Abstract
Glycine max (L.) Merr. (soybean) crop plants have been found to have high lead (Pb) levels in aerial organs; however, knowledge about the processes involved in the incorporation, and subsequent translocation and accumulation of the metal in the plants is scarce. Considering the toxicity of this heavy metal, the aim of the present study was to evaluate Pb uptake and translocation, and their toxic effects on soybean seedlings via experiments of ionic competition with Ca2+ (2.5 mM, Ca:Pb 1:1) and alteration of the transpiration flow [0.25 mM Pb(NO3)2]. The following variables were analyzed: biomass, leaf area (morphological parameters), photosynthetic efficiency, biochemical response (considered physiological stress markers: antioxidant power, chlorophylls, carotenoids, starch, proteins, sugars, and malondialdehyde), and Pb content. Results showed that soybean seedlings can accumulate high Pb concentration in its organs; however, in general, no morpho-physiological Pb stress symptoms were observed, except for lipid peroxidation and antioxidant power. The treatment with Ca ions was not effective in reducing Pb entry into root over time when both Ca and Pb where present in the grow solution. Alteration of the transpiration rate in soybean showed that the air flow increased the consumption of solutions, regardless of the treatments. However, Pb accumulation was lower in seedlings exposed to air flow, indicating a selective exclusion of the metal in the solution. In both experiments, soybean seedlings showed to be tolerant to high Pb concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, the concentrations of pollutants in different environmental compartments, such as air, water, and soil, have rapidly increased due to industrial activities. This is the case of lead (Pb), which has been used for more than 5000 years and is currently widely employed in numerous industrial processes due to its low corrosion, high density, ductility, and malleability (Liu et al. 2018; Pourrut et al. 2013; Zhang et al. 2015). Therefore, Pb can be found as a pollutant in all environmental compartments, affecting living organisms. The main problems related to this heavy metal lie in its persistence, accumulation, and toxicity in the environment and trophic levels, since it has no biological function in living organisms (Carocci et al. 2016; Maestri et al. 2010). Thus, exposure to Pb can produce toxic effects, even at low concentrations, due to the absence of adequate defense mechanisms to mitigate their toxic effects (Nakata et al. 2017; Pourrut et al. 2013).
Contamination of agricultural soils with Pb has been widely reported, with indications of a potential toxicological risk through the consumption of crops grown in these soils. Thus, food consumption has been identified as the main source of exposure to metals in humans (El-Kady and Abdel-Wahhab 2018), and its accumulation in agricultural crops is of great interest in terms of both environment and food security. In this sense, high levels of Pb have been reported in different crops, such as Glycine max (L.) Merr. (soybean, fabaceae family), which has been mentioned as a crop plant that accumulates Pb and other toxic elements in its aerial organs, particularly in seeds (Blanco et al. 2017; Rodriguez et al. 2011; Rodriguez et al. 2014; Salazar et al. 2012; Vergara Cid et al. 2020; Zhao et al. 2014). Knowledge about the processes that involve the uptake of Pb into plants is scarce and under constant revision. Some studies indicate that Pb can passively enter the roots and then move through the apoplastic pathway for water transport. This process, however, is limited by the barrier generated by the endodermis (Pourrut et al. 2011). This passive mechanism would be the main Pb uptake route from radical hairs, to be accumulated later in the cell wall (Kabata-Pendias 2011). However, the molecular mechanism by which Pb enters the roots is still unknown. Pb might enter the roots through several pathways, with the ionic channels having been proposed as the most important (Kohli et al. 2020). Several investigations indicate the Ca2+ ionic channels as the main pathway of Pb uptake in roots (Pourrut et al. 2011). The competition with Ca2+ and Mg2+ was found to significantly reduce Pb concentration in rice roots (Oryza sativa L.), especially at the root tip (Kim et al. 2002). Wang et al. (2007) demonstrated that the use of Ca2+ channel blockers (such as lanthanum) significantly reduces the entry of Pb into corn roots (Zea mays L.). In addition, the voltage-dependent Ca2+ channels were found to be the main Pb uptake pathway through the cell membrane (Sharma and Dubey 2005). Considering the toxicity of Pb and the scarce knowledge of the uptake processes in soybean crops, further studies are necessary to help elucidate these topics.
On the other hand, once heavy metals enter the plant, one part can be stored and fixed in different sites of the root, whereas another part of the metals can cross the root and be transported to higher organs, in a process called translocation. Thus, translocation of toxic metals from roots to higher organs (leaves, seeds, fruits) is one of the processes of the greatest concern in edible plants, due to the toxicological risk associated with food security, either by direct or indirect consumption (Salazar et al. 2012). In this sense, the Casparian strip in the roots has been frequently proposed as the main barrier to the transport of metals to the upper vessels of plants (Sharma and Dubey 2005). The transport of toxic metals would be driven by negative pressures of transpiration flow (Liao et al. 2006; Sheoran et al. 2016). Thus, with the entry of water, Pb could also enter the roots and then be translocated by mass flow to the aerial organs. In addition, the rate of transpiration is a climatic-dependent parameter that could affect the absorption (Sheoran et al. 2016). Rucińska-Sobkowiak (2016) indicates that the transport of water from roots to the aerial organs occurs through the xylem in more than 99.5% of plants, being driven mainly by the negative pressures originated in the transpiration process. Thus, these authors suggest that the inclusion of an external air flow to a plant could increase the transpiration rate, which could be accompanied by an increase of the uptake of metals (including contaminants), as proposed in the present study. Therefore, to elucidate the efficiency of a plant to transfer the metal, the translocation factor is calculated, i.e., the relationship between accumulation in upper organs (stem, leaves, seeds, etc.) and lower organs (roots).
The aim of the present study was to evaluate Pb uptake and translocation in soybean seedlings via experiments of ionic competition with Ca and alteration of the transpiration flow by assessing physiological and biochemical variables.
Materials and methods
Plant material and growth conditions
Seeds of G. max Alim 3.14 GM III, provided by the Instituto Nacional de Tecnología Agropecuaria (INTA - Argentina) seed bank, were surface sterilized with 1% NaClO for 10 min and then washed with ultrapure water. Then, the seeds were germinated for 4 days in vermiculite under controlled conditions of temperature (28 °C) and daily watering with ultrapure water. After this period, seedlings were grown in a culture chamber under controlled hydroponic conditions of light-dark period of 16–8 h respectively (luminance > 300 PAR, MASTER TL5 HO 54 W/830 SLV/40 Philips), 70% relative humidity, and an average temperature of 28 °C; in a modified Hoagland nutrient solution containing the following nutrients: Ca(NO3)2 5 mM, KNO3 5 mM, KH2PO4 1 mM, MgSO4·7H2O 2 mM, H3BO3 47 μM, ZnSO4·7H2O 0.2 μM, CuSO4·5H2O 0.32 μM, Na2MoO4·2H2O 0.09 μM, MnCl2·4H2O 10 μM, Fe-EDTA 24 μM. At V1 development stage according to Fehr and Caviness (1977), the seedlings were transplanted to hydroponic cultivations under different treatments (see below).
Competition between calcium and lead ions (Ca2+–Pb2+)
Soybean seedlings were grown in dark 750-mL containers with the corresponding culture solution at pH 5.5, with three replicates for each of the four treatments: (1) control (NH4NO3 2.5 mM); (2) control + Ca (NH4NO3 2.5 mM + CaCl2 2.5 mM); (3) Pb (Pb(NO3)2 2.5 mM); and (4) Pb + Ca (Pb(NO3)2 2.5 mM + CaCl2 2.5 mM). A high concentration of Pb, and consequently of Ca, was selected according to previous studies in polluted sites cultivated with soybean (Blanco et al. 2017; Vergara Cid et al. 2020). In addition, three plants per pot, which was considered an experimental unit, were cultivated in order to increase the plant biomass. Finally, seedlings were harvested on days 3 and 6 of exposure.
Plant growth was monitored at the different stages of development by periodically measuring quantum efficiency of PSII photochemistry (ФPSII) under ambient light conditions (Maxwell and Johnson 2000), using pulse of amplitude modulated fluorometer (FMS2, Hansatech Instruments, Pentney King’s Lynn, United Kingdom).
ФPSII was calculated as follows:
where Fm’ is the maximum fluorescence in the light, and Ft is the steady-state fluorescence yield.
After each harvest, roots, stems, and leaves were separated, fresh weight (FW) was recorded, and plant samples were oven-dried at 50 °C until constant dry weight (DW) for biomass assessment. Moreover, leaf area (LA) was measured from photographs using the ImageJ software at the beginning and end of the exposure treatments on each harvest day to calculate the percentage of leaf growth (% LG), as follows:
where LAi and LAf are the initial and final leaf area, respectively, measured at the beginning and end of the treatment exposure.
In addition, three disks (1 cm of diameter) of the first trifoliate leaf were obtained using a round punch hole toll and stored at − 80 °C until biochemical analysis.
During this experiment, the solutions were not replenished during cultivation in order to compute the difference in consumption among treatments.
Alteration of transpiration rate
To assess if the alteration of the transpiration rate could change the Pb uptake and translocation, soybean seedlings were transplanted to dark containers, which were placed on a lid support that also prevented evaporation of the solution. Each container had 8 seedlings and the experiment was performed in triplicate. Seedlings were subjected to the following treatments with or without air flow (AF) with the culture solutions at pH 5.5: control (NH4NO3 0.25 mM), Pb (Pb(NO3)2 0.25 mM), control + AF (NH4NO3 0.25 mM + air flow), Pb + AF (Pb(NO3)2 0.25 mM + air flow) (Fig. 1). In this experiment, a lower concentration of Pb was selected with the purpose to not saturate the accumulation of Pb in the seedlings, but to stimulate a differential incorporation with the air flow, as mentioned in other studies (Liao et al. 2006). The experiments with or without air flow were carried out under controlled hydroponic conditions (light-dark period of 16–8 h respectively, 70% relative humidity, and an average temperature of 28 °C) in consecutive weeks. The air flow was generated with two fans (each with an air flow of 58.39 m3 min) located on each side of the plant growth chambers during the first two days of treatment. Then, during the 2 days later (4 days from the start of the experiment), the rest of the plants did not receive air flow so that they could potentially recover from stress, and were then harvested according to Liao et al. (2006). Four plants per container were collected in each harvest. On each collection day, samples of roots, stems, and leaves were separated, and FW and LA were measured. In this case, specific leaf area was calculated as a ratio between LA and leaves DW. Moreover, first trifoliate leaf samples were also collected for further biochemical analysis.
Pb determination in plants
Lead content was analyzed according to Arshad et al. (2008) and Rosenkranz et al. (2017). Briefly, each sample was ground in a stainless mill, and 1 g was washed at 430 °C for 8 h and then digested using H2O2 and HNO3 65% (1:4 v/v) for 24 h, with the digestion being completed in Hach® tubes by heating over a hot plate at 120 °C. Finally, the digested samples were filtered (Whatman no. 542) and the volume was adjusted to 10 mL with ultrapure water. The Pb concentrations were measured by atomic absorption spectrophotometer (AAS; Perkin-Elmer AA3110). As a quality control, blanks and samples of the standard reference material (hay IAEA-V-10, and beech leaf WEPAL -IPE 2012.1) were prepared as described above. The quality control resulted in recoveries of over 93% of the certified material value. The amount of Pb accumulated by each plant organ was expressed as a concentration value (μg Pb g-1 DW), and as absolute Pb content (μg Pb), taking into account the biomass for the latter. The absolute Pb content allows to evaluate the accumulation of the metal in relation to the total quantity of biomass of that organ. This measure is useful to evaluate the potentiality of a species as a phytoextractor and phytoaccumulator of heavy metals (Salazar et al. 2016).
Biochemical analyses
Ferric-reducing ability of plasma, chlorophylls, carotenoids, malondialdehyde, and soluble sugars
The ferric-reducing ability of plasma (FRAP), chlorophylls, carotenoids, and malondialdehyde (MDA), as well as total leaf soluble sugars, were measured from a single plant extract, following a sequential extraction technique (Miranda Pazcel et al. 2018; Ergo et al. 2018). Briefly, first trifoliate leaf samples were ground using liquid nitrogen with a mortar and pestle, and then suspended in 750 μL of ethanol (80% v/v). This homogenate was centrifuged at 14,000 RPM at 4 °C for 10 min. Subsequently, an aliquot of the supernatant was used to calculate the FRAP value (Robert et al. 2014), by diluting the sample in a reaction mix consisting of a 0.3 M sodium acetate buffer (pH 3.6), 2,4,6-tri-2-pyridyl-s-triazine 10 mM (TPTZ), and FeCl3 200 mM. Then, a calibration curve was performed with 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) as standard reference; finally, the samples were measured by spectrophotometry in microplates at 600 nm (Thermo Scientific-Multiskan Spectrum).
The pellet was re-suspended with the remaining supernatant and incubated at 80 °C for 20 min; then, it was stabilized at room temperature and centrifuged again at 14,000 RPM for 10 min to be used for measurement of the remaining parameters.
MDA was quantified according to Heath and Packer (1968), using a digestion of the supernatant with 0.5% TBA and 20% TCA at 90 °C for 20 min. Then, it was centrifuged at 14,000 RPM for 5 min, and the absorption was read at 532 and 600 nm, being MDA concentration calculated as follows:
where ε is the extinction coefficient of 155 mM−1 cm−1, l is the length of the optical path, and Abs is the absorbance at a certain wavelength.
Chlorophyll (Chl) and carotenoid determination was performed using the same supernatant as that used for the determination of MDA content; absorbance was measured at 470, 645, 654, and 663 nm, and calculated according to Lightenthaler (1987) and Palta (1990), as follows:
For the measurement of total soluble sugars, the supernatant was oven-dried for 24 h and then resuspended in water and mixed with antrone reagent. Then, it was placed at 80 °C for 30 min, and measured at 620 nm, using a glucose calibration curve (Leyva et al. 2008).
Starch and proteins
Starch content was determined as described by Guan and Janes (1991), and protein content according to Lowry et al. (1951), with some modifications; in both cases, the supernatant was the same as that used for the determination of the previous parameters (2.5.1.). The pellet was oven-dried at 60 °C for 24 h and then partitioned equally for total starch and protein determinations. Regarding total starch content, the pellet was re-suspended by shaking it in hot water (100 °C) for 30 min. Then, a digestion with the enzyme amyloglucosidase was performed overnight at 55 °C. Finally, it was centrifuged at 12,000 RPM and the sample was suspended in summer reagent (NaOH, NaK tetrahydrate, 3,5-dinitrosalicylic acid) to be immediately read at 550 nm.
For the total protein content, the pellet was re-suspended by shaking it in water containing 0.1% v/v tween 20 (Sigma-Aldrich, St. Louis, USA) and heated at 100 °C for 5 min, and then centrifuged at 14,000 RPM for 10 min at room temperature. Finally, the supernatant was mixed with a solution composed of 10 parts of 2% sodium carbonate, 0.1% sodium hydroxide, and 1% tween 20; 0.25 part of 1% sodium potassium tartrate (aqueous); and 0.25 parts of 1% Cu sulfate (aqueous). The total leaf protein value was calculated using bovine serum albumin (Sigma-Aldrich, St. Louis, USA) as the standard reference. The samples were incubated for 15 min and then the reaction was stopped with Folin-Ciocalteu reagent. Finally, it was measured by spectrophotometry in microplates at 578 nm.
Relative water content
The leaf relative water content (RWC) was calculated as follows (Kholodova et al. 2011):
where FW is fresh weight, DW is dry weight, and SW is the saturated water weight of a leaf (obtained after immersing a leaf in ultrapure water for 24 h).
Data analyses
Translocation factor
The translocation factor (TF) was calculated using the ratio of Pb concentration in aerial organs (stem and/or leaves) and roots or stems, according to the following formula (Rodriguez et al. 2011):
TF values higher than 1 indicate that the metal was easily translocated (effective translocation).
Statistical analysis
Data were analyzed using the R 3.3.3 software (R Core Team 2017), Tydiverse package (Wickham 2017). A principal component analysis (PCA) was performed using the calcium treatments as the classification criteria in order to assess the relationship among them, the accumulation of Pb, and morpho-biochemical parameters in G. max. The assumptions of PCA were met (with continuity of the variables and the number of elements observed being greater than the number of original variables). Moreover, multifactorial analysis of variance, and a Tukey post-hoc test for multiple means comparisons, was employed. Normality assumptions were corroborated, and for cases without homogeneity, the results were adjusted using the Infostat ® Software (Di Rienzo et al. 2019).
Results and discussion
Lead calcium competition assays
Lead accumulation
The results of Pb accumulation in different soybean organs showed great differences between control and Pb treatments, as expected, since control values were below the detection limit of Pb measurement by AAS (< 0.01 mg L-1). Therefore, in order to evaluate the effect of Ca2+ as a competitor ion, only Pb treatments were compared (Fig. 2). The presence of Ca significantly decreased Pb accumulation in roots after 3 days of exposure. This result agrees with a decrease in Pb concentration in rice roots after 24 h of exposure to 10 μM of Pb(NO3)2 and Ca(NO3)2 reported by Kim et al. (2002). A partial recovery of Pb accumulation was found after 6 days of treatment. Thus, the result indicates a decrease in Pb absorption by roots in the presence of Ca2+ at the beginning of treatment, but an increase of Pb over longer exposure periods, probably due to the effect of depletion of the competitor ion (Fig. 2). A significant reduction of Pb accumulation was observed in roots and shoots of Typha latifolia L. exposed to three times more Ca2+ than Pb2+, without a reversion of Pb incorporation after 10 days of exposure (Rodriguez-Hernandez et al. 2015), which was probably due to the higher concentration of Ca2+ ions in the nutrient solution. Antosiewicz (2005) showed a consistent decrease of Pb accumulation, especially in roots, in four species treated with Ca ions for 7 days.
Pb accumulation in stems and leaves did not vary significantly (Fig. 2), indicating that Pb translocation to the aerial organs remained unchanged. Thus, our findings suggest that the ability of soybean roots to translocate Pb to aerial organs could be saturated at low concentrations of the metal; Pb would be accumulated mainly in the roots, probably as a protective mechanism, as previously reported (Fahr et al. 2013; Khan et al. 2015; Tangahu et al. 2011).
In agreement with the results of Pb accumulation, the TF between roots and aerial parts of soybean seedlings indicates ineffective translocation (TF < 1) (supplementary material Fig. S1).
Biomass, %LG, and ФPSII
To evaluate if the incorporation of Ca2+ reverted the stress symptoms due to Pb exposure, different morpho-physiological parameters were analyzed. Table 1 shows the biomass values of soybean organs and the %LG. Results of the accumulated biomass (DW) between harvests (3 or 6 days) showed no differences for any treatments with Pb, with a significant increase being observed only in the control (Table 1). Regarding leaf growth (%LG), although the development was not altered by the addition of Ca ions, a decrease in the treatments with Pb on the second harvest day was observed (Table 1). By contrast, Wojas et al. (2007) demonstrated a significant effect of Ca on tolerance of tobacco plants measured as root elongation, using 750 more times Ca than Pb in the culture solution.
On the other hand, the analysis of ФPSII did not show significant differences among treatments (data not shown). In contrast, there is much evidence of the deleterious effect of Pb on photosynthesis, in particular on PSII efficiency (Zhou et al. 2018). Thus, soybean would seem to have a higher tolerance to Pb stress than other species.
Principal component analyses
The results of the biochemical parameters (FRAP, MDA, Chl, proteins, starch, soluble sugars, RWC, ΦPSII), morphological parameters (biomass, LA, %LG), and Pb accumulation (in roots and leaves) were analyzed by a PCA (Fig. 3; supplementary material Table S1). Results of the first harvest showed a strong association between control treatments, with or without Ca, with Chl, accumulation of starch, RWC, and growth leaf (Fig. 3a). Heavy metal effects in Chl content has been widely reported, since it can replace Mg2+ in the molecule (Rai et al. 2016). This effect on Chl impacts on less effective photosynthesis, being starch one of the main primary products. Pb could also generate a drop in RWC and growth, by a reduction in the plasticity of the cell wall (Kohli et al. 2020).
Biplot based on the first two components of the principal components analysis for Pb in leaves and biochemical parameters measured in G. max leaves exposed to different treatments (control and Pb with/without Ca) on two harvest days: a 3 days of exposure; b 6 days of exposure. PC, principal component; var., variance; C, control; LA, leaf area; % LG, percentage of leaf growth; MDA, malondialdehyde; Chl, total chlorophyll; Pb_root, Pb concentration in roots; Pb_leaf, Pb concentration in leaves; ФPSII, quantum efficiency of PSII; RWC, relative water content in leaves; DW_root, biomass dry weight of root; DW_leaf, biomass dry weight of leaf
Our results showed that Pb treatments were associated with Pb content in roots and leaves (as was discussed in the “Lead accumulation” section), leaf biomass, and MDA concentration, indicating lipid peroxidation (Fig. 3a, Table S2). Indeed, the increase of MDA has been widely reflected in studies of plants exposed to Pb, even at low concentrations (Kumar et al. 2017; Malar et al. 2016). Moreover, a weak association with FRAP, proteins, and soluble sugars was observed in Pb + Ca treatment, showing the ability of the plant to respond to oxidative stress (Fig. 3a). Plants can remobilize starch in order to generate sugar as available energy source in conditions of abiotic stress (Thalmann and Santelia 2017). This process could explain an increase in soluble sugar content under Pb + Ca treatment (Table S2). FRAP values are generally linked to the presence of reducing substances, which donate hydrogen protons to free radicals and thereby break their chains and destabilize them (Meriño-Gergichevich et al. 2015). FRAP increased in Pb treatment, which was expected, but also increased even more in Pb + Ca treatments (Table S2). Similar results were reported for blueberry leaves treated with Al + Ca after a longer exposure time (15 days) by Meriño-Gergichevich et al. (2015). Several studies indicate an increase in the calmodulin proteins bound to calcium ions and as a signal transduction in response to toxic metals, which control the activation of genes associated with detoxification of these metals (Jain et al. 2018).
On the second harvest day (Fig. 3b), a decrease in the protective effect of Ca was observed, with the differences between treatments with and without Ca being less evident (Fig. 3b; Table S1). MDA content was higher in Pb + Ca treatment, showing a tendency of more stress. Moreover, a decrease of the photosynthetic pigments, and the consequent consumption of starch as energy storage, was observed for Pb + Ca treatment (Table S2), This behavior could be a response of the plant to ensure a normal physiological function as mentioned by Santos et al. (2015). In summary, our results showed that the inefficiency of the competitor ion was directly related to the exposure time, causing it to deplete, while the Pb availability increased being the stress effect more significant.
Alteration of transpiration rate
Consumption of culture solutions
To evaluate if the treatment with external air flow could modify the transpiration rate of soybeans and, consequently, the consumption of the culture solutions [control, NH4NO3; and Pb, Pb(NO3)2], the volume of each experimental container was measured every day during the experiment. The results showed that the air flow increased the solution consumption by the plant, with approximately 25% higher reduction of the volume than in the containers corresponding to the treatments without air flow (Fig. 4). Thus, our results suggest a higher consumption of solutions by plants exposed to air flows. In addition, plants grown in Pb + air flow treatments absorbed less solution than those exposed to the control treatment + air flow. Similar results were observed by Mensah et al. (2008), who reported a significant decrease in transpiration in cabbage, carrot, and lettuce crops grown in solutions with Pb. Heavy metals were frequently reported to produce an effect on water relations, with a negative influence on water consumption in root and, therefore, in the hole plant (Rucińska-Sobkowiak 2016).
Pb concentration in soybean
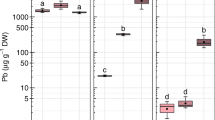
Results of Pb concentration and absolute Pb content in roots and aerial organs (stem and leaves) for the Pb treatments are shown in Fig. 5. Regarding Pb concentration, an earlier accumulation in the roots after 2 days of exposure was observed; however, this accumulation was significantly lower in the treatment with air flow (Fig. 5a). In the aerial organs, the earlier concentration of Pb was also lower at higher transpiration rate (air flow), but a recovery in the concentration after 4 days of treatment was observed (Fig. 5b). Similar results were obtained for absolute Pb content, but in this case, it was increased by a significant rise in biomass on the second harvest day (see the “Biomass, %LG, and ФPSII” section). However, a very low translocation factor was observed between roots and aerial organs and between stems and leaves in soybeans (< 1) (supplementary material Fig. S2).
Pb concentration (left), and absolute Pb content per plant (right) (mean ± SE) corresponding to roots (a) and aerial organs (b) of G. max plants exposed to treatments with Pb [0.25 mM Pb(NO3)2] with and without air flow (AF) on two harvest days (2 or 4 days of exposure). Different letters indicate significant differences at p < 0.05
In summary, the application of air flow generated a decrease in Pb concentration, whereas during the recovery period, the accumulation of Pb increased again in the aerial organs. Indeed, the effect of Cd on transpiration rate was widely studied (Kaznina et al. 2011; Kaznina and Titov 2014; Mensah et al. 2008), but few studies have evaluated other toxic metals such as Pb. In this context, based on a study carried out with three species, Ahkter and Macfie (2012) concluded that the dependence or not on the xylematic pathway for metal translocation (in particular for Cd) would be species-specific.
On the other hand, the analysis of the accumulation in the whole plant on the final harvest day (4 days) showed that Pb content in the treatments with air flow was lower (Fig. 6). This behavior could be explained either by a limitation in the absorption of the roots (saturation of Pb), or by a selective absorption of ions in the culture solution, with Pb ions being excluded. The latter assumption was supported by the final Pb concentration in the remaining solution, which was significantly higher in the treatments with air flow (Fig. 6). It is important to note that if the plants had absorbed the culture solutions equally, the volume would have decreased, with the concentrations in the solutions being the same. However, the Pb concentration remaining in the culture solution of the treatment without air flow was lower than the one with air flow, indicating a Pb exclusion mechanism.
Morpho-physiological response of soybean
In presence of air flow, both control and Pb treatments showed an increase in the biomass of the aerial organs, mainly on the second harvest day (4 days) (Table 2). However, the biomass of roots remained constant among treatments. These results suggest that the plants would be consuming other nutrients of the solutions, with the consequent increase in the aerial biomass. However, in contrast to our results, Liao et al. (2006) did not observe an increase in aerial biomass of lettuce exposed to a high rate of transpiration, with values between 0.3 mM and 1 mM of Pb(NO3)2 in the culture solution.
On the other hand, the tolerance of soybean seedlings to Pb concentration (0.25 mM) is notable, since they did not show differences in biomass compared to the controls (results not shown). Regarding LA, a tendency to an increase in plants exposed to air flow was observed (Table 2). However, when the specific leaf area (SLA) was analyzed, considering the leaf DW, a decrease in all treatments was observed in the second harvest, except for control (Table 2). These results indicate a reduction of leaf thickness due to the effect of Pb and air flow, as reported in other studies involving plants exposed to high Cd levels (Nazarian et al. 2016; Yilmaz et al. 2009).
Results of biochemical parameters (MDA, FRAP, sugars, starch, proteins, chlorophylls, carotenoids, and RWC) are shown in Table 3. Significant differences among treatments were found only for starch, FRAP, MDA, and carotenoid concentrations. However, only FRAP increased as a consequence of the air flow, regardless of the treatment, and no clear results were observed for the remaining parameters. These results are mainly related to the fact that the seedlings excluded the incorporation of Pb when air flow was increased, as a protective response.
Conclusions
Our findings indicate that the concentrations of the competitor ion Ca2+ (1:1, Ca:Pb) were not high enough to reverse Pb accumulation in soybean seedlings over time, although the greatest accumulation was recorded in roots, with a minimal translocation to the aerial organs. This result is attributed to a decrease of the Ca2+ ion in the culture solution over time, which is used by the plant, as opposed to the Pb ions, which have no essential function. This behavior was demonstrated by assessing morpho-physiological and biochemical parameters, which showed a protective effect of calcium at the beginning of exposure where the symptoms of physiological stress decreased but were reversed over time.
On the other hand, the alteration of the transpiration rate in soybean seedlings showed that at a higher air flow, the consumption of all the culture solutions increased, followed by an increase in biomass, regardless of the Pb treatment, whereas Pb accumulation in seedlings exposed to air flow was even lower. This indicates that soybean seedlings exposed to Pb and air flow selectively excluded the metal in the solution, absorbing only water and nutrients, which was also confirmed by the absence of physiological stress.
In summary, soybean seedlings showed to be highly tolerant to Pb; further research is necessary to elucidate the processes and factors that influence the translocation of toxic metals such as Pb from roots to the aerial organs.
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Ahkter MF, Macfie SF (2012) Species-specific relationship between transpiration and cadmium translocation in lettuce, barley and radish. Journal of Plant Studies 1(1):1–13. https://doi.org/10.5539/jps.v1n1p2
Antosiewicz DM (2005) Study of calcium-dependent lead-tolerance on plants differing in their level of Ca-deficiency tolerance. Environ Pollut 134(1):23–34. https://doi.org/10.1016/j.envpol.2004.07.019
Arshad M, Silvestre J, Pinelli E, Kallerhoff J, Kaemmerer M, Tarigo A, Shahid M, Guiresse M, Pradere P, Dumat C (2008) A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere 71(11):2187–2192. https://doi.org/10.1016/j.chemosphere.2008.02.013
Blanco A, Salazar MJ, Vergara Cid C, Pignata ML, Rodriguez JH (2017) Accumulation of lead and associated metals (Cu and Zn) at different growth stages of soybean crops in lead-contaminated soils: food security and crop quality implications. Environ Earth Sci 76(4):182. https://doi.org/10.1007/s12665-017-6508-x
Carocci A, Catalano A, Lauria G, Sinicropi MS, Genchi G (2016) Lead toxicity, antioxidant defense and environment. In: Reviews of Environmental Contamination and Toxicology, vol 238. Issue December. Springer, New York, pp 45–67. https://doi.org/10.1007/398_2015_5003
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2019) InfoStat versión 2019. Grupo InfoStat, FCA. Universidad Nacional de Córdoba, Argentina URL http://www.infostat.com.ar
El-Kady AA, Abdel-Wahhab MA (2018) Occurrence of trace metals in foodstuffs and their health impact. Trends Food Sci Technol 75:36–45
Ergo VV, Lascano HR, Vega CRC, Parola R, Carrera CS (2018) Heat and water stressed field-grown soybean: a multivariate study on the relationship between physiological-biochemical traits and yield. Environ Exp Bot 148:1–11. https://doi.org/10.1016/j.envexpbot.2017.12.023
Fahr M, Laplaze L, Bendaou N, Hocher V, Mzibri ME, Bogusz D, Smouni A (2013) Effect of lead on root growth. Front Plant Sci 4(6):1–7. https://doi.org/10.3389/fpls.2013.00175
Fehr, WR, Caviness CE (1977) Stages of soybean development. In: Special report, Vol. 80, Issue March. Iowa St. Univ.
Guan HP, Janes HW (1991) Light regulation of sink metabolism in tomato fruit: I. Growth and sugar accumulation. Plant Physiol 96(3):916–921. https://doi.org/10.1104/pp.96.3.916
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Jain S, Muneer S, Guerriero G, Liu S, Vishwakarma K, Chauhan DK, Dubey NK, Tripathi DK, Sharma S (2018) Tracing the role of plant proteins in the response to metal toxicity: a comprehensive review. Plant Signal Behav 13(9):e1507401
Kabata-Pendias A (2011) Trace elements in soils and plants. CRC press. https://doi.org/10.1201/b10158-25
Kaznina NM, Titov AF, Laidinen GF, Batova YV (2011) Cadmium effect on water metabolism in barley. Tr Karel Nauchn Tsentra, Ross Akad Nauk 3:57–61
Kaznina NM, Titov AF (2014) The influence of cadmium on physiological processes and productivity of Poaceae plants. Biol Bull Rev 4:335–348. https://doi.org/10.1134/S2079086414040057
Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut R 22(18):13772–13799. https://doi.org/10.1007/s11356-015-4881-0
Kholodova V, Volkov K, Abdeyeva A, Kuznetsov V (2011) Water status in Mesembryanthemum crystallinum under heavy metal stress. Environ Exp Bot 71(3):382–389. https://doi.org/10.1016/j.envexpbot.2011.02.007
Kim YY, Yang YY, Lee Y (2002) Pb and Cd uptake in rice roots. Physiol Plant 116(3):368–372. https://doi.org/10.1034/j.1399-3054.2002.1160312.x
Kohli SK, Handa N, Bali S, Khanna K, Arora S, Sharma A, Bhardwaj R (2020) Current scenario of Pb toxicity in plants: unraveling plethora of physiological responses. In: P. de Voogt (Ed.) Reviews of Environmental Contamination and Toxicology, Vol 249. Springer International Publishing, pp 153–197. https://doi.org/10.1007/398_2019_25
Kumar A, Pal L, Agrawal V (2017) Glutathione and citric acid modulates lead- and arsenic-induced phytotoxicity and genotoxicity responses in two cultivars of Solanum lycopersicum L. Acta Physiol Plant 39(7):1–12. https://doi.org/10.1007/s11738-017-2448-z
Leyva A, Quintana A, Sánchez M, Rodríguez EN, Cremata J, Sánchez JC (2008) Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: method development and validation. Biologicals 36(2):134–141. https://doi.org/10.1016/j.biologicals.2007.09.001
Liao YC, Chien SWC, Wang MC, Shen Y, Hung PL, Das B (2006) Effect of transpiration on Pb uptake by lettuce and on water soluble low molecular weight organic acids in rhizosphere. Chemosphere 65(2):343–351. https://doi.org/10.1016/j.chemosphere.2006.02.010
Lightenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu C, Chang C, Fei Y, Li F, Wang Q, Zhai G, Lei J (2018) Cadmium accumulation in edible flowering cabbages in the Pearl River Delta, China: critical soil factors and enrichment models. Environ Pollut 233:880–888. https://doi.org/10.1016/j.envpol.2017.08.092
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68(1):1–13. https://doi.org/10.1016/j.envexpbot.2009.10.011
Malar S, Shivendra Vikram S, Jc Favas P, Perumal V (2016) Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot Stud 55(1):54. https://doi.org/10.1186/s40529-014-0054-6
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51(345):659–668
Mensah E, Odai SN, Ofori E, Kyei-Baffour N (2008) Influence of transpiration on cadmium (Cd) and lead (Pb) uptake by cabbage, carrots and lettuce from irrigation water in Ghana. Asian J Agric Res 2(2):56–60. https://doi.org/10.3923/ajar.2008.56.60
Meriño-Gergichevich C, Ondrasek G, Zovko M, Šamec D, Alberdi M, Reyes-Díaz M (2015) Comparative study of methodologies to determine the antioxidant capacity of Al-toxified blueberry amended with calcium sulfate. J Soil Sci Plant Nutr 15(4):965–978
Miranda Pazcel EM, Wannaz ED, Pignata ML, Salazar MJ (2018) Tagetes minuta L. variability in terms of lead phytoextraction from polluted soils: is historical exposure a determining factor? Environ Process 5(2):243–259. https://doi.org/10.1007/s40710-018-0293-8
Nakata H, Nakayama SMM, Oroszlany B, Ikenaka Y, Mizukawa H, Tanaka K, Harunari T, Tanikawa T, Darwish WS, Yohannes YB, Saengtienchai A, Ishizuka M (2017) Monitoring lead (Pb) pollution and identifying Pb pollution sources in Japan using stable Pb isotope analysis with kidneys of wild rats. Int J Environ Res Public Health 14:56. https://doi.org/10.3390/ijerph14010056
Nazarian H, Amouzgar D, Sedghianzadeh H (2016) Effects of different concentrations of cadmium on growth and morphological changes in basil (Ocimum Basilicum L.). Pak J Bot 48(3):945–952
Palta JP (1990) Leaf chlorophyll content. Remote Sens Rev 5(1):207–213. https://doi.org/10.1080/02757259009532129
Pourrut B, Shahid M, Douay F, Dumat C, Pinelli E (2013) Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In: Heavy Metal Stress in Plants. Springer, Berlin Heidelberg, pp 121–147. https://doi.org/10.1007/978-3-642-38469-1_7
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. In: Reviews of Environmental Contamination and Toxicology Volume 213. Springer, pp 113–136.
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rai R, Agrawal M, Agrawal SB (2016) Impact of heavy metals on physiological processes of plants: with special reference to photosynthetic system. Plant Responses to Xenobiotics. Springer, In, pp 127–140
Robert G, Muñoz N, Melchiorre M, Sánchez F, Lascano R (2014) Expression of animal anti-apoptotic gene Ced-9 enhances tolerance during Glycine max L.-Bradyrhizobium japonicum interaction under saline stress but reduces nodule formation. PLoS One 9(7):e101747. https://doi.org/10.1371/journal.pone.0101747
Rodriguez-Hernandez MC, Bonifas I, Alfaro-De la Torre MC, Flores-Flores JL, Bañuelos-Hernández B, Patiño-Rodríguez O (2015) Increased accumulation of cadmium and lead under Ca and Fe deficiency in Typha latifolia: a study of two pore channel (TPC1) gene responses. Environ Exp Bot 115:38–48. https://doi.org/10.1016/j.envexpbot.2015.02.009
Rodriguez JH, Klumpp A, Fangmeier A, Pignata ML (2011) Effects of elevated CO2 concentrations and fly ash amended soils on trace element accumulation and translocation among roots, stems and seeds of Glycine max (L.) Merr. J Hazard Mater 187(1):58–66
Rodriguez JH, Salazar MJ, Steffan L, Pignata ML, Franzaring J, Klumpp A, Fangmeier A (2014) Assessment of Pb and Zn contents in agricultural soils and soybean crops near to a former battery recycling plant in Córdoba, Argentina. J Geochem Explor 145:129–134
Rosenkranz T, Kisser J, Wenzel WW, Puschenreiter M (2017) Waste or substrate for metal hyperaccumulating plants — the potential of phytomining on waste incineration bottom ash. Sci Total Environ 575:910–918. https://doi.org/10.1016/j.scitotenv.2016.09.144
Rucińska-Sobkowiak R (2016) Water relations in plants subjected to heavy metal stresses. Acta Physiol Plant 38(11). https://doi.org/10.1007/s11738-016-2277-5
Salazar MJ, Rodriguez JH, Nieto GL, Pignata ML (2012) Effects of heavy metal concentrations (Cd, Zn and Pb) in agricultural soils near different emission sources on quality, accumulation and food safety in soybean [Glycine max(L.) Merrill]. J Hazard Mater 233–234:244–253
Salazar MJ, Rodriguez JH, Vergara Cid C, Bernardelli CE, Donati ER, Pignata ML (2016) Soil variables that determine lead accumulation in Bidens pilosa L. and Tagetes minuta L. growing in polluted soils. Geoderma 279:97–108. https://doi.org/10.1016/j.geoderma.2016.06.011
Santos RW, Schmidt ÉC, Vieira IC, Costa GB, Rover T, Simioni C, Barufi JB, Soares CHL, Bouzon ZL (2015) The effect of different concentrations of copper and lead on the morphology and physiology of Hypnea musciformis cultivated in vitro: a comparative analysis. Protoplasma 252(5):1203–1215. https://doi.org/10.1007/s00709-014-0751-8
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17(1):35–52. https://doi.org/10.1590/S1677-04202005000100004
Sheoran V, Sheoran AS, Poonia P (2016) Factors affecting phytoextraction: a review. Pedosphere 26(2):148–166. https://doi.org/10.1016/S1002-0160(15)60032-7
Thalmann M, Santelia D (2017) Starch as a determinant of plant fitness under abiotic stress. New Phytol 214:943–951. https://doi.org/10.1111/nph.14491
Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N (2011) Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 2011:1–31. https://doi.org/10.1155/2011/939161
Vergara Cid C, Pignata ML, Rodriguez JH (2020) Effects of co-cropping on soybean growth and stress response in lead-polluted soils. Chemosphere 246:125833. https://doi.org/10.1016/j.chemosphere.2020.125833
Wang H, Shan X, Wen B, Owens G, Fang J, Zhang S (2007) Effect of indole-3-acetic acid on lead accumulation in maize (Zea mays L.) seedlings and the relevant antioxidant response. Environ Exp Bot 61(3):246–253. https://doi.org/10.1016/j.envexpbot.2007.06.004
Wickham H (2017) tidyverse: Easily Install and Load the ‘Tidyverse’. R package version 1.2.1. https://CRAN.R-project.org/package=tidyverse
Wojas S, Ruszczyńska A, Bulska E, Wojciechowski M, Antosiewicz DM (2007) Ca2+-dependent plant response to Pb2+ is regulated by LCT1. Environ Pollut 147(3):584–592. https://doi.org/10.1016/j.envpol.2006.10.012
Yilmaz K, Akinci İE, Akinci S (2009) Effect of lead accumulation on growth and mineral composition of eggplant seedlings (Solarium melongena). New Zeal J Crop Hort 37(3):189–199. https://doi.org/10.1080/01140670909510264
Zhang R, Wilson VL, Hou A, Meng G (2015) Source of lead pollution, its influence on public health and the countermeasures. Int J Health Anim Sci Food Saf 2(1):18–31. https://doi.org/10.13130/2283-3927/4785
Zhao Y, Fang X, Mu Y, Cheng Y, Ma Q, Nian H, Yang C (2014) Metal pollution (Cd, Pb, Zn, and As) in agricultural soils and soybean, Glycine max, in Southern China. B Environ Contam Tox 92(4):427–432. https://doi.org/10.1007/s00128-014-1218-5
Zhou J, Zhang Z, Zhang Y, Wei Y, Jiang Z (2018) Effects of lead stress on the growth, physiology, and cellular structure of privet seedlings. PLoS One 13(3):e0191139. https://doi.org/10.1371/journal.pone.0191139
Acknowledgments
We would like to thank to Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba; Fondo para la Investigación Científica y Técnica; Consejo de Investigaciones Científicas y Técnicas for the support in this study. We thank Biol. Alejandro Enet and Dr. Rodrigo Parola for providing advice in stress analysis. We are especially thankful to the English translator Jorgelina Brasca for language revision.
Funding
The following funding have contributed to the design of the study, laboratory analysis, interpretation of the data, and writing of the manuscript: Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba, UNC, (funding holder: Salazar M.J. 30820150100435CB; funding holder: Rodriguez J.H. PID Consolidar 33620180100151CB), Fondo para la Investigación Científica y Técnica (funding holder: Pignata M.L. PICT 2013-0988; PICT 2016-2061); and Consejo de Investigaciones Científicas y Técnicas (funding holder: Rodriguez J.H. PIP 11220120100402CO). A. Blanco was funded by CONICET through a scholarship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. AB, MJS, and JHR performed material preparation, data collection and analysis. JHR, MLP, and HRL performed the experimental design, and supervision of experiments. The first draft of the manuscript was written by AB and JHR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Fig. S1
Translocation factor (TF) (mean ± SE) from roots to aerial organs in soybean seedlings exposed to different treatments of Pb [Pb(NO3)2 and Pb(NO3)2 + CaCl2] (PNG 140 kb)
Fig. S2
Translocation factor (TF) (mean ± SE) from roots to aerial organs (a), and from stem to leaves (b) in soybean seedlings exposed to different treatments of Pb [Pb(NO3)2 and Pb(NO3)2 + air flow (AF)] (PNG 175 kb)
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Blanco, A., Pignata, M.L., Lascano, H.R. et al. Lead uptake and translocation pathways in soybean seedlings: the role of ion competition and transpiration rates. Environ Sci Pollut Res 28, 20624–20636 (2021). https://doi.org/10.1007/s11356-020-11901-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11901-x