Abstract
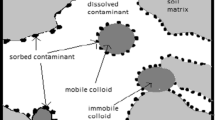
Colloid mobilization is a significant process governing colloid-associated transport of heavy metals in subsurface environments. It has been studied for the last three decades to understand this process. However, colloid mobilization and heavy metal transport in soil solutions have rarely been studied using soils in South Korea. We investigated the colloid mobilization in a variety of flow rates during sampling soil solutions in sand columns. The colloid concentrations were increased at low flow rates and in saturated regimes. Colloid concentrations increased 1000-fold higher at pH 9.2 than at pH 7.3 in the absence of 10 mM NaCl solution. In addition, those were fourfold higher in the absence than in the presence of the NaCl solution at pH 9.2. It was suggested that the mobility of colloids should be enhanced in porous media under the basic conditions and the low ionic strength. In real field soils, the concentrations of As, Cr, and Pb in soil solutions increased with the increase in colloid concentrations at initial momentarily changed soil water pressure, whereas the concentrations of Cd, Cu, Fe, Ni, Al, and Co lagged behind the colloid release. Therefore, physicochemical changes and heavy metal characteristics have important implications for colloid-facilitated transport during sampling soil solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colloids are the ubiquitous particles in subsurface environments in the range from 1 nm to 10 μm (Buddemeier and Hunt 1988; Puls et al. 1992; Dai et al. 1999) and play an important role in transporting nutrients and contaminants in subsurface environments due to their high surface area and charge intensity (Amrhein et al. 1993; de Jonge et al. 2004). In particular, colloids facilitate the transport of contaminants such as heavy metals (Cr, Cd, Cu, Ni, Pb, and Zn), radionuclides (Cs, Pu, and Am), and pesticides (atrazine) (Ryan et al. 1998; Novikov et al. 2006; Cheng and Saiers 2010; Qi et al. 2014), and therefore, those may trigger rapid contamination and public health risks due to viruses, bacteria, and protozoa (Redman et al. 2001; Klitzke et al. 2015). These contaminants have been observed with colloids in saturated (Sprajue et al. 2000; Chen et al. 2005; Graham et al. 2006; Miller et al. 2011) and unsaturated porous media (Mills et al. 1991; Ryan et al. 1998; Sprague et al. 2000; Pawlowska et al. 2017). In addition, colloid-binding metals have been released and mobilized over long distances rather than dissolved metals in the subsurface (Zhou et al. 2011). This is because the interactions between these colloids and contaminants can change their physical and chemical compositions (Amrhein et al. 1993; Kaplan et al. 1993; de Jonge et al. 2004).
The mobilization of colloids is significantly affected by physical perturbations related to the flow rate and water content (Gamerdinger and Kaplan 2001). Rapid flows such as rainfall infiltration and sampling for soil solutions and groundwater increase the colloid concentrations in the subsurface environment (Backhus et al. 1993; Kaplan et al. 1993; Ryan and Elimelech 1996). The increase in colloid concentrations is due to the fact that high flow velocity forms a high hydrodynamic shear force between the colloids and soils leading to an increase in the colloid concentrations (Puls et al. 1992). However, studies have reported that colloid concentrations in soil solutions decreased with increasing flow rate (Biddle et al. 1995; Ryan et al. 1999). The relationship between colloid mobilization and flow rate during the sampling of soil solutions has not been fully understood.
Chemical perturbations such as pH and ionic strength play an important role in colloid mobilization in saturated and unsaturated regimes (Grolimund and Borkovec 1999; Saiers and Lenhart 2003). A high pH and low ionic strength typically enhance the colloid mobilization in subsurface environments (McDowell-Boyer 1992; Ryan and Gschwend 1994; Grolimund and Borkovec 1999) with a further increase in pH (> point of zero charge, PZC) resulting in the enhancement of repulsion and detachment energy (Ryan and Gschwend 1994; Seta and Karathanasis 1997; Mohanty et al. 2016). Infiltration events (e.g., rainfall, snow melting, and irrigation) are known to decrease the ionic strength, leading to the electrostatic repulsion of negatively charged colloids and soil (Saiers and Lenhart 2003). However, Karathanasis and Johnson (2006) reported that colloid mobilization and stability were not significantly affected by the pH value. Many studies have reported the colloid mobilization on the effect of physical and chemical perturbations under infiltration systems; however, it is not unclear during sampling soil solutions. The objectives of this study are to: (1) investigate the colloid mobilization at a variety of flow rates and the effect of pH and ionic strength during sampling soil solutions and (2) examine that colloids facilitate heavy metal transport in real field soils during the sampling of soil solutions.
Materials and methods
Experimental setup
The experimental column was made from a clear acryl plastic pipe having a 14 cm inside diameter and 55.5 cm height (Fig. 1). The tensionic sampler consisted of a ceramic cup, capillary tube, and needle sensor (Model STCP 850, SDEC, France). The ceramic cup had a porous structure, with a pore size of approximately 2 μm. The tensionic sampler collected soil solutions using a capillary tube, and a needle sensor was used to measure the SWP. The capillary tube was connected using Tygon lab tubing (Model 064, Masterflex) for pumping the soil solutions, which was then transferred to the fraction collector using a peristaltic pump. A portable micrologger (ML, Model EC600, Fourier Systems Inc., USA) was used to record physical parameters such as temperature (°C), SWP, and humidity; measurements were made over a 21 h, with SWP measured every 10 s.
Column experiments
Sand produced in Joomoonjin, Gangwon province, South Korea, was air-dried at room temperature (24 ± 1 °C), and disturbed surface materials were removed. The sample was then passed through a 2 mm sieve to obtain the uniform sand less than 2 mm particle diameter. To saturate air-dried sands, Joomoonjin sand was packed into a 10 mM NaCl solution prepared using ultrapure Milli-Q water (18.2 MΩ·cm, Millipore,). Briefly, the sand was poured slowly in 1 cm increments into the electrolyte solution while being gently tapped to avoid air bubbles and to establish uniformly packing. The tensionic sampler was installed at 24.5 cm from the bottom of a column. 9878 g of sand and 2400 mL of electrolyte solution were added into the column. The average bulk density and porosity were 1.63 ± 0.01 kg L−1 and 0.38 ± 0.004, respectively, assuming an average particle density of 2.65 kg L−1 (Table 1). After packing, the needle sensor was connected onto the top of the tensionic sampler and linked to the micrologger. The top of the column was covered by a punctured para film membrane to prevent evaporation of the soil solutions. Tygon tubing was connected to the capillary tube of the tensionic sampler, and to investigate the relationship between colloids and flow rate a peristaltic pump was operated at a variety of flow rates. The soil solutions were collected through the capillary tube using a peristaltic pump, after equilibration of the SWP. The effluent was collected in 15 mL high-density polyethylene (HDPE) tubes at regular intervals. Simultaneously, the SWP was automatically measured every 10 s and recorded by the micrologger. 6 mM of NaOH and 10 mM of NaCl were prepared in order to investigate the effect of pH and ionic strength on the colloid mobilization in saturated and unsaturated sand regimes. The sand was packed, with NaOH and NaCl solution added while measuring the pH and electrical conductivity as described above. After packing the sand and solution, the soil solutions were left to equilibrate for about 24 h.
Real field soils were collected from near the abandoned Duckum mine tailings, a gold-mine area in South Korea (34°57′00″N, 126°35′00″E) (Fig. 5a). The column experiment was conducted to evaluate the colloid and heavy metal concentrations, SWP, and colloid shapes during sampling the soil solutions. Duckum soil was sieved (< 0.2 mm) and packed into the column. Overall, 9378 g of Duckum soil was used with 2300 mL of 10 mM NaCl solution and mixed to ensure saturation. The packing of the Duckum soil was performed using same methods as for the Joomoonjin sand.
Analytical methods
Ten milliliters of the collected soil solutions was required in order to analyze the colloid size, morphology, and colloid concentration. The cumulative volume and flow rate were determined by weighting and measuring the collected soil solution. The pH and electrical conductivity of the effluent were measured during sampling soil solutions using a multi-parameter instrument (W20 model, Horiba, Japan). Colloid concentrations were determined using the gravimetrical method, as previously described (El-Farhan et al. 2000; Karathanasis and Johnson 2006). In brief, soil samples (5 g) were added to centrifuge Teflon bottles that contained Milli-Q water (25 mL). The samples were then shaken at 150 rpm for 24 h, followed by being centrifuged at 750 rpm for 20 min. A known volume of the supernatant solution was passed through a preweighed 0.2 μm filter, which was dried at 100 °C for 24 h. The mass of particles retained on the filter was calculated by the mass of the preweighed and dried filters. Then colloid stock solutions (~ 2000 mg L−1) were obtained. The colloid concentrations in the soil solutions were determined by the following calibration procedures (Seta and Karathanasis 1997; Nunana et al. 1998-Ultraviolet absorbance (280 nm) of compounds released from Karathanasis and Johnson 2006; Liu et al. 2013). The calibration curve was prepared by diluting the supernatant solution, and the absorbance values of the diluted solutions were measured at 280 nm by an UV spectrophotometer (UV 1601PC, Shimadzu, Japan). The soil solutions collected from the soil column were sonicated for 15 min to prevent the aggregation and then measured to obtain the absorbance which was converted to colloid concentration using the calibration curve. For transmission electron microscopy (TEM), soil solutions were collected and prepared on 200 mesh copper grids. The morphology and size of colloidal particles were analyzed by TEM (JEM-2100, JEOL, Japan). Parallel blanks were prepared using Milli-Q water (18.2 MΩ cm) to examine the particulate contamination.
The soil solutions from the Duckum soil column were collected, and then prepared as for the Joomoonjin sand procedures described above, in order to analyze the concentration, size, and morphology of the colloids. The collected soil solutions were filtered using a 0.22-µm cellulose acetate filter and then amended with HNO3 at ~ 2% (v/v). Finally, the heavy metal concentrations were determined using an inductively coupled plasma mass spectrometer (ICP/MS, Agilent 7500CE, USA).
Results and discussion
Effect of flow rate on colloid mobilization
In order to examine the relationship among colloid mobilization, flow rate, and SWP, we measured the colloid concentrations, volumetric flow rate, and SWP during sampling soil solutions from the Joomoonjin sand column (Fig. 2). The colloid concentrations and absolute SWP increased at a 40 min of extraction time after the equilibrium state of SWP (~ − 5 kPa). The colloid concentrations were 0.23, 0.003, 0.002, 0.0035, 0.0023, and 0.0006 mg L−1 at flow rates of 2.3, 3.7, 5.3, 6.5, 9.4, and 15.6 mL min−1, respectively. This is because hydrodynamic shear stress between colloids and soils causes colloid mobilization in subsurface environments (Hubbe 1985; Ryan and Elimelech 1996). Upon the initial sampling of soil solutions, the momentarily changed SWP may be related to the colloid mobilization in saturated regimes. These results were consistent with observations in previous studies, in which the initial transient flow was deemed to be significantly important for improving the mobilization of colloids (Kaplan et al. 1993; El-Farhan et al. 2000; Mohanty et al. 2015, 2016). The colloid concentrations and mass fluxes increased with the rising and falling moments of the water flow, or at the inflow after its interruption (El-Farhan et al. 2000; Zhu et al. 2014). The colloid concentrations were much higher at the flow rate of 2.3 mL min−1 than at high flow rates (> 2.3 mL min−1), indicating that the colloid mobilization did not increase with an increase in the steady flow rates or SWP in saturated regimes or near the boundary between saturated and unsaturated regimes (Fig. 2). Previous studies similarly reported that colloids were smaller and more mobile at low flow rates than those at high flow rates, thereby facilitating colloid mobilization (Kapplan et al. 1993; Ryan and Elimelech 1996; Ryan et al. 1998; Mishurov et al. 2008).
Effect of flow rate on colloid mobilizations in Joomoonjin sand columns during sampling soil solutions. Flow rates in saturated regimes that reflect the SWP were: a 2.3 mL min−1, b 3.7 mL min−1, c 5.3 mL min−1, d 6.5 mL min−1, e 9.4 mL min−1, and f 15.6 mL min−1. The vertical dashed lines indicate the boundary between saturated and unsaturated regimes
The colloid concentrations in the saturated regime were higher than in the unsaturated regime (Fig. 2). The water content was ~ 21.3% in the saturated regimes, whereas it decreased to the range of ~ 11.8 to 13.6% in the unsaturated regimes (Table 1). The low water content increased the colloid deposition rate via film straining and air–water interfaces (AWI) attachment (Chen and Mao 2007; Zhang et al. 2010; Knappenberger et al. 2015), indicating that the decrease in water content inhibits colloid mobilization in unsaturated regimes (Kaplan et al. 1993; Keller and Sirivithayapakorn 2004). The colloid deposition rates were higher in the unsaturated regimes due to the additional retention at the air–water-solid (AWS) interfaces and the formation of low flow rate regimes in the presence of air, even though the infiltration rates in the unsaturated regimes were three times higher than in the saturated regimes (Sang et al. 2013). In addition, TEM images showed that colloids (~ 40–60 nm) in saturated regimes (Fig. 3a, b) were smaller and more spherical than those (> ~ 300 nm) in unsaturated regimes (Fig. 3c, d). In particular, the overall sizes of colloids became large in unsaturated regimes due to the aggregation, leading to the deposition and then reduction in porosity (Jassby et al. 2012; Roth et al. 2015). The presence of positively charged metals such as aluminum oxide nanoparticles neutralizes the negatively charged surface of colloids, resulting in the formation of amorphous aggregates, induced by reducing the electrostatic repulsive forces (Kretzschmar and Schaefer 2005; Thompson et al. 2006; Wang et al. 2013; Mui et al. 2016).
Effect of pH and ionic strength on colloid mobilization
To examine the effect of pH and ionic strength on colloid mobilization, colloid concentrations and SWP in soil solutions were analyzed under a variety of pH and ionic strength conditions. The colloid concentrations and SWP increased with increasing pH at the range from pH 4.7 to 9.3 (Fig. 4). The colloid concentrations were two orders magnitude higher in a neutral solution (pH 7.3–7.6) than an acidic solution (pH 4.7–5.0) (Fig. 4a–d). In addition, the colloid concentration was 10.3 mg L−1 in a basic solution (pH 9.2) and without adding NaCl, which was the highest colloid concentration extracted from the Joomoonjin sand column (Fig. 4e, f). This result was consistent with previous findings, in which the mobility and stability of colloids were found to be increased in porous media when the pH of the soil solution was higher than the PZC due to an increase in the repulsion and detachment energy (Ryan and Gschwend 1994; Seta and Karathanasis 1997; Mohanty et al. 2016). Hydrogen from colloids dissociates into the soil solution, and the surface of colloids forms a negatively charged hydroxyl (OH) group (Brady and Weil 2008). Thus, the electrostatic repulsion between colloids and soils occurs, thereby increasing the colloid mobilization under subsurface soil conditions.
Effect of pH and ionic strength on colloid mobilization during sampling soil solutions: a low pH (pH 4.97) without adding NaCl (EC 0.94 mS cm−1), b low pH (pH 4.69) and 10 mM NaCl (EC 2.61 mS cm−1), c neutral pH (pH 7.31) without adding NaCl (EC 0.91 mS cm−1), d neutral pH (pH 7.64) and 10 mM NaCl (EC 2.21 mS cm−1), e high pH (pH 9.23) without adding NaCl (EC 0.89 mS cm−1); and f high pH (pH 9.33) and 10 mM NaCl (EC 1.86 mS cm−1)
The colloid mobilization increased with a decrease in the ionic strength of the soil solutions, particularly, in the basic solution where colloids are abundant in soil solutions (Fig. 4e, f). This is consistent with previous findings, in which a decrease in the ionic strength due to infiltrations such as rainfall, snow melting, and irrigation increased the absolute values for the zeta (ζ) potential (DeNovio et al. 2004; Haliena et al. 2016) and the electrostatic repulsion of negatively charged colloids and soil, which thus enhanced the colloid mobilization (Saiers and Lenhart 2003). This suggestion is supported by the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, in which a lower ionic strength leads to an increase in the diffuse double layer thickness, followed by an increase in the repulsive force (Torkzaban et al. 2007; Zhang and Selim 2007; Mesticou et al. 2014; Mohanty et al. 2016). Conversely, the increase in the ionic strength results in the increase in the deposition rate coefficients, and subsequently the colloid retention (Cheng and Saiers 2010; Zhang et al. 2010). Overall, the colloid mobilization and deposition were found to be dominantly dependent on the chemical perturbation of the soil solution (i.e., pH and ionic strength) in porous media.
Colloid mobilization and heavy metal transport in real field soils
To investigate the colloid mobilization and heavy metal transport in real field soils (Duckum soil) during sampling soil solutions, colloid and heavy metal concentrations were measured at the time intervals (Fig. 5). The concentrations of heavy metals (As, Cr, and Pb) increased with an increase in the colloid concentration (~ 15.4 mg L−1) at 40 min (Fig. 5b, c). These results indicate that organic matter in the colloids could adsorb heavy metal ions, as effective carriers in porous media (Grolimund et al. 1998; Graham et al. 2006; Hartland et al. 2015). Colloidal particles showed correlations with colloidal As, rather than truly dissolved As, in the groundwater between streams and aquifers (Hartland et al. 2015). On the other hand, the concentrations of Cd, Cu, Fe, Ni, Al, and Co were high at 53 and 100 min, and did not increase with increases in the colloid concentrations (Fig. 5c). It has been posited that truly dissolved organic carbons could be significantly correlated with heavy metals such as Cd, Cu, and Ni, predominant fractions in the total dissolved fractions, indicating that these heavy metals may not be correlated with colloids (Dai et al. 1995; Oursel et al. 2013). Over the range from 120 to 240 min, the Mn and Zn concentrations increased in the absence of colloids, which was consistent with the observation in previous literature (Miller et al. 2011; Kim and Kim 2015). This result suggests that the colloid-facilitated transport can be affected by the characteristics of the colloids and heavy metals in subsurface environments. TEM images revealed the independent and spherical shapes of colloids (Fig. 6). These shapes may be attributed to the high pH value (pH 7.8) of the soil solution which increased the electrostatic repulsive force. Large-sized colloids (~ 310 nm) were released and mobilized at 40 min (momentarily changed SWP) due to the shear stress between the colloids and soil surface. In addition, the presence of Si, Al, and Fe indicated the existence of silicates, aluminosilicates, and iron (or –oxy hydroxide) oxides (Liu et al. 2013). Therefore, individual colloids at the momentarily changed SWP may adsorb heavy metals and facilitate the transport of toxic heavy metals in the subsurface environments (Amrhein et al. 1993; Grolimund and Borkovec 1999; Kim and Kim 2015).
Conclusions
This study investigated the variation of colloid concentrations, flow rate, and SWP during the sampling of soil solutions in soils of South Korea. Colloid concentrations increased with decreasing flow rates corresponding to SWP in the soil solution, while those remained low at high flow rates. Colloid concentrations were higher in saturated regimes than in unsaturated regimes in sand column. The decrease in the water content in the unsaturated regime may result in an increase in the colloid deposition rate due to film straining, AWI attachment, and additional retention at the AWS interfaces. The colloid concentrations significantly increased with increasing pH in suggesting that the mobility of colloids was enhanced in the basic solution. In addition, the colloid concentrations increased with a decrease in the ionic strength. Infiltrations such as rainfall, snow melting, and irrigation decrease the ionic strength and thereby may increase the colloid mobilization in subsurface environments.
The concentrations of As, Cr, and Pb increased with an increase in the colloid concentrations during momentarily changed SWP in real field soil, indicating that colloids could adsorb positively charged heavy metal ions as effective carriers. However, the mobilization of Cd, Cu, Fe, Ni, Al, and Co lagged behind the colloid mobilization, suggesting that colloid-facilitated transport could be affected by the characteristics of heavy metals. Therefore, physicochemical perturbations and heavy metal properties were deemed to play a significant role in accelerating or inhibiting the colloid mobilization in subsurface environments.
References
Amrhein, C., Mosher, P. A., & Strong, J. E. (1993). Colloid-assisted transport of trace metals in roadside soils receiving deicing salts. Soil Science Society of America Journal, 57(5), 1212–1217.
Backhus, D. A., Ryan, J. N., Groher, D. M., MacFarlane, J. K., & Gschwend, P. M. (1993). Sampling colloids and colloid-associated contaminants in ground water. Ground Water, 31(3), 466–479.
Biddle, D. L., Chittleborough, D. J., & Fitzpatrick, R. W. (1995). Field monitoring of solute and colloid mobility in a gneissic sub-catchment, South Australia. Applied Clay Science, 9(6), 433–442.
Brady, N. C., & Weil, R. R. (2008). The nature and properties of soils. Upper Saddle River, NJ: Pearson Prentice Hall.
Buddemeier, R. W., & Hunt, J. R. (1988). Transport of colloidal contaminants in groundwater: Radionuclide migration at the Nevada test site. Applied Geochemistry, 3(5), 535–548.
Chen, G., Flury, M., Harsh, J. B., & Lichtner, P. C. (2005). Colloid-facilitated transport of cesium in variably saturated hanford sediments. Environmental Science and Technology, 39(10), 3435–3442.
Chen, X., & Mao, S. S. (2007). Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chemical Reviews, 107(7), 2891–2959.
Cheng, T., & Saiers, J. E. (2010). Colloid-facilitated transport of cesium in vadose-zone sediments: The importance of flow transients. Environmental Science and Technology, 44(19), 7443–7449.
Dai, Z., Fornasiero, D., & Ralston, J. (1999). Particle-bubble attachment in mineral flotation. Journal of Colloid and Interface Science, 217(1), 70–76.
Dai, M., Martin, J.-M., & Cauwet, G. (1995). The significant role of colloids in the transport and transformation of organic carbon and associated trace metals (Cd, Cu and Ni) in the Rhône delta (France). Marine Chemistry, 51(2), 159–175.
de Jonge, L. W., Kjaergaard, C., & Moldrup, P. (2004). Colloids and colloid-facilitated transport of contaminants in soils. Vadose Zone Journal, 3(2), 321.
DeNovio, N. M., Saiers, J. E., & Ryan, J. N. (2004). Colloid movement in unsaturated porous media. Vadose Zone Journal, 3(2), 338–351.
El-Farhan, Y. H., DeNovio, N. M., Herman, J. S., & Hornberger, G. M. (2000). Mobilization and transport of soil particles during infiltration experiments in an agricultural field, shenandoah valley, Virginia. Environmental Science and Technology, 34(17), 3555–3559.
Gamerdinger, A. P., & Kaplan, D. I. (2001). Physical and chemical determinants of colloid transport and deposition in water-unsaturated sand and Yucca Mountain tuff material. Environmental Science and Technology, 35(12), 2497–2504.
Graham, M. C., Vinogradoff, S. I., Chipchase, A. J., Dunn, S. M., Bacon, J. R., & Farmer, J. G. (2006). Using size fractionation and Pb isotopes to study Pb transport in the waters of an organic-rich upland catchment. Environmental Science and Technology, 40(4), 1250–1256.
Grolimund, D., & Borkovec, M. (1999). Long-term release kinetics of colloidal particles from natural porous media. Environmental Science and Technology, 33(22), 4054–4060.
Grolimund, D., Elimelech, M., Borkovec, M., Barmettler, K., Kretzschmar, R., & Sticher, H. (1998). Transport of in situ mobilized colloidal particles in packed soil columns. Environmental Science and Technology, 32(22), 3562–3569.
Haliena, B., Zheng, H., Melson, N., Kaplan, D. I., & Barnett, M. O. (2016). Decreased salinity and actinide mobility: Colloid-facilitated transport or pH change? Environmental Science and Technology, 50(2), 625–632.
Hartland, A., Larsen, J. R., Andersen, M. S., Baalousha, M., & O’Carroll, D. (2015). Association of arsenic and phosphorus with iron nanoparticles between streams and aquifers: Implications for arsenic mobility. Environmental Science and Technology, 49(24), 14101–14109.
Hubbe, M. A. (1985). Detachment of colloidal hydrous oxide spheres from flat solids exposed to flow 2. Mechanism of release. Colloids and Surfaces, 16(3), 249–270.
Jassby, D., Farner Budarz, J., & Wiesner, M. (2012). Impact of aggregate size and structure on the photocatalytic properties of TiO2 and ZnO nanoparticles. Environmental Science and Technology, 46(13), 6934–6941.
Kaplan, D. I., Bertsch, P. M., Adriano, D. C., & Miller, W. P. (1993). Soil-borne mobile colloids as influenced by water flow and organic carbon. Environmental Science and Technology, 27(6), 1193–1200.
Karathanasis, A. D., & Johnson, D. M. C. (2006). Stability and transportability of biosolid colloids through undisturbed soil monoliths. Geoderma, 130(3–4), 334–345.
Keller, A. A., & Sirivithayapakorn, S. (2004). Transport of colloids in unsaturated porous media: Explaining large-scale behavior based on pore-scale mechanisms. Water Resources Research, 40(12), W12403.
Kim, I., & Kim, G. (2015). Role of colloids in the discharge of trace elements and rare earth elements from coastal groundwater to the ocean. Marine Chemistry, 176, 126–132.
Klitzke, S., Schroeder, J., Selinka, H.-C., Szewzyk, R., & Chorus, I. (2015). Attenuation and colloidal mobilization of bacteriophages in natural sediments under anoxic as compared to oxic conditions. Science of the Total Environment, 518–519, 130–138.
Knappenberger, T., Aramrak, S., & Flury, M. (2015). Transport of barrel and spherical shaped colloids in unsaturated porous media. Journal of Contaminant Hydrology, 180, 69–79.
Kretzschmar, R., & Schaefer, T. (2005). Metal retention and transport on colloidal particles in the environment. Elements, 1(4), 205–210.
Liu, W., Wang, T., Borthwick, A. G. L., Wang, Y., Yin, X., Li, X., et al. (2013). Adsorption of Pb2+, Cd2+, Cu2+ and Cr3+ onto titanate nanotubes: Competition and effect of inorganic ions. Science of the Total Environment, 456–457, 171–180.
McDowell-Boyer, L. M. (1992). Chemical mobilization of micron-sized particles in saturated porous media under steady flow conditions. Environmental Science and Technology, 26(3), 586–593.
Mesticou, Z., Kacem, M., & Dubujet, P. (2014). Influence of ionic strength and flow rate on silt particle deposition and release in saturated porous medium: Experiment and modeling. Transport in Porous Media, 103(1), 1–24.
Miller, J. O., Karathanasis, A. D., & Matocha, C. J. (2011). In situ generated colloid transport of Cu and Zn in reclaimed mine soil profiles associated with biosolids application. Applied and Environmental Soil Science, 2011, 9.
Mills, W. B., Liu, S., & Fong, F. K. (1991). Literature review and model (COMET) for colloid/metals transport in porous media. Ground Water, 29, 199–208.
Mishurov, M., Yakirevich, A., & Weisbrod, N. (2008). Colloid transport in a heterogeneous partially saturated sand column. Environmental Science and Technology, 42(4), 1066–1071.
Mohanty, S. K., Saiers, J. E., & Ryan, J. N. (2015). Colloid mobilization in a fractured soil during dry-wet cycles: Role of drying duration and flow path permeability. Environmental Science and Technology, 49(15), 9100–9106.
Mohanty, S. K., Saiers, J. E., & Ryan, J. N. (2016). Colloid mobilization in a fractured soil: Effect of pore-water exchange between preferential flow paths and soil matrix. Environmental Science and Technology, 50(5), 2310–2317.
Mui, J., Ngo, J., & Kim, B. (2016). Aggregation and colloidal stability of commercially available Al2O3 nanoparticles in aqueous environments. Nanomaterials, 6(5), 90.
Novikov, A. P., Kalmykov, S. N., Utsunomiya, S., Ewing, R. C., Horreard, F., Merkulov, A., et al. (2006). Colloid Transport of plutonium in the far-field of the Mayak Production Association, Russia. Science, 314(5799), 638.
Oursel, B., Garnier, C., Durrieu, G., Mounier, S., Omanović, D., & Lucas, Y. (2013). Dynamics and fates of trace metals chronically input in a Mediterranean coastal zone impacted by a large urban area. Marine Pollution Bulletin, 69(1–2), 137–149.
Pawlowska, A., Sznajder, I., & Sadowski, Z. (2017). The colloid hematite particle migration through the unsaturated porous bed at the presence of biosurfactants. Environmental Science and Pollution Research International, 24(21), 17912–17919.
Puls, R. W., Clark, D. A., Bledsoe, B., Powell, R. M., & Paul, C. J. (1992). Metals in ground water: Sampling artifacts and reproducibility. Hazardous Waste and Hazardous Materials, 9(2), 149–162.
Qi, Z., Hou, L., Zhu, D., Ji, R., & Chen, W. (2014). Enhanced transport of phenanthrene and 1-naphthol by colloidal graphene oxide nanoparticles in saturated soil. Environmental Science and Technology, 48(17), 10136–10144.
Redman, J. A., Grant, S. B., Olson, T. M., & Estes, M. K. (2001). Pathogen filtration, heterogeneity, and the potable reuse of wastewater. Environmental Science and Technology, 35(9), 1798–1805.
Roth, E. J., Gilbert, B., & Mays, D. C. (2015). Colloid deposit morphology and clogging in porous media: Fundamental insights through investigation of deposit fractal dimension. Environmental Science and Technology, 49(20), 12263–12270.
Ryan, J. N., Aiken, G. R., Backhus, D. A., Villholth, K. G., & Hawley, C. M. (1999). Investigating the potential for colloid-and organic matter-facilitated transport of polycyclic aromatic hydrocarbons in crude oil-contaminated ground water. US geological survey toxic substances hydrology program–Proceedings of the Technical Meeting (pp. 211–222), Charleston, South Carolina.
Ryan, J. N., & Elimelech, M. (1996). A collection of papers presented at the symposium on colloidal and interfacial phenomena in aquatic environments colloid mobilization and transport in groundwater. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 107, 1–56.
Ryan, J. N., & Gschwend, P. M. (1994). Effects of ionic strength and flow rate on colloid release: Relating kinetics to intersurface potential energy. Journal of Colloid and Interface Science, 164(1), 21–34.
Ryan, J. N., Illangasekare, T. H., Litaor, M. I., & Shannon, R. (1998). Particle and plutonium mobilization in macroporous soils during rainfall simulations. Environmental Science and Technology, 32(4), 476–482.
Saiers, J. E., & Lenhart, J. J. (2003). Ionic-strength effects on colloid transport and interfacial reactions in partially saturated porous media. Water Resources Research, 39(9), 1256.
Sang, W., Morales, V. L., Zhang, W., Stoof, C. R., Gao, B., Schatz, A. L., et al. (2013). Quantification of colloid retention and release by straining and energy minima in variably saturated porous media. Environmental Science and Technology, 47(15), 8256–8264.
Seta, A. K., & Karathanasis, A. D. (1997). Atrazine adsorption by soil colloids and co-transport through subsurface environments. Soil Science Society of America Journal, 61(2), 612–617.
Sprague, L. A., Herman, J. S., Hornberger, G. M., & Mills, A. L. (2000). Atrazine adsorption and colloid-facilitated transport through the unsaturated zone. Journal of Environmental Quality, 29(5), 1632–1641.
Thompson, A., Chadwick, O. A., Boman, S., & Chorover, J. (2006). Colloid mobilization during soil iron redox oscillations. Environmental Science and Technology, 40(18), 5743–5749.
Torkzaban, S., Bradford, S. A., & Walker, S. L. (2007). Resolving the coupled effects of hydrodynamics and DLVO forces on colloid attachment in porous media. Langmuir, 23(19), 9652–9660.
Wang, T., LaMontagne, D., Lynch, J., Zhuang, J., & Cao, Y. C. (2013). Colloidal superparticles from nanoparticle assembly. Chemical Society Reviews, 42(7), 2804–2823.
Zhang, J., Li, Y., Zhang, X., & Yang, B. (2010). Colloidal self-assembly meets nanofabrication: from two-dimensional colloidal crystals to nanostructure arrays. Advanced Materials, 22(38), 4249–4269.
Zhang, H., & Selim, H. M. (2007). Colloid mobilization and arsenite transport in soil columns: Effect of ionic strength. Journal of Environmental Quality, 36(5), 1273–1280.
Zhou, D., Wang, D., Cang, L., Hao, X., & Chu, L. (2011). Transport and re-entrainment of soil colloids in saturated packed column: effects of pH and ionic strength. Journal of Soils and Sediments, 11(3), 491–503.
Zhu, Y., Ma, L. Q., Dong, X., Harris, W. G., Bonzongo, J. C., & Han, F. (2014). Ionic strength reduction and flow interruption enhanced colloid-facilitated Hg transport in contaminated soils. Journal of Hazardous Materials, 264, 286–292.
Acknowledgements
This work was supported by the “Climate Technology Development and Application” research project from International Environmental Research Institute (IERI) at Gwangju Institute of Science and Technology (GIST), Korea, in 2017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S., Ko, IW., Yoon, IH. et al. Colloid mobilization and heavy metal transport in the sampling of soil solution from Duckum soil in South Korea. Environ Geochem Health 41, 469–480 (2019). https://doi.org/10.1007/s10653-018-0099-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0099-7