Abstract
Roadside dust can contain particulates enriched with potentially toxic elements (PTEs) as a result of the degradation of mechanical vehicular parts, tyre wear and combustion processes. To assess the potential accumulation of these metals in roadside areas, a snapshot study was carried out, investigating metal content at rural and urban locations in central Scotland. Samples of road dust were collected at six sites representing low, medium and high traffic intensity at rural and urban locations. The samples were separated based on particle size and analysed for heavy metal content using inductively coupled plasma optical emission spectroscopy (ICP-OES) after acid digestion. The metals analysed were aluminium (Al), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), magnesium (Mg), manganese (Mn), lead (Pb) and zinc (Zn). The rural area measurements were carried out in West Lothian, approximately 13 to 17 miles west of the city of Edinburgh (UK). The urban area measurements were carried out in the southern part of the Edinburgh city district (UK). Concentrations of Cu, Cr and Zn were found to correlate with traffic intensity, although only Cu and Zn concentrations exceed recommended EC directive 86/278/EEC guidelines for urban runoff materials. The metal concentrations of small particles (0.45–20 μm) were exceedingly high in both Cu and Zn at areas of high traffic intensity, indicating potential areas of concern regarding health impacts for pedestrians and cyclists who are exposed to roadside dust on a regular basis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mass utilisation of mechanised vehicles used to transport people and goods in modern day society has become a common part of everyday modern life; however, it brings with it serious consequences for both human health and the wider environment. As well as harmful air pollution emitted as the direct result of the combustion of fossil fuels (i.e. nitrogen oxides (NOx), volatile organic compounds (VOCs) and particulate matter (PM) in the form of soot (DEFRA 2019)), the degradation of vehicles via operational wear and tear results in the production of PM enriched with a variety of potentially toxic elements (PTEs) (DEFRA 2019; Duong and Lee 2011; Pagotto et al. 2001; Wei and Yang., 2010). As a consequence, the PTE content of roadside dust can increase considerably in areas with high traffic flow. Human exposure then occurs via inhalation or ingestion of such dusts (Du et al. 2013; Li et al. 2013; Soltani et al. 2015).
Since the Industrial Revolution in the midnineteenth century, smelting of metal ores, fossil fuel combustion and, from the early twentieth century, combustion of leaded petrol, released large quantities of PTEs to the atmosphere which were then transferred to UK soils and waters via both wet and dry deposition (Nriagu 1996; Renberg et al. 2001). Since the 1980s, however, atmospheric emissions of PTEs have fallen dramatically due to introduction of industrial regulations established to protect human health (DEFRA 2019) (e.g. the 1982 EC Directive 82/884/EEC and the 1985 EC Directive 85/210/EEC which limited lead in the air and introduced unleaded petrol). Although the acute toxicity of exposure to many of these elements is well understood, the long-term impacts of low and sustained exposure to these compounds is less defined (Fortoul et al. 2015; Kampa and Castanas 2008; Singh et al. 2011). There are many diseases and illnesses, e.g. Alzheimer’s, Parkinson’s, cancer, and numerous cardiovascular diseases that are believed to be linked to elevated heavy metal concentrations in the body (Ashley 2012; Järup 2003; Rehman et al. 2017; Tchounwou et al. 2012). As the number of vehicles in use continues to rise at a global scale in an increasingly urbanised world (especially in Asia, e.g. Arora et al. 2011; Huo et al. 2007), human exposure to heavy metals in road dust is likely to increase as a result.
In environments where the PTE content of PM is elevated, exposure to fine particulates (< 2.5 μm) is of greater concern as these particles are not so easily cleared from the lungs via the mucociliary response (the natural process which removes PM from the body (Kreyling et al. 2002)). Smaller particles are more likely to be fully ingested and thus transfer heavy metals into vital organs and the blood stream where PTEs may cause harm in a number of ways. Smaller particles are also more likely to stick to skin, clothes and other surfaces than larger particles, resulting in increased ingestion of particulate matter in foods and especially by young children who frequently put their hands in their mouths (Bekö et al. 2013; Ferreira-Baptista and De Miguel 2005) and tend to ingest higher quantities of soil (US-EPA 2017). The human bioaccessibility and potential harm posed by particulates in the < 20 μm size fraction is still poorly understood. Traditionally, inhalation is believed to be the primary route of ingestion of road dust, with focus typically limited to the < 2.5 and < 10 μm particulate fractions (Khan and Strand 2018). These small size fractions are considered to cause respiratory and cardiovascular health problems; however, this simplistic assessment has been criticised as it does not account for deposition of particles in the lungs and in vivo availability (Kastury et al. 2017). Ingestion of larger particles via the stomach is also well documented with variables such as pH and organic matter content both thought to be important in determining bioaccessibility (Poggio et al. 2009; Broadway et al. 2010).
The most significant contributors of PTE pollution from vehicles are considered to be brake wear, tyre erosion, exhaust emissions and oil losses (Napier et al. 2008), although there are also a large variety of other sources. Brakes pads, which degrade over time, are often classed as semimetallic materials since they contain ~ 30–65% metal by weight, e.g. including iron (Fe) and copper (Cu) together with lead (Pb), chromium (Cr), zinc (Zn) and antimony (Sb) compounds as friction modifiers (Garg et al. 2000; Thorpe and Harrison 2008). One reason that brake wear can have considerable health effects is that the brake pad materials often degrade into very small (< 2.5 μm) high-density particles which can be easily inhaled deep into our lower respiratory system. Therefore their toxicity has to be considered with more attention than larger particles (> 20 μm) (Garg et al. 2000).
Tyre erosion is also a significant source of heavy metal traffic pollution. Tyre tread contains heavy metals such as manganese (Mn), Fe, cobalt (Co), nickel (Ni), Cu, Zn, cadmium (Cd) and Pb (Adachi and Tainosho 2004; Ellis and Revitt 1982; Johansson et al. 2009). The vast quantities of tyre rubber that is released on to the roads each year (estimated at 0.23 to 4.7 kg/year per capita in Kole et al. 2017) is cause for concern as it increases the mass of metal rich roadside dust, and thus human exposure to inhalable and ingestible particulate matter. Exhaust emissions from traffic contain harmful heavy metals such as mercury, nickel, cadmium and lead from the burning of fossil fuels, and catalytic converters also degrade with time, resulting in losses of platinum, palladium and rhodium, released onto the roadside in the form of small high density particulate metallic dust (Johansson et al. 2009; Zereini et al. 1997).
Other sources of heavy metal pollution from traffic include the many potentially toxic liquids used in vehicle maintenance. Antifreeze, oil, transmission fluid, power steering fluid, brake fluid and windscreen wash all leak from traffic sources onto roads. Metals present in these fluids include chromium from coolants, nickel and zinc in motor oil and lead from lubricating oil (Fox and Cramer 1997). The general corrosion of automobiles also contributes to heavy metal pollution. Aluminium (Al), Fe, Cr, magnesium (Mg), Ni, Zn, Cu and titanium (Ti) are all used in car parts that degrade over time. Corrosion of the road itself can release particulates containing heavy metals as asphalt contains bitumen which is known to contain Ni, vanadium (V), Pb, Cr, mercury (Hg) and other potentially toxic elements (Legret et al. 2005). Road works, abrasion of surfaces, weathering and frost damage loosens asphalt materials which in turn releases particulate matter containing heavy metals into the environment.
The potential increase in concentrations of heavy metal content in roadside areas presents with it several long-term environmental and health problems (Namdeo and Bell 2005). As PM is mobile in the environment in both the atmosphere and aquatic systems, it can potentially transport heavy metal materials in road dust over long distances in the air (after initial release and resuspension) and in moving water bodies via colloidal interactions (Frimmel et al. 2007; Karathanasis 1999; Lee et al. 2018). These routes of transportation allow PTEs to disperse far from the roadsides given time, and contaminate nearby agricultural and residential areas where ingestion of heavy metals is likely to increase as a result of contamination of water supplies and crop uptake.
Major hosts for contaminant trace metals in roadside dusts and sediments are iron-rich spherical glass grains derived from high temperature combustion processes and iron oxide particles derived from the corrosion of steel (reported as > 25% of the total mass of road dust in Taylor and Robertson 2009). These materials have a high binding affinity for heavy metals and are easily mobilised into water sources by rain water. PTEs bound to particulates are cause for concern in drainage from urban areas as they can pollute drinking water and damage ecological systems (Davis and Birch 2009). Once the heavy metal contaminant particles have deposited in sediment it is also very difficult to remove them without incurring large economic costs, therefore it is inevitable that most roadside areas will eventually end up with harmful concentrations of heavy metals if left long enough without remediation efforts (Mulligan et al. 2001).
This small scale study aims to examine a snapshot of the heavy metal content of roadside dust at six locations of varying traffic intensity in central Scotland, identifying the concentrations of nine common heavy metals, chosen to represent the mechanical degradation of metallic parts, as well as tyre dust and combustion sources associated with traffic (aluminium (Al), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), magnesium (Mg), manganese (Mn), lead (Pb) and zinc (Zn)). The study also aims to quantify the metal content in varying particle size fractions, identifying where heavy metal content of smaller size fractions is higher as a result of the influence of traffic. Due to the uncertainty in current literature about the true risk of exposure to PTEs in regard to particle size, we use the < 20 μm size fractions in this study to identify potential human exposure to PTEs in terms of both inhalation and ingestion.
Methodology
Data in this study was analysed using the statistical software ‘R’ (R Core Team 2017), and presented using the ‘ggplot2’ package (Wickham 2016).
Sample collection
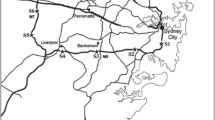
Approximately 500 g of roadside dust was taken from the top 2-cm of the soil surface using a small trowel from 6 different sampling sites with varying environmental characteristics (Fig. 1, Table 1). Samples were collected from each site (within a 5 m radius) on a weekly basis (Fridays) for 5 weeks (n = 5), from 1st to the 29th of October in 2010. Protective gloves and face masks were worn during collection to prevent exposure to contaminated dust. Three of the sites chosen were in rural areas, and three were chosen in urban areas. In both areas, samples were taken at low, medium (med) and heavy traffic sites, using local knowledge and Department for Transport: Road Traffic Statistics (for 2010: https://roadtraffic.dft.gov.uk) to distinguish these characteristics. The rural sites were located in West Lothian, approximately 13 to 17 miles west of the city of Edinburgh (UK). The urban area measurements were carried out in the southern part of the Edinburgh City district (UK). Post codes are provided to exclude exact measurement sites for the privacy of local residents. Samples were collected approximately 1 m from the kerbside of the road. None of the sites chosen in the study shared proximity (within 10 miles) to local sources of potential metal contamination other than traffic (i.e. incinerators or refineries …); thus, in this study, we assume that long-term metal deposition in the roadside dust is not influenced significantly by nearby industry.
Samples were collected from six sites. The samples sites classed as urban were situated in the south of the city of Edinburgh, while the rural sites were sampled from the West Lothian region to the west of the city. Locations classed as low, medium and high traffic were chosen for both urban and rural areas based on local knowledge and traffic data
Sample preparation
Subsamples of 400 g were weighed into aluminium trays and placed into a drying oven held at a constant 105 °C. Dry sample mass was recorded after drying for 24 h. The dried samples were then passed through a sequential sieve-stack (Endecotts Ltd. Stainless steel laboratory test sieve) with gradually decreasing pore diameters of 2 mm, 125 μm, 53 μm and 20 μm. The samples were dry sieved until the majority of particles had been separated. Two litres of deionised water were passed through all stacked sieves to aid separation. The water and particulates passing through the 20-μm sieve was collected in a 2-L flask. The < 20-μm fraction was then passed through a 0.45-μm filter (Whatman Cellulose Nitrate Membrane Filters 0.45 μm) paper and the 0.45–20-μm particulate fraction was retained on the filter. After separation, each size fraction was put in an aluminium tray and placed in a drying oven overnight at a constant 105 °C. The dry mass of each size fraction was recorded after it was dried. The dissolved fraction that had passed through the 0.45-μm filters was evaporated down to about 1 L. Aliquots (25 mL) of the solution were transferred into 3 replicate labelled 30-mL plastic tubes (Sterilin universal container, Scientific Laboratory Supplies Ltd). The volume of the remaining solution was measured before being discarded.
Approximately 1 g of each size fraction was ground into a fine powder using a mortar and pestle. Approximately 0.25 g of the ground sample was accurately weighed into a 50-ml pyrex beaker. The beakers were covered with a watch glass and put in a furnace at 450 °C for 4 h. The samples were allowed to cool prior to weighing. Loss on ignition and then percentage organic content were calculated to establish the ratio of mineral to organic material in the sample, and to investigate relationship with PTEs.
Microwave digestion
Microwave digestion was carried out via MARS5 (CEM) microwave digestion apparatus using the methodology and variables as follows. Ashed samples were transferred into a clean microwave vessel (HP-500, CEM), and the remaining residue was washed into the vessel from the glass beaker using 10 ml of concentrated HNO3 (ARISTAR, 69% w/v, VWR Ltd.). In order to dissolve silicate materials in the sample, 1 ml of hydrofluoric acid (HF) was also added to the vessel (used as supplied, ARISTAR grade, 48% w/v, VWR Ltd.). Twelve soil samples, a blank and a reference material vessel were incorporated into each microwave run. The EPA3052H - HP500 method (#3052) was used to microwave the samples.
After the apparatus had cooled, the vessels were removed from the carousel and vented with care. The solutions were transferred into clean Teflon beakers and the vessels rinsed with 1–2 ml of 2% ARISTAR HNO3 to ensure complete sample transfer. The Teflon beakers were heated on a hot plate until around 1–2 ml of solution remained. The flasks were made up to the 25 ml mark using 2% (v/v) ARISTAR HNO3. The contents of the flasks were transferred into labelled 30-ml plastic tubes and stored at room temperature for further analysis.
ICP-OES analysis
The concentrations of Al, Cd, Cr, Cu, Fe, Mg, Mn, Pb and Zn in the digestates were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) using a Perkin Elmer Optima 5300 DV, employing an RF forward power of 1400 W, with argon gas flows of 15, 0.2 and 0.75 L min−1 for plasma, auxiliary and nebuliser flows, respectively. Using a peristaltic pump, sample solutions were taken up into a Gem Tip cross-flow nebuliser and Scotts spray chamber at a rate of 1.50 ml min−1. The selected wavelengths for each element were analysed in fully quantitative mode (three points per peak). After the analysis was completed, the wavelengths which recorded the most accurate results for the reference materials and which gave the most consistent data for each element were selected for reporting results (Table 2).
A range of calibration standards were prepared using single element 1000 mg L−1 stock solutions (Al, Cd, Cu, Fe, Mg, Mn, Pb and Zn: Fisher Scientific UK LTD; Cr: Spectrosol Standard Solution, BDH Lab Supplies) diluted with 2% v/v HNO3 (Aristar). Standard solutions of 0.1, 1, 10, 20 and 50 mg L−1 were prepared for each Al, Fe, Mg and Mn. Standard solutions of 0.01, 0.1, 0.25, 1 and 10 mg L−1 were prepared for each Cd, Cr, Cu, Pb and Zn. Using these standards, calibration lines were calculated to cover the range of concentrations found in the digestates.
Results
Particle size distribution
The particle size distribution of the individual samples from each location was dominated by the presence of larger particles (i.e. greater than 125 μm in diameter) (Fig. 2). The 125-μm–2-mm fraction contributes to the majority of the dry mass of most of the samples, with larger contributions from the > 2-mm fractions in the rural areas when compared with urban. The mean ratio of the 0.45–20-μm fraction at the different sites ranges from 1.6 to 5.5% of the dry mass, with the largest fraction at the high traffic urban location. The high traffic urban location had a greater proportion of PM with diameter less than 125 μm than any other site, exceeding a quarter of the total mass of the samples.
Organic matter content
The organic matter content (OM) at the different sites (bulk sample) was broadly similar (5.0 ± 2.4% OM), with the exception of the urban low traffic location (19.7 ± 8.0% OM), where samples were collected near a tree line at the edge of the road (Fig. 3). With the exception of the urban low traffic location, a similar trend in OM within the different particle size ranges is observed, with OM approximately 5 times higher in the smallest particle size fraction when compared with the largest particle size fraction. Excluding the urban low traffic location, OM ranged between 15 and 25% for the 0.45–20-μm size fraction and between 3 and 5% for the > 2-mm size fraction (0.25-g samples for all measurements).
Metal content by size fraction
Nine metals in total were measured after microwave digestion of the road dust samples (Al, Cd, Cr, Cu, Fe, Mg, Mn, Pb and Zn) (Fig. 4). Four of these (Al, Fe, Mg and Mn) are major soil constituents which we know to be present in soils (especially the clay fraction) in naturally high concentrations. In the case of Fe and to some extent Mg, a fairly uniform distribution of metal content is observed across the different measurement sites and throughout the particle size ranges. But, elevated concentrations in the 0.45–20-μm size fraction are evident for these metals when compared with the larger size fractions. Concentrations of Al are very high in the 0.45–20-μm size fraction, averaging 31.8 ± 5.6 g kg−1 when compared with the mean concentration of the other size fractions 11.8 ± 4.1 g kg−1. There is little observable difference between the metal content of Al, Fe and Mg at the sites with different traffic intensities or the urban/rural divide, suggesting that traffic and other local human activity is unlikely to contribute significantly to the total metal content at the sites.
Concentrations of Cd in the size fractions greater than 20 μm vary little between the sites, with a mean metal content of 0.38 ± 0.8 mg kg−1 across all sites. However, the Cd content of the 0.45–20-μm size fraction varies more significantly, with mean values ranging from 4.3 to 24.3 mg kg−1. The highest Cd concentrations are observed at in the 0.45–20 μm size fraction at the low traffic rural location, suggesting a local specific source.
There seems to be a large source of Pb contamination at the medium traffic rural site, which skews the comparison between the different sites. Although this appears to be an outlier of sorts, repeated measurements from the same location (samples taken within 1 m of each other) show that the Pb content of the samples were consistently high. With the exception of the medium traffic rural site, samples from the other rural locations appear to contain less Pb than those measured in the urban locations. Concentrations of Pb were generally highest in the 0.45–20-μm size fraction, decreasing in concentration with increasing particle size, although there was some variability in the Pb content of the samples in the larger size fractions.
The Cu, Cr and Zn content of the samples follow the trend with traffic intensity more than the other metals, especially in the 0.45–20-μm size fraction (see Fig. 4). For each of these metals, concentrations were much higher in the smaller size fractions, following a negative trend as particle size increased.
Metal concentrations in the dissolved fractions of the samples were very low in comparison with those in the solid size fractions (Fig. 5). This can be attributed to the low solubility of the metals in their various oxidation states and the near to neutral pH conditions (see Table 1). The concentrations in the dissolved fraction do not correlate with the metal content in the solid size fractions in the corresponding samples, with the exception of Cu. Cu in the dissolved fraction appears to correlate with traffic intensity, as does the metal content of the solid size fractions.
Total metal content by location
No temporal patterns were observed in the metal content of individual samples taken over the 5-week period at each site (Fig. 6). Although metal concentrations were generally highest in the 0.45–20-μm size fraction, in most cases, these particles contributed only a small fraction to the overall metal content of the road dust as samples primarily consisted of particulates greater than 125 μm in diameter (see Fig. 2). The dissolved fraction contribution to total metal content of the samples was negligible at all sites. In the cases of the high abundance metals (Al, Fe, Mg and Mn), there were no observable trends between metal content and traffic intensity, with differences in elemental composition most likely dominated by natural variation between the sites.
Total Cd content was similar across the sites, with slightly higher concentrations at the urban locations (rural mean = 1.98 mg kg−1 and urban mean = 2.46 mg kg−1). The Pb contamination at the medium traffic rural location is clearly observable in the results, with a mean concentration of 250 mg kg−1 at this site when compared with a mean of 49 mg kg−1 at all other sites (see Fig. 6). Excluding this site, the concentrations of Pb in the urban sites were higher than those at the remaining two rural locations (rural mean = 26.8 mg kg−1 and urban mean = 64.2 mg kg−1, t test p = 0.09); however, there appears to be no direct correlation with traffic intensity.
Concentrations of Cu, Cr and Zn in the road dust correlates well with traffic intensity at the sites, with large differences between low and high traffic locations for each element. Concentrations of Cu exceeding 150 mg kg of Cu are measured on several occasions, with means of 55.0, 83.1 and 145.5 mg kg−1 for all low, medium and high traffic sites, respectively. This pattern is similar in the observed Zn concentrations, but there appears to be an additional effect caused by the rural/urban divide with concentrations at the high traffic intensity rural site being similar in magnitude to the low traffic urban site. Concentrations of Cr are higher at the medium and high intensity traffic sites than the low traffic locations; however, there is little difference between high and medium sites.
Relationship between OM and metal content
For several of the metals, there are consistent trends between metal content and OM in the road dust (Fig. 7). This relationship differs to some extent with the traffic intensity at each of the sites, with a larger metal to OM ratio at the high traffic sites. Due to the very high OM at the low traffic urban site and the relatively similar OM at the other sites, it is difficult to assess the direct effect of traffic intensity on this relationship; however, differences are most notable for Cu, Cr and Zn. The OM % is largest in the 0.45–20-μm size fraction, decreasing with increasing particle size (see Fig. 3). Our previous results show that metal concentrations increase as particle size decreases at our sites (see Fig. 4), thus OM % and metal content correlate well for most of the metals. However, our results cannot determine if high OM results in accumulation of metals in smaller particles or if the two variables are correlated for other reasons (i.e. increased surface area interactions).
Discussion
The metal concentrations of the road dust reported in this study are of the same order of magnitude of those reported in previous studies, although measurements carried out in large cities can be considerably higher than what was observed in the Edinburgh district. A summary of 15 studies reported by Charlesworth et al. (2003) showed that in large cities, Cd content varied from 1 to 6250 (but generally remained below 10) mg kg−1, Cu varied from 69 to 1987 mg kg−1, Pb varied from 60 to 2583 mg kg−1 and Zn varied from 152 to 3358 mg kg−1. When comparing our results with these studies, and multiple more recent studies (e.g. Duong and Lee 2011; Wei and Yang 2010), we find that our observations are within the range of reported results in studies from locations with similar traffic intensities. As Edinburgh is a fairly small city (population < 0.5 million) and is not considered a heavy industrial polluter in comparison with the megacities of the world, it stands to reason that metal concentrations would be closer to the lower end of the scale when compared directly (Table 3).
The metals that appeared to be most influenced by traffic intensity in this study were Cu, Cr and Zn. This agrees with what we might expect from previous studies in which the highest concentrations of heavy metals in road dust were found at busy junctions where traffic was often stopped by traffic lights and crossings (Charlesworth et al. 2003). This is because brake and tyre wear in these locations is much higher than open road, and thus deposition increases considerably. There will also be additional combustion emissions while the cars are stationary or moving slowly. In our study, the medium and high intensity traffic locations were all at junctions, which would explain the higher metal contents observed in comparison to the lower intensity site. An estimated 90% of Cu emissions from traffic are as a result of brake wear (Johansson et al. 2009) which explains why copper correlated so well with traffic intensity in this study. Cu has been correlated with Zn and Cr in previous studies, highlighting the importance of brake wear on the deposition of these metals as well (Johansson et al. 2009).
Concentrations of Cd and Pb in our study did not correlate well with traffic intensity; however, there were differences observed between rural and urban locations (when excluding the Pb contamination at the medium traffic rural site). It has previously been reported that Pb concentrations are higher in urban areas when compared with rural locations (Aelion et al. 2012). These differences can be attributed to a number of factors, particularly legacy deposition (as unlike other pollutants, metals cannot degrade), and disturbance of soils that was affected prior to the banning of Pb additives in fuel in the year 2000, thus construction activity can result in rerelease of Pb in dust and runoff. The larger number of construction projects in and around cities are a likely cause of elevated Pb in urban areas. The cause for the Pb contamination at the medium traffic rural site has not been identified in this study, but highlights that significant heavy metal contamination is possible in seemingly unexpected locations. As Cd contamination is typically associated with industrial activities and fossil fuel burning (Hutton 1983), and in some cases mineral fertilizers (Roberts 2014), it is not surprising that there was little difference in Cd content at the sites. Cd is known to be emitted from combustion sources (DEFRA, 2018); however, heavy metals released in this manner may disperse further from the roadside than the metal particulates associated with vehicle wear. This study does not conclusively show a difference in Cd content of roadside dust as a result of traffic or of an urban/rural divide.
The metal content was highest in the 0.45–20-μm size fraction for all metals. This was also the case for the metals with a naturally high abundance (Al, Fe, Mg and Mn), suggesting that the high concentrations of metal in the small size fractions for the metals more influenced by traffic intensity (Cu, Cr, Zn) is not solely due to anthropogenic factors. The correlation between organic matter and heavy metal content is well documented in previous studies (Lin and Chen 1998; Quenea et al. 2009; Yang et al. 2010), which have detailed the affinity for different metals in different sediment types and resultant changes in microbial carbon cycling. Due to the similarity of the organic matter size distributions at our sites (with the exception of the low traffic urban site), measurements suggest that the concentrations of Cu and Zn in the 0.45–20-μm size fraction are especially high as a result of traffic activity and not due to organic matter accumulation. This agrees with previous studies that suggest brake wear can result in large amounts of fine metallic dust gathering at busy traffic junctions (Charlesworth et al. 2003; Johansson et al. 2009).
Other factors to take into consideration that we cannot quantify in this study are the physical barriers/interactions at the different locations of the sampling. Road side areas are often lined with trees, buildings or other infrastructure such as barriers and bridges. These barriers (especially trees) may filter airborne particulates, altering deposition from traffic and surrounding areas (McDonald et al. 2007). The impact of fallen (deciduous) leaves are also likely to have impacted road dust abundance, absorbing particulates on the surface of the leaves which may be removed via street sweeping which would also have a major impact. Of the six sites sampled from, the most affected by these physical interactions would be the low traffic urban site with the nearby treeline and regular street sweeping; however, these conditions are not abnormal, and the road dust present is still an accurate assessment of the area during sampling.
Based on the EC Directive 86/278/EEC, the guideline threshold metal concentrations of sewage sludge (urban runoff) deemed safe for regular human exposure are presented in Table 4. Although not specifically used to determine the risks posed by the metal content of roadside dusts, this directive has been derived specifically to prevent harmful effects on soil, vegetation, animals and people (via ingestion of food grown on sludge-amended soil). We therefore use the guidelines as a proxy for environmental and health risks in this study as we do not have data to provide either the atmospheric concentrations or occupational exposure to the metals in question. The only metals in exceedance of these guidelines are Cu and Zn, the two metals which correlated strongly with traffic intensity. At the low traffic sites, metals fall well within the guideline values. The exceedance of these guidelines, paired with the high metal content in the smallest particle sizes highlights a potential health hazard at areas of high traffic intensity in urban and rural areas. The general public in Edinburgh (and the UK as a whole) are very unlikely to wear face masks (which may only offer limited protection anyway, Shakya et al. 2016) due to the relatively low atmospheric pollution in the city when compared with other global mega cities. This combined with the relatively large number of road cyclists in the city likely means that heavy metal pollution is having some degree of negative health impact, especially when exposure to Cu and Zn is concerned. This exposure may also be higher for other metals not investigated in this study that are also present in tyres and brake pads (i.e. cobalt and nickel).
However, the seriousness of these health impacts, especially that of inhaled metal elements, remains poorly understood. Oral exposure to ingested metals such as copper, zinc and nickel is typically not considered a human health concern, and few studies have set out to explicitly identify the direct impacts on human health of low exposure to particular elements that are not considered acutely toxic (such as arsenic, lead and cadmium) (Noonan et al. 2003; Taylor et al. 2020; Genchi et al. 2020). As this study was limited in scale, further wide scale work would be required to better identify the dangers that heavy metal pollution from vehicles presents to traffic heavy populated areas in the UK. Future studies should also investigate smaller size fractions where resources allow, such as PM10 and PM2.5 that are more traditionally associated with human health effects.
Conclusions
This snapshot study shows that the heavy metal content of road dust in the Edinburgh and West Lothian regions is generally below thresholds considered harmful to human health, although hotspots of contamination exist where traffic intensity is high and brake and tyre wear is greater than normal. Concentrations of Cu, Cr and Zn were found to correlate with traffic intensity in this study, with contamination considerably higher in both rural and urban environments where traffic intensity increased. Both Cu and Zn concentrations exceed recommended guidelines and are dangerously high in small particles (0.45–20 μm) which humans can ingest through inhalation or consumption, indicating areas of concern for pedestrians and cyclists. These concentrations are not as high as reported in many other larger cities with serious traffic pollution issues; however, as the long-term effects of exposure to these metals is not fully understood, it is difficult to assess the true impact of this pollution on human health even in low doses. Future studies in this field should attempt to quantify human exposure more clearly, as measuring dust concentrations may not be the best way to directly quantify this. Further research identifying the risk of exposure to different particle sizes > PM10 would also clarify the true risk posed by elevated concentrations of PTEs in road dust.
References
Adachi K, Tainosho Y (2004) Characterization of heavy metal particles embedded in tire dust. Environ Int 30:1009–1017. https://doi.org/10.1016/j.envint.2004.04.004
Adamiec E, Jarosz-Krzemińska E, Wieszała R (2016) Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ Monit Assess 188:369. https://doi.org/10.1007/s10661-016-5377-1
Aelion CM, Davis HT, Lawson AB, Cai B, McDermott S (2012) Associations between soil lead concentrations and populations by race/ethnicity and income-to-poverty ratio in urban and rural areas. Environ Geochem Health 35:1–12. https://doi.org/10.1007/s10653-012-9472-0
Arora S, Vyas A, Johnson LR (2011) Projections of highway vehicle population, energy demand, and CO2 emissions in India to 2040. Nat Res Forum 35:49–62. https://doi.org/10.1111/j.1477-8947.2011.01341.x
Ashley IB (2012) The metal theory of Alzheimer’s disease. JAD 33:S277–S281. https://doi.org/10.3233/JAD-2012-129011
Bekö G, Weschler CJ, Langer S, Callesen M, Toftum J, Clausen G (2013) Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS One 8:e62442. https://doi.org/10.1371/journal.pone.0062442
Broadway A, Cave MR, Wragg J, Fordyce FM, Bewley RJF, Graham MC, Ngwenya BT, Farmer JG (2010) Determination of the bioaccessibility of chromium in Glasgow soil and the implications for human health risk assessment. Sci Total Environ 409:267–277. https://doi.org/10.1016/j.scitotenv.2010.09.007
Charlesworth S, Everett M, McCarthy R, Ordóñez A, de Miguel E (2003) A comparative study of heavy metal concentration and distribution in deposited street dusts in a large and a small urban area: Birmingham and Coventry, West Midlands, UK. Environ Int 29:563–573. https://doi.org/10.1016/s0160-4120(03)00015-1
Chon H-T, Kim K-W, Kim J-Y (1995) Metal contamination of soils and dusts in Seoul metropolitan city, Korea. Environ Geochem Health 17:139–146. https://doi.org/10.1007/bf00126082
Davis BS, Birch GF (2009) Catchment-wide assessment of the cost-effectiveness of stormwater remediation measures in urban areas. Environ Sci Pol 12:84–91. https://doi.org/10.1016/j.envsci.2008.09.004
DEFRA (2019) Emissions of Air Pollutants in The UK, 1970 To 2017. National statistical release 15th February, 2019. Department for Environment, Food and Rural Affairs. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/778483/Emissions_of_air_pollutants_1990_2017.pdf
Du Y, Gao B, Zhou H, Ju X, Hao H, Yin S (2013) Health risk assessment of heavy metals in road dusts in urban parks of Beijing, China. Procedia Environ Sci 18:299–309. https://doi.org/10.1016/j.proenv.2013.04.039
Duong TTT, Lee B-K (2011) Determining contamination level of heavy metals in road dust from busy traffic areas with different characteristics. J Environ Manag 92:554–562. https://doi.org/10.1016/j.jenvman.2010.09.010
Ellis BJ, Revitt DM (1982) Incidence of heavy metals in street surface sediments: solubility and grain size studies. Water Air Soil Pollut 17:87–100. https://doi.org/10.1007/BF00164094
Fergusson J, Ryan D (1984) The elemental composition of street dust from large and small urban areas related to city type, source and particle size. Sci Total Environ 34:101–116. https://doi.org/10.1016/0048-9697(84)90044-5
Ferreira-Baptista L, De Miguel E (2005) Geochemistry and risk assessment of street dust in Luanda, Angola: a tropical urban environment. Atmos Environ 39:4501–4512. https://doi.org/10.1016/j.atmosenv.2005.03.026
Fox JW, Cramer DR (1997) Hypercars: a market-oriented approach to meeting lifecycle environmental goals, in: SAE technical paper series. Presented at the International Congress & Exposition, SAE International https://doi.org/10.4271/971096
Fortoul TI, Rodriguez-Lara V, Gonzalez-Villalva A, Rojas-Lemus M, Colin-Barenque L, Bizarro-Nevares P, García-Peláez I, Ustarroz-Cano M, López-Zepeda S, Cervantes-Yépez S, López-Valdez N, Meléndez-García N, Espinosa-Zurutuza M, Cano-Gutierrez G, Cano-Rodríguez MC (2015) Health effects of metals in particulate matter, in: current air quality issues. InTech. https://doi.org/10.5772/59749
Frimmel, F.H., Von Der Kammer, F., Flemming, H.-C. (Eds.), 2007. Colloidal transport in porous media. Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-540-71339-5
Garg BD, Cadle SH, Mulawa PA, Groblicki PJ, Laroo C, Parr GA (2000) Brake wear particulate matter emissions. Environ Sci Technol 34:4463–4469. https://doi.org/10.1021/es001108h
Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A (2020) Nickel: human health and environmental toxicology. IJERPH 17:679. https://doi.org/10.3390/ijerph17030679
Hutton M (1983) Sources of cadmium in the environment. Ecotoxicol Environ Saf 7:9–24. https://doi.org/10.1016/0147-6513(83)90044-1
Huo H, Wang M, Johnson L, He D (2007) Projection of Chinese motor vehicle growth, oil demand, and CO2 emissions through 2050. Transp Res Rec 2038:69–77. https://doi.org/10.3141/2038-09
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Johansson C, Norman M, Burman L (2009) Road traffic emission factors for heavy metals. Atmos Environ 43:4681–4688. https://doi.org/10.1016/j.atmosenv.2008.10.024
Kampa M, Castanas E (2008) Human health effects of air pollution. Environ Pollut 151:362–367. https://doi.org/10.1016/j.envpol.2007.06.012
Karathanasis AD (1999) Subsurface migration of copper and zinc mediated by soil colloids. Soil Sci Soc Am J 63:830–838. https://doi.org/10.2136/sssaj1999.634830x
Kastury F, Smith E, Juhasz AL (2017) A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal (loid) s from ambient particulate matter or dust. Sci Total Environ 574:1054–1074. https://doi.org/10.1016/j.scitotenv.2016.09.056
Khan RK, Strand MA (2018) Road dust and its effect on human health: a literature review. Epidemiol Health 40:e2018013. https://doi.org/10.4178/epih.e2018013
Kole PJ, Löhr AJ, Van Belleghem F, Ragas A (2017) Wear and tear of Tyres: a stealthy source of microplastics in the environment. Int J Environ Res Public Health 14:1265. https://doi.org/10.3390/ijerph14101265
Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Oberdörster G, Ziesenis A (2002) Minute translocation of inhaled ultrafine insoluble iridium particles from lung epithelium to extrapulmonary tissues. Ann Occup Hyg 46(Issue suppl_1):223–226. https://doi.org/10.1093/annhyg/46.suppl_1.223
Legret M, Odie L, Demare D, Jullien A (2005) Leaching of heavy metals and polycyclic aromatic hydrocarbons from reclaimed asphalt pavement. Water Res 39:3675–3685. https://doi.org/10.1016/j.watres.2005.06.017
Lee S, Ko I-W, Yoon I-H, Kim D-W, Kim K-W (2018) Colloid mobilization and heavy metal transport in the sampling of soil solution from Duckum soil in South Korea. Environ Geochem Health 41:469–480. https://doi.org/10.1007/s10653-018-0099-7
Li H, Qian X, Hu W, Wang Y, Gao H (2013) Chemical speciation and human health risk of trace metals in urban street dusts from a metropolitan city, Nanjing, SE China. Sci Total Environ 456–457:212–221. https://doi.org/10.1016/j.scitotenv.2013.03.094
Li X, Poon C, Liu PS (2001) Heavy metal contamination of urban soils and street dusts in Hong Kong. Appl Geochem 16:1361–1368. https://doi.org/10.1016/s0883-2927(01)00045-2
Lin J-G, Chen S-Y (1998) The relationship between adsorption of heavy metal and organic matter in river sediments. Environ Int 24:345–352. https://doi.org/10.1016/s0160-4120(98)00012-9
McDonald AG, Bealey WJ, Fowler D, Dragosits U, Skiba U, Smith RI, Donovan RG, Brett HE, Hewitt CN, Nemitz E (2007) Quantifying the effect of urban tree planting on concentrations and depositions of PM10 in two UK conurbations. Atmos Environ 41:8455–8467. https://doi.org/10.1016/j.atmosenv.2007.07.025
Miazgowicz A, Krennhuber K, Lanzerstorfer C (2020) Metals concentrations in road dust from high traffic and low traffic area: a size dependent comparison. Int J Environ Sci Technol 17:3365–3372. https://doi.org/10.1007/s13762-020-02667-3
de Miguel E, Llamas JF, Chacón E, Berg T, Larssen S, Røyset O, Vadset M (1997) Origin and patterns of distribution of trace elements in street dust: unleaded petrol and urban lead. Atmos Environ 31:2733–2740. https://doi.org/10.1016/s1352-2310(97)00101-5
Mulligan CN, Yong RN, Gibbs BF (2001) An evaluation of technologies for the heavy metal remediation of dredged sediments. J Hazard Mater 85:145–163. https://doi.org/10.1016/s0304-3894(01)00226-6
Nakajima F, Aryal R (2018) Heavy metals in Urban dust, in: heavy metals. InTech. https://doi.org/10.5772/intechopen.74205
Namdeo A, Bell MC (2005) Characteristics and health implications of fine and coarse particulates at roadside, urban background and rural sites in UK. Environ Int 31:565–573. https://doi.org/10.1016/j.envint.2004.09.026
Napier F, D’Arcy B, Jefferies C (2008) A review of vehicle related metals and polycyclic aromatic hydrocarbons in the UK environment. Desalination 226:143–150. https://doi.org/10.1016/j.desal.2007.02.104
Noonan CW, Kathman SJ, Sarasua SM, White MC (2003) Influence of environmental zinc on the association between environmental and biological measures of lead in children. J Expo Sci Environ Epidemiol 13:318–323. https://doi.org/10.1038/sj.jea.7500286
Nriagu, J.O., 1996. A history of global metal pollution. Science 272, 223–0. https://doi.org/10.1126/science.272.5259.223, 220
Pagotto C, Rémy N, Legret M, Le Cloirec P (2001) Heavy metal pollution of road dust and roadside soil near a major rural highway. Environ Technol 22:307–319. https://doi.org/10.1080/09593332208618280
Poggio L, Vrščaj B, Schulin R, Hepperle E, Ajmone Marsan F (2009) Metals pollution and human bioaccessibility of topsoils in Grugliasco (Italy). Environ Pollut 157:680–689. https://doi.org/10.1016/j.envpol.2008.08.009
Quenea K, Lamy I, Winterton P, Bermond A, Dumat C (2009) Interactions between metals and soil organic matter in various particle size fractions of soil contaminated with waste water. Geoderma 149:217–223. https://doi.org/10.1016/j.geoderma.2008.11.037
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Rehman K, Fatima F, Waheed I, Akash MSH (2017) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119:157–184. https://doi.org/10.1002/jcb.26234
Renberg I, Bindler R, Brännvall M-L (2001) Using the historical atmospheric lead-deposition record as a chronological marker in sediment deposits in Europe. The Holocene 11:511–516. https://doi.org/10.1191/095968301680223468
Roberts TL (2014) Cadmium and phosphorous fertilizers: the issues and the science. Procedia Eng 83:52–59. https://doi.org/10.1016/j.proeng.2014.09.012
Shakya KM, Noyes A, Kallin R, Peltier RE (2016) Evaluating the efficacy of cloth facemasks in reducing particulate matter exposure. J Expo Sci Environ Epidemiol 27:352–357. https://doi.org/10.1038/jes.2016.42
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharm 43:246–253. https://doi.org/10.4103/0253-7613.81505
Soltani N, Keshavarzi B, Moore F, Tavakol T, Lahijanzadeh AR, Jaafarzadeh N, Kermani M (2015) Ecological and human health hazards of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in road dust of Isfahan metropolis, Iran. Sci Total Environ 505:712–723. https://doi.org/10.1016/j.scitotenv.2014.09.097
Suryawanshi PV, Rajaram BS, Bhanarkar AD, Chalapati Rao CV (2016) Determining heavy metal contamination of road dust in Delhi, India. Atm 29:221–234. https://doi.org/10.20937/atm.2016.29.03.04
Taylor KG, Robertson DJ (2009) Electron microbeam analysis of urban road-deposited sediment, Manchester, UK: improved source discrimination and metal speciation assessment. Appl Geochem 24:1261–1269. https://doi.org/10.1016/j.apgeochem.2009.03.011
Taylor AA, Tsuji JS, Garry MR et al (2020) Critical review of exposure and effects: implications for setting regulatory health criteria for ingested copper. Environ Manage 65:131–159. https://doi.org/10.1007/s00267-019-01234-y
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment, in: Experientia Supplementum. Springer Basel, pp. 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Thorpe A, Harrison RM (2008) Sources and properties of non-exhaust particulate matter from road traffic: a review. Sci Total Environ 400:270–282. https://doi.org/10.1016/j.scitotenv.2008.06.007
US EPA (2017) Update for chapter 5 of the exposure factors handbook soil and dust ingestion. United States Environmental Protection Agency Report EPA/600/R-17/384F. National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Washington, DC 20460
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107. https://doi.org/10.1016/j.microc.2009.09.014
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York. https://ggplot2.tidyverse.org
Yang X, Xiong B, Yang M (2010) Relationships among heavy metals and organic matter in sediment cores from Lake Nanhu, an Urban Lake in Wuhan, China. J Freshw Ecol 25:243–249. https://doi.org/10.1080/02705060.2010.9665074
Zereini F, Skerstupp B, Alt F, Helmers E, Urban H (1997) Geochemical behaviour of platinum-group elements (PGE) in particulate emissions by automobile exhaust catalysts: experimental results and environmental investigations. Sci Total Environ 206:137–146. https://doi.org/10.1016/s0048-9697(97)80005-8
Funding
This work was funded by the University of Edinburgh School of Chemistry MSc research fund for students, supported by contribution from the Natural Environment Research Council award number NE/R016429/1 as part of the UK-SCaPE programme delivering National Capability.
Author information
Authors and Affiliations
Contributions
N. Cowan carried out the field work, sample collection, data analysis and writing of the manuscript. D. Blair assisted in sample preparation and carried out analysis of metal concentrations using ICP-MS instrumentation. H. Malcolm assisted with writing of the manuscript, and contributed to the discussion section of the study. M. Graham supervised the logistics and scope of the study, analysis laboratory and manuscript preparation. All coauthors assisted in the production of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
Data will be submitted to the Environmental Information Data Centre (EIDC) upon publication of the study, and will subsequently be freely available in the public domain.
Consent to participate and publication
Not applicable.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cowan, N., Blair, D., Malcolm, H. et al. A survey of heavy metal contents of rural and urban roadside dusts: comparisons at low, medium and high traffic sites in Central Scotland. Environ Sci Pollut Res 28, 7365–7378 (2021). https://doi.org/10.1007/s11356-020-11081-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11081-8