Abstract
Arsenic (As) is a toxic metalloid that can cause significant health issues through drinking water. The present study was aimed to evaluate As distribution and the related health risks from drinking groundwater in rural areas of Hashtroud, Iran. Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were also applied to better explain relationship pattern between different resources. The samples were taken from 51 locations in 37 villages. Arsenic concentration was determined by a polarograph device, and the corresponding carcinogenic and non-carcinogenic health risks were calculated based on US Environmental Protection Agency (EPA) guideline. PCA analysis extracted four main components that explained nearly 62% of data variance. Results pointed severe As contamination in the studied area, where As was detected in 78% of the samples ranging from less than 0.001 to 0.250 mg/L. Forty percent of the contaminated places violated guideline value of 10 μg/L suggested by EPA and institute of standards and industrial research of Iran (ISIRI). Based on our findings, 1329 people including 239 children were living in the areas with higher As contamination. Hazard quotient (HQ) in 72%, 59%, and 33% of the samples was higher than one for children, adolescent, and adult age groups, respectively. Excess life time cancer risk (ELCR) in almost 80% of all age groups was significantly higher than EPA recommended guideline (10−4 or 10−6). In summary, from the view point of arsenic HQ and ELCR, water resources in the studied areas were not appropriate for drinking and hygienic purposes; necessary and urgent management strategies to guarantee water supply and health safety for local residents should be considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, due to severe water scarcity, providing safe and healthy drinking water is one of the most important challenges in most of the societies (Rasool et al. 2016). In developing countries, more than 18 million people lose their life every year because of waterborne diseases (Beatrice et al. 2019; WHO 2006). So, water quality is becoming a major problem in both rural and urban areas of developing countries (Chalchisa et al. 2018). Infiltration may increase contaminants, rendering groundwater resources unsuitable for drinking purposes (Zietz et al. 2007). Contamination of water resources with heavy metals is a major health concern (Gul et al. 2015; Khan et al. 2016). These pollutants enter to the environment through natural activities such as weathering, erosion, and geothermal processes or anthropogenic activities such as petroleum refining, the use of pesticides, paints, drugs, and metals, which finally find their ways into the drinking water resources (Jadoon et al. 2019; Liang et al. 2016; Rasool et al. 2016). Arsenic (As) is one of the heavy metals that can be found everywhere in the earth’s crust (Rasheed et al. 2016). People might be exposed to high arsenic through various pathways, including drinking water, air, and food (Brahman et al. 2016). Among them, drinking water is considered as one of the most important pathway which people may receive every contaminant (Hopenhayn-Rich et al. 1996). The international agency for research on cancer (IARC) has listed arsenic in drinking water as a group 1 carcinogen (Papillomaviruses 2011). Furthermore, in the classification of agency for toxic substances and disease registry (ATSDR), among the 20 most dangerous substances, As ranks first (ATSDR 2007). The U.S. environmental protection agency (USEPA) recommended the maximum contaminant level (MCL) of 10 μg/L as a guideline for drinking purposes (Bizier and DeBarry 2003; Standard methods 1915).
Physical, chemical, and microbial properties are main parameters determining safety and health of drinking water, as well as its acceptability (Babaei et al. 2014; Jain et al. 2018). The quality of drinking water including various physical and chemical parameters should be monitored at regular time intervals. The choice of testing parameters mainly depends on the purpose of use, amount, and quality of required water (Patil et al. 2012). A common way to assess water quality and its sustainability for drinking purposes is to use water quality index (WQI) that simply states overall water quality (Abbasnia et al. 2018; Călmuc et al. 2018); in other words, WQI plays a significant role in water resources management (Wu et al. 2018).
Multivariate statistical analysis is a manner to assess groundwater geochemistry variations (Cloutier et al. 2008). Principal component analysis (PCA) and hierarchical cluster analysis (HCA) are two of the multivariate statistical methods widely used to classify groundwater samples, create a correlation between variables, and dimension reduction or structure detection (Dehghanzadeh et al. 2015). PCA can help to find the relationship among different variables and explains the pattern of correlations within a set of variables (Manbohi and Gholamipour 2020; Zhong et al. 2018). HCA, by grouping samples according to their different chemical characteristics, is a strong tool used to identify chemical relationships between the samples (Dhanasekarapandian et al. 2016; Zhang et al. 2012).
Some studies have shown that arsenic concentrations in natural waters range from less than 0.5 to over 5000 μg/L, worldwide (Ayotte et al. 2015; Lalwani et al. 2006). Arsenic is toxic even at low concentrations (Wongsasuluk et al. 2014) and can cause chronic health risks (McGrory et al. 2017). In Asia, chronic arsenic poisoning is becoming an emergency epidemic, with more than 100 million people exposed to high levels of As through groundwater (Saint-Jacques et al. 2014). Some of the non-cancerous effects of As include skin injuries, vascular diseases, diabetes, neurotoxicity, liver toxicity, chronic cough, and adverse pregnancy complications (Bakhat et al. 2017; D’Ippoliti et al. 2015). Severe health effects have been reported in people who received arsenic-rich waters for long periods (WHO 2001). Therefore, arsenic health risk assessment from contaminated groundwater consumption and understanding the potential threat to consumers is quite vital. Hazard quotient (HQ) and excess lifetime cancer risk (ELCR), recommended by the USEPA, have widely been used to estimate the potential non-carcinogenic and carcinogenic risks from arsenic exposure (Liang et al. 2018; USEPA 1998).
Studies emphasizing the contamination of groundwater with arsenic have been of interest in different parts of the world, as well as in Iran. Health risk assessment in drinking water resources and rice in Meydavood area, Khuzestan province, showed that the risk level was worrying and unsafe in all three groups of children, men, and women (Kolahkaj1 et al. 2018). The prevalence of hyperpigmentation and hyperkeratosis due to high concentration of arsenic was reported in a study in the Bijar region, Kurdistan, Iran (Mosaferi et al. 2008). Ecological risk assessment and origin of heavy metals including arsenic in surface sediments of Khiyav River, northwestern of Iran, showed that arsenic contamination was due to various factors such as geothermal activities, hot springs, and mineral activities (Fard et al. 2018).
Regarding arsenic contamination in drinking water and associated health risk, no research has been carried out in the study area. This study was conducted for the first time to analyze human health risks caused by groundwater arsenic in the area. The study aims to determine the following: (a) arsenic spatial distribution and the related health risk in drinking water of Hashtroud and its rural communities, (b) water quality assessment using WQI, (c) PCA and HCA were also applied to better understand relationship patterns between variable and resources. Given the crucial role of unpolluted water on human health, the results of this study may be of interest to various stakeholders.
Material and method
Study area
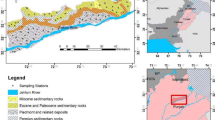
Hashtroud (37° 39′ N, 47° 19′ E) is a county in East Azerbaijan province located northwest of Iran. This county has two cities, Hashtroud and Nazarkahriz, and 198 villages. According to the latest information provided by Iranian statistical center in 2017, Hashtroud, with the area of 1990 km2, has 57,199 population which 38.09% and 61.91% of them were living in urban and rural areas, respectively. Figure 1 shows the study area and sampling points. Hashtroud’s climate is relatively cold and dry. The average temperature in the study ranges between 24 ° C for the hottest month and − 2 ° C for the coldest month of the year. The county is located 1150 m above sea level. The selected villages were representatively covered all geographical directions, i.e., north, south, east, and west.
Hydrogeology of the study area
The study area in terms of hydrogeological divisions is part of Caspian Sea drainage basin and Sefidrud River sub-basin. This area is one of the young volcanic-sedimentary zones. The geological formations in the region are divided into two major sections of upper red and pyroclastic deposits. Pyroclastic deposits have the most expansion in the study area, and their ages range from Miocene to Quaternary. The lithology of the area includes limestones, sandstones, conglomerate, and volcanic rocks. The study area lacks a large and extensive aquifer; surface and groundwaters are interconnected in a way that water from precipitation and snowmelt penetrates the ground. Much of the area’s groundwater is drained through inter-layer seams through the springs, run-off, and permeation. Geological studies show that these springs must be of the mineral type.
Sample collection and analysis
A total of 51 samples of drinking groundwater were randomly taken from 37 different locations. Replicate samples were taken in places where As concentration violated the recommended standards. Samples were collected in clean polyethylene bottles of 1500-mL capacity, in 2018. Sampling points were selected in a way that cover the whole study area. All samples were analyzed based on the standard methods for the examination of water and wastewater (Standard methods 2017). Sampling containers were washed with double distilled water and rinsed with sample water, before sampling. Each sample bottle was labeled and the necessary information was provided. Groundwater samples were transferred to the laboratory and the physicochemical parameters were measured immediately. To measure the As concentration, a subsample was separated from each sample and acidified using nitric acid (97% w/v) to ensure As fixation. Acidified samples were stored in the refrigerator at temperatures below 4 °C. Arsenic concentration was determined using the polarograph device (Metrohm Co., Switzerland), which was capable of measuring all heavy metals at the level of ppb and ppt. The main advantage of the polarographic measurement method is its repeatability and high detection limit.
Calculation of water quality index
The WQI is useful for assessing groundwater quality (Kalaivanan et al. 2018). Since the groundwater is the main source of drinking water supply in the studied villages, groundwater quality index (GWQI) was used to assess groundwater quality. The GWQI is calculated by Eq. (1):
where Ci is the concentration of each chemical parameter in each sample (mg/L); Si is the WHO drinking water standard for each chemical parameter (mg/L); wi is the assigned weight according to its relative importance in the overall quality of water for drinking purposes, which based on the WHO standard (2011) ranges from 1 to 5; n is the number of parameters; qi is the water quality rating; Wi is the relative weight; and SIi is the sub index of ith parameter (WHO 2011). The assigned weight (wi) and relative weights (Wi) for all the chemical parameters are shown in Table 1. Computed WQI values for drinking purposes are usually classified into five categories that are presented in Table 2 (Sahu and Sikdar 2008).
Multivariate statistical analysis
In this study, principal component analysis (PCA) was applied to extract significant principal components (PCs). The PCs lie along the directions of maximum variance. Principal components are linear combinations of original variables. In the PCA prioritization, the most important parameters place in the first component, and other parameters with lower importance place in the next components, respectively. Along with the acquisition of PCs, PCA provides a correlation matrix that describes the relationship of each parameter to the others (Bhakar and Singh 2019; Faryadi et al. 2012; Zhong et al. 2018). Also, hierarchical cluster analysis (HCA) was used to identify relatively homogeneous groups of variables based on their intrinsic properties. In HCA, a set of variables is combined such that the variables of each group, known as clusters, are more similar to each other than the variables in other clusters (Jiang et al. 2015; Monjerezi et al. 2011; Singh et al. 2005). In this study, the data were evaluated by conducting PCA and HCA using the SPSS software for windows, version 20.
Human health risk assessment
The non-carcinogenic and carcinogenic health risks due to groundwater contamination by arsenic were estimated using the USEPA method (USEPA 2005). Arsenic average daily intake is obtained from Eq. (2):
where ADD is the average daily dose from ingestion of As (mg/kg day); C is the As concentration in the groundwater (mg/L); IR is the daily water intake rate (L/day); ED is the exposure duration (years); EF is the exposure frequency (365 days/year); BW is body weight (kg), and AT is averaging time of life expectancy (days). The parameters required to determine the average daily dose from ingestion of As through water intake (for three age groups of children, adolescents, and adults) are presented in Table 3.
Non-carcinogenic risk calculation
Hazard quotient (HQ) is the ratio between the exposure to a heavy metal and its reference dose, which is used to express non-carcinogenic exposure. It can be obtained from Eq. (3):
where RfD is the reference dose (mg/kg day). The oral RfD value for As is 0.0003 mg/kg day (USEPA 2005). If the calculated HQ is less than one, it indicates that there is no adverse health effect, but if the calculated HQ is greater than 1, there will be unacceptable non-carcinogenic effect to consumer health.
Carcinogenic risk calculation
Health risk for carcinogenic exposure was calculated as an excess lifetime cancer risk (ELCR). The ELCR is calculated by using Eq. (4):
where CSF is the cancer slope factor (mg/kg day). According to USEPA database, the CSF value for arsenic is 1.5 mg/kg day (USEPA 2005). In this study, the estimated amount of ELCR was compared with the maximum acceptable risk recommended by the EPA, which is 10−4 or 10−6.
Statistical analysis
All data were statistically analyzed by Microsoft Excel 2016 and SPSS for windows V.21. Arsenic contamination dispersion and HQ interpolation were performed using inverse distance weighting (IDW) in the Arc GIS 10.3 software.
Results and discussion
Physicochemical characteristics
Some of the most important physicochemical parameters of drinking water to be tested include pH, total dissolved solids (TDS), alkalinity, hardness, chloride, nitrate, nitrite, sulfate, sodium, and potassium. Descriptive statistics for all the parameters analyzed are shown in Table 4.
pH value gives the amount of hydrogen ion concentration and determines the corrosive nature of water (Patil et al. 2012), which is an important factor in water quality assessment (Sujatha et al. 2012). The pH of drinking water in the surveyed villages was in accordance with the standard recommended by WHO (6.5–8.5). In a similar study in the Maragheh-Bonab region of northwestern Iran, the pH was in the range of 7.6–8.4, which corresponds to our findings and WHO standard (Fijani et al. 2017). Total dissolved solids (TDS) mainly comprised of inorganic salts (including calcium, magnesium, potassium, sodium, bicarbonates, chloride, and sulfate) and some small amount of organic substances that are dissolved in water and affect water quality (Lanjwani et al. 2020; Vijaya Kumar et al. 2020). The concentrations of TDS in the studied areas were between 93 and 612 mg/L with an average value of 302.25 mg/L. The TDS of all samples were within the limits suggested by WHO (1000 mg/L). A study conducted in northwestern part of Iran reported that the TDS level in groundwater was ranged from 193.8 to 2238 mg/L. The maximum TDS level in that region was higher than the WHO standard and also does not correspond to the present study (Rostami et al. 2019). Different studies have shown that pH and TDS are good indicators of groundwater pollution (Abdullahi et al. 2017; Nwankwoala et al. 2014). Sulfate (SO4) is a prevalent anion in natural water bodies which, in concentrations higher than the recommended values, threatens both human health and ecological balance (Wang and Zhang 2019). The concentration of sulfate in the studied villages’ varied between 1 and 120 mg/L. The analyzed values were within the WHO recommended limit (250 mg/L). According to a literature, the concentration of SO4 during 2003 and 2014 was in the range of 0.08–22.13 mg/L (Asadi et al. 2019). High concentration of chloride (Cl) creates a salty taste in water which is known as a factor of contamination. Excessive chlorine concentration also have laxative properties and may cause vascular disease (Lanjwani et al. 2020; Verma et al. 2017). The concentration of chloride in groundwater of the study zone varied from 5 to 130 mg/L. All analyzed values were lower than permissible limit recommended by WHO (250 mg/L). Groundwater pollution arising from extreme use of fertilizers, animal waste or seepage of sewage, increases nitrate (NO3) and nitrite (NO2) concentrations in groundwaters (Sabo and Christopher 2014). High concentration of nitrate causes methaemoglobinemia in children, abortion, malformation, and stomach cancer (Sevda et al. 2018). The concentration of nitrate and nitrite in the studied areas were found from 0.01 to 44.5 mg/L and 0 to 3 mg/L, respectively. According to the WHO guideline for short-term exposure, the nitrate and nitrite concentration should not exceed 50 and 3 mg/L, respectively. In a similar study, analytical results of water quality in a part of Hashtroud County close to Sahand volcanic mountains showed that the concentration of nitrate was in the range of 4.96–1372.06 mg/L (Mosaferi et al. 2017).
Sodium (Na) is present in all groundwaters and its high concentration definitely cause saline taste; furthermore, hypertension and cardiovascular disease are another consequences of Na presence, reported previously (Lanjwani et al. 2020; Logeshkumaran et al. 2015). Based on the WHO guideline, the maximum permissible limit of sodium is 200 mg/L. The concentration of sodium was varied between 0.005 and 94 mg/L that for all samples were less than WHO limit. Potassium (K) naturally exists in many rocks, and with the gradual dissolution of these rocks, the concentration of potassium in groundwater increases over time (Chaurasia et al. 2018). The concentration of K was between 0 and 5.15 mg/L in the groundwater of the studied areas that all of them were lower than the permissible limit recommended (12 mg/L). Our findings in this regard are in well accordance with those reported previously (Rostami et al. 2019). Total hardness (TH) is a main factor that arises due to the presence of calcium and magnesium along with their carbonates, sulfates, and chlorides anions (Arumugam 2013). The desirable limit of 500 mg/L has been recommended for total hardness by WHO. Hardness ranged from 100 to 328 mg/L, which was within the permissible limits. Alkalinity is usually a function of the amounts of carbonate, bicarbonate, and hydroxide ions present. Alkalinity acts as a stabilizer for pH (Vijaya Kumar et al. 2020), which in higher amount causes an unpleasant taste (Chaurasia et al. 2018). Alkalinity values in the studied areas ranged from 40 to 320 mg/L. WHO has set 200 mg/L alkalinity as an acceptable value in drinking water. It should be noted that the majority of the samples exceeded the desirable limit.
Groundwater quality index
Water quality index (WQI), due to its ease of use and scientific basis, has become a common tool in water quality assessment. The WQI is a single dimensionless number that expresses the overall quality of water, based on different water quality parameters, at a given location and time. The results of this index can be used to determine the sanitation level of water resources (Sutadian et al. 2016). In this study, the GWQI for 51 groundwater samples were calculated using TDS, SO4, Cl, NO3, NO2, Na, K, hardness, and pH. These parameters have been widely suggested for WQI calculation (Adimalla and Qian 2019; Howladar et al. 2018; Rabeiy 2018). The calculated GWQI values ranged from 17.167 to 88.134 with an average of 45.049 that is represented in Table 5. Considering this table, 60.8% and 39.2% of studied samples showed excellent and good quality, respectively. According to the results, it can be said that from the physical-chemical point of view, the quality of groundwaters in the studied areas was suitable for drinking purposes.
Multivariate statistical analysis
PCA is the most commonly applied multivariate method which is designed to convert the original variables into new variables, called the principal components (PCs) (Bhakar and Singh 2019). In this study, the PCA was carried out to assess groundwater quality. The PCA results of 10 physicochemical parameters from 51 groundwater samples are shown in Tables 6 and 7. Four major principle components (PC1, PC2, PC3, and PC4) affecting quality of groundwaters are identified. The first principal component (PC1) after varimax rotation, accounts for more than 21% of the total variance (Table 6) and has very high loadings on SO4, Cl, NO3, and TH. The second principal component (PC2), which accounts for more than 16% of the total variance, has very high loading on K and alkalinity. The third principal component (PC3), which accounts for more than 13% of the total variance, has high loadings on pH, TDS, and Na. The PC4, which accounts for more than 11% of the total variance, has significant loadings on NO2.
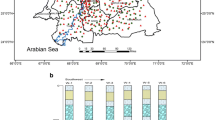
The result of HCA for 51 groundwater samples is shown in Fig. 2. In HCA, clusters are generated sequentially with the most similar pairs of variables, and more clusters are gradually produced. The dendrogram provides a graphical summary of the clustering procedures (Dhanasekarapandian et al. 2016; Manbohi and Gholamipour 2020). In this study, four different clusters were obtained which had their own water quality. The samples from nearby locations fall in the same cluster because of the appropriate involvement of sampling sites in the development of cluster. More than 82% of the water samples were classified in cluster 4. The average values of Cl, Na, and K were higher in cluster 1 than other three clusters. Groundwaters in clusters 1 and 4 were characterized by high TDS concentrations. So, the groundwaters grouped in cluster 1 and 4 were the most saline groundwaters in the study area. The value of total hardness is higher in clusters 1, 3, and 4 than cluster 2, with the mean values of 210 mg/L hardness as CaCO3 in cluster 1, 196 mg/L as CaCO3 in cluster 3, 194.6 mg/L as CaCO3 in cluster 4, and 94 mg/L as CaCO3 in cluster 2. Samples in cluster 3 are characterized by higher NO3 concentration with mean values of 28.5 mg/L. Therefore, cluster 3 shows the relatively more toxic state than other three clusters. In the study area, pH values ranged between 6.8 and 7.8. Groundwaters in cluster 3 have slightly higher pH value than clusters 1, 2, and 4. The first cluster comprises of two groundwater samples (S1 and S29) which have the lowest alkalinity.
Arsenic concentration in the study area
Concentration of As in water resources and the amount of water taken daily are two main factors affecting the amount of arsenic ingested through drinking water. It has been stated that higher arsenic concentration along with higher water consumption will lead to arsenic poisoning in the population (Bhowmick et al. 2018). In all studied villages, groundwater is the main source of drinking water supply.
Excessive variations of arsenic levels were found between different studied villages (see Fig. 1). Table 8 shows the As concentration in the studied groundwater samples. Arsenic was detected in 78% (40 out of 51 samples) of the samples. Among the contaminated places, As concentration in 60% of the samples was less than the EPA and ISIRI guideline value (10 μg/L), while 40% of the samples violated the recommended standard. The highest concentration of As was measured in the Saadatlu village (sample no. 39) with a concentration of 0.245 mg/L, which was 24.5 times higher than the recommenced standard.
Health risk assessment
Arsenic is a toxic metalloid which presents in both organic and inorganic forms, whose inorganic form was far more toxic and typically is widely spread in natural environments such as soil and groundwater (Jiang et al. 2019; Kumar et al. 2016). Arsenic poisoning can have acute or chronic effects. Acute poisoning that usually happens rarely leads to shock, multi-organ deficiency, and death. Prolonged human exposure to arsenic will cause chronic health problems including a variety of diseases and cancers (Gunduz et al. 2017). The response to arsenic exposure varies from person to person, but some vulnerable groups, such as pregnant women, infants and children, the elderly, and those with poor immune systems, are at greater risk of health effects (Alexander et al. 2009; Hong et al. 2014; Rasheed et al. 2016). Due to the volcanism of the study area and the high volcanic ash distribution, the origin of the contamination can be attributed to these compounds. Previous studies in the adjacent area also confirmed the role of volcanic activity in increasing arsenic levels in these areas (Behbahaninia and Farahani 2016; Mosaferi et al. 2008). Since there is no industrial or urban contaminant activities in the study area, the only anthropogenic source that can be assumed for this contamination is the application of arsenic-containing fertilizers and pesticides; however, agricultural activity in the studied area is typically in the form of dry farming, and fertilizers or pesticides are not widely used, so they cannot be the main cause of contamination (Behbahaninia and Farahani 2016). Due to the natural origin of arsenic in the study area and the use of groundwater by local residents for hygienic, drinking, and irrigation purposes, arsenic accumulation during the time is very likely. Our findings showed that there are obvious health risks for some residents in some areas. Based on the results presented in Table 9, 1329 people including 239 children, 192 adolescents, and 898 adults in the studied rural areas were exposed to arsenic concentrations above the recommended values (10 μg/L). Non-carcinogenic and carcinogenic health risks of arsenic are calculated and deeply discussed in the following section.
Carcinogenic and non-carcinogenic risks
The evaluated HQs through Eq. (3) varied from 0 to 62.82, 0 to 43.75, and 0 to 26.34 for children, adolescent, and adults, respectively. Statistics regarding the hazard quotient (HQ) in different age groups are listed in Table 10. According to the table, the average HQ in three age groups is higher than 1. So, the occurrence of non-carcinogenic risk is very likely. The maximum HQ and ELCR value was calculated for children age group. The HQ values in different age groups were in the following order: children > adolescent > adults. Table 11 shows the carcinogenic and non-carcinogenic risks for studied locations. Italicized values in this table indicate risks higher than permissible level. The HQ values in 37 (72.5%) drinking water sources were above the standard recommended for children age group, which must be considered as a serious risk factor. In addition, in nearly 59% (30 out of 51) and 33% (17 out of 51) of the samples, the calculated HQ were higher than the guide line value for both adolescents and adult age groups, respectively. Because children consume more water per unit of body weight than adults, they are more vulnerable to non-carcinogenic arsenic toxicity. The non-carcinogenic effects of arsenic include cardiovascular disease and diabetes (Phung et al. 2017). Based on arsenic contamination dispersion map and interpolation of HQ by IDW method in GIS software (Fig. 3), arsenic health risk from drinking water resources was estimated for the age groups of children, adolescents, and adults. According to Fig. 3, it is clear that for children age group, most areas are at higher risk.
The ELCR of arsenic exposure via drinking water was calculated by Eq. (4). The evaluated ELCR varied from 0 to 0.028, 0 to 0.019, and 0 to 0.011 for children, adolescent, and adults, respectively. According to Table 10, the average ELCR calculated via arsenic exposure in the three age groups was in the order of as follows: children > adolescent > adults. The ELCR values more than the recommended standard are indicated as italics in Table 11. According to this Table, 80% of the population of children and adolescents and 78.4% of adults in rural areas were exposed to ELCR values higher than the range defined by the USEPA (10−4). In other words, in the study area, 40 drinking water resources had ELCR higher than those defined by the USEPA for all three groups of children, adolescents, and adults. In contaminated areas, there is carcinogenic potential due to drinking water contaminated with arsenic; the common types of cancer in chronic arsenic exposure are skin, kidney, bladder, gastrointestinal, and lung cancers (Sadeghi et al. 2018). Therefore, arsenic in drinking water is a serious threat to residents of the area.
A similar study was conducted in Shabestar, northwest of Iran, and indicated that As with 95% contribution in the total noncarcinogenic risk and HQ content greater than 1 was the most effective element to induce health risk in both children and adults (Barzegar et al. 2019). Also, Sadeghi et al. (2018) reported that all the ELCR values for each age group were higher than the recommended limits due to contaminated drinking water in Ardabil, northwest of Iran (Sadeghi et al. 2018).
Conclusions
The physicochemical characteristics and arsenic concentration of groundwater of Hashtroud villages were evaluated. The physicochemical parameters in the studied areas were within the WHO standard. Also, the WQI showed excellent and good water quality. By PCA, four significant principal components (PCs) were extracted that explained 61.7% of the data variance. Also, HCA played a key role in identifying the spatial similarity between the groundwater samples in the study area. The hazard quotient (HQ) and the ELCR were used to define priorities for the health risk assessment. The maximum HQ was found for children age group. Also, 80% of the population of children and adolescents and nearly 78% of adults were exposed to ELCR higher than the USEPA recommended standard. According to the results of this study, continuous monitoring of drinking water resources is recommended as the necessity of earth nature of the contamination. Unlike WQI results, risk assessment results indicated that most of the resources were not appropriate for drinking purposes. As the population were at higher risk of As contamination, local authorities must take some supervisory actions such as using either alternative water resources or efficient treatment techniques to purify water used for drinking and hygienic purposes. Epidemiologic investigations to find As health outcomes in the population living in the contaminated villages is suggested for future studies.
References
Abbasnia A, Alimohammadi M, Mahvi AH, Nabizadeh R, Yousefi M, Mohammadi AA, Pasalari H, Mirzabeigi M (2018) Assessment of groundwater quality and evaluation of scaling and corrosiveness potential of drinking water samples in villages of Chabahr city, Sistan and Baluchistan province in Iran. Data in brief 16:182–192. https://doi.org/10.1016/j.dib.2017.11.003
Abdullahi S, Ameh I, Abubakar A (2017) Assessment of surface and groundwater quality in Gussoro Gold Mining Site, Niger State. NJPAS 9:138–147
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol 176:153–161. https://doi.org/10.1016/j.ecoenv.2019.03.066
Alexander J et al (2009) Scientific opinion on arsenic in food EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J 7. https://doi.org/10.2903/j.efsa.2009.1351
Arumugam K (2013) Assessment of groundwater quality in Tirupur region. Shodhganga: a reservoir of Indian theses http://hdl.handle.net/10603/10388
Asadi E, Isazadeh M, Samadianfard S, Ramli MF, Mosavi A, Shamshirband S, Chau K-W (2019) Groundwater quality assessment for drinking and agricultural purposes in Tabriz Aquifer, Iran. Preprints. https://doi.org/10.20944/preprints201907.0339.v2
ATSDR (Agency for Toxic Substances and Disease Registry) (2007) US Department of Health and Human Services, Centers for Disease Control and …,
Ayotte JD, Belaval M, Olson SA, Burow KR, Flanagan SM, Hinkle SR, Lindsey BD (2015) Factors affecting temporal variability of arsenic in groundwater used for drinking water supply in the United States. Sci Total Environ 505:1370–1379. https://doi.org/10.1016/j.scitotenv.2014.02.057
Babaei A, Ghafarizadeh F, Nourmoradi H, JANGALI K, Moslemnia M, Salimi J (2014) Investigating the microbial quality of water treatment centers in the city of Abadan. J Ilam Univ Med Sci 22:132–140 (In Persian)
Bakhat HF, Zia Z, Fahad S, Abbas S, Hammad HM, Shahzad AN, Abbas F, Alharby H, Shahid M (2017) Arsenic uptake, accumulation and toxicity in rice plants: possible remedies for its detoxification: a review. Environ Sci Pollut Res 24:9142–9158. https://doi.org/10.1007/s11356-017-8462-2
Barzegar R, Moghaddam AA, Soltani S, Fijani E, Tziritis E, Kazemian N (2019) Heavy metal (loid) s in the groundwater of Shabestar area (NW Iran): source identification and health risk assessment. Exposure Health 11:251–265. https://doi.org/10.1007/s12403-017-0267-5
Beatrice NA et al (2019) Assessment of physicochemical and heavy metal properties of groundwater in Edéa (Cameroon). J Am Water Resour Assoc 7:1–10. https://doi.org/10.12691/ajwr-7-1-1
Behbahaninia A, Farahani M (2016) Investigation of natural sources contamination with arsenic in the suburbs of Hashtrood City, East Azerbayjan Province. JEnv Sci Tech 18:471–475 (In Persian)
Bhakar P, Singh AP (2019) Groundwater quality assessment in a hyper-arid region of Rajasthan, India. Nat Resour Res 28:505–522. https://doi.org/10.1007/s11053-018-9405-4
Bhowmick S, Pramanik S, Singh P, Mondal P, Chatterjee D, Nriagu J (2018) Arsenic in groundwater of West Bengal, India: a review of human health risks and assessment of possible intervention options. Sci Total Environ 612:148–169. https://doi.org/10.1016/j.scitotenv.2017.08.216
Bizier P, DeBarry PA. World Water Congress (2003), understanding your water quality analysis. American Society of Civil Engineers. https://www.ideals.illinois.edu/bitstream/handle/2142/94506/ISWSC-185.pdf?sequence=1&isAllowed=y
Brahman KD, Kazi TG, Afridi HI, Arain SS, Kazi AG, Talpur FN, Baig JA, Panhwar AH, Arain MS, Ali J, Arain MB, Naeemullah (2016) Toxic risk assessment of arsenic in males through drinking water in Tharparkar Region of Sindh, Pakistan. Biol Trace Elem Res 172:61–71. https://doi.org/10.1007/s12011-015-0567-1
Călmuc VA, Călmuc M, Țopa MC, Timofti M, Iticescu C, Georgescu LP (2018) Various methods for calculating the water quality index. Analele Universității” Dunărea de Jos” din Galați Fascicula II, Matematică, fizică, mecanică teoretică/Annals of the” Dunarea de Jos” University of Galati Fascicle II, Mathematics, Physics, Theoretical Mechanics 41:171-178. https://doi.org/10.35219/ann-ugal-math-phys-mec.2018.2.09
Chalchisa D, Megersa M, Beyene A (2018) Assessment of the quality of drinking water in storage tanks and its implication on the safety of urban water supply in developing countries. Environ Syst Res 6:1–6. https://doi.org/10.1186/s40068-017-0089-2
Chaurasia AK, Pandey H, Tiwari S, Prakash R, Pandey P, Ram A (2018) Groundwater quality assessment using water quality index (WQI) in parts of Varanasi District, Uttar Pradesh, India. J Geol Soc India 92:76–82. https://doi.org/10.1007/s12594-018-0955-1
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353:294–313. https://doi.org/10.1016/j.jhydrol.2008.02.015
D’Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, Michelozzi P (2015) Arsenic in drinking water and mortality for cancer and chronic diseases in Central Italy, 1990-2010. PLoS One 10:e0138182. https://doi.org/10.1371/journal.pone.0138182
Dehghanzadeh R, Hir NS, Sis JS, Taghipour H (2015) Integrated assessment of spatial and temporal variations of groundwater quality in the eastern area of Urmia Salt Lake Basin using multivariate statistical analysis. Water Resour Manag 29:1351–1364. https://doi.org/10.1007/s11269-014-0877-7
Dhanasekarapandian M, Chandran S, Devi DS, Kumar V (2016) Spatial and temporal variation of groundwater quality and its suitability for irrigation and drinking purpose using GIS and WQI in an urban fringe. Journal of African Earth Sciences 124:270–288. https://doi.org/10.1016/j.jafrearsci.2016.08.015
Fard MS, Shakeri A, Mehrabi B (2018) Ecological risk assessment and origin of heavy metals and arsenic in surface sediments of Khiyav River in Sabalan Geothermal Area, Northwest of Iran. J Adv Appl Geol. https://doi.org/10.22055/AAG.2018.23768.1779
Faryadi S, Shahedi K, Nabatpoor M (2012) Investigation of water quality parameters in Tadjan River using multivariate statistical techniques. Journal of watershed management research 3:75–92
Fijani E, Moghaddam AA, Tsai FT-C, Tayfur G (2017) Analysis and assessment of hydrochemical characteristics of Maragheh-Bonab plain aquifer, northwest of Iran. Water Resour Manag 31:765–780. https://doi.org/10.1007/s11269-016-1390-y
Gul N, Shah MT, Khan S, Khattak NU, Muhammad S (2015) Arsenic and heavy metals contamination, risk assessment and their source in drinking water of the Mardan District, Khyber Pakhtunkhwa, Pakistan. Journal of Water and Health 13:1073–1084. https://doi.org/10.2166/wh.2015.011
Gunduz O, Bakar C, Simsek C, Baba A, Elci A, Gurleyuk H, Mutlu M, Cakir A (2017) The health risk associated with chronic diseases in villages with high arsenic levels in drinking water supplies. Exposure and Health 9:261–273. https://doi.org/10.1007/s12403-016-0238-2
Hong Y-S, Song K-H, Chung J-Y (2014) Health effects of chronic arsenic exposure. J Prev Med Public Health 47:245–252. https://doi.org/10.3961/jpmph.14.035
Hopenhayn-Rich C, Biggs ML, Fuchs A, Bergoglio R, Tello EE, Nicolli H, Smith AH (1996) Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology 7:117–124 https://www.jstor.org/stable/3703023
Howladar MF, Al Numanbakth MA, Faruque MO (2018) An application of water quality index (WQI) and multivariate statistics to evaluate the water quality around Maddhapara Granite Mining Industrial Area, Dinajpur, Bangladesh. Environ Syst 6:13. https://doi.org/10.1186/s40068-017-0090-9
Jadoon S, Hilal Z, Ali M, Muhammad S (2019) Potentially toxic elements in drinking water and associated health risk assessment in Abbottabad city, northern Pakistan. Desalin Water Treat 151:392–402. https://doi.org/10.5004/dwt.2019.23909
Jain C, Sharma S, Singh S (2018) Physico-chemical characteristics and hydrogeological mechanisms in groundwater with special reference to arsenic contamination in Barpeta District, Assam (India). Environ Monit Assess 190:417. https://doi.org/10.1007/s10661-018-6781-5
Jiang Y, Guo H, Jia Y, Cao Y, Hu C (2015) Principal component analysis and hierarchical cluster analyses of arsenic groundwater geochemistry in the Hetao basin, Inner Mongolia. Geochemistry 75:197–205. https://doi.org/10.1016/j.chemer.2014.12.002
Jiang Z, Li P, Wang Y, Liu H, Wei D, Yuan C, Wang H (2019) Arsenic mobilization in a high arsenic groundwater revealed by metagenomic and Geochip analyses. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-49365-w
Kalaivanan K, Gurugnanam B, Pourghasemi HR, Suresh M, Kumaravel S (2018) Spatial assessment of groundwater quality using water quality index and hydrochemical indices in the Kodavanar sub-basin, Tamil Nadu, India. Sustain Water Resour Manag 4:627–641. https://doi.org/10.1007/s40899-017-0148-x
Khan S, Rauf R, Muhammad S, Qasim M, Din I (2016) Arsenic and heavy metals health risk assessment through drinking water consumption in the Peshawar District, Pakistan. HUM ECOL RISK ASSESS 22:581–596. https://doi.org/10.1080/10807039.2015.1083845
Kolahkajl M, SB-L, HA, SM (2018) Health Risk Assessment of Exposure to Arsenic in Drinking Water, Irrigation Water, and Rice from Meydavood in Khoozestan Province, Iran. Health Syst Res 13:292–296. https://doi.org/10.22122/jhsr.v13i3.2876
Kumar M, Rahman MM, Ramanathan A, Naidu R (2016) Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci Total Environ 539:125–134. https://doi.org/10.1016/j.scitotenv.2015.08.039
Lalwani S, Dogra T, Bhardwaj D, Sharma R, Murty O (2006) Study on arsenic level in public water supply of Delhi using hydride generator accessory coupled with atomic absorption spectrophotometer. Indian J Clin Biochem 21:70–76
Lanjwani MF, Khuhawar MY, Khuhawar TMJ (2020) Groundwater quality assessment of Shahdadkot, Qubo Saeed Khan and Sijawal Junejo Talukas of District Qambar Shahdadkot, Sindh. Appl Water Sci 10:26. https://doi.org/10.1007/s13201-019-1098-2
Liang C-P, Wang S-W, Kao Y-H, Chen J-S (2016) Health risk assessment of groundwater arsenic pollution in southern Taiwan. Environ Geochem Health 38:1271–1281. https://doi.org/10.1007/s10653-016-9794-4
Liang C-P, Chen J-S, Chien Y-C, Chen C-F (2018) Spatial analysis of the risk to human health from exposure to arsenic contaminated groundwater: a kriging approach. Sci Total Environ 627:1048–1057. https://doi.org/10.1016/j.scitotenv.2018.01.294
Logeshkumaran A, Magesh N, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343. https://doi.org/10.1007/s13201-014-0196-4
Manbohi A, Gholamipour S (2020) Utilizing chemometrics and geographical information systems to evaluate spatial and temporal variations of coastal water quality. Reg Stud Mar Sci 34:101077. https://doi.org/10.1016/j.rsma.2020.101077
McGrory ER et al (2017) Arsenic contamination of drinking water in Ireland: a spatial analysis of occurrence and potential risk. Sci Total Environ 579:1863–1875. https://doi.org/10.1016/j.scitotenv.2016.11.171
Monjerezi M, Vogt RD, Aagaard P, Saka JD (2011) Hydro-geochemical processes in an area with saline groundwater in lower Shire River valley, Malawi: an integrated application of hierarchical cluster and principal component analyses. J Appl Geochem 26:1399–1413. https://doi.org/10.1016/j.apgeochem.2011.05.013
Mosaferi M, Yunesian M, Dastgiri S, Mesdaghinia A, Esmailnasab N (2008) Prevalence of skin lesions and exposure to arsenic in drinking water in Iran. Sci Total Environ 390:69–76. https://doi.org/10.1016/j.scitotenv.2007.09.035
Mosaferi M, Nemati S, Armanfar F, Nadiri A, Mohammadi A (2017) Geogenic arsenic contamination in northwest of Iran; role of water basin hydrochemistry. JEHSD 2:205–216
Nwankwoala H, Amadi A, Oborie E, Ushie F (2014) Hydrochemical factors and correlation analysis in groundwater quality in Yenagoa, Bayelsa State, Nigeria. Appl Ecol Env Res 2:100–105. https://doi.org/10.12691/aees-2-4-3
Papillomaviruses H (2011) IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France
Patil P, Sawant D, Deshmukh R (2012) Physico-chemical parameters for testing of water-A review. Int J Environ Sci 3:1194
Phung D, Connell D, Rutherford S, Chu C (2017) Cardiovascular risk from water arsenic exposure in Vietnam: application of systematic review and meta-regression analysis in chemical health risk assessment. Chemosphere 177:167–175. https://doi.org/10.1016/j.chemosphere.2017.03.012
Rabeiy RE (2018) Assessment and modeling of groundwater quality using WQI and GIS in Upper Egypt area. Environ Sci Pollut Res 25:30808–30817. https://doi.org/10.1007/s11356-017-8617-1
Rasheed H, Slack R, Kay P (2016) Human health risk assessment for arsenic: a critical review. Crit Rev Environ Sci Technol 46:1529–1583. https://doi.org/10.1080/10643389.2016.1245551
Rasool A, Farooqi A, Masood S, Hussain K (2016) Arsenic in groundwater and its health risk assessment in drinking water of Mailsi, Punjab, Pakistan. HUM ECOL RISK ASSESS 22:187–202. https://doi.org/10.1080/10807039.2015.1056295
Rostami AA, Isazadeh M, Shahabi M, Nozari H (2019) Evaluation of geostatistical techniques and their hybrid in modelling of groundwater quality index in the Marand Plain in Iran. Environ Sci Pollut Res 26:34993–35009. https://doi.org/10.1007/s106591-019-1356z
Sabo A, Christopher EO (2014) Physicochemical and bacteriological quality of ground water at Abubakar Tatari Ali Polytechnic Bauchi, Nigeria. Eur Sci J 10. https://doi.org/10.19044/esj.2014.v10n18p25p
Sadeghi F, Nasseri S, Yunesian M, Nabizadeh R, Mosaferi M, Mesdaghinia A (2018) Carcinogenic and non-carcinogenic risk assessments of arsenic contamination in drinking water of Ardabil city in the Northwest of Iran. J Environ Sci Heal A 53:421–429
Sahu P, Sikdar P (2008) Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal, India. Environ Geol 55:823–835. https://doi.org/10.1007/s00254-007-1034-x
Saint-Jacques N, Parker L, Brown P, Dummer TJ (2014) Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environmental health 13:44. https://doi.org/10.1186/1476-069X-13-44
Sevda S, Sreekishnan T, Pous N, Puig S, Pant D (2018) Bioelectroremediation of perchlorate and nitrate contaminated water: a review. Bioresour Technol 255:331–339. https://doi.org/10.1016/j.biortech.2018.02.005
Singh KP, Malik A, Singh VK, Mohan D, Sinha S (2005) Chemometric analysis of groundwater quality data of alluvial aquifer of Gangetic plain, North India. Anal Chim Acta 550:82–91. https://doi.org/10.1016/j.aca.2005.06.056
Standard methods for the examination of water and wastewater (1915) vol 2. American Public Health Association Washington, D C
Standard methods for the examination of water and wastewater (2017) vol 2. American Public Health Association Washington, D C
Sujatha M, Gopalakrishnayya A, Satyana-rayana DT (2012) Assessment Of groundwater quality in rural areas of Vijayawada, AP. Int j eng 2:645–648
Sutadian AD, Muttil N, Yilmaz AG, Perera B (2016) Development of river water quality indices—a review. Environ Monit Assess 188:58. https://doi.org/10.1007/s10661-015-5050-0
USEPA (1998) Human health evaluation manual, supplemental guidance: “standard default exposure factors”. U.S. Environmental Protection Agency, Washington D C
USEPA (2005) Guidelines for carcinogenic risk assessment. U.S. Environmental Protection Agency, Washington D C
Verma D, Bhunia GS, Shit PK, Kumar S, Mandal J, Padbhushan R (2017) Spatial variability of groundwater quality of Sabour block, Bhagalpur district (Bihar, India). Appl Water Sci 7:1997–2008. https://doi.org/10.1007/s13201-016-0380-9
Vijaya Kumar H, Patil NS, Prabhu N (2020) Analysis of water quality parameters of groundwater near Ranebennur Industrial Area, Haveri district, Karnataka, India. In: AIP Conference Proceedings, vol 1. AIP Publishing LLC, p 020025. https://doi.org/10.1063/1.5141562
Wang H, Zhang Q (2019) Research Advances in Identifying Sulfate Contamination Sources of Water Environment by Using Stable Isotopes. Int J Environ Res Public Health 16:1914. https://doi.org/10.3390/ijerph16111914
WHO (2001) Arsenic in drinking water, World Health Organization2:2007
WHO (2006) Guidelines for drinking water quality, 3th edn. World Health Organization, Geneva
WHO (2011) Guidelines for drinking water quality. World Health Organization 216:303–304
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36:169–182. https://doi.org/10.1007/s10653-013-9537-8
Wu Z, Wang X, Chen Y, Cai Y, Deng J (2018) Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci Total Environ 612:914–922. https://doi.org/10.1016/j.scitotenv.2017.08.293
Zhang B, Song X, Zhang Y, Han D, Tang C, Yu Y, Ma Y (2012) Hydrochemical characteristics and water quality assessment of surface water and groundwater in Songnen plain, Northeast China. Water Res 46:2737–2748. https://doi.org/10.1016/j.watres.2012.02.033Get
Zhong M, Zhang H, Sun X, Wang Z, Tian W, Huang H (2018) Analyzing the significant environmental factors on the spatial and temporal distribution of water quality utilizing multivariate statistical techniques: a case study in the Balihe Lake, China. Environ Sci Pollut Res 25:29418–29432. https://doi.org/10.1007/s11356-018-2943-9
Zietz BP, Laß J, Suchenwirth R (2007) Assessment and management of tap water lead contamination in Lower Saxony, Germany. Int J Environ 17:407–418. https://doi.org/10.1080/09603120701628719
Acknowledgments
This study was part of an MSc thesis in environmental engineering. The authors wish to thank Hashtroud and Tabriz health centers for their comprehensives support of the study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Funding
This study was supported by East Azerbaijan province health center.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Samira Sheikhi, Zahra Faraji, and Hassan Aslani. The first draft of the manuscript was written by Samira Sheikhi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable for this study.
Consent to participate
No human and/or animals participated in this study.
Consent for publication
Not applicable for this study.
Additional information
Responsible Editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• HQ and ELCR violated standards in most of the studied villages.
• Children in the contaminated places were at most risk.
• In nearly 80% of the resources, ELCR was higher than the recommended standard.
• As concentration in drinking water of 1329 individual was higher than the standard.
• Emergency supervisory actions must be considered to replace water resources.
Rights and permissions
About this article
Cite this article
Sheikhi, S., Faraji, Z. & Aslani, H. Arsenic health risk assessment and the evaluation of groundwater quality using GWQI and multivariate statistical analysis in rural areas, Hashtroud, Iran. Environ Sci Pollut Res 28, 3617–3631 (2021). https://doi.org/10.1007/s11356-020-10710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10710-6