Abstract
This work describes a special case of pollution potential assessment applied to an abandoned sulfide tailings impoundment located in the Riotinto mining district (Huelva), near the Tinto River. Three overlapping levels of discharged tailings were recognized in the impoundment, from deeper to upper: pale yellow to white, red, and brownish-yellow. Mineralogical, physical, and chemical characteristics of tailings, water leachates, water, and sulfate efflorescent salts were analyzed. The total toxic element content and the leachate concentration were respectively used to calculate two indices that support potential toxicity assessment: the Index of Contamination (IC) and the Hazard Average Ratio (HAQ). According to the IC values, all tailings samples showed a high potential for contaminating soils and sediments, especially the intermediate tailings with up to As (8.6 g kg−1), Pb (14.8 g kg−1), and Cu (1 g kg−1). Deeper tailings leachate was extremely saline and acidic, with a very high concentration of sulfates and toxic elements, exceeding the values: 2600, 980, 30, and 17 mg L−1 for SO4, Fe, Al, and Cu, respectively. For this reason, these deeper tailings were linked to the saline and acid seepage, and also to the sulfate acid efflorescences formed at the dike toe. In conclusion, the studied abandoned tailings impoundment is related with a high likelihood of polluting the environment, represented by very high IC and HAQ values. However, when the Tinto River is considered the receiving water body, the severity of the potential contamination must be judged as very low.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Old mining waste storage facilities abandoned without rehabilitation, or where the rehabilitation has been incomplete or negligent, often represent a permanent potential risk for the population and the environment, especially when they contain potentially toxic elements. The Directive 2006/21/CE of the European Parliament and the Council (European Parliament and Council 2006), on the management of waste from extractive industries, sets down that “it is necessary for Member States to ensure that an inventory of closed, including abandoned, waste facilities located on their territory is drawn up in order to identify those which cause serious negative environmental impacts or have the potential of becoming in the medium or short term a serious threat to human health or the environment.” Within this framework, the Geological Survey of Spain (Instituto Geológico y Minero de España, IGME) developed a methodology for the preliminary risk assessment of abandoned mine waste facilities (Alberruche del Campo et al. 2016). The complete methodology has been systematically applied to Spanish mining waste facilities, for the development and update of the national inventory under article 20 of the Mining Waste Directive, from 2011 to the present.
The methodology was designed to identify the most problematic tailings impoundments or mining heaps, assessing the likelihood and the severity of the consequences for various risk scenarios of contamination and physical instability. The assumption is that this risk assessment should be the first step for prioritizing abandoned mine waste facilities for rehabilitation. In addition to the site-specific factors that define the exposure pathways and receptors, the potential contamination risks caused by mining wastes depend firstly on their chemical properties. Concerning contamination processes, the methodology judges the total content of toxic elements and the water-soluble fraction, accepting that chemical contamination of soils, sediments, and water is the result of a combination of the dispersion of particles and dissolved elements by wind and water when they act on mining waste (Arranz-González et al. 2016). When the IGME methodology was designed, it was decided to assess the hazard of water pollution linked to soluble elements of mining wastes through leaching tests because, in Spain, water affected by mining wastes can rarely be sampled, especially in dry seasons.
The purpose of this work is to investigate and evaluate the contamination potential of a special case of tailings impoundment, using part of the overall evaluation methodology designed for abandoned mining waste facilities. This selected impoundment stores three different types of tailings, perfectly distinguishable from each other. From a practical point of view, part of the tailings will be more relevant as a source of pollution linked to eroded materials, and another will be more important due to the dissolution of pollutants. This circumstance is uncommon or, at least, it is the only case found among the facilities included in the Spanish inventory. The impoundment is located in the Riotinto mining district (Huelva, Spain, Fig. 1). It is one of the numerous abandoned mining waste facilities present in the Iberian Pyrite Belt (IPB). As is known, the exploitation of metallic minerals in the IPB, since pre-Roman times, has left a huge legacy of abandoned mines and one of the world’s largest accumulation of orphan mining wastes and sources of acid mine drainage (AMD) (Sánchez-España 2008). The work focuses on the application of two indices for potential toxicity assessment, which are part of the mentioned methodology: the Index of Contamination (IC) and the Hazard Average Quotient (HAQ) (Alberruche del Campo et al. 2016; Arranz-González et al. 2016). The IC index is involved in estimating the probability of erosion contamination scenarios. The HAQ index takes part in the estimation of the probability of water pollution by effluents generated in mine wastes. These indices allow obtaining a synthetic measure of the polluting potential of the mining wastes. By using these indices, this work aims to study whether the tailings stored at this facility have a great potential to contaminate nearby water bodies, even, from the perspective of the Tinto River, the severity of these effects may not be high.
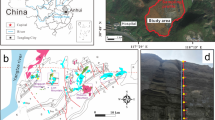
Simplified location map and images of the Represa I-II impoundment. a Aerial image. Modified from an image downloaded from the web page http://sigpac.mapa.es/fega/visor/. Superimposed numbers refer to UTM coordinates (ETRS89/UTM zone 29N). b Front view of the tailings impoundment, showing the three types of mining waste stored, and the sulfate efflorescences associated with seepage
Materials and methods
Site description
The study area is located in the Riotinto mining district (province of Huelva, Fig. 1), belonging to the well-known Iberian Pyrite Belt (IPB). There are several published papers that provide pertinent geological information (e.g., García-Palomero 1990; Leistel et al. 1998; Tornos 2006; Mellado et al. 2006, and references therein). The ores that have mainly mined in the Iberian Pyrite Belt consist of massive polymetallic sulfides (e.g., Leistel et al. 1998), containing pyrite (FeS2), with minor chalcopyrite (CuFeS2), arsenopyrite (FeAsS), sphalerite (ZnS), and galena (PbS). Another important ore exploited since very early in the IPB is gossan, which was mined for gold, copper, silver, and gold. It was formed by the weathering of massive sulfide mineralizations. Gossan appears enriched on secondary iron oxyhydroxides, oxides, and oxy-sulfates: goethite (α-FeO(OH)), hematite (Fe2O3), and minerals of the jarosite group.
The average annual temperature in the study area is over 17.1 °C. The average annual precipitation is 756 mm, and annual potential evapotranspiration reaches 758 mm. Despite the similarity of the two annual values, the distribution of precipitation and temperature throughout the year causes a marked dry summer, as is typical of the Mediterranean climate. This dry period, identified with the Thornthwaite water balance (Thornthwaite and Mather 1955), extends from June to September. Hydrology reflects this seasonal water balance with a marked water flow variability from summer to winter, as well as between different years. Theoretically, the original vegetation was a forest of holm oak (Quercus rotundifolia). Dominant land cover in the surroundings consists of spoil heaps, slag dumps, other tailings impoundments, industrial mining ruins, pine forests, and pastures.
The tailings impoundment named Represa I-II store flotation wastes from the Riotinto Mine. Recently, it was included in the inventory performed by the Geological Survey of Spain with the code 938-IV-4-003. It seems that the impoundment was operative until the beginning of the 50s. It is located in the municipality of Minas de Riotinto, within the area known as Zarandas-Naya. This area became the center of mineral processing from the 19th century until relatively recent times. The mineral-based processes carried out were three: copper concentration by flotation, concentration of gold and silver by oxidation, leaching and cyanidation, and copper cementation on iron scrap. The Zarandas-Naya area is the southernmost part of the Riotinto mining district, which is crossed by the Tinto River after passing the “Peña del Hierro” Mine at its source. Throughout this whole course, the river receives abundant inputs of sulfate, iron, metals, and metalloids from many mine adits, spoil heaps, ore stockpiles, tailings, and slags.
Represa I-II was the subject of research by Martín-Crespo et al. (2011) and Arranz-González and Cala-Rivero (2011). Likewise, it is located in the vicinity of a slag dump studied by Lottermoser (2005), and next to another abandoned tailings facility studied by Arranz-González et al. (2012). It is mostly a side-hill impoundment, although in its central part it can be considered a cross-valley type (Vick 1990) because it sits over the bed of an ephemeral creek. This ephemeral stream runs for about 350 m until joining the stream named Barranco del Pie de la Sierra, and the waters of both converge with the Tinto River about 200 m downstream. The impoundment can also be qualified as an upstream raised embankment (Vick 1990), formed with the thickest fractions of the tailings. Decant towers were installed inside the impoundment but are non-functional, probably for a long time. At the top, centered from north to south, a large trench was dug to drain any water that might be accumulated on the surface. The foot of the dam is located about 280 m above sea level, and the maximum height reaches about 26 m. The impoundment stores about 350,000 m3 of three different types of tailings, perfectly distinguishable from each other. This circumstance is uncommon or, at least, it is the only case found among the facilities included in the cited inventory. In the eastern half of the facility are recognized three thick overlapping levels (Fig. 2): (a) pale yellow to white tailings, located at the bottom and covered with a slag cover, like a stone mulch (deeper tailings, DT); (b) red tailings in the middle (intermediate tailings, IT); and (c) brownish-yellow tailings at the top (upper tailings, UT). The two deeper layers are no longer observed approximately from the buried creek of the central part to the west, that is, the upper layer overlies the middle layer at the eastern half and the natural terrain at the west. The dam sits on impermeable bedrock formed mainly by Devonian shales. At present, the slope of the dam is heavily eroded, except for the lower layer covered with slag. However, the slag cover is broken in the area overlying the stream, where the white tailings are apparent (Fig. 1 and Fig. 2).
There is no specific information available about the treatment processes from which derived such old mining wastes. Everything seems to indicate that, in this case, UT and DT consist of flotation wastes due to its small particle size (see below), even in the samples taken on the dike (DT). The intermediate tailings (IT) were probably produced by crushing and cyanide leaching of gossan. It seems that they are the rejects of a process that involves crushing, milling, and flotation/leaching of three different mineralizations, respectively, for DT, IT, and UT (Solomon 1976; Mellado et al. 2006; Tornos 2006): massive pyrites or massive sulfide ore (constituted by fine-grained pyrite and subordinate sphalerite, chalcopyrite and galena); gossan (a product of long-term supergene alteration of massive sulfides dominated by goethite and hematite); and stockwork (composed of anastomosed sulfide veins of pyrite, with quartz chalcopyrite and some chlorite). The history of the facility and the deposition of the layers are not well documented, but by observing old aerial photos, it follows that filling was completed before 1957. Therefore, the most superficial layers have undergone oxidation and erosion processes for more than 60 years. Although it is not possible to know the exact moment, a large drainage ditch was constructed approximately aligned with the buried channel of the creek. Similarly, during the 1990s, some of the easternmost red tailings were removed for reprocessing.
Sampling
Tailings samples were obtained in October 2013. Sampling was done following the methods described by Smith et al. (2000) and Hageman and Briggs (2000). The sampling method applied during the inventory consists of the collection of a composite sample, which is the product of the combination and mixture of 30 or more subsamples, taken following a random walk on the surface of each mine waste facility. At the top of the impoundment (Fig. 2), subsamples were obtained up to a depth of 20 cm, assuming that this surface horizon is the main source of dissolved elements and eroded particles. The composite sample was named UT. During the fieldwork, water was found emerging at the lowest point of the dam (corresponding with the bed of the buried creek). This water was extremely acid (see below). It was suspected, from the work of Arranz-González and Cala-Rivero (2011), that the upper tailings could not be responsible for this acidity. For this reason, it was also decided to take a sample of the white tailings. In order not to alter the slag cover, this sample was taken in the area of the stream, joining a total of five subsamples (Fig. 2). This sample was labeled DT (deeper tailings). During the same field visit, it was also decided to take a sample of the red tailings from the eastern intermediate section, to complete the characterization of all stored waste. This sample was identified as IT (intermediate tailings).
On new fieldwork developed in July 2014 (dry season), sulfate efflorescence formation was observed over the substrate, probably associated with seepage at the base of the impoundment dike (Fig. 2). Efflorescences of hydrated acid sulfates are produced by the evaporation of acidic waters generated from the oxidation of sulfide minerals (Hammarstrom et al. 2005). The dissolution of these efflorescent salts after the first rains following the dry period releases acidity and trace elements that can produce short-term environmental damage to water and soil (Alpers et al. 1994; Jambor et al. 2000). Four individual samples of these efflorescences were gently collected with a spatula, preserved in polyethylene bags, and immediately refrigerated in a portable fridge. The samples were named EF1, EF2, EF3, and EF4, with EF1 being the closest to the dam foot and EF4 the furthest.
In both October 2013 and July 2014, it was possible to sample water emerging at the lowest point of the dam (corresponding with the bed of the buried creek). These samples were named W1 and W2 for October and July, respectively. The flow was not measured, but was appreciably small in October and practically a droplet in July. A water sample was taken from the Tinto River in July 2014, downstream from the mining area. Another sample from the river, taken in February 2014, was also available. Water samples were divided into two parts. One part was filtered in the field with nitrocellulose filters (0.45 μm) and acidified with supra pure HNO3 for cation analysis. The other part was carried to the laboratory for analysis of Na, K, Ca, Mg, anions, pH, and electrical conductivity. The water samples were kept for a few days in a portable fridge until they were delivered to the laboratory.
Analytical procedures
Total and soluble toxic elements present in tailings samples were analyzed, with the support of other physical, mineralogical, and chemical characterizations. The solid samples were air-dried and homogenized. Then, tailings samples were sieved using a 2-mm stainless-steel sieve, discarding the > 2-mm fraction. According to Smith et al. (2000), the use of the < 2 mm size fraction appears to be a good option because the majority of the chemically reactive potential of the weathered surface of historic mine-waste facilities is encompassed on this fraction. Before physical and chemical analysis, moisture content was measured with a standard oven drying of the solid samples at 105 ± 5 °C over a period of 24 h. Then, particle size distribution was measured by standard dry sieving (ASTM 5000 series) for 2–0.063-mm fractions, combined with a Sedigraph 5100 particle size analyzer (Micromeritics, Norcross, USA) for < 0.063-mm fractions.
Solid samples were gently ground before chemical and mineralogical analysis, except for pH, electrical conductivity, and leaching tests. Minerals were identified by powder X-ray diffraction (XRD), using an X’PertPro equipment (PANalytical®, Malvern, UK), with monochromated CuKα radiation. Data were collected scanning from 3° to 62° 2θ with a counting time of 0.5 s per step. Minerals identification was developed considering areas under the peaks and d-spacings, employing the software of the diffractometer and the ICDD database.
Major elements (Si, Al, Fe, Ca, Ti, Mn, K, Mg, and P) were analyzed using a MagiX X-ray fluorescence spectrometer (PANalytical®, Malvern, UK). Na was determined by lithium tetraborate fusion and analysis by a Varian Spectra AA 220FS atomic absorption spectrophotometer (Varian Inc., Palo Alto, USA). An Orion pH-meter (Thermo-Fisher Scientific, Waltham, USA) was used to measure pH in a 1:1 soil-water mixture (Peech 1965; Price 1997). Total sulfur (wt% Stotal) was determined by ignition in a pure oxygen atmosphere, using an induction furnace with elemental CS-800 infrared analyzer (Eltra, Haan, Germany). The analysis of Ssulfate (wt%) was performed by digestion in 10% HCl, and adding an excess of precipitating reagent (BaCl2) to precipitate BaSO4, which was determined by gravimetry. The percent of Ssulfide content was obtained by the difference between Stotal and Ssulfate. The potential for acidity generation (AP) (as kg of equivalent CO3Ca t−1) of wastes is obtained by multiplying the % of S by 32.25. This method may underestimate AP if jarosite or other acid-producing sulfate minerals are present. The neutralization potential (NP) of tailings was determined by digestion of 2 g of sample in hot HCl and then titrated with NaOH (Sobek et al. 1978).
The analysis of total trace elements (except Hg) was achieved by digestion with a concentrated mixture of HF-HNO3-HClO4 heated to dryness, and then dissolving the residue with HNO3. The concentration of 22 elements was measured by ICP-MS (inductively coupled plasma-mass spectrometry), in a 7500ce equipment (Agilent®, Santa Clara, USA), or by ICP-AES (inductively coupled plasma atomic emission spectrometry), in a Vista MPX equipment (Varian Inc., Palo Alto, USA). Mercury was analyzed by cold vapor atomic absorption spectrometry (EPA 7471 method, USEPA 1994) that is: a part of each sample was digested with aqua regia (three volumes of concentrated HCl plus one volume of concentrated HNO3), and KMnO4 was added to maintain the metal in an oxidized state. The released Hg was measured using a continuous flow of cold vapor with the SpectrAA 220FS equipment (Varian Inc., Palo Alto, USA).
The potential solubilization of trace elements from tailings and efflorescences was evaluated using leaching extracts (in a solid-water ratio of 1:10), as described by the European standard EN 12457-2 (ECS 2002), without particle size reduction. The leachates were analyzed by ICP-MS with the above mentioned 7500ce spectrometer (Agilent®, Santa Clara, USA). A Multimeter MM41 (Crison, Alella, Spain) was employed to determine electric conductivity, Eh, and pH of the leachates. An UV-Vis absorption spectrophotometry was used or anions determination, employing an Alliance-Integral-Plus autoanalyzer (AMS-Alliance, Frepillon, France) of continuous flow. The determination of Na, K, Mg, Ca, and Fe was performed by atomic absorption spectrophotometry (Flame) with a SpectrAA 220FS instrument (Varian Inc., Palo Alto, USA). The same analytical techniques and instruments used for leachates were applied to water samples.
All the obtained results were evaluated with an internal quality control system of the IGME labs, involving analysis of replicates, blanks, and Standard Reference Materials (SRM). For accuracy verification, the SRM employed were GBW 077103, GBW 07114, and GBW 07104 (China National Analysis Center for Iron and Steel, NACIS), for major determinations by XRF; GBW 077105 and GBW 07106 (NACIS), for Na determination by AAS; AR 4016, AR 4018, and AR 4019 (Alpha Resources, Inc.) for S determination by elemental analyzer; GBW 07103, GBW 07105, and GBW 071038 (NACIS) for trace element determinations by ICP-MS or ICP-AES; GBW 07404, and GBW 07405 (NACIS) for Hg determination by AAS; custom standards supplied by Inorganic Ventures for anions determinations with an Autoanalyzer, and Na, K, Mg, Ca, and Fe determinations by AAS; TM-9.2, and TM-62.2 (Environment and Climate Change Canada) for trace element determinations by ICP-MS (liquid samples). The maximum relative error was 10% for spectrometric analysis. All other maximum relative errors were between 1 and 5%. The limits of detection (LOD) (in μg mL−1) were 1 for Al; 0.5 for Fe; 0.05 for Ca, and K; 0.025 for Na, and Mg. The LODs for trace elements were (μg L−1): 0.05 for As, Co, Cr, and V; 0.2 for Cd, Cu, Mo, Pb, and Zn; and 0.5 for Ni, and Se. LOD for SO4 2− was 0.02 g L−1. Other limits of detection are shown in the respective tables.
Index of Contamination
The erosion of mining wastes can affect nearby soils and sediments. When high concentrations of potentially toxic elements are present in the wastes, the potential to contaminate soils and sediments, due to the dispersion of particulate material, is best assessed by measuring the total content of these elements. This does not exclude that the mobility or bioavailability of trace elements transported in the eroded particles will depend on the pH, organic matter content, redox potential, and temperature of the deposition site (Alloway 2013). The assessment of the potential for contamination of particulate matter emitted from mining waste facilities was based on the examination of potentially toxic elements concentration in waste. As, Cd, Co, Cr, Cu, Hg, Mo, Ni, Pb, V, and Zn were the studied potentially toxic elements (PTE), according to European Commission Decision 2009/359/EC (European Commission 2009), to which Se was added. The methodology for the regular classification of abandoned mining waste facilities in Spain (Alberruche del Campo et al. 2016; Arranz-González et al. 2016) employs an aggregation data procedure called Index of Contamination (IC), which is proposed as a measure of the potential of mining waste to contaminate soils and sediments due to erosion processes. It is determined by the average of the relationship between total concentrations measured in a mining waste and the regional soil background:
where X is the measured total metal concentration in the mining waste, BLX is the geochemical background value for the X element, and n is the number of elements in which concentration measured in mining waste is higher than BLX. The value of this index is always higher than 1. According to Arranz-González et al. (2016), the greater the value of IC, the greater is the likelihood of pollutants emission to nearby soils and sediments. Therefore, the values obtained from the index, together with the assessment of other circumstances specific to each case, can provide the first element of judgment to establish a ranking of the potential for contamination of different types of mining waste.
Hazard Average Quotient
Knowledge about the total contents of potentially toxic elements is not sufficient to assess the hazard of mining wastes. The chemical speciation present in mining waste is more determinant of mobility than the total concentration of the toxic elements (Arranz-González and Cala-Rivero 2011). Accordingly, leaching tests are helpful for geochemical characterization of the soluble constituents that can be mobilized from mining wastes (Hageman and Briggs 2000). With a view to water pollution likelihood, the methodology of the Spanish Geological Survey (Alberruche del Campo et al. 2016; Arranz-González and others Arranz-González et al. 2016) focuses on the elements included in European Commission Decision 2009/359/EC, but adding Al and Se. Another index, named “Hazard Average Quotient” (HAQ) was created to assess the potential toxicity factor for water due to the presence of soluble PTEs in abandoned mining wastes. The reference values were selected levels for the toxic elements, Al, As, Cd, Co, Cr, Cu, Hg, Mo, Ni, Se, Pb, V, and Zn, obtained from various water quality standards (Alberruche del Campo et al. 2016; Arranz-González et al. 2016). This set of values is very conservative, both from the perspective of the health of aquatic ecosystems and for any other possible use of water. The HAQ is calculated as:
where [XLEACH]i is the concentration of the ith element in the leachate, WQLx is the water quality standard for the element X, and n is the number of elements for which the resulting fractions are greater than one. WQL is a demanding standard, formed by the lowest concentration values among those contemplated by European Council (1998), USEPA (2002), USEPA (2012), and Puura and D’Alessandro (2005).
It is interesting to note that, apart from the use of these indices for the evaluation of abandoned mining waste facilities inventoried in Spain, their application has also inspired other recent works (Del Rio-Salas et al. 2019; Peña-Ortega et al. 2019, Guzmán-Martínez et al. 2020).
Results and discussion
Grain size and mineralogy
The particle size fraction below 2 mm was 100% for IT and DT, and 99.54% for UT. The sand (2–0.063 mm) content of the samples was 44.5, 55.2, and 60.17% dry weight, respectively, for UT, IT, and DT samples. UT was more clayey with (20%), compared to 10.4% for DT and 8.1% for IT.
Table 1 shows the mineralogical composition obtained by powder XRD. For the comments that follow, it has been considered that the term primary refers to the minerals probably present in the fresh tailings stored in the impoundment, and secondary refers to the minerals originated by the weathering processes (Jambor 1994). As mentioned, tailings of the upper part of the impoundment (UT) have been subjected to weathering for a long time. Therefore, these materials offer the typical appearance of weathered pyritic tailings, with a brownish yellow color (Munsell notation 10YR 6/8), which is characteristic of the presence of secondary minerals like jarosite and goethite. Jarosite precipitates at low pH, high SO42− ion concentration, high levels of Fe3+, and oxidizing environments (Jambor 1994). Goethite is the most stable secondary Fe mineral and is mostly formed under slightly acidic conditions, or it may also form through the transformation of less stable oxyhydroxides and hydroxysulfates (Murad and Rojik 2005). No other secondary minerals were detected by XRD analysis in the UT sample due probably to their low crystallinity. The presence of quartz and chlorite in these tailings indicates the probable origin of the exploited material: stockworks. The exploited stockworks of Riotinto Mine were composed of anastomosing veinlets of quartz with disseminated sulfides (Nehlig et al. 1998). The quartz-sulfide veins of stockworks fit on chloritized host rocks. The mineralogical results were consistent with those obtained by Martín-Crespo et al. (2011) for mostly oxidized UT samples taken in a profile located in the face of the central trench. These authors found some pyrite and sphalerite only at 2.5 m depth.
IT red tailings (Munsell notation 10R 4/4), quartz, goethite, jarosite, and hematite were identified. All of these minerals could be primary. Indeed, the IPB gossans are dominated by goethite and hematite, with quartz and some members of the jarosite group, like jarosite, plumbojarosite, or beudantite (García-Palomero et al. 1986).
The residues at the base of the impoundment (DT) were pale yellow to very light greenish-gray in the field (Munsell notations 5Y 8/1 to 10Y 8/1). In the DT sample, the identified minerals were quartz, jarosite, pyrite, elemental S, and a not well-identified phyllosilicate (probably chlorite). This combination of minerals is likely to result from the alteration of massive sulfides. The presence of other secondary hydroxides or Fe-sulfates cannot be ruled out since the XRD technique is not sensitive enough to detect low concentrations of these minerals, or because they may be minerals of low crystallinity.
All efflorescence samples were complex sulfate assemblages. The presence of quartz on EF2 and EF3 may be explained by the presence of substrate particles carried-ever during sampling, or because quartz grains provided sites for sulfate mineral formation, and were thus inevitably included during sampling (Grover et al. 2016). Except for gypsum, all the detected secondary minerals in efflorescences are highly soluble (Hammarstrom et al. 2005). Copiapite was detected in all the efflorescence samples. This is the most common iron-sulfate mineral associated with oxidized mine wastes (Blowes et al. 2004). According to Jambor et al. (2000), in the field, the paragenesis indicates an evolutive sequence from the formation of the divalent salts of iron (rozenite) to minerals containing both ferrous and ferric iron (copiapite). Indeed, rozenite is only present in the two samples closest to the dam foot. Pickeringite was also present in three samples. This sulfate is formed mainly at low pH, by the oxidation of pyrite or other sulfides that are found together with Al-rich minerals. Other divalent cation minerals detected in efflorescent salts were gypsum and hexahydrite. It is reasonable to think that the flow of acidified water through the slag cover can also influence the subsequent precipitation of these minerals, as Lottermoser (2005) showed in his study of a nearby slag dump.
Bulk chemical composition
The major oxides analysis indicated that UT and DT tailings were characterized by a high percentage of SiO2, and a relatively important concentration of Fe2O3 (Table 2). The Al2O3 percentage was much lower in both materials, although slightly more significant in UT. About 18% in DT and 6% in UT corresponded to Loss on Ignition (LOI). Other oxides present in these materials were negligible. Unlike the previous ones, IT was dominated by Fe2O3, with a certain percentage of SiO2, and lower amounts for the remaining oxides. LOI was intermediate in this sample. SiO2 content follows the order UT > DT > IT, like in the expected sequence: stockworks > massive sulfides > gossan. The Fe2O3 content follows the order IT > DT > UT, like in the expected sequence: gossan > massive sulfides > stockworks.
The efflorescent salts were dominated clearly by Fe2O3, with similar values than DT. As stated above, the presence of relatively more silica content in two of the salts can be interpreted as contamination, or by the inclusion of quartz grains into the efflorescences (Grover et al. 2016). The content of MgO was also significant. The magnitude reached by the LOI was remarkable, exceeding 63% in all cases, which is mainly attributable to water from hydrated minerals.
In general, the chemical composition agrees quite well with the mineralogy. The efflorescence salts concentrate Mg compared to the three types of mine wastes. As set out below, the DT residue provided the largest measure of soluble Mg. In the EF2 sample, the presence of hexahydrite would explain this. In the other samples, this mineral may be present in a concentration that makes it undetectable. It is also possible, as mentioned, that the acidic water emerging from the impoundment toe solubilizes some Mg when passing through the slag cover. This element can substitute Fe in melanterite and copiapite. Regarding pH values, the extraordinary acidity of the DT sample, as well as such of all the efflorescences, should be highlighted. This fact can be interpreted as the first evidence of the relationship between the chemical characteristics of DT tailings and the seepage water, which is the origin of the efflorescences.
Table 3 gives the results of the sulfur forms and the calculation of the acid-base account ABA based on Sobek et al. (1978). Total S ranged from 0.24 wt% in UT to 19.3 wt% in DT. Total sulfur measured in efflorescence samples ranged from 14.1 to 18.8%. Whereas a material can be considered as acid-generating when its net neutralization potential (NNP) is lesser than -20 kgCaCO3 equivalent/t (Ferguson and Morin 1991), neither UT nor IT can be qualified as acid-generating wastes. Nevertheless, the calculated net neutralization potential for DT was strongly negative as a consequence of the acid generation caused by the sulfide oxidation, and the lack of suitable acid-neutralizing minerals. To this, it must be added the unevaluated presence of secondary acid-generating minerals, such as jarosite.
The total contents levels of toxic elements measured in waste samples are shown in Table 4. IC values were calculated taking into account these total element concentrations, and adopting as reference the background soil levels established by Junta de Andalucía (2004) for the Sud-Portuguese Zone (ZSP). The IPB is located in this big geologic dominium. For comparison purposes, the values obtained in the profile studied by Martín-Crespo et al. (2011) are also used. Thinking about the evident erosion of the slopes of UT and IT tailings layers, and the subsequent emission of sediments to watercourses, the results were also compared to some data from the Tinto River sediments, taken in the final section of the mining area (Galán et al. 2003).
There were neither regional background values for Mo and V, but the measured contents were not of great concern: Mo (3.2–16.1 mg kg−1) and V (1.8–23.6 mg kg−1). For Se, while no references are provided in Table 4, the results obtained for the three tailings types are considered very high (21–67.1 mg kg−1) according to RCT (1988), which admits a maximum of 3 mg kg−1 for suitable surficial materials on mine-soils. Cr and Ni concentrations measured in all samples were below the soil background level, and Cd concentration was under the detection limit. The Zn content was high in DT and IT samples, above the values accepted by RCT (1988). Co was high only in DT, and Hg in the other tailings samples. According to RCT (1988), Hg was unacceptable in DT and IT samples. The total contents of As, Cu, and Pb were higher than the background levels. Especially remarkable were the values achieved in the IT sample: 8689 mg kg−1, 1012 mg kg−1, and 14,822 mg kg−1, for As, Cu, and Pb, respectively. The total contents of Pb and As measured in this sample are much higher than those measured in sediments from the Tinto River taken in the final part of the mining area as ochreous precipitates. This is a clear consequence of the origin of IT, which undoubtedly derived from gossan. For example, the iron content of gossan was around 50% in Cerro Colorado, with 0.7 of arsenic (Williams 1934). The contents of iron and arsenic in the IT sample are 50.5% and 0.86%, respectively. The contents of Pb also point in this direction.
According to the IC values, all the tailings samples exhibit a high potential to contaminate surrounding soils and sediments, especially IT. The calculated IC for IT sample (237.8) is the highest ever published (Arranz-González et al. 2016; Arranz-González et al. 2017; Arranz-González et al. 2019; Del Rio-Salas et al. 2019; Peña-Ortega et al. 2019; Fernández-Naranjo et al. 2020; Guzmán-Martínez et al. 2020). When assessing the pollution due to eroded tailings dispersion (by water or wind erosion processes), the exposed tailings surface must be taken into account, which is maximum for UT and minimum for DT. Besides, the slag cover over the slope formed in DT tailings would reduce the possibility of erosion to almost zero. In addition to the climatic characteristics of the area, all these considerations should be taken into account to evaluate the likelihood of pollution by the emission of eroded particles from this impoundment. But it should be borne in mind that, in the IGME methodology, the comparison of the contents of potentially toxic elements of mining wastes with the corresponding soil background levels constitutes the primary yardstick for assessing the pollution potential connected with erosive processes. Nevertheless, in this special case, if the Tinto River is seen as the main final receptor, the contamination risk would be much lower, as can be recognized from the sediment data (Galán et al. 2003). Even so, it would still be appreciable for As and Pb coming from intermediate tailings (IT). The real effect on the river can only be known by studying the sediments or precipitates along the entire pathway from the impoundment.
Leaching behavior
Tailings and efflorescences were subjected to the leaching test according to European standard EN 12457-2. Leaching results are shown in Table 5, which also includes water data from the seepage and the Tinto River. The values measured in the Tinto River samples were in general agreement with those published by de la Torre et al. (2011) and Cánovas et al. (2014), for the final segment of the river within the Riotinto mining district, somewhat further south than the Zarandas-Naya area.
The waste material type, influencing the solubility of elements, dictated the pH of the leachates. In general terms, it is worth mentioning that there was very limited solubilization of all the elements into the leachates of UT and IT samples. Only the percentage of soluble aluminum in UT is remarkable (23.5). The soluble percentage is between 0 and 4.7 for the rest of the elements. For the UT sample, only the values obtained for Al, Cu, Pb, and Zn exceed the standard. For IT, only Al, Hg, Se, and Zn exceed it. It should be remembered that this is a very demanding water standard. Only Hg is prominent in IT tailings. According to Arranz-González et al. (2019), the Hazard Average Quotient (HAQ) values obtained for these samples can be qualified as very low, which represents a low likelihood of contaminating water in the short term.
Thinking specifically of the Tinto River, which will sooner or later be the receptor of the dissolved elements, it does not seem likely that the input of soluble elements it might receive from these wastes will affect it. Even Hg, which has the potential to dissolve appreciably, appears below the detection limit in both seepages and efflorescences, which could mean attenuation through precipitation or sorption reactions in the tailings impoundment themselves. On the other hand, the soluble Fe, Al, Cu, Zn, and sulfate contents measured in these tailings cannot be the origin of the concentrations measured for the same parameters in seepage and efflorescence samples.
Certain very high levels of total elements in the UT and IT samples were not reflected in the corresponding leachates. This is the case for As, Se, and Pb. In particular, the negligible Pb content in the leachate of the IT sample is striking, despite the very high total content. A plausible explanation for the reduced solubility of As and Se could be the incorporation of arsenate and selenate by SO42− substitution in jarosite, and the retention in goethite. On the other hand, it is possible the incorporation of Pb in jarosite, resulting in a solid solution of jarosite-plumbojarosite (Fendorf et al. 1997; Dutrizac and Jambor 2000; Savage et al. 2000). Furthermore, the presence of undetected anglesite (PbSO4) and/or beudantite PbFe3(SO4)(AsO4)(OH)6 (a typical mineral in gossan) cannot be excluded.
On the other hand, the leachate of the DT sample was, by far, the highest concentrate on sulfate and all the elements studied. Leachates were extremely saline and acidic, (EC = 2.64 mS cm−1 at 25°, and pH 1.57). The elements Al, As, Cu, and Zn were particularly high (Table 5). The percentages that represent the soluble concentrations of Al, As, Co, Cr, Cu, Fe, Ni, and V are moderate, exceeding 5%. The percentages that represent the soluble concentrations are low for the rest of the elements. The soluble concentrations of Ca (7 mg L−1) and Mg (6.22 mg L−1) were also significant, being much higher than those measured in the other two tailings samples. It was considered interesting the comparison of the total release results (expressed in mg kg−1) with the waste acceptance criteria for landfills. The results obtained for the DT sample were 40 mg kg−1 and 174 mg kg−1 of As and Cu, respectively, which are above the limits for hazardous waste (25 mg kg−1 for As, and 100 mg kg−1 for Cu). According to Arranz-González et al. (2019), the HAQ value obtained for the DT (237.8) can be qualified as very high, showing a very high potential for emitting contaminating effluents to water. However, reviewing the literature providing data for this index, calculated with the same leaching method, it is possible to find up to 11 wastes from metal and coal mining that exceed the value obtained for DT (Arranz-González et al. 2016; Arranz-González et al. 2019; Guzmán-Martínez et al. 2020).
As expected, in the case of efflorescence samples, the percentages of soluble elements were very high for almost all elements. However, the elements As, Cr, Pb, Se, and V were less soluble, with percentages between 14.8 and 79.7. They are probably associated with minerals of the jarosite group, which were not detected. The percentage of Mo could not be calculated in three of the four samples, and Hg was practically insoluble in all of them.
Seepage water samples have pH values ranging from 2.44 to 2.76. As expected, the concentrations of seepage samples were greater than in DT leachate, and higher in the dry season (July) than in October for all the elements and sulfate, except for As and Se. The most abundant elements in solutions produced by leaching the efflorescent salts were Fe, Al, Cu, and Zn. On the other hand, the formation of efflorescent minerals removed SO4− and metals from the solution. It is reasonable to think that infiltration water (and seepage) from the waste repository is the main contributor to efflorescences nature. Assuming that seepage data from July can be quite directly related to those obtained for the efflorescences closest to the foot of the impoundment, the ratios between the concentrations of potentially toxic elements in efflorescence leachates and those in the seepage were calculated. The ratios were as follows: Se (4.6) > As = Pb = Zn (3.6) > Ni (2.9) > Co (2.5) > Fe (2.4) > V (2) > Al ≈ Cd (1.7) > Cu (1.2). As and Pb were more concentrated in most distal efflorescent salts, probably incorporated to solid-solution of copiapite, a typical mineral of hyper-acid environment (pH 0.94–1.12). In any case, starting from a solution that could resemble DT leachate, the result of efflorescence salts formation is an important enrichment of elements. This is difficult to explain because efflorescence formation involves a series of complex processes: dissolution, seepage through the base of the dike, soaking of the substrate and interaction with it, drying, and mineral precipitation. The final consequence is that the soluble concentration of SO42− was very high on all the dissolved salts (up to 39,000 mg L−1), and soluble Fe, Al, Zn, As, Cu, and Co were extremely high. Indeed, these efflorescent salts are solid solutions that incorporate a variety of ions (e.g., Hammarstrom et al. 2005) and scavenge these elements during precipitation. However, this retention is brief because it is well known that the dissolution of metal-sulfate salts during storm-runoff events causes an abrupt increase in dissolved metal and sulfate concentrations, even as the water flow increases (Dagenhart Jr 1980). Undoubtedly, the quality of the waters that intermittently flow through the small stream buried by the impoundment has been affected for decades. However, looking at the seepage data, and not considering the probable dilution and attenuation from the sampling points to the confluence with the Tinto River, only Fe, Pb, and perhaps Co content would be slightly increased.
It should be emphasized that the sampling of water and efflorescences is not always feasible when carrying out inventories, as it depends on the season, the weather, and the nature of the mining waste. In Spain, water and efflorescent salts associated with waste facilities have rarely been sampled. In this sense, when the methodology was designed, it was decided to assess each potential for water pollution by leaching tests, even if the existence of other data would facilitate understanding and judgment. From the perspective of the inventories and assessments made, the HAQ has proved valuable. However, in the exceptional case studied, it was considered necessary to assess the hazard based on leaching data for deeper tailings (DT) rather than those dominating in volume and surface area exposed (UT).
Conclusions
The tailings impoundment named Represa I-II represents a special case among the abandoned mining waste facilities in Spain. It stores three very different types of waste: UT, IT, and DT. Erosion processes have produced and will continue to produce the emission of toxic elements (As, Pb, and Cu) linked to the eroded particles. The application of a multi-pollutant index, named Index of Contamination (IC) to the total content data, allows characterizing the toxicity of these tailings as very high. The IC calculated for IT sample is the highest ever published. Furthermore, decades of tailings weathering have generated surficial oxidation. As a result, the surface layers of wastes are enriched in readily soluble weathering products such as sulfate and toxic elements, which are also present in seepage water. The solubility of some metals in leachates obtained by the European Standard EN-12457-2 was very high, especially for Al, As, Cu, Pb, and Zn for deeper tailings (DT). Regarding soluble elements, an additional index named Hazard Average Quotient (HAQ) was applied to leachates data, resulting very high for DT tailings.
Finally, Represa I-II embodies a high hazard for soils, sediments, and water contamination, represented by very high IC and HAQ values. Other information on pathways and receptors is necessary for assessing the risk of this facility: distance to water bodies, water uses, people’s presence and access, nearby land uses, etc. However, if the Tinto River is considered the main receiving water body, the severity of the effects of this potential contamination must be judged as very low. In this sense, for remediation priorities, this abandoned tailings impoundment cannot be regarded as a priority, especially when the exceptional characteristics of the Tinto River have made it worthy of protection in its current state. This does not detract that Represa I-II is undoubtedly one of many abandoned mining facilities that have contributed to making the Tinto River what it is today. Similar studies could be extended to the many other abandoned facilities in the Riotinto mining district, to obtain a clearer idea of the influence of historical mining on the Tinto River nature.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Alberruche del Campo ME, Arranz-González JC, Rodríguez-Pacheco R, Vadillo-Fernández L, Rodríguez-Gómez V, Fernández-Naranjo FJ (2016) Simplified guide for closed/abandoned mining waste facilities risk assessment MAGRAMA-IGME. Madrid. https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/publicaciones/guiasimplificadaevaluacionriesgoseninglesversion2_tcm30-185046.pdf
Alloway BJ (2013) Heavy metals in soils. In: Environmental pollution, 3rd edn. Springer Netherlands, Dordrecht. https://doi.org/10.1007/978-94-007-4470-7
Alpers CN, Blowes DW, Nordstrom DK, Jambor JL (1994) Secondary minerals and acid mine-water chemistry. In: Jambor JL, Blowes DW (eds.), Environmental geochemistry of sulfide mine-wastes, Mineral Assoc Can Short Course Ser 22, pp 247–270
Arranz-González JC, Cala-Rivero V (2011) Evaluación de la movilidad de metales pesados en residuos mineros de flotación de minería metálica en la provincia de Huelva. Bol Geol Min 122(2):203–220
Arranz-González JC, Cala-Rivero V, Iribarren-Campaña I (2012) Geochemistry and mineralogy of surface pyritic tailings impoundments at two mining sites of the Iberian Pyrite Belt (SW Spain). Environ Earth Sci 65:669–680
Arranz-González JC, Rodríguez-Gómez V, Alberruche-del Campo ME, Vadillo-Fernández L, Fernández-Naranjo FJ, Reyes-Andrés J, Rodríguez-Pacheco R (2016) A methodology for ranking potential pollution caused by abandoned mining wastes: application to sulfide mine tailings in Mazarrón (Southeast Spain). Environ Earth Sci 75(8):656–666
Arranz-González JC, Vadillo-Fernández L, Alberruche-delCampo E, Rodríguez-Gómez V, Fernández-Naranjo FJ, Rodríguez-Pacheco R (2017) Metodología para clasificar la contaminación potencial causada por residuos mineros abandonados. Aplicación a los residuos mineros del distrito Linares-La Carolina. XII Congreso Nacional-XI Ibérico de Geoquímica. Linares
Arranz-González JC, Rodríguez-Gómez V, Rodríguez-Pacheco R, Fernández-Naranjo FJ, Vadillo-Fernández L, Alberruche del Campo E (2019) Guía para la rehabilitación de instalaciones abandonadas de residuos mineros. MITECO. Madrid. https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/publicaciones/guiarehabilitacioninstalacionesresiduosminerosabandonadas2019_tcm30-496582.pdf
Blowes DW, Ptacek CJ, Jambor JL, Weisner CG (2004) The geochemistry of acid mine drainage. In: Holland, H.D, Turekian, KK, Lollar BS (eds.), Treatise on geochemistry, environmental geochemistry, vol. 9. Elsevier, pp 149–204
Cánovas CR, Olías M, Nieto JM (2014) Metal (loid) attenuation processes in an extremely acidic river: the Río Tinto (SW Spain). Water Air Soil Pollut 225. https://doi.org/10.1007/s11270-013-1795-7
Dagenhart TV Jr (1980) The acid mine drainage of Contrary Creek, Louisa County, Virginia: factors causing variations in stream water chemistry. MSc thesis, Univ. Virginia, Charlottesville
De la Torre ML, Grande JA, Graiño J, Gómez T, Cerón JC (2011) Characterization of AMD pollution in the River Tinto (SW Spain). Geochemical comparison between generating source and receiving environment. Water Air Soil Pollut 216(3):3–19
Del Rio-Salas R, Ayala-Ramírez Y, Loredo-Portales R, Romero F, Molina-Freaner F, Minjarez-Osorio C, Pi-Puig T, Ochoa–Landín L, Moreno-Rodríguez V (2019) Mineralogy and geochemistry of rural road dust and nearby mine tailings: a case of ignored pollution hazard from an abandoned mining site in semi-arid zone. Nat Resour Res 28. https://doi.org/10.1007/s11053-019-09472-x
Dutrizac JE, Jambor JL (2000) Jarosites and their application in hydrometallurgy. In: Alpers CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals: crystallography, mineralogy and environmental significance. Reviews in Mineralogy and Geochemistry. Mineralogical Society of America and the Geochemical Society, vol 40. Washington, D.C., pp 405–452
ECS (European Committee for Standardization) (2002) EN 12457-2 Standard: Characterización of waste-Leaching-Compliance test for leaching of granular waste materials and sludges-part 2: one stage batch test at a liquid to solid ratio of 10l/kg for materials with high solid content and with particle size below 4 mm (without or with size reduction), Brussels
European Commission (2009) Commission Decision of 30 April 2009 completing the definition of inert waste in implementation of Article 22(1) (f) of Directive 2006/21/EC of the European Parliament and the Council concerning the management of waste from extractive industries. Off J Eur Union L110:46–47
European Council (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Commun L330:32–54
European Parliament and Council (2006) Union Directive 2006/21/EC of 15 March 2006 on the management of waste from extractive industries. Off J Eur Commun L102:15–33
Fendorf S, Eick MJ, Grossl P, Sparks DL (1997) Arsenate and chromate retention mechanisms on goethite. 1. Surface structure. Environ Sci Technol 31(2):315–320
Ferguson K, Morin K (1991) The prediction of acid rock drainage: lessons from the data base. In: Proceedings of the second international conference on the abatement of acidic drainage, vol 3. CANMET, Ottawa, pp 83–106
Fernández-Naranjo FJ, Arranz-González JC, Rodríguez-Gómez V, Rodríguez-Pacheco RL, Vadillo L (2020) Geochemical anomalies for the determination of Surface stream sediments pollution: case of Sierra de Cartagena-La Unión mining district, Spain. Environ Monit Assess 192:247. https://doi.org/10.1007/s10661-020-8199-0
Galán E, Gómez-Ariza JL, González I, Fernández-Caliani JC, Morales E, Giráldez I (2003) Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Appl Geochem 18:409–421
García-Palomero F (1990) Río Tinto deposits. Geology and geological models for their exploration and ore reserves evaluation. In: Sulphide deposits: their origin and processing. The Institute of Mining and Metallurgy, London, pp 17–35
García-Palomero F, Bedia-Fernández JL, García-Magariño M, Sides EJ (1986) Nuevas investigaciones y trabajos de evaluación de reservas de gossan en minas de Río Tinto. Bol Geol Min Sp 97-5:622–642
Grover BPC, Johnson RH, Billing DG, Weiersbye IM, Tutu H (2016) Mineralogy and geochemistry of efflorescent minerals on mine tailings and their potential impact on water chemistry. Environ Sci Pollut Res 23:7338–7348. https://doi.org/10.1007/s11356-015-5870-z
Guzmán-Martínez F, Arranz-González JC, Ortega MO, García-Martínez MJ, Rodríguez-Gomez V (2020) A new ranking scale for assessing leaching potential pollution from abandoned mining wastes based on the Mexican official leaching test. J Environ Manag 273:11139
Hageman PL, Briggs PH (2000) A simple field leach for rapid screening and qualitative characterization of mine waste material on abandoned mine lands. ICARD 2000, Fifth International Conference on Acid Rock Drainage. Society for Mining, Metallurgy and Exploration Inc., Denver, pp 1463–1475
Hammarstrom JM, Seal RR II, Meier AL, Kornfeld JM (2005) Secondary sulfate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments. Chem Geol 215:407–431. https://doi.org/10.1016/j.chemgeo.2004.06.053
Jambor JL (1994) Mineralogy of sulphide rich tailings and their oxidation products. In: Jambor JL, Blowes DW (eds) Environmental geochemistry of sulfide mine wastes. Miner Assoc Can Short Course Ser 22:103–132
Jambor JL, Blowes DW, Ptacek CJ (2000) Mineralogy of mine wastes and strategies for remediation. In: Vaughan DJ, Wogelius RA (eds) European Mineralogical Union Notes in Mineralogy, Environmental Mineralogy, Budapest University Press, chap. 7, vol. 2, pp 255–290
Junta de Andalucía (2004) Estudio de Elementos Traza en Suelos de Andalucía. Serie Informes, Estudios, Trabajos y Dictámenes. Consejería de Medio Ambiente de la Junta de Andalucía, Sevilla
Leistel JM, Marcoux E, Thiéblemont D, Quesada C, Sánchez A, Almodóvar GR, Pascual E, Sáez R (1998) The volcanic-hosted massive sulphide deposits of the Iberian Pyrite Belt. Mineral Deposita 33:2–30
Lottermoser BG (2005) Evaporative mineral precipitates from a historical smelting slag dump, Rio Tinto, Spain. Neues Jahrbuch Fur Mineralogie-Abhandlungen 181:183–190
Martín-Crespo T, Martín-Velázquez S, Gómez-Ortiz D, De Ignacio-San José C, Lillo-Ramos J (2011) A geochemical and geophysical characterization of sulfide mine ponds at the Iberian Pyrite Belt (Spain). Water Air Soil Pollut 217:387–405
Mellado D, González Clavijo E, Tornos F, Conde C (2006) Geología y estructura de la Mina de Río Tinto (Faja Pirítica Ibérica, España). Geogaceta 40:231–234
Murad E, Rojik P (2005) Iron mineralogy of mine-drainage precipitates as environmental indicators: review of current concepts and a case study from the Sokolov Basin, Czech Republic. Clay Miner 40:427–440
Nehlig P, Cassard D, Marcoux E (1998) Geometry and genesis of feeder zones of massive sulphide deposits: constraints from the Río Tinto ore deposits (Spain). Mineral Deposita 33:137–149
Peech M (1965) Hydrogen-ion activity. In: Black CA (ed) Methods of soil analysis, Part II, chemical and microbiological properties. American Society of Agronomy, Madison, pp 914–926
Peña-Ortega M, Del Rio-Salas R, Valencia-Sauceda J, Mendívil-Quijada H, Minjarez-Osorio C, Molina-Freaner F, de la O-Villanueva M, Moreno-Rodríguez V (2019) Environmental assessment and historic erosion calculation of abandoned mine tailings from a semi-arid zone of northwestern Mexico: insights from geochemistry and unmanned aerial vehicles. Environ Sci Pollut Res 26:203–215. https://doi.org/10.1007/s11356-019-05849-w
Price WA (1997) Draft guidelines and recommended methods for the prediction of metal leaching and acid rock drainage at minesites in British Columbia, British Columbia Ministry of Energy and Mines, Smithers
Puura EM, D’Alessandro M (2005) A classification system for environmental pressures related to mine water discharges. Mine Water Environ 24-1:43–52
Railroad Commission of Texas (RCT) (1988) Technical release SA-2: suitability criteria for topsoil substitutes and materials suitable for placement in the top four feet of leveled minespoil. Railroad Commission of Texas, Surface Mining and Reclamation Division, Austin
Sánchez-España J (2008) Acid mine drainage in the Iberian Pyrite Belt: an overview with special emphasis on generation mechanisms, aqueous composition and associated mineral phases. Macla 10:34–43
Savage KS, Tingle TN, O’Day PA, Waychunas GA, Bird DK (2000) Arsenic speciation in pyrite and secondary weathering phases, Mother Lode Gold District, Tuolumne County, California. Appl Geochem 15:1219–1244
Smith KS, Ramsey CA, Hageman PL (2000) Sampling strategy for the rapid screening of mine-waste dumps on abandoned mine lands. ICARD 2000, Fifth International Conference on Acid Rock Drainage. Society for Mining, Metallurgy and Exploration Inc., Denver, pp 1463–1475
Sobek AA, Shuller WA, Freeman JR, Smith RM (1978) Field and laboratory methods applicable to overburdens and minesoils. EPA Report EPA600/2–78-054. US EPA, Cincinnati
Solomon M (1976) Volcanic massive sulphide deposits and their host rocks: a review and an explanation. In: Wolf KH (ed) Handbook of strata-bound and stratiform ore deposits, II: regional studies and specific deposits. Elsevier, Amsterdam, pp 1307–1328
Thornthwaite W, Mather JR (1955) The water balance. Publications in climatology, VIII (1). Laboratory of Climatology, Centerton, p 104
Tornos F (2006) Environment of formation and styles of volcanogenic massive sulfides: the Iberian Pyrite Belt. Ore Geol Rev 28:259–307
USEPA (1994) Method 7471B (SW-846): Mercury in solid or semisolid waste (manual cold-vapor technique), Revision 2. U.S. Environmental Protection Agency, Washington DC
USEPA (2002) National Recommended Water Quality Criteria. EPA 822-R-02-047. U.S. Environmental Protection Agency, Washington DC
USEPA (2012) Drinking water standards and health advisories, 2012 Edition. EPA 822-S-12-001. Washington DC: U.S. Environmental Protection Agency. Washington DC
Vick SG (1990) Planning, design and analysis of tailings dams. BiTech Publishers Ltd., Richmond
Williams D (1934) The geology of the Rio Tinto mines, Spain. Bull Inst Min Metal 335:593–678
Acknowledgments
The authors acknowledge the support provided by Jesús Reyes de Andrés for the chemical analysis. The authors are also grateful to two anonymous reviewers for helpful comments on the manuscript.
Funding
This research was funded by the Instituto Geológico y Minero de España (Geological Survey of Spain) within the internal project Support and actions in environmental impact assessment, risk assessment, and mining waste management (Code: 78.5.00.01.00).
Author information
Authors and Affiliations
Contributions
Julio César Arranz-González: Conceptualization, sampling, methodology, writing-original draft, formal analysis, and investigation. Virginia Rodríguez-Gómez: Sampling, methodology, writing-review and editing, formal analysis, and investigation. Francisco Javier Fernández-Naranjo: Sampling, methodology, and writing-review and editing. Lucas Vadillo-Fernández: Supervision.
Corresponding author
Ethics declarations
Ethical approval, consent to participate, and consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arranz-González, J.C., Rodríguez-Gómez, V., Fernández-Naranjo, F.J. et al. Assessment of the pollution potential of a special case of abandoned sulfide tailings impoundment in Riotinto mining district (SW Spain). Environ Sci Pollut Res 28, 14054–14067 (2021). https://doi.org/10.1007/s11356-020-11473-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11473-w