examine developmental information. Death of zebrafish was defined according to four apical observations: coagulation of fertilized eggs, lack of somite formation, lack of detachment of the tail bud from the yolk sac, and lack of heartbeat (Braunbeck et al. 2005, 2015; Busquet et al. 2014). All fish were treated humanely and with regard for alleviation of suffering. Heartbeat measurement was recorded for 30 s on 2 dpf with the inverted microscope, as heart rate is an important toxicological endpoint in embryonic test (De Luca et al. 2014). The generation of reactive oxygen species (ROS) was determined through a membrane-permeable fluorescent dye 2′,7′-dichlorodihydrofluorescin diacetate (H2DCFDA) (Aladdin, China) at 60 hpf (Hermann et al. 2004; Olivari et al. 2008). Briefly, larvae were washed twice in culture medium and maintained with H2DCFDA solution (0.5 mg/L) for 30 min in the dark, then washed with culture medium three times, anesthetized with 0.03% tricaine (Aladdin, China), and observed under a fluorescence microscope (Nikon, Japan). Ten live larvae per group were used in the ROS staining and analyzed by an image processing program ImageJ (National Institutes of Health, USA).
Biochemical analysis
To investigate whether the activity of common biomarkers relative to oxidative stress and tricarboxylic cycle was changed in zebrafish, 4 dpf live larvae of DMSO control and IPZ treat groups (0.05, 0.1, 0.2, and 0.3 mg/L) were prepared for measured glutathione S-transferase (GST) activity, glutathione (GSH) concentration, superoxide dismutase (SOD) activity, malondialdehyde (MDA) level, and SDH activity. These biomarkers were determined and calculated using the relevant assay kits from Nanjing Jiancheng Bioengineering Institute (Jiancheng, China) according to the manufacturer’s directions. Moreover, soluble protein content was determined according to the method of Bradford (1976) with bovine serum albumin (Aladdin, China) as a standard. All biomarker concentrations were normalized to its protein content respectively. This assay was run in triplicate.
Gene expression analysis
A total of 30 larvae were anesthetized and collected per concentration on 4 dpf, and washed three times with physiological saline. To isolate total RNA from tissue of zebrafish, these larvae were homogenized in TRIzol Reagent (Invitrogen, USA) according to the manufacturer’s protocols. After RNA extraction, quality of total RNA was evaluated by the ratio of absorption at 260 and 280 nm using a ND5000 spectrophotometer (BioTeke, China). Reverse transcription reactions to synthesize cDNA were performed with ReverTra Ace qPCR RT Kit (Toyobo, Japan), according to the manufacturer’s instructions. Quantitative real-time PCR was carried out on Mastercycler® ep realplex real-time PCR (Eppendorf, Germany), with the THUNDERBIRD SYBR® qPCR Mix kit (Toyobo, Japan). The amplification protocol consisted of the following phases: initial denaturation for 1 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The primer sequences (Table S1) for β-actin, catalase (cat), glutathione peroxidase 1a (gpx1a), superoxide dismutase 1 (sod1), superoxide dismutase 2 (sod2), and succinate dehydrogenase complex, subunit B, iron sulfur (Ip) (sdhb) were based on previous reports (Cambier et al. 2009; Jin et al. 2010). The relative expression levels were calculated with the “2−ΔΔCt method” according to a previous report (Livak and Schmittgen 2001), and β-actin was used to standardize the results as an internal control.
Statistical analysis
The data were analyzed by IBM SPSS Statistics 25 (IBM, USA). Mortality data was used to statistically estimate the LC50 and calculated by probability regression analysis. All values were illustrated as the means with standard deviation (SD) except for special instructions. For biomarker concentration measurements, one unit (U) of GST activity was expressed as the quantity of the enzyme that reduced the glutathione concentration of the reaction system by 1 μM per minute. One U of SOD activity was expressed as the quantity of the enzyme that inhibited half of the photoreduction of luminol of the reaction system per minute. One U of SDH activity was expressed as the quantity of the enzyme that reduces the absorbance of the reaction system by 0.01 per minute. The statistically significant differences between DMSO control and treatment groups were evaluated by one-way analysis of variance, followed by Dunnett’s post hoc test. Differences were considered significant if p < 0.05 between control and treatment.
Results
Developmental toxicity
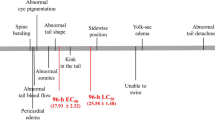
A large suite of developmental abnormalities and mortality induced by IPZ were observed for 4 dpf exposure experiment. No significant difference was found between the results of the culture medium control group and the DMSO group (data not shown). Here, the solvent group was used for the control group. A concentration-response effect on mortality was shown in zebrafish embryos exposed to IPZ within 4 days, with mortality rate increased with exposure concentration (Fig. 2). As the data show, average percent mortality significantly increased to higher than 50% after exposure to 0.2 mg/L or higher concentrations. Besides, 0.025- and 0.05-mg/L IPZ exposure did not show a significant difference in mortality with control. The statistical result showed that the 96-h LC50 value of IPZ on zebrafish embryos was 0.234 (0.217–0.253) mg/L within 95% confidence intervals. In general, the visible developmental effect of IPZ on zebrafish embryo was concentration and time dependently.
At 72 hpf, significant difference was found of hatching rate between control and IPZ exposure groups (Fig. 3). Results shown that no significant difference between 0.1 mg/L and lower concentration groups with control at 72 hpf, with almost embryos were hatched. Meanwhile, a significant decrease was found in 0.15 mg/L and higher concentration groups, within a lower 80% hatching rate. No hatched embryo could be found in the 0.4-mg/L group.
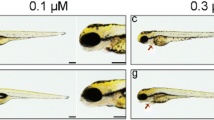
Obvious embryo and larvae malformations were observed from IPZ exposed groups from 0.05 mg/L and higher concentration groups, including edema, microcephaly, body deformation (spinal deformity and tail tip curved), incomplete swim bladder inflation, and hypopigmentation (Figs. 4 and 5). Although no evident malformation in the control and 0.025-mg/L groups, 55% showed deformity in the 0.05-mg/L group, and all fish showed morphological abnormality in 0.1-, 0.2-, and 0.3-mg/L groups. The heart rate was significantly influenced after IPZ exposure at 48 hpf (Fig. 6 and Video S1). Embryo heart rates increased in the 0.025- and 0.05-mg/L groups, and decreased in the 0.1, 0.2, and 0.3-mg/L groups, were on average 74, 84, 91, 64, 51, and 46 times/30 s of ten embryos from the control, 0.025-, 0.05-, 0.1-, 0.2- and 0.3-mg/L groups, respectively.
(AVI 40887 kb)
(AVI 43487 kb)
(AVI 38370 kb)
(AVI 45548 kb)
(AVI 38772 kb)
(AVI 44921 kb)
ROS in vivo analysis
As shown in Fig. 7, green fluorescence emitted by 2′,7′-dichlorofluorescin (DCF) in larvae was shown in all test groups. The results demonstrated that ROS production detected by H2DCFDA staining was apparent higher in IPZ exposure groups, especially around the heart, intestinal, and brain area at 60 hpf. The DCF fluorescence in the 0.025-, 0.05-, 0.1-, 0.15-, and 0.2-mg/L groups were about 1.7-, 2.1-, 2.2-, 2.8-, and 2.9-fold of the control, respectively.
Biotransformation and antioxidant enzymes
As shown in Fig. 8, biomarkers which related to oxidative stress and intracellular energy conversion system, GST, SDH, SOD, GSH, and MDA were changed in live zebrafish larvae of IPZ exposure relative to the control group on 4 dpf. The MDA concentration in larvae after exposure to IPZ increased with fungicide concentration and was significantly higher (p < 0.05) in all IPZ exposure groups. Especially in 0.1- and 0.2-mg/L exposure groups, the MDA concentrations were about 2.3- and 3.0-fold over the control, respectively. SOD activity in exposure groups was significantly higher than that in the control group, except the 0.2-mg/L group, and displayed a clear increase with fungicide concentration in lower concentrations. Activity of GST in 0.025-, 0.05-, and 0.1-mg/L exposure groups showed a significant increase, about 1.3, 1.6, and 1.3 times that in the control group, respectively, except that in the 0.2-mg/L group. The GSH concentration in larvae showed a significant increase of 0.05- and 0.1-mg/L IPZ groups, at about 1.4- and 1.3-fold over the control. However, no significant difference in the concentration of GSH in live larvae was found between 0.025 and 0.2 mg/L compared with the control on 4 dpf. The SDH activity showed significant decrease in exposure groups: 27.80, 28.24, 18.68, and 17.46 U/mg protein of 0.025 mg/L, 0.05 mg/L, 0.1 mg/L and 0.2 mg/L IPZ, respectively, while in the control group was 32.86 U/mg protein.
Gene expression
As shown in Fig. 9, the transcription level of the oxidative stress-associated gene cat, gpx1a, sod1, and sod2 was upregulated, and the SDH expression-associated gene sdhb decreased after IPZ exposure compared to the control group on 4 dpf. The expression levels were about 1.55- and 1.16-fold of cat, 1.08- and 1.11-fold of gpx1a, 1.07- and 1.12-fold of sod1, 1.08- and 1.13-fold of sod2, and 0.93- and 0.92-fold of sdhb in the 0.05- and 0.1-mg/L IPZ exposure groups, respectively, compared to the control group.
Discussion
In the present study, we provide evidence that zebrafish embryos exposed to IPZ triggered a series of developmental effects, even lethality. Although there is no significant difference of hatch and survival between the 0.05 mg/L and control group, 55% of fish in the 0.05 mg/L group showed malformation, which demonstrated that malformation in the embryo provides a more sensitive endpoint than hatching rate and lethality.
The results revealed that IPZ caused edema, arrhythmia, and blood flow reduction in developing zebrafish, which indicated that this substance possibly influences cardiac development of the zebrafish embryo. The enlargement of the heart became evident over time, resulting in an enormously big heart which even came out with a string-like structure and fibrillate. We also found that the heart contracts in a chaotic and irregular fashion in the higher exposure groups. Pericardial edema was a sensitive endpoint of zebrafish embryo following exposure to IPZ. These symptoms may be related to changes in expression of genes like weak beat (web), stretched (str), santa (san), legong (leg), and slip jig (sli), as reported (Chen et al. 1996).
In addition, we observed that IPZ exposure induced a noticeable change of oxidative stress-related biomarkers. An obviously concentration-dependent increase in MDA, indicating that lipid peroxidation had occurred, and oxidative stress intensity was increasing with IPZ concentration. An interesting result was that, the activity of SOD was clearly increased after IPZ exposure, but showed a noticeable decrease on 0.2 mg/L group to the 0.1 mg/L. SOD is produced as a by-product of oxygen metabolism, which plays a crucial role in catalyzing ROS into ordinary molecular oxygen and hydrogen peroxide (McCord and Fridovich 1969; Stowe and Camara 2009). Meanwhile, a significantly decrease of GST and GSH activity on 0.2 mg/L group to the 0.1 mg/L were observed. GST can catalyze GSH to xenobiotic substrates, and GSH is an important antioxidant which can prevent damage to important cellular components caused by reactive oxygen species (Sies 1999; Townsend and Tew 2003; Massarsky et al. 2017). Above phenomenon indicated that IPZ exposure possibly induced an antioxidant response to toxicant stress, and 0.2-mg/L exposure induced overwhelmed antioxidant capacity in zebrafish larvae, leading to a downregulation of the detoxifying system (Sies 1986; Blahová et al. 2013). Zhu et al. (2014) reported that fluconazole, myclobutanil, and triflumizole caused the SOD activity of rare minnow embryos increased after exposure to 1.0 mg/L, while was decreased when the exposure dose reached 2.0 mg/L. Mu et al. (2016) found that difenoconazole caused expression level of Zn/Cu-SOD increased in the 0.5 mg/L group, while downregulated for the 2.0 mg/L group at 96 hpf. Similarly, Wiegand et al. (2001) reported that 5 mg/L atrazine induced GST activity increased in zebrafish, while decreased in higher concentrations. In addition, testing the expression of the encoding genes is considered to be an effective method of determining oxidative stress, and here the upregulated transcription level of gene which relates to CAT, GPx, GST, and SOD also indicated that IPZ could trigger oxidative stress response of zebrafish embryos. It was the same as the result of in vivo ROS images. It should be mentioned that the 0.2 mg/L is close to the 96-h LC50 value of IPZ on zebrafish embryos (0.234 mg/L). Oxidative stress is believed to occur when there is an imbalance in the biological oxidant-to-antioxidant ratio, which is a complex phenomenon involved in physiological and pathological processes (Mittler 2002; Jones 2006; Sies 2015). Recently, a number of reports focused on the link between oxidative stress enzyme activities and the related gene expression, proved that pesticides can induce oxidative stress in zebrafish and other aquatic test models (Lushchak 2011; Han et al. 2016; Moura et al. 2017).
Moreover, we found that both SDH enzyme activity and relative RNA transcription level in zebrafish larvae decreased with increasing IPZ exposure, which provide evidence for the first time, to our knowledge, that intracellular energy conversion system of zebrafish was affected by IPZ. Similarly, a recent study found that the thifluzamide, one of SDHIs, induced abnormal spontaneous movement, slow heartbeat, hatching inhibition, growth regression, morphological deformities, and oxidative stress on zebrafish embryos (Yang et al. 2016a). The negative changes in SDH activity and mitochondrial structural damage were suggested and might be responsible for oxidative damage, cell apoptosis, and inflammation on zebrafish lead by thifluzamide, which would facilitate the action of these factors in cell death and might play a crucial role during toxic events (Yang et al. 2016a, b).
Increased oxidative stress can induce damage to the cellular structure and potentially destroy tissues. However, ROS are needed for adequate cell function, including the production of energy by the mitochondria (Uttara et al. 2009). On the one hand, compounds which induce apoptosis are either oxidants or stimulators of cellular oxidative metabolism; on the other hand, many inhibitors of apoptosis have antioxidant activities or enhance cellular antioxidant defenses (Buttke and Sandstrom 1994). SDH embedded in the internal membrane of mitochondrion is involved in the key route of glucose metabolism, which is the only enzyme that participates in both the citric acid cycle and the electron transport chain, and is essential in energy support system of many organisms (Hederstedt and Rutberg 1981; Oyedotun and Lemire 2004). Reports showed that change of SDH in organisms could impair several normal functions even result in disease, including ROS formation (Hatefi 1985; Niemann and Müller 2000; Yankovskaya et al. 2003; Xiao et al. 2016). Thus, it can be speculated that zebrafish development could be affected by IPZ, including lethal effect. Besides, SDH activity decrease, oxidative stress, and abnormality were accompanied with this effect. However, further studies should be performed to help understand the mechanisms of IPZ effects on zebrafish development.
Conclusion
There is limited knowledge about the effects of IPZ exposure, and this study finds the adverse effects on early-life zebrafish. These results indicate that zebrafish embryos are sensitive to low levels of fungicide in aquatic environment, and IPZ is very toxic to aquatic life which should be labeled as acute toxicity category 1 with a 96-h LC50 lower than 1 mg/L (EPA 2004). Exposure to IPZ could alter the morphological and physiological abnormalities of zebrafish, which may affect its populations. The present study provides information on the heart rate disruption and oxidative stress of zebrafish triggered by IPZ. In addition, it provides evidence of IPZ induced decrease of SDH activity in zebrafish larvae. In conclusion, these study results will help our understanding of IPZ effects on early-life zebrafish. Furthermore, further studies are recommended for understanding how IPZ effects on zebrafish and other non-target organisms.
References
Abad-Fuentes A, Ceballos-Alcantarilla E, Mercader JV, Agulló C, Abad-Somovilla A, Esteve-Turrillas FA (2015) Determination of succinate-dehydrogenase-inhibitor fungicide residues in fruits and vegetables by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 407(14):4207–4211
Andrade TS, Henriques JF, Almeida AR, Machado AL, Koba O, Giang PT, Soares AMVM, Domingues I (2016) Carbendazim exposure induces developmental, biochemical and behavioural disturbance in zebrafish embryos. Aquat Toxicol 170:390–399
APVMA (2018) Australian pesticides and veterinary medicines authority. Proposed registration of Seguris Flexi Fungicide containing the new active constituent isopyrazam. https://apvma.gov.au/node/28996. Accessed 21 April 2018
Avenot HF, Michailides TJ (2010) Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot 29(7):643–651
Avetta P, Marchetti G, Minella M, Pazzi M, De Laurentiis E, Maurino V, Minero C, Vione D (2014) Phototransformation pathways of the fungicide dimethomorph ((E, Z) 4-[3-(4-chlorophenyl)-3-(3, 4-dimethoxyphenyl)-1-oxo-2-propenyl] morpholine), relevant to sunlit surface waters. Sci Total Environ 500:351–360
Blahová J, Plhalová L, Hostovský M, Divišová L, Dobšíková R, Mikulíková I, Stěpánová S, Svobodová Z (2013) Oxidative stress responses in zebrafish danio rerio after subchronic exposure to atrazine. Food Chem Toxicol 61(6):82–85
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Braunbeck T, Böttcher M, Hollert H, Kosmehl T, Lammer E, Leist E, Rudolf M, Seitz N (2005) Towards an alternative for the acute fish LC50 test in chemical assessment: the fish embryo toxicity test goes multi-species—an update. ALTEX 22(2):87–102
Braunbeck T, Kais B, Lammer E, Otte J, Schneider K, Stengel D, Strecker R (2015) The fish embryo test (FET): origin, applications, and future. Environ Sci Pollut Res 22(21):16247–16261
Bunzel K, Schäfer RB, Thrän D, Kattwinkel M (2015) Pesticide runoff from energy crops: a threat to aquatic invertebrates? Sci Total Environ 537:187–196
Busquet F, Strecker R, Rawlings JM, Belanger SE, Braunbeck T, Carr GJ, Cenijn P, Fochtman P, Gourmelon A, Hübler N, Kleensang A, Knöbel M, Kussatz C, Legler J, Lillicrap A, Martínez-Jerónimo F, Polleichtner C, Rzodeczko H, Salinas E, Schneider KE, Scholz S, van den Brandhof EJ, van der Ven LT, Walter-Rohde S, Weigt S, Witters H, Halder M (2014) OECD validation study to assess intra-and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul Toxicol Pharmacol 69(3):496–511
Buttke TM, Sandstrom PA (1994) Oxidative stress as a mediator of apoptosis. Immunol Today 15(1):7–10
Cambier S, Benard G, Mesmer-Dudons N, Gonzalez P, Rossignol R, Brethes D, Bourdineaud JP (2009) At environmental doses, dietary methylmercury inhibits mitochondrial energy metabolism in skeletal muscles of the zebra fish (Danio rerio). Int J Biochem Cell Biol 41(4):791–799
Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, Van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nüsslein-Volhard C (1996) Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development 123(1):293–302
De Luca E, Zaccaria GM, Hadhoud M, Rizzo G, Ponzini R, Morbiducci U, Santoro MM (2014) ZebraBeat: a flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci Rep 4:649–652
Di Giulio RT, Washburn PC, Wenning RJ, Winston GW, Jewell CS (1989) Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 8(12):1103–1123
Driessen M, Vitins AP, Pennings JL, Kienhuis AS, van de Water B, van der Ven LT (2015) A transcriptomics-based hepatotoxicity comparison between the zebrafish embryo and established human and rodent in vitro and in vivo models using cyclosporine A, amiodarone and acetaminophen. Toxicol Lett 232(2):403–412
EPA (2004) United States Environmental Protection Agency. Chemical hazard classification and labeling: comparison of OPP requirements and the GHS. https://www.epa.gov/sites/production/files/2015-09/documents/ghscriteria-summary.pdf. Accessed 11 Dec 2017
EPA (2017) United States Environmental Protection Agency. Isopyrazam Fact Sheet. https://www3.epa.gov/pesticides/chem_search/reg_actions/pending/fs_PC-129222_05-Oct-11.pdf. Accessed 20 April 2018
Fernández D, Voss K, Bundschuh M, Zubrod JP, Schäfer RB (2015) Effects of fungicides on decomposer communities and litter decomposition in vineyard streams. Sci Total Environ 533:40–48
Fong HCH, Ho JCH, Cheung AHY, Lai KP, Tse WKF (2016) Developmental toxicity of the common UV filter, benophenone-2, in zebrafish embryos. Chemosphere 164:413–420
FRAC (2017) FRAC Code List 2017: Fungicides sorted by mode of action (including FRAC Code numbering). http://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2017-final.pdf. Accessed 11 December 2017
Glickman NS, Yelon D (2002) Cardiac development in zebrafish: coordination of form and function. Semin Cell Dev Biol 13(6):507–513
Gulkowska A, Buerge IJ, Poiger T (2014) Online solid phase extraction LC–MS/MS method for the analysis of succinate dehydrogenase inhibitor fungicides and its applicability to surface water samples. Anal Bioanal Chem 406(25):6419–6427
Han Y, Liu T, Wang J, Wang J, Zhang C, Zhu L (2016) Genotoxicity and oxidative stress induced by the fungicide azoxystrobin in zebrafish (Danio rerio) livers. Pestic Biochem Physiol 133:13–19
Hand LH, Moreland HJ (2014) Surface water mineralization of isopyrazam according to OECD 309: observations on implementation of the new data requirement within agrochemical regulation. Environ Toxicol Chem 33(3):516–524
Hand LH, Oliver RG (2010) The behavior of isopyrazam in aquatic ecosystems: implementation of a tiered investigation. Environ Toxicol Chem 29(12):2702–2712
Hatefi Y (1985) The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem 54(1):1015–1069
He L, Cui K, Ma D, Shen R, Huang X, Jiang J, Mu W, Liu F (2017) Activity, translocation and persistence of isopyrazam for controlling cucumber powdery mildew. Plant Dis 101(7):1139–1144
Hederstedt L, Rutberg L (1981) Succinate dehydrogenase—a comparative review. Microbiol Rev 45(4):542–555
Hermann AC, Millard PJ, Blake SL, Kim CH (2004) Development of a respiratory burst assay using zebrafish kidneys and embryos. J Immunol Methods 292(1):119–129
Hermsen SA, van den Brandhof EJ, van der Ven LT, Piersma AH (2011) Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol in Vitro 25(3):745–753
Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86(1):6–19
Jin Y, Zhang X, Shu L, Chen L, Sun L, Qian H, Liu W, Fu Z (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78(7):846–852
Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8(9–10):1865–1879
Keon JP, White GA, Hargreaves JA (1991) Isolation, characterization and sequence of a gene conferring resistance to the systemic fungicide carboxin from the maize smut pathogen, Ustilago maydis. Curr Genet 19(6):475–481
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101(1):13–30
Massarsky A, Kozal JS, Di Giulio RT (2017) Glutathione and zebrafish: old assays to address a current issue. Chemosphere 168:707–715
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8(6):2265–2303
Moreno-González R, Campillo JA, García V, León VM (2013) Seasonal input of regulated and emerging organic pollutants through surface watercourses to a Mediterranean coastal lagoon. Chemosphere 92(3):247–257
Moura MA, Oliveira R, Jonsson CM, Domingues I, Soares AM, Nogueira AJ (2017) The sugarcane herbicide ametryn induces oxidative stress and developmental abnormalities in zebrafish embryos. Environ Sci Pollut Res:1–10
Mu X, Chai T, Wang K, Zhu L, Huang Y, Shen G, Li Y, Li X, Wang C (2016) The developmental effect of difenoconazole on zebrafish embryos: a mechanism research. Environ Pollut 212:18–26
Niemann S, Müller U (2000) Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet 26(3):268–270
Noyes PD, Garcia GR, Tanguay RL (2016) Zebrafish as an in vivo model for sustainable chemical design. Green Chem 18(24):6410–6430
OECD (2013) Fish embryos acute toxicity (FET) test, Test Guideline No. 236, OECD guidelines for testing of chemicals. Organization for Economic Co-operation and Development, Paris
Olivari FA, Hernández PP, Allende ML (2008) Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res 1244:1–12
Oyedotun KS, Lemire BD (2004) The quaternary structure of the saccharomyces cerevisiae succinate dehydrogenase homology modeling, cofactor docking, and molecular dynamics simulation studies. J Biol Chem 279(10):9424–9431
Plummer SM, Wright J, Currie RA (2018) Dose-dependent effects on rat liver miRNAs 200a/b and 429: potential early biomarkers of liver carcinogenesis. Toxicol Rep 5:309–313
Rasmussen JJ, McKnight US, Loinaz MC, Thomsen NI, Olsson ME, Bjerg PL, Binning PJ, Kronvang B (2013) A catchment scale evaluation of multiple stressor effects in headwater streams. Sci Total Environ 442:420–431
Reilly TJ, Smalling KL, Orlando JL, Kuivila KM (2012) Occurrence of boscalid and other selected fungicides in surface water and groundwater in three targeted use areas in the United States. Chemosphere 89(3):228–234
Rouquie D, Tinwell H, Blanck O, Schorsch F, Geter D, Wason S, Bars R (2014) Thyroid tumor formation in the male mouse induced by fluopyram is mediated by activation of hepatic CAR/PXR nuclear receptors. Regul Toxicol Pharmacol 70(3):673–680
Sarmah S, Marrs JA (2016) Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. Int J Mol Sci 17:2123
Scalliet G, Bowler J, Luksch T, Kirchhofer-Allan L, Steinhauer D, Ward K, Niklaus M, Verras A, Csukai M, Daina A, Fonné-Pfister R (2012) Mutagenesis and functional studies with succinate dehydrogenase inhibitors in the wheat pathogen Mycosphaerella graminicola. PLoS One 7(4):e35429
Selderslaghs IWT, Blust R, Witters HE (2012) Feasibility study of the zebrafish assay as an alternative method to screen for developmental toxicity and embryotoxicity using a training set of 27 compounds. Reprod Toxicol 33(2):142–154
Sies H (1986) Biochemistry of oxidative stress. Angew Chem Int Ed 25(12):1058–1071
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27(9):916–921
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183
Smalling KL, Kuivila KM, Orlando JL, Phillips BM, Anderson BS, Siegler K, Huntb JW, Hamilton M (2013) Environmental fate of fungicides and other current-use pesticides in a central California estuary. Mar Pollut Bull 73(1):144–153
Song Y, Zhang Z, Chen L, He L, Lu H, Ren Y, Mu W, Feng L (2016) Baseline sensitivity of botrytis cinerea to the succinate dehydrogenase inhibitor isopyrazam and efficacy of this fungicide. Plant Dis 100(7):1314–1320
Stengel D, Wahby S, Braunbeck T (2018) In search of a comprehensible set of endpoints for the routine monitoring of neurotoxicity in vertebrates: sensory perception and nerve transmission in zebrafish (Danio rerio) embryos. Environ Sci Pollut Res:1–19
Stowe DF, Camara AKS (2009) Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal 11(6):1373–1414
Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T (2012) Zebrafish embryos as an alternative to animal experiments—a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol 33(2):128–132
Tanabe A, Mitobe H, Kawata K, Yasuhara A, Shibamoto T (2001) Seasonal and spatial studies on pesticide residues in surface waters of the Shinano River in Japan. J Agric Food Chem 49(8):3847–3852
Tinwell H, Rouquié D, Schorsch F, Geter D, Wason S, Bars R (2014) Liver tumor formation in female rat induced by fluopyram is mediated by CAR/PXR nuclear receptor activation. Regul Toxicol Pharmacol 70(3):648–658
Townsend DM, Tew KD (2003) The role of glutathione-s-transferase in anti-cancer drug resistance. Oncogene 22(47):7369–7375
Tsuda T, Nakamura T, Inoue A, Tanaka K (2009) Pesticides in water and sediment from littoral area of Lake Biwa. Bull Environ Contam Toxicol 82(6):683–689
Tu W, Niu L, Liu W, Xu C (2013) Embryonic exposure to butachlor in zebrafish (Danio rerio): endocrine disruption, developmental toxicity and immunotoxicity. Ecotoxicol Environ Saf 89:189–195
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74
Wang Y, Yang G, Dai D, Xu Z, Cai L, Wang Q, Yu Y (2017) Individual and mixture effects of five agricultural pesticides on zebrafish (Danio rerio) larvae. Environ Sci Pollut Res 24(5):4528–4536
Westerfield M (1995) The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). University of Oregon Press, Eugene
Wiegand C, Krause E, Steinberg C, Pflugmacher S (2001) Toxicokinetics of atrazine in embryos of the zebrafish (Danio rerio). Ecotoxicol Environ Saf 49(3):199–205
Wu S, Lei L, Liu M, Song Y, Lu S, Li D, Shi H, Raley-Susman KM, He D (2018) Single and mixture toxicity of strobilurin and sdhi fungicides to xenopus tropicalis embryos. Ecotoxicol Environ Saf 153:8–15
Xiao W, Sarsour EH, Wagner BA, Doskey CM, Buettner GR, Domann FE, Goswami PC (2016) Succinate dehydrogenase activity regulates PCB3-quinone-induced metabolic oxidative stress and toxicity in HaCaT human keratinocytes. Arch Toxicol 90(2):319–332
Yang Y, Liu W, Mu X, Qi S, Fu B, Wang C (2016a) Biological response of zebrafish embryos after short-term exposure to thifluzamide. Sci Rep 6:38485
Yang Y, Qi S, Wang D, Wang K, Zhu L, Chai T, Wang C (2016b) Toxic effects of thifluzamide on zebrafish (Danio rerio). J Hazard Mater 307:127–136
Yankovskaya V, Horsefield R, Törnroth S, Luna-Chavez C, Miyoshi H, Léger C, Byrne B, Cecchini G, Iwata S (2003) Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299(5607):700–704
Yoshida M, Inoue K, Takahashi M (2015) Predictive modes of action of pesticides in uterine adenocarcinoma development in rats. J Toxicol Pathol 28(4):207–216
Zhu B, Liu L, Gong YX, Ling F, Wang GX (2014) Triazole-induced toxicity in developing rare minnow (Gobiocypris rarus) embryos. Environ Sci Pollut R 21(23):13625–13635
Acknowledgements
The authors sincerely thank Jason Martin (United States Department of Agriculture) for paper improvement.
Funding
This study received grants from the Project of Science and Technology by Zhejiang Food and Drug Administration (grant number SP201717), the Project by Science Technology Department of Zhejiang Province (grant number LGF18B070003), and the National Natural Science Foundation of China (grant number 21277126).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Table S1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Yao, H., Xu, X., Zhou, Y. et al. Impacts of isopyrazam exposure on the development of early-life zebrafish (Danio rerio). Environ Sci Pollut Res 25, 23799–23808 (2018). https://doi.org/10.1007/s11356-018-2449-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2449-5