Abstract
In the Basin of Mexico, one of the most important economic regions in the country with over 22 million inhabitants, peri-urban streams have been transformed into sewers, drains, and pipes to avoid flooding or unsanitary conditions; the change affects not only the ecosystem’s goods and services but also the aquatic communities that support the natural ecological processes. We aimed to develop a diatom-based diagnosis of the ecological quality of these aquatic ecosystems through the study of epilithic diatom response to regional environmental gradients. Samples of epilithic diatoms and water were collected in 45 sites representing 12 perennial streams, and multivariate analyses were performed on environmental and biological data. The ecological quality gradient to which diatoms responded was related to oxygen saturation, soluble reactive phosphorous, dissolved inorganic nitrogen, and hydromorphological quality. Three species groups were recognized according to their ecological preferences along CCA1 axis, indicators of high and low ecological quality, and tolerant species. By assigning an indicator value to each species group, we calculated the DEQI using the formula adapted from Pantle and Buck, indicating five different ecological quality classes. This index is proposed for complementing the ecological quality evaluation as a biological metric that responds to multiple regional stressors of the structure and function of these peri-urban streams in the Basin of Mexico.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecological quality (EQ) in aquatic ecosystems is defined by its structure and function, and it is evaluated using physical, chemical, hydromorphological, and biological indicators (EC 2000). In this sense, different biological groups have been used for assessing and monitoring freshwater ecosystems. Among them, epilithic diatoms are recognized worldwide as valuable indicators of environmental conditions as they respond directly and sensitively to specific physical, chemical, and biological changes (Wetzel 1983; EC 2000; Hill et al. 2001; Lobo et al. 2004, 2016; Stevenson et al. 2010). A substantial number of diatom indices have been developed in various geographic areas in the world based on species relative abundance and their sensitivity to environmental gradients, allowing numeric values to be assigned as measures of the response of diatom species to abiotic factors in order to obtain a quantitative, cost-effective, fast, and accurate EQ bioassessment (Kelly and Whitton 1995; Gómez and Licursi 2001; Lobo et al. 2004, 2015, 2016, 2019; Ector and Rimet 2005; Álvarez-Blanco et al. 2013).
Diatom distribution patterns and some species ecological preferences respond not only to changes in water quality but also to regional and physiographical factors, affecting the effectiveness of indices when these are applied in regions other than that where species-environment relationships were originally assessed to construct the metrics (Potapova and Charles 2007; Álvarez-Blanco 2013). Consequently, to improve bioassessments, it is important to perform studies on an ecoregional level (areas with similar climate, landscape, vegetation, or land use), recognizing regional floras and its species distributions along local environmental gradients using datasets representative of the areas where metrics want to be applied to assess aquatic ecosystems EQ (Solninen et al. 2004; Leira and Sabater 2005; Álvarez-Blanco et al. 2011). The foregoing is extremely important in Latin America, where regional studies continue exploring diatom biodiversity, species ecological preferences, and some less conventional metrics to measure changes in diatom assemblages in urban-impacted streams like nuclear abnormalities or ecological guilds (Lobo et al. 2019; Nicolosi-Gelis et al. 2020).
In the Basin of Mexico (BM), with over 22 million inhabitants, aquatic ecosystems have been transformed into drains, converted into sewers or piped to avoid flooding and unsanitary conditions (Legorreta 2009; CONAGUA 2018). Streams in this region flow from natural protected areas (PA) at high elevations, being exposed to different human activities along their course through the BM. Not only the ecosystem’s goods and services are affected but also the aquatic communities that support the natural processes in these environments (Mazari-Hiriart et al. 2014). According to the Mexican National Monitoring Network established by the National Water Commission (CONAGUA), the assessments of contamination levels in aquatic systems are based on physical, chemical, and bacteriological variables (CONAGUA 2018), not necessarily integrating the EQ (Rocha 1992; Karr et al. 2000; Carmona-Jiménez and Caro-Borrero 2017). Recently, through a study of macroscopic algae and macroinvertebrate communities in streams of BM, Carmona-Jimenez and Caro-Borrero (2017) established a preliminary characteristic baseline of the potential ecological reference conditions, defined as a set of conditions that are similar to those finding in ecosystems free from anthropogenic influences, with a “natural” biota. In this context, the research aimed at developing the Diatom Ecological Quality Index (DEQI) for mountain streams located in the Basin of Mexico, providing a scientific basis for the implementation of diatom-based diagnoses of these ecosystems ecological quality.
Material and methods
Study area
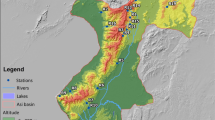
The BM (Fig. 1) is an endorheic basin located in Central Mexico within the morphotectonic region of the Trans-Mexican Volcanic Belt. It occupies territories from five states: Mexico City, Mexico, Hidalgo, Tlaxcala, and Puebla. Considering the total surface of 9600 km2, 57% are mountainous terrains that rise above 2400 m a.s.l. Also, the basin contains 45 perennial or ephemeral streams (Ferrusquía-Villafranca 1998; Legorreta 2009). The climate of the region is sub-humid, with an annual median temperature of 13.4 °C, abundant rains from June to October, and dry season from November to May (annual median precipitation between 1200 and 1500 mm) (García 2004). Its geological traits consist of alternating andesitic to basaltic lavas (Ferrusquía-Villafranca 1998). Forests of Abies religiosa (Kunth) Schltdl. & Cham., Pinus hartwegii Lindl., and Quercus spp. grow in the upper area of the watershed, with mixed forests in the middle and lower areas (Ávila-Arkerberg 2010).
The Basin of Mexico, land use, and distribution of the 12 sampled streams and 45 sites. (1) Coatlaco (CT1–2), (2) Coaxcacoaco (CX1–2), (3) San Rafael (SR1–8), (4) Ameca (AM1–3), (5) Las Regaderas (LR1–3), (6) Eslava (ES1–2), (7) Magdalena (MG1–7), (8) Santo Desierto (SD1–5), (9) Tlalnepantla (TL1–3), (10) La Caldera (LC1), (11) Cuautitlán (CU1–7), and (12) San Ildefonso (SI1–2)
Sampling and data analysis
Twelve perennial streams were selected in the BM, and through several campaigns during dry seasons (2013–2017), we collected 45 sites in order to represent all different conditions from pristine areas to human-altered urban areas (each site was visited once). Samples of epilithic diatoms and water were collected simultaneously. The following physicochemical parameters were recorded in situ (whit daylight) with a Hanna Multiparameter probe 991300 (Dallas, USA): water temperature, specific conductivity, and pH. In addition, oxygen saturation as a proxy for organic pollution (YSI-85 meter, YSI, OH, USA) and water velocity (Global Water FP111, TX, USA) were recorded. At each sampling site, 500 ml samples of water were filtered in situ and analyzed in the laboratory according to the criteria established by the official Mexican guidelines and international standards (DOF 2003; Baird et al. 2017). Nitrite nitrogen (NO2-N), nitrate nitrogen (NO3-N), ammonium nitrogen (NH4-N), dissolved inorganic nitrogen (DIN), and soluble reactive phosphorous (SRP, PO4-P) were analyzed with a DR 3900 laboratory spectrophotometer (Hach, Loveland, CO; Hach 2003). The trophic status was recognized as oligo-mesotrophic or mesotrophic-eutrophic according to Dodds et al. (1998) and Dodds (2003). Hydromorphological quality (HQ) was evaluated with the Ecological Quality of Andean Rivers Index (Acosta et al. 2009), method that assesses 24 basin attributes, hydrology, reach, and riverbed and that fluctuates from 24 to 120 points (> 96 high, 76–95 good, 51–75 moderate, 26–50 poor, < 25 bad). The conservation status of riparian vegetation was evaluated according to local descriptions (Ávila-Akerberg 2010; Espinosa and SarukÁn 1997; Rzedowski and Rzedowski 2001).
Epilithic diatom samples were collected by brushing off 10 cm2 with a toothbrush from the upper surface of five submerged stones along a 10-m transect and fixed with ethanol (70%) in independent samples. Before chemical treatments, we observed the samples to make sure that empty frustules were always rare (< 1%) to avoid overestimating species abundances. The 225 samples were oxidized with the hydrogen peroxide method following Kelly (2001), and three subsamples were mounted into permanent preparations (675 slides) in the high refraction index mounting medium Naphrax® for species quantification. In each slide, we counted 500 valves following random transects using an Olympus BX51 microscope. Microphotographs were taken with an Olympus DP12 digital camera (Olympus, Tokyo, Japan). Plates were edited using Adobe® Photoshop Elements 14.1. For species identification, the following taxonomic references were used: Krammer and Lange-Bertalot (1986–1991), Metzeltin and Lange-Bertalot (1998, 2007), Krammer (2000), Rumrich et al. (2000), Metzeltin and García-Rodríguez (2003), Metzeltin et al. (2005), and Cantonati et al. (2017). The abundant species were selected for analyses following the criterion of Lobo and Leighton (1986), which consider those species whose number of individuals is higher than the average (statistically) value of the total number of individuals in a sample. Since this was a regional study, we made a presence/absence matrix of species by stream to calculate the mean distribution of species in the basin and selected those with higher values than the average. Lastly, voucher samples were stored in the F.C.M.E. Herbarium at the Science Faculty, UNAM.
DEQI development
The DEQI was developed by analyzing environmental differences in sampling sites and diatom ecological preferences using multivariate techniques following Bahls et al. (2008) and Lobo et al. (2015). All statistical analyses were performed using RStudio (RStudio team 2019) with the packages vegan (Oksanen et al. 2019) and ggplot2 (Wickham 2016). Environmental factors were tested for collinearity to avoid correlation among variables, and those selected for further analyses were transformed by log(x + 1), except for pH.
For environmental characterization, we searched for groups of sites with similar environmental characteristics using hierarchical agglomerative clustering analysis based on the Ward method (Ward 1963). A principal component analysis (PCA) was performed to analyze which environmental gradients were ordering the sites among groups.
We analyzed how species (previously selected by their abundance and distribution) were ordered along environmental gradients with a canonical correspondence analysis (CCA) to recognize diatom ecological preferences. CCA global results, by axis and by variable, were tested for significance by 999 permutations. The variance partitioning was calculated to understand the contribution of physicochemical and hydromorphological aspects in diatom response. Species score distributions along axis 1 in CCA were used to separate them in three groups according to their ecological preferences, and an indicator value was assigned to each group. These were used to calculate the DEQI using the formula adapted from Pantle and Buck (1955): \( \mathrm{DEQI}=\frac{\sum \left(v\ h\right)}{\sum h}, \) where v is the species indicator value and h is the species relative abundance in the sample.
DEQI validation
We used a new test set with twelve samples from seven streams within the BM collected during dry season in 2017. The HQ, physicochemical parameters, and diatom samples were obtained as previously described, and the DEQI was calculated for each site. To validate the index performance summarizing the EQ, we calculated Spearman’s rank correlation coefficients of the DEQI with nutrient concentrations (DIN and SRP), oxygen saturation, specific conductivity, and HQ.
Results
Environmental characterization
The mountain streams in the BM were collected at an elevation range of 2345–3448 m a.s.l. (2941.8 ± 311.3) and presented water with cold to temperate temperature, 5–16.9 °C (11.5 ± 2.6); circumneutral pH, 5.7–8.0 (7.0 ± 0.4); mostly highly oxygenated, 40–100% (81.8 ± 19.2), and mostly electrolyte poor, 41–546 μS cm−1 (102.9 ± 81.6); with variable concentrations of SRP, 0.1–7.7 mg L−1 (0.7 ± 0.3) and DIN, 0.04–17.03 mg L−1 (1.1 ± 2.6); and a water flow that ranged from slow laminar to fast flowing, 0.1–1.0 m s−1 (0.4 ± 0.2). The HQ in sites ranged from 52 to 120 pts. (94.2 ± 18.7), with 23 sites recognized with high, 14 sites with good, and eight sites with moderate HQ, and four sites with oligo-mesotrophic conditions (DIN < 0.7 mg L−1 and SRP < 0.25 mg L−1) and seven sites with meso-eutrophic accordingly (DIN > 1.5 mg L−1 and SRP > 0.75 mg L−1).
After analyzing collinearity among environmental variables, we removed the elevation and dissolved oxygen, since these strongly reflected water temperature and oxygen saturation; no other environmental variable was removed for further analyses, as their collinearity values were below 10 (1.3–3.7). Through the hierarchical agglomerative clustering, sites were grouped in five different clusters based on their environmental characteristics (Fig. 2). Cluster 1 contained only one site (CX2), which presented the highest values for K25, DIN, and SRP. Cluster 2 grouped eight sites (lower reaches) with high concentrations of K25, DIN, and SRP and low OS and HQ. Cluster 3 included 15 sites (some headwaters and middle reaches) with intermediate concentrations of K25, low DIN and SRP concentrations, and high OS and HQ. Cluster 4 grouped eight sites (middle reaches) with low K25, moderate DIN and SRP concentrations, high OS, and moderate HQ. Finally, cluster 5 included 13 sites (headwaters) characterized by high OS, low K25, DIN, and SRP concentrations, and very good HQ.
Hierarchical agglomerative cluster recognizing five clusters of sites based on environmental data. Site codes in Table 1
The ordination of sites in the PCA revealed 78.66% of total data variance explained in the first two components. The first component indicated 54.43% of explained variance (λ = 0.3), mainly related to DIN (0.69), SRP (0.47), and K25 (0.43) and less to OS (− 0.23) and HQ (− 0.16). The second component explained 24.24% of variance (λ = 0.2), with K25 (− 0.87) and DIN (0.39) as the main related variables. The PCA biplot (Fig. 3) shows a gradient left to right along PC1 of decreasing environmental EQ: increased DIN and SRP concentrations and K25 and decreased OS and HQ; while the gradient from top to bottom along axis 2 represents how K25 increases and NID concentrations decreases in some sites, especially in those from the streams San Rafael-Tlalmanalco and Ameca-Canal Nacional grouped in cluster 3.
PCA ordination biplot of sites along PC1 and PC2, based on environmental data with overlaid clustering results. See Table 1 for sites and environmental variables codes
Diatom ecological preferences
We recognized a diverse diatom flora of 503 infrageneric diatom taxa distributed in 80 genera. The average abundance in the assemblages ranged from 0.6 to 3.1%, and the average distribution of species was 3.5 streams. We selected 160 abundant and frequent taxa above those averages for further analysis and to develop the DEQI. Ordination of sites and species along the CCA first two axis revealed 50.09% of total constrained variance being globally significant (p < 0.001) (Fig. 4a). Axis 1 explained 27.75% of variance (λ = 0.41; p < 0.001), showing a response from diatoms towards a decrease in EQ (left to right) given by a decrease in OS (− 0.49; p < 0.05) and HQ (− 0.77; p < 0.05), and an increase in SRP (0.76; p < 0.05) and DIN (0.92; p < 0.05) concentrations. Along this axis, species correlated with high HQ and OS and low concentrations of SRP and DIN are positioned in the left side of the biplot, and species correlated with low HQ and OS and high concentrations of SRP and DIN are positioned towards the right side. Axis 2 explained 22.34% of variance (λ = 0.31; p < 0.001), showing a physical response of diatoms to K25 (0.77; p < 0.05), pH (0.36; p < 0.05), V (0.34; p < 0.05), and T (− 0.20; p > 0.05). Along this axis, species correlated with high K25, pH, V, and T are positioned in the upper part of the biplot, while those correlated with low values of these variables are positioned in the lower part. All variables except for T had p values < 0.05. We focused on diatom response along axis 1 since it explains more variation related to EQ.
CCA scatterplot showing the ordination of species and sites along axis 1 and 2. a Ordination of species clustered in three groups according to their ecological optima along axis 1 EQ gradient (H, high; T, tolerant; B, bad). b Box plot of species scores in axis 1, species scores below 25% quartile indicated high EQ, those in the interquartile range were considered tolerant species, and those above the 75% quartile indicated bad EQ. Environmental variable codes in Table 1
After variation partitioning from the physicochemical and HQ sets in diatom response, we found that both sets of explanatory variables contributed to the explanation of the species data, although the unique contribution of the physicochemical set (fraction [a], R2adj = 0.118) was much larger than that of HQ (fraction [c], R2adj = 0.022). The variation explained jointly by the two sets (fraction [b], R2adj = 0.023) indicated that the physicochemical and hydromorphological variables were low intercorrelated. Still, HQ was considered a part of the EQ gradient.
We used species scores along axis 1 (EQ gradient) to define species ecological preferences (Fig. 4b). Species with scores below the 25% quartile (− 0.41) were considered indicators of high EQ (n = 44), as these represent the species whose preferences were restricted to the lowest values in the EQ gradient: low nutrient concentrations (SRP and DIN) and high OS and HQ. Those species with scores within the 25% and 75% percentile (n = 77) were considered tolerant species, with preferences in the intermediate values in the EQ gradient. And those species with scores above the 75% percentile (0.15) were considered indicators of low EQ (n = 40), as these represent the species whose preferences were among the highest values in the EQ gradient: high nutrient concentrations and low OS and HQ. An indicator value (v) was assigned to each species group as follows: v = 1 to species representing high EQ, v = 3 to tolerant species, and v = 5 to species representing low EQ. Species and their indicator value are presented in Table 2, and its microphotographs are in Figs. 5, 6, 7, 8, 9, and 10 in order to properly use the DEQI. The five most common and frequent in each group are mentioned below.
Abundant and frequent diatoms in the BM included in the DEQI. 1–2, Achnanthes coarctata; 3–4, Achnanthidium exiguum; 5–6, A. minutissimum; 7, Adlafia minuscula; 8, Amphora copulata; 9, A. pediculus; 10, Aulacoseira ambigua; 11, Caloneis bacillum; 12, C. fontinalis; 13, C. silicula; 14, C. stauroneiformis; 15, Cavinula cocconeiformis; 16, C. lapidosa; 17, C. pseudoscutiformis; 18, Chamaepinnularia submuscicola; 19–20, Cocconeis placentula; 21, Craticula subminuscula; 22, Cyclotella menenghiniana; 23, Cymbella mexicana; 24, C. tumida; 25, Cymbopleura naviculiformis; 26, Decussata placenta. Scale bar = 10 μm
Abundant and frequent diatoms in the BM included in the DEQI. 1, Diploneis smithii; 2, D. subovalis; 3, Discostella pseudostelligera; 4, Encyonema lange-bertalotii; 5, E. minutum; 6, E. muelleri; 7, E. silesiacum; 8, E. ventricosum; 9, Encyonema sp.; 10, Epithemia adnata; 11, E. turgida; 12, Eunotia arcus; 13, E. bilunaris; 14, E. implicata; 15, E. minor; 16, E. paratridentula; 17, Fragilaria capucina; 18, F. crotonensis; 19, F. vaucheriae; 20, Frankophila sp.; 21, Frustulia crassinervia; 22, F. vulgaris; 23, Geissleria acceptata; 24, Gomphonema acuminatum; 25, G. angustatum; 26, G. capitatum; 27, G. clavatum; 28, G. commutatum. Scale bar = 10 μm
Abundant and frequent diatoms in the BM included in the DEQI. 1, Gomphonema exillisimum; 2, G. gracile; 3, G. lagenula; 4, G. minutum f. pachypus; 5, G. parvulum; 6, G. subclavatum; 7, G. tenuissimum; 8, Halamphora montana; 9, H. veneta; 10, Hantzschia amphioxys; 11, H. calcifuga; 12, Humidophila contenta; 13, H. perpusilla; 14, Iconella linearis; 15–16, Lemnicola hungarica; 17, Luticola goeppertiana; 18, L. mutica; 19, L. nivalis; 20, Mayamaea atomus; 21, Melosira varians; 22, Meridion constrictum; 23, Navicula angusta; 24, N. aurora; 25, N. cryptocephala; 26, N. cryptotenella; 27, N. gregaria; 28, N. lundii; 29, N. radiosa. Scale bar = 10 μm
Abundant and frequent diatoms in the BM included in the DEQI. 1, Navicula rhynchocephala; 2, N. seibigiana; 3, N. symmetrica; 4, N. tenelloides; 5, N. veneta; 6, N. vilaplanii; 7, Neidium ampliatum; 8, Neidium sacoense; 9, Nitzschia acidoclinata; 10, N. acula; 11, N. archibaldii; 12, N. bacillariaeformis; 13, N. clausii; 14, N. communis; 15, N. costei; 16, N. dissipata; 17, N. fonticola; 18, N. frustulum; 19, N. linearis; 20, N. palea; 21, N. paleacea; 22, N. pusilla; 23, N. recta; 24, N. solgensis; 25, N. soratensis; 26, N. supralitorea; 27, N. umbonata; 28–29, Nupela praecipua; 30, Odontidium mesodon; 31, O. rostratum; 32, Orthoseira roeseana; 33, Pinnularia acoricola; 34, P. anglica; 35, P. appendiculata var. amaniana; 36, P. bertrandii; 37, P. borealis. Scale bar = 10 μm
Abundant and frequent diatoms in the BM included in the DEQI. 1, Pinnularia divergens; 2, P. divergentissima; 3, P. erratica; 4, Pinnularia frequentis; 5, P. inconstans; 6, P. johndonatoi; 7, P. microstauron; 8, P. nodosa; 9, P. peragalli; 10, P. reichardtii; 11, P. sinistra; 12, P. subcommutata var. nonfasciata; 13–14, Planothidium biporomum; 15–16, P. cryptolanceolatum; 17–18, P. frequentissimum; 19–20, Platessa conspicua; 21, Prestauroneis protractoides; 22, Pseudostaurosira brevistriata; 23, Pseudostaurosira sp.; 24, Reimeria sinuata; 25, Rhoicosphenia sp.; 26, Rhopalodia rupestris; 27–28, Rossithidium nodosum; 29, Sellaphora cosmopolitana; 30, S. laevissima; 31, S. nigri; 32, S. pseudopupula; 33, S. pupula. Scale bar = 10 μm
Abundant and frequent diatoms in the BM included in the DEQI. 1, Pinnularia sp.; 2, S. saugerresii; 3, Sellaphora sp. 1; 4, Sellaphora sp. 2; 5, Simonsenia delognei; 6, Stauroneis kriegeri; 7, S. phoenicenteron; 8, S. subgracilis; 9, S. thermicola; 10, Staurosira venter; 11, Staurosirella sp.; 12, Stephanodiscus minutulus; 13, S. niagarae; 14, S. oregonicus; 15, Surirella angusta; 16, S. muscicola; 17, Ulnaria acus; 18, U. ulna. Scale bar = 10 μm (a, 1 and 7; b, the others)
We recognized Achnanthidium minutissimum, Cocconeis placentula, Gomphonema lagenula, Planothidium cryptolanceolatum, and Rhoicosphenia sp. among the species that indicated high EQ (v = 1); Fragilaria capucina, Navicula cryptotenella, N. gregaria, N. rhynchocephala, and Reimeria sinuata in the tolerant species group (v = 3); and Craticula subminuscula, Nitzschia palea, N. soratensis, Sellaphora cosmopolitana, and S. saugerresii as species that indicated low EQ (v = 5).
Species relative abundances and their indicator value were used to calculate the Diatom Ecological Quality Index (DEQI) using the formula adapted from Pantle and Buck (1955). The DEQI for a given site was calculated by ascribing an indicator value of 1 to those species not included in Table 4. The index values ranged from 1 to 5 indicating five different EQ following those established by the WFD (EC 2000) (Table 3). High EQ represents almost pristine conditions in reference sites, good EQ represents that there is still a natural structure and function in the aquatic ecosystem, moderate EQ indicates slightly disturbance given by nutrient increase, organic pollution, or hydromorphological alterations, poor EQ indicates severe disturbance, where the structure and function in the ecosystem are almost lost, and bad EQ represents a total loss of these.
From the training set used to develop the DEQI, the index was calculated resulting in four sites with a high EQ, 23 sites with good EQ, 11 sites had moderate EQ, three sites had poor EQ, and four had bad EQ (Table 4).
DEQI validation
We tested the DEQI to prove its performance summarizing the EQ in a new test set from twelve sites which represented the different conditions in the BM including pristine areas in headwaters to human-altered urban areas in lower reaches. It presented a strong positive correlation with DIN, moderate positive correlations with K25 and SRP, and strong negative correlation with OS and moderate negative correlation with HQ (p < 0.05 for all correlations) (Fig. 11). In this sense, as the DEQI scores increased, it reflected an increase in nutrient concentrations (especially DIN over SRP), mineralization (K25), organic pollution (OS as a proxy), and hydromorphological alterations.
Discussion
Different human activities were detected as the streams flowed through the BM, including the presence of hydraulic infrastructure (channels and dams), the construction of bridges or roads, fish farming, agriculture, cattle raising, unregulated tourism, or irregular human settlements, resulting in nutrient enrichment (phosphorous and nitrogen), decrease in dissolved oxygen concentration, and low hydromorphological quality (due to the loss of velocity and continuity of flow). This condition highlights the increase in the degradation of ecological quality, which leads to the loss of habitat and ecosystem services, and in some cases to a risk to human health (Mazari-Hiriart et al. 2014). Yet, we still found some sites (some headwaters) with oligotrophic conditions, permanent water flow, and good hydromorphological quality, recognized as reference conditions in the BM by Carmona-Jiménez and Caro-Borrero (2017), where they found some macroinvertebrate families and macroalgae species associated with these conditions, establishing a preliminary baseline for bioassessment in these aquatic ecosystems in Central Mexico. Since different biological groups respond to different stressors (EC 2000), it would be desired to construct metrics based on these other biological groups to perform an integrated evaluation of the ecosystems ecological quality.
Organic pollution and eutrophication (using orthophosphate as a proxy) are some factors to which diatoms respond, and thus, several biotic indices have been developed to monitor such factors (Prygiel and Coste 1993; Whitton and Kelly 1995; Kelly and Whitton 1995). Many scientists combine these referring to them as eutrophication in a broad sense as organic pollution and eutrophication of the water are highly cyclical correlated events (Branco 2005; Esteves 2011; Schneider et al. 2014; Dupas et al. 2015; Lobo et al. 2015). Other physical factors dependent of hydromorphology, such as water flow, influence diatom community structure as well (Gomà et al. 2005). In this sense, indices like the Trophic Water Quality Index (Lobo et al. 2015) and the DEQI have been developed using multivariate analysis, to reflect a more consistent, robust, and objective way to determine the ordination of species along local environmental gradients defined by physical, chemical, and hydromorphological factors acting jointly in the ecosystems.
The suitability of a biotic index relies greatly on how many species are included in it from those integrating the local flora from the region where we want to use it; besides, species distribution and their ecological preferences respond not only to water quality but also to regional and physiographical factors (Álvarez-Blanco et al. 2011). In this matter, it was important to recognize the regional diatom flora in the BM, thus identifying the frequent and abundant species that significantly contributed for determining the community structure. And even though most of the species included in the DEQI are widely distributed in other temperate regions and included in other metrics (Cantonati et al. 2017), it was important to study species response to local environmental gradients, without previous assumptions.
Physicochemical variables explained more variation in diatom assemblages, since these aquatic photosynthetic organisms respond directly to eutrophication gradients (Wetzel 1983; Hill et al. 2001; Lobo et al. 2015; Stevenson et al. 2010). Yet, for a more solid diatom-based assessment, it has been denoted that it is important to quantify the influence of other hydromorphological stressors affecting these ecosystems (Bere et al. 2016) like the native vegetation cover, presence of dams, channel modifications, or human activities, which are included in the HQ evaluation. The greatest example of this was the development of some common planktonic species like Cyclotella menenghiniana, Discostella stelligera, or Fragilaria crotonensis (observed with cell content before chemical oxidation of samples) in sites post-dams. For better understanding of the influence of hydromorphological characteristics in diatom assemblages in the BM, individual variables should be considered to develop a more complete dataset in further campaigns.
The five most abundant species and with grater distribution, representing each indicator group, were used to discuss the ecology of the species present in the index. Within the species included in the group representing high EQ, we found Achnanthidium minutissimum, considered a representative of oligotrophic environments and with low tolerance to eutrophication (Kelly and Whitton 1995; Segura-García et al. 2012; Lobo et al. 2015). Cocconeis placentula has been reported as a species tolerant to nutrient enrichment by Van Dam et al. (1994), yet, it also has been reported in low nutrient concentration environments (Kelly and Whitton 1995; Abuhatab-Aragón and Donato-Rondón 2012; Carmona-Jiménez et al. 2016). This species has been described with several varieties, and some authors do not differentiate between them, so the subsuming of them has blurred the autoecology of these taxa (Jahn et al. 2009). In the BM, C. placentula was a widespread species, but it was abundant in headwaters with low nutrient concentration. Therefore, until further analyses to distinguish between the Cocconeis taxa within the placentula species complex in the BM, we considered it as indicator of high EQ. Gomphonema lagenula has been recently separated from the broad Gomphonema parvulum complex by morphological and molecular data, with an epitype from a close region in Central Mexico (Abarca et al. 2014). In that study, the authors mention that this species occurred in an anthropogenic influenced river, yet no physicochemical information nor abundance information are provided, although they point it with a tropical distribution. In the BM, it was widespread and abundant in headwaters with low nutrient concentrations and high OS and HQ; hence, this is an important opportunity to contribute towards the understanding of this tropical species ecological preference. Another common species in this group was Planothidium cryptolanceolatum; this species was used to be identified as P. lanceolatum (Brébisson ex Kützing) Lange-Bertalot in the BM (Ramírez-Vázquez et al. 2001; Carmona-Jiménez et al. 2016), a species with a wide ecological amplitude and widely distributed (Cantonati et al. 2017). Yet, Jahn et al. (2017) recently described P. cryptolanceolatum through a polyphasic approach including populations from Central Mexico, and our populations are consistent with its morphological and morphometrical characteristics. Our populations were distributed in headwaters and middle reaches of these mountain streams with low nutrient concentrations and good HQ as previously reported by Carmona-Jiménez et al. (2016) (as P. lanceolatum). In this matter, we can contribute towards the understanding of the species biogeography and autoecology. Rhoicosphenia sp. has previously been recorded as R. abbreviata (C. Agardh) Lange-Bertalot in other studies in the Basin of Mexico. Yet, the morphology, morphometry, and autoecology of Rhoicosphenia sp. differ from R. abbreviata, since the latter has been described to be widely distributed in mesotrophic to eutrophic rivers and thus used as an indicator of eutrophication and pollution (Van Dam et al. 1994; Kelly and Whitton 1995; Levkov et al. 2010). This species was abundant in most headwaters with low nutrient concentration and good hydromorphological quality; therefore, the identity of these taxa should be investigated.
Within the tolerant species, we recognized Fragilaria capucina, a species with an ecological amplitude not well defined, found in oligotrophic to meso-eutrophic freshwater habitats (Cantonati et al. 2017). Navicula cryptotenella, a cosmopolitan species with a wide spectrum of tolerance to nutrient enrichment and organic pollution (Van Dam et al. 1994; Kelly and Whitton 1995; Lobo et al. 2015), has been reported from oligo to mesotrophic conditions in the upper Lerma Basin, in Central Mexico (Segura-García 2010), which coincides with its distribution within the Basin of Mexico. N. gregaria is considered a tolerant species to eutrophication and pollution, also associated with high conductivity (Kobayasi and Mayama 1989; Van Dam et al. 1994; Kelly and Whitton 1995; Lobo et al. 2015; Mora et al. 2017). Navicula rhynchocephala has been considered tolerant to eutrophication (Van Dam et al. 1994; Segura-García et al. 2010; Carmona et al. 2016), occurring in some headwaters and most middle reaches with increased nutrient concentration. Moreover, Reimeria sinuata is a widely distributed species in siliceous streams over a wide range of trophic levels (Cantonati et al. 2017).
Within the species included in the group with preference for low EQ, we found Craticula subminuscula, considered highly tolerant to eutrophication and with preference for heavily polluted environments (Kobayasi and Mayama 1989; Van Dam et al. 1994; Segura-García et al. 2012; Lobo et al. 2015; Mora et al. 2017). Nitzschia palea is a widespread diatom recognized as highly tolerant to organic pollution (found in mesosaprobic to polysaprobic environments) and eutrophication (indicator of hypereutrophic conditions) (Lange-Bertalot 1979; Kobayasi and Mayama 1989; Van Dam et al. 1994; Potapova and Charles 2003; Lobo et al. 2010, 2015; Segura et al. 2012; Mora et al. 2017). N soratensis has been reported previously as N. inconspicua Grunow in some streams of the BM (Carmona-Jiménez et al. 2016), yet with scanning electron microscopy, we identified every population as N. soratensis. These two species are easily confused because of their similar valve shape, but they have different environmental preferences. N. soratensis is a strictly freshwater species with preference for slightly eutrophic waters (Morales and Vis 2007), whereas N. inconspicua extends from freshwater into brackish and marine waters (Trobajo et al. 2013). Sellaphora cosmopolitana is described as a tolerant species to both eutrophication and organic pollution (Bey and Ector 2013). Moreover, Sellaphora saugerresii is a species occurring in highly eutrophic freshwater habitats (Cantonati et al. 2017).
Comparing the EQ obtained using the DEQI with the trophic status and HQ of the sites from the training set, we could conclude that the trophic status and HQ evaluation were insufficient on their own to reflect all the possible stressors of the ecological status of these systems. In addition, a calibration is needed to establish the reference conditions for nutrient concentrations and HQ, highlighting that the use of biological metrics could provide a suitable framework (Skoulikidis et al. 2004, 2006). Moreover, as the DEQI was developed from the ordering of species along an environmental gradient of multiple factors acting jointly together, it reflected a consistent, robust, and objective way to determine the species response to the local stressors occurring in these peri-urban streams, covering a gap in the need of these methods in Latin America (Lobo et al. 2019).
Conclusions
We found a rich diatom flora in the mountain streams in the Basin of Mexico (BM), and through the analysis of species ecological preferences within the region, we proposed a new metric to assess the ecological quality (EQ) of these aquatic ecosystems. Even though the objective of the campaigns in this study was to cover a large area in the BM to have a representation of different scenarios that occur in the basin (from “pristine” conditions within PAs, rural areas with agricultural, livestock, or fish farming activities, to urban areas), there is still work to do to understand the specific influence of hydromorphological characteristics and seasonal effects on diatom assemblages, which could be integrated in a future calibration of the index. Still, we propose the use of the DEQI to complement the EQ evaluation and improve sustainable management of rivers and streams in Mexico, while filling the gap in the need of biological methods for environmental biomonitoring in Latin America. Finally, we would like to denote that the evaluation of EQ is not confined to a single taxonomic group but requires understanding of different groups within the basin to conduct an integrative assessment, since different groups respond to different pressures.
Data availability
Not applicable.
References
Abarca N, Jahn R, Zimmermann J, Enke N (2014) Does the cosmopolitan diatom Gomphonema parvulum (Kützing) Kützing have a biogeography? PLoS One 9(1):e86885. https://doi.org/10.1371/journal.pone.0086885
Abuhatab-Aragón YA, Donato-Rondón JC (2012) Cocconeis placentula y Achnanthidium minutissimum especies indicadoras de arroyos oligotróficos andinos. Caldasia 34(1):205–212 (in Spanish)
Acosta R, Ríos B, Rieradevall M, Prat N (2009) Propuesta de un protocolo de evaluación de la calidad ecológica de ríos andinos (CERA) y su aplicación a dos cuencas en Ecuador y Perú. Limnetica 28(1):35–64 (in Spanish)
Álvarez-Blanco I, Cejudo-Figueiras C, Bécares E, Blanco S (2011) Spatiotemporal changes in diatom ecological profiles: implications for biomonitoring. Limnology 12(2):157–168. https://doi.org/10.1007/s10201-010-0333-1
Álvarez-Blanco I, Blanco S, Cejudo-Figueiras C, Bécares E (2013) The Duero Diatom Index (DDI) for river water quality assessment in NW Spain: design and validation. Environ Monit Assess 185:969–981. https://doi.org/10.1007/s10661-012-2607-z
Ávila-Akerberg VD (2010) Forest quality in the southwest of México City. Assessment towards ecological restoration of ecosystem services. Dissertation, University of Freiburg, Germany: Faculty of Forest and Environmental Sciences, Albert-Ludwigs-Universitat
Bahls L, Teply M, Sada de Suplee R, Suplee MW (2008) Diatom bio criteria development and water quality assessment in Montana: a brief history and status report. Diatom Research 23(2):533–540
Baird RB, Eaton AD, Rice EW (Eds.) (2017) Standard methods for examination of water and wastewater (23rd Ed.). American Public Health Association (APHA), American Water Works Association and Water Environment Federation, Washington, D.C.
Bere T, Mangadze T, Mwedzi T (2016) Variation partitioning of diatom species data matrices: understanding the influence of multiple factors on benthic diatom communities in tropical streams. Sci Total Environ 566:1604–1613. https://doi.org/10.1016/j.scitotenv.2016.06.058
Bey M, Ector L (2013) Atlas des diatomées des cours déau de la région Rhône-Alpes. Tome 3. Direction régionale de lÉnvironnment, de lÁménagement et du Logement Rhône-Alpes, Caluire
Branco SM (2005) Água: Origem, uso e preservação. Editora Moderna, São Paulo (in Portuguese)
Cantonati M, Kelly MG, Lange-Bertalot H (2017) Freshwater benthic diatoms of Central Europe: over 800 common species used in ecological assessment. Koeltz Botanical Books, Schmitten-Oberreifenberg
Carmona-Jiménez J, Caro-Borrero A (2017) The last peri-urban rivers of the Mexico Basin: establishment of the reference conditions through the evaluation of ecological quality and biological indicators. Revista Mexicana de Biodiversidad 88(2):425–436. https://doi.org/10.1016/j.rmb.2017.03.019
Carmona-Jiménez J, Ramírez-Rodríguez R, Bojorge-García MG, González-Hidalgo B, Cantoral-Uriza EA (2016) Estudio del valor indicador de las comunidades de algas bentónicas: una propuesta de evaluación y aplicación en el río Magdalena, Ciudad de México. Revista Internacional de Contaminación Ambiental 32(2):139–152 (in Spanish). https://doi.org/10.20937/RICA.2016.32.02.01
Comisión Nacional del Agua (CONAGUA) (2018) Estadísticas del agua en México, edición 2018. Secretaría del Medio Ambiente y Recursos Naturales. Comisión Nacional del Agua, Ciudad de México (in Spanish)
Diario Oficial de la Federación (DOF) (2003) [Norma Oficial MexicanaNOM-001-SEMARNAT-1996 (aclaración a la NOM-001-ECOL-1996), que establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales en aguas y bienes nacionales] (in Spanish). http://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/agenda/DOFsr/60197.pdf. Accessed 20 August 2019
Dodds WK (2003) Misuse of inorganic N and soluble reactive P concentrations to indicate nutrient status of surface waters. J N Am Benthol Soc 22(2):171–181. https://doi.org/10.2307/1467990
Dodds WK, Jones JR, Welch EB (1998) Suggested classification of stream trophic state: distributions of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Res 32(5):1455–1462. https://doi.org/10.1016/S0043-1354(97)00370-9
Dupas R, Delmas M, Dorioz JM, Garnier J, Moatar F, Gascuel-Odoux C (2015) Assessing the impact of agricultural pressures on N and P loads and eutrophication risk. Ecol Indic 48:396–407. https://doi.org/10.1016/j.ecolind.2014.08.007
Ector L, Rimet F (2005) Using bioindicators to assess rivers in Europe: an overview. In: Lek S, Scardi M, Verdonschot PFM, Decsy JP, Park YS (eds) Modelling community structure in freshwater ecosystems. Springer, Berlin, pp 7–19
Espinosa G, SarukÁn J (1997). Manual de malezas del Valle de México. Ciu- dad de México, México: Universidad Nacional Autónoma de México/Fondo de Cultura Económica
Esteves FA (2011). Fundamentos de Limnologia. Interciência LTDA: Rio de Janeiro (in Portuguese)
European Commission (EC) (2000) Directive 2000/60/ EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Official Journal of the European Communities L 327, 22/12/2000, 1–73
Ferrusquía-Villafranca F (1998) Geología de México: una sinopsis. In: Ramamoorthy TP, Bye R, Lot A, Fa J (Eds.) Diversidad biológica de México. Orígenes y distribución (pp. 3–108). Instituto de Biología, UNAM, Ciudad de México, pp 3–108 (in Spanish)
García E (2004) Modificaciones al sistema de clasificación climática de Köppen. Instituto de Geografía, Ciudad de México (in Spanish)
Gomà J, Rimet F, Cambra J, Hoffmann L, Ector L (2005) Diatom communities and water quality assessment in mountain rivers of the upper Segre basin (La Cerdanya, Oriental Pyrenees). Hydrobiologia 551(1):209–225. https://doi.org/10.1007/s10750-005-4462-1
Gómez N, Licursi M (2001) The Pampean Diatom Index (IDP) for assessment of rivers and streams in Argentina. Aquat Ecol 35(2):173–181. https://doi.org/10.1023/A:1011415209445
Hach (2003) Water analysis handbook, 4th edn. Hach Co, Loveland
Hill BH, Stevenson RJ, Pan Y, Herlihy AT, Kaufmann PR, Johnson CB (2001) Comparison of correlations between environmental characteristics and stream diatom assemblages characterized at genus and species levels. J N Am Benthol Soc 20(2):299–310. https://doi.org/10.2307/1468324
Jahn R, Kusber WH, Romero OE (2009) Cocconeis pediculus Ehrenberg and C. placentula Ehrenberg var. placentula (Bacillariophyta): Typification and taxonomy. Fottea 9(2):275–288. https://doi.org/10.5507/fot.2009.027
Jahn R, Abarca N, Gemeinholzer B, Mora D, Skibbe O, Kulikovskiy M, Gusev E, Kusber WH, Zimmermann J (2017) Planothidium lanceolatum and Planothidium frequentissimum reinvestigated with molecular methods and morphology: four new species and the taxonomic importance of the sinus and cavum. Diatom Research 32(1):75–107. https://doi.org/10.1080/0269249X.2017.1312548
Karr JR, Allen JD, Benke AC (2000) River conservation in the United States and Canada. In: Boon PJ, Davies BR, Petts GE (eds) Global perspectives on river conservation: science, policy and practice. Wiley, New York, pp 3–39
Kelly MG (2001) Use of similarity measures for quality control of benthic diatom samples. Water Res 35(11):2784–2788. https://doi.org/10.1016/S0043-1354(00)00554-6
Kelly MG, Whitton BA (1995) The trophic diatom index: a new index for monitoring eutrophication in rivers. J Appl Phycol 7(4):433–444. https://doi.org/10.1007/BF00003802
Kobayasi H, Mayama S (1989) Evaluation of river water quality by diatoms. The Korean Journal of Phycology 4:121–133
Krammer K (2000) Diatoms of Europe. Diatoms of the European inland waters and comparable habitats. The genus Pinnularia. Vol. 1. Koeltz Scientific Books, Koenigstein
Krammer K, Lange-Bertalot H (1986–1991) Bacillariophyceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (Eds.) Sußwasserflora von Mitteleuropa, Vol. 2 /1 (1986), 2/2 (1988), 2/3 (1991a), 2/4 (1991b). Gustav Fischer Verlag, Sttutgart-New York (in German)
Lange-Bertalot H (1979) Pollution tolerance of diatoms as a criterion for water quality estimation. Nova Hedwig Beih 64:285–304
Legorreta J (2009) Ríos, lagos y manantiales del valle de México. Universidad Autónoma Metropolitana, Mexico City (in Spanish)
Leira M, Sabater S (2005) Diatom assemblages distribution in catalan rivers, NE Spain, in relation to chemical and physiographical factors. Water Res 39:73–82. https://doi.org/10.1016/j.watres.2004.08.034
Levkov Z, Mihalić KC, Ector L (2010) A taxonomical study of Rhoicosphenia Grunow (Bacillariophyceae) with a key for identification of selected taxa. Fottea 10(2):145–200. https://doi.org/10.5507/fot.2010.010
Lobo E, Leighton G (1986) Estructuras comunitarias de las fitocenosis planctonicas de los sistemas de desembocaduras de rios y esteros de la zona central de Chile. Rev Biol Mar Oceanogr 22(1):1–29 (in Spanish)
Lobo EA, Callegaro VL, Hermany G, Bes D, Wetzel CE, Oliveira MA (2004) Use of epilithic diatoms as bioindicator from lotic systems in Southern Brazil, with special emphasis on eutrophication. Acta Limnologica Brasiliensia 16:25–40
Lobo EA, Wetzel CE, Ector L, Katoh K, Blanco S, Mayama S (2010) Response of epilithic diatom communities to environmental gradients in subtropical temperate Brazilian rivers. Limnetica 29(2):323–340. https://doi.org/10.23818/limn.29.27
Lobo EA, Schuch M, Heinrich CG, Da Costa AB, Düpont A, Wetzel CE, Ector L (2015) Development of the Trophic Water Quality Index (TWQI) for subtropical temperate Brazilian lotic systems. Environ Monit Assess 187:354. https://doi.org/10.1007/s10661-015-4586-3
Lobo EA, Heinrich CG, Schuch M, Wetzel CE, Ector L (2016) Diatoms as bioindicators in rivers. In: Necci O (ed.) River algae. Springer, pp. 245-271
Lobo EA, Weber-Freitas N, Salinas VH (2019) Diatomeas como bioindicadores: Aspectos ecológicos de la respuesta de las algas a la eutrofización en América Latina. Mexican Journal of Biotechnology 4(1):1–24 (in Spanish). https://doi.org/10.29267/mxjb.2019.4.1.1
Mazari-Hiriart M, Pérez-Ortíz G, Orta-Ledesma MT, Armas-Vargas F, Tapia MA, Solano-Ortiz R, Silva MA, Yañez-Noguez I, López-Vidal Y, Díaz-Ávalos C (2014) Final opportunity to rehabilitate an urban river as a water source for Mexico City. PLoS One 9(7):1–17. https://doi.org/10.1371/journal.pone.0102081
Metzeltin D, García-Rodríguez F (2003) Las diatomeas uruguayas. DI.R.A.C. – Facultad de Ciencias, Montevideo (in Spanish)
Metzeltin D, Lange-Bertalot H (1998) Tropische Diatomeen in Südamerika I. 700 überwiegend wenig bekannte oder neue Taxa repräsentativ als Elemente der neotropischen Flora. In: Lange-Bertalot H (ed.) Iconographia Diatomologica, 5. A. R. G. Gantner Verlag, Konigstein (in German)
Metzeltin D, Lange-Bertalot H (2007) Tropical diatoms of South America II. Special remarks on biogeographic disjunction. In: Lange-Bertalot H (ed.) Iconographia Diatomologica, 18. A. R. G. Gantner Verlag, Konigstein
Metzeltin D, Lange-Bertalot H, García-Rodríguez F (2005) Diatoms of Uruguay. Compared with other taxa from South America and elsewhere. In: Lange-Bertalot H (ed.) Iconographia Diatomologica, 15. A. R. G. Gantner Verlag, Konigstein
Mora D, Carmona-Jiménez J, Jahn R, Zimmermann J, Abarca N (2017) Epilithic diatom communities of selected streams from the Lerma-Chapala Basin, Central Mexico, with the description of two new species. PhytoKeys 88:39–62. https://doi.org/10.3897/phytokeys.88.14612
Morales EA, Vis ML (2007) Epilithic diatoms (Bacillariophyceae) from cloud forest and alpine streams in Bolivia, South America. Proc Acad Natl Sci Phila 156:123–155. https://doi.org/10.1635/0097-3157(2007)156[123:EDBFCF]2.0.CO;2
Nicolosi-Gelis MM, Cochero J, Donadelli J, Gómez N (2020) Exploring the use of nuclear alterations, motility and ecological guilds in epipelic diatoms as biomonitoring tools for water quality improvement in urban impacted lowland streams. Ecological Indicators 110. https://doi.org/10.1016/j.ecolind.2019.105951
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) vegan: community ecology package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan
Pantle R, Buck H (1955) Die biologische Überwachung der Gewässer und die Darstellung der Ergebnisse. Gas- und Wasserfach Wasser und Abwasser 96:609–620 (in German)
Potapova M, Charles DF (2003) Distribution of benthic diatoms in U.S. rivers in relation to conductivity and ionic composition. Freshw Biol 48:1311–1328. https://doi.org/10.1046/j.1365-2427.2003.01080.x
Prygiel J, Coste M (1993) The assessment of water quality in the Artois-Picardie water basin (France) by the use of diatom indices. Hydrobiologia 269(1):343–349. https://doi.org/10.1007/BF00028033
Ramírez-Vázquez M, Beltran-Magos Y, Bojorge-García MG, Carmona-Jiménez J, Cantoral-Uriza EA, Valadez-Cruz F (2001) Flora algal del río la Magdalena Distrito Federal, México. Boletín de la Sociedad Botánica de México 68:45–67 (in Spanish). https://doi.org/10.17129/botsci.1635
Rocha A (1992) Algae as biological indicators of water pollution. In: Cordeiro-Marino M, Azebedo MTP, Sant’Anna CL, Tomita NY, Plastino EM (Eds.) Algae and environment: a general approach. Sociedade Brasileira de Ficologia, CETEBS, São Paulo, pp. 34–52
RStudio Team (2019) RStudio: integrated development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/
Rumrich U, Lange-Bertalot H, Rumrich M (2000) Diatomeen der Anden. Von Venezuela bis Patagonien/Feuerland. In: Lange-Bertalot H (ed.) Iconographia Diatomologica, 9. A. R. G. Gantner Verlag, Konigstein (in German)
Rzedowski CG, Rzedowski J (2001). Flora fanerogÁmica del Valle de Méx- ico (2nd ed.). PÁtzcuaro, MichoacÁn: Instituto de Ecología A.C./Comisión Nacional para el Conocimiento y Uso de la Biodiversidad
Schneider SC, Cara M, Eriksen TE, Goreska BB, Imeri A, Kupe L, Lokoska T, Patceva S, Trajanovska S, Trajanovski S, Talevska M, Sarafiloska EV (2014) Eutrophication impacts littoral biota in Lake Ohrid while water phosphorus concentrations are low. Limnologica 44:90–97. https://doi.org/10.1016/j.limno.2013.09.002
Segura-García V, Israde-Alcántara I, Maidana NI (2010) The genus Navicula sensu stricto in the upper Lerma Basin, Mexico. I. Diatom Research 25(2):367–383. https://doi.org/10.1080/0269249X.2010.9705857
Segura-García V, Cantoral-Uriza EA, Israde-Alcántara I, Maidana NI (2012) Diatomeas epilíticas como indicadores de la calidad del agua en la cuenca alta del río Lerma México. Hidrobiológica 22(1):16–27 (in Spanish)
Skoulikidis NT, Gritzalis KC, Kouvarda T, Buffagni A (2004) The development of an ecological quality assessment and classification system for Greek running waters based on benthic macroinvertebrates. Hydrobiologia 516:149–160. https://doi.org/10.1023/B:HYDR.0000025263.76808.ac
Skoulikidis NT, Amaxidis Y, Bertahas I, Laschou S, Gritzalis K (2006) Analysis of factors driving stream water composition and synthesis of management tools—a case study on small/medium Greek catchments. Sci Total Environ 362(1–3):205–241. https://doi.org/10.1016/j.scitotenv.2005.05.018
Solninen J, Paavola R, Muotka T (2004) Benthic diatom communities in boreal streams: community structure in relation to environmental and spatial gradients. Ecography 27:330–342. https://doi.org/10.1111/j.0906-7590.2004.03749.x
Stevenson RJ, Pan Y, Van Dam H (2010) Assessing environmental conditions in lakes and streams with diatoms. In: Stoermer EF, Smol JP (eds) The diatoms: application for environmental and earth sciences. Cambridge University Press, Cambridge, pp 57–85
Trobajo R, Rovira L, Ector L, Wetzel CE, Kelly M, Mann DG (2013) Morphology and identity of some ecologically important small Nitzschia species. Diatom Research 28(1):37–59. https://doi.org/10.1080/0269249X.2012.734531
Van Dam H, Mertens A, Sinkeldam J (1994) A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Neth J Aquat Ecol 28(1):117–133. https://doi.org/10.1007/BF02334251
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58(301):236–244
Wetzel RG (1983) Limnology, 2nd edn. Saunders College Publishing, New York
Whitton BA, Kelly MG (1995) Use of algae and other plants for monitoring rivers. Aust J Ecol 20(1):45–56. https://doi.org/10.1111/j.1442-9993.1995.tb00521.x
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Funding
The authors would like to thank the Posgrado en Ciencias del Mar y Limnología, UNAM, and to CONACYT for the scholarship granted to the first author (747202). JCJ received financial support through the Research Grant PAPIIT-UNAM (IN220115 and IN307219) and PAPIME-UNAM (PE201118). We also thank MSc Arantza Ivonne Daw Guerrero for her help in preparing the map.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Victor Hugo Salinas Camarillo and Javier Carmona-Jiménez. Eduardo Lobo drafted the work and revised it critically for important intellectual content. The first draft of the manuscript was written by Victor Hugo Salinas, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Ethical approval
The submitted work contains original data and has not been published elsewhere in any form or language (partially or in full). As well, we agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Consent to participate and publish
All authors agreed with the content and all gave explicit consent to submit/publish and that they obtained consent from the responsible authorities at the University on where the work has been carried out, before the work is submitted.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salinas-Camarillo, V.H., Carmona-Jiménez, J. & Lobo, E.A. Development of the Diatom Ecological Quality Index (DEQI) for peri-urban mountain streams in the Basin of Mexico. Environ Sci Pollut Res 28, 14555–14575 (2021). https://doi.org/10.1007/s11356-020-11604-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11604-3