Abstract
Microplastics (MPs) with an average size of less than 5 mm, along with nanoplastics (NPs) of an average size of fewer than 0.1 μm are the result of huge plastic waste fragmentation or straight environmental emissions. Pollution from micro- and nanoplastics is a worldwide paradigm that raises environmental and human health concerns. They may also comprise very harmful chemicals that are implemented in plants and animals when MPs/NPs are used that may lead to higher accumulation of these compounds in food chains. In addition, higher surface area-to-volume ratio, characteristic of MPs/NPs can contribute to their potentially harmful impact as other pollutants, like continuous organic contaminants, can also be bio-accumulated and adsorbed. A complex issue correlated with MPs/NPs is their ability to absorb and interact with other common pollutants in the environment, such as metals, pharmaceuticals, and other contaminants. Thus, MPs/NPs can directly influence on destiny and toxicity of these substances to the environment and organisms. In this review, first, we introduce possible sources and formation, their destinies, and environmental impact of MPs/NPs and then explain feasible paths of all these particles entering the human body. Then, the review highlights the effect of MPs/NPs on human health. Finally, it provides a brief summary of the potential as well as the neurological toxicity of MPs/NPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, plastics have been commonly used for our purposes and feasibility since the last century and have considerably improved. Currently, industrial manufacturing of plastics reaches 320 million tons in a year, which is used as single-use packaging by more than 40%, leading to plastic waste. The plastic products shelf life may differ from 1 to more than 50 years before disposal as plastic waste, depending on their use. In which, 9% of recycled energy was recovered by 12%, and 8% was disposed of in the land, or 71% was lost to the environment. Plastic products were mostly degradable due to weathering and aging, i.e., ultraviolet (UV) exposure catalyzes plastic photo-oxidation. The plastic degradation process develops with an exaggerated period within the surroundings and should ultimately contribute to nanoparticles being formed. They have accumulated and persisted for years to centuries in aquatic habitats (Jeong et al. 2016) and are the main problem for the toxicity of plastic waste (Bergmann et al. 2015). An approximately 4.8 to 12.7 million tons (MT) of plastic waste reached the oceanic ecosystem in 2010 and is forecast to improve significantly more than 100–250 MT by 2025 (Peng et al. 2018).
Microplastics (MPs) are commonly known as plastic debris 0.1–1000; whereas, plastic particles are categorized as nanoplastics (NPs) ≤ 0.1 μm (Peng et al. 2018). MPs are intentionally manufactured for various applications, like microbeads in exfoliates for personal care products (Farrell and Nelson 2013). Many of the substances contain nanoplastics like electronics, paints, drug delivery systems, and adhesives (Kosuth et al. 2018). For instance, 3D printing can emit polymer nanoparticles (Stephens et al. 2013). This substance is emitted continuously from textile washing machines into the public wastewater containing plastic microfibers (Browne et al. 2008, 2011). It is characterized by MPs as smaller or larger MPs when their size was less than or greater than 1 mm, respectively (Eriksen et al. 2014). Whereas a further study detected particle resolution level to categorize MPs or NPs by a 100-μm increase. They are commonly found in the ocean and aquatic areas, such as river water (Wang et al. 2017), beaches (Van et al. 2012), sediments (Dekiff et al. 2014), marine water (Woodall et al. 2014), and sometimes in polar zones (Kanhai et al. 2018). The smaller size and the highly accurate surface area of MPs or NPs allow living beings to consume and increases the chances of adsorbing and desorbing harmful substances in tissues of organisms or in water (Chae et al. 2017, Farrell and Nelson 2013). Current researches have shown that NPs have enhanced negative impacts relative to MPs (Chen et al. 2017), although, owing to analytical challenges in their identification, there is still a comprehension of the existence of NPs in the water (Hidalgo-Ruz and Thiel 2013). Overall representation of the environmental fate of microplastics is shown in Fig. 1.
Plastic ocean pollution has evidently become a primary environmental problem. While long-term effects in the aquatic ecosystem of micro- and nanoplastics remain a challenge to estimate, this element could be a major enterprise for our community (Villarrubia-Gómez et al. 2018). A risk that has been underestimated so far is the effect on the terrestrial environment of microplastics in sediments and soils (De Souza Machado et al. 2018). Recently, research has started in this path, as 80% of calculable microplastic pollution in the seas comes from territory (Rochman 2018; Awet et al. 2018) (Fig. 2). Microplastics are readily accumulated in aquatic environments by various species because of their smaller size (Cole et al. 2013). So, MPs are spread at different tropic levels; microplastic levels can improve at greater tropic levels through bioaccumulation in the body. MPs are also transmitted to humans through the food chain (Gong et al. 2009, Bouwmeester et al. 2015). It, thus, indicates individuals may face the most destructive impacts of microplastic toxicity. Besides microplastic toxicity itself, it also causes unknown toxicity due to the absorption of toxins (Li et al. 2016), comprising poly nuclear aromatic hydrocarbons (Teuten et al. 2007), organochlorine (OC) pesticides, likes DDT (Ivar Do Sul and Costa 2014), and polychlorinated biphenyls (Zarfl and Matthies 2010) from environment and transferred to the food chain (Reisser et al. 2014). Microplastic consumption can contribute to oxidative stress, abrasion, satiation, ulcers, decreased growth rate, and decreased fitness for reproduction (Miao et al. 2011, Fossi et al. 2016). Therefore, it is essential to assess microplastic hazards to human health.
Nanoplastic communication with the environment, especially with living organisms on behalf of the assessment of prospective health risks is very crucial, especially as nanoplastic particles react other than their microsized counterparts. On the other hand, the present study directed at solving plastic findings focuses mostly on the marine environment and only restricted information can be acquired on the effects of NPs on human health (Wright and Kelly 2017; Revel et al. 2018). We identify the most appropriate sources and formation of MPs/NPs in this review and give some outlook on their fate once it is released into the environment (Fig. 2).
Formation of micro/nanoplastics
This study examines the physical and chemical processes leading to the MP formation, followed by NPs. These processes correlate with the tendency of NPs/MPs to occur in water as single particles or agglomerates. MPs are categorized as primary (10) MPs, once it finds itself within the surroundings as microsized particles and secondary (20) MPs once produced from plastic waste that is already a presence in the environment.
The fragmentation of huge plastic things happens following totally different mechanisms, severally or put together, like a photo oxidation by chemical reaction, ultraviolet light, and mechanical shock, leading to soil abrasion or mechanical turbulence in water or biological assimilation by microorganisms (Neves et al. 2015; Gewert et al. 2015). Oxidation of plastic pellets like poly(lactic acid) (PLA), terephthalate (PET), poly(ethylene) (PE), poly(styrene) (PS), and poly(propylene) (PP) happens when subjected to ultraviolet light in soil and water environments (Cai et al. 2018; Lambert and Wagner 2016a, b). Hydrolysis is one among the first processes of degradation of heteroatom polymers like poly(urethane) (PU) and PET (Lambert and Wagner 2016a, b). The effect on the atmosphere is increasing as concentrations fall (in the case of chlorofluorocarbons (CFCs)) or rise (in the case of hydrofluorocarbons (HFCs), which are used as replacements for CFCs in refrigeration and insulation) into the global setting. Trifluoroacetic acid, a minor product of the atmospheric decomposition of certain HCFCs and HFCs and the pyrolysis of fluoropolymers, is distributed uniformly in seawater of over 4000 m (Suquet et al. 2018; McCulloch 2003). In the aqueous world, trifluoroacetate is widespread; it is present in fog, rain, rivers and lakes, groundwater, and, most importantly, in seawater (Yadav et al. 2020; Naik et al. 2000; Hayman and Derwent 1997).
The bond breakage of organic compound results in the chemical group formation creating the autocatalysis, subsequently increasing the hydrolysis rate of the acidic conditions. Both processes of photo-oxidation and degradation of the response trigger breaks split and trap to form on the surface of items, plastic induction embrittlement. Plastic fragments are so weakened and mechanical stress like abrasion or friction breaks into microplastic particles (Cai et al. 2018). The fragmentation process relies on the environmental circumstances, the polymer substance, as well as plastic additives that can impact the material’s physico-chemical characteristics (Veresoglou et al. 2015; Gewert et al. 2015). It is therefore possible to rapidly generate MPs of distinct sizes, forms, densities, and chemical and mechanical properties based on environmental circumstances and plastic products ( Lambert and Wagner 2016a, b). However, the mechanism and fragmentation rate of MPs in the environment are still uncertain and desires to be studied in order to assess the rate of MPs generated by fragmentation.
Nanoplastics formation
It is also anticipated that MPs will be fragmented into NPs as well as the formation of MPs. Even though NPs are difficult to identify, discharge of NPs up to 30 nm in size was recorded after being exposed to the outdoor aquatic microcosm of PP, PS, and PE pellets and thus demonstrated by the absence of bulk material (Zhao et al. 2018; Lambert et al. 2013). The nanofragment’s number improved by 5 orders of magnitude after being exposed to outdoor circumstances compared with PE mention not subjected to weathering (Lambert and Wagner 2016a, b). The existence of NPs proves that theory of degradation of big plastic products into MPs can be expanded to the degradation of MPs into small products like NPs. This research proposed that the physical breakage of MPs in cosmetics during manufacturing or use could lead to the discharge of produced NPs in water, and 2° MPs/NPs could lead to plastic pollution. As stated previously for big fragments of plastic, the process of manufacturing has an impact on the fragmentation of MPs because, as a consequence of their production, defects can be introduced in particles. Primary MPs like powders, fillers and pellets are commonly generated through comminution (Chadwick 1988). In which the solid particle size reduced by effect, compression, or shear force is intentionally (Somani et al. 2017). The comminution is performed generally by milling and grinding to trigger defect like breaks on the particles that propagate into fragments until they are totally broken (Yigit 1976). In the case of MPs ending up in water, these abnormalities would damage particle constructions and improve the ESC owing to environmental variables including the turbulence of water, resulting in the conversion of MPs to NPs. No experimental information was recorded demonstrating the breakdown of MPs into NPs in water owing to the proliferation of abnormalities.
Environmental impact of micro/nanoplastics
Microplastics are rapidly growing contaminant to the environment, and their biological effects have attracted extensive attention in the last year (De Sá et al. 2018). Land systems have obtained much less scientific exposure than water counterparts. Nevertheless, microplastic pollution on territory could be 4 to 23 times than the ocean (Horton et al. 2017). MP-littered environment extends from the equator to the pole (Barnes et al. 2009). The highest quantity of MPs in groundwater could exceed 100,000 products/m3 and 100,000 products /m3 on the shores (Desforges et al. 2014). Pollution by MPs have spread all over the world, distributed widely in soil ecosystems, coastal waters, marine waters (Barnes et al. 2009), freshwater systems (Li et al. 2018; Peng et al. 2018), and even polar regions (Obbard et al. 2014).
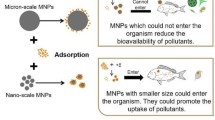
Some considerations have been given to the environmental impacts of MPs/NPs in freshwater environments (Eerkes-Medrano et al. 2015). Due to its small size, MPs/NPs can be ingested by aquatic species more readily immediately and indirectly than larger particles, sometimes consumed for food, resulting in adverse physical impact. For instance, data from marine research shows that MP/NP intake can lead to shock, blocked digestive tract, organ damage, weakening, and eventually death (Derraik 2002). Furthermore, MPs/NPs can absorb persistent organic pollutants (POPs), which could lead to toxicity across the food chain (Bakir et al. 2012). This could finally reach people through bioaccumulation. Desorption of POPs, as well as other producing additives, may boost the concentration of pollutants in water and enhance the susceptibility of bigger parts to degradation (Dubaish and Liebezeit 2013). However, data on MP leaching and sorption of POPs is limited and almost all of the toxicity knowledge comes from marine and laboratory studies (Eerkes-Medrano et al. 2015), even while freshwater information is still restricted. In addition, MP/NP surfaces can give natural habitats both for microbial colonization and biofilm development, enabling opportunistic microorganisms and invasive species to migrate (Zettler et al. 2013). The latter can be important to WWTP as it may impact the working of therapeutic procedures and improve the transportation of WWT bacteria from these facilities to water (Tagg et al. 2015; Zettler et al. 2013).
The combined microplastic impacts can influence plants through physicochemical modifications in soil appearance and composition, resulting in water cycling and the working of the ecosystem in terrestrial environments and different feedback from plants (Bergmann et al. 2016). In this sense, microplastic-driven modifications in soil hydrological characteristics could affect soil microbial growth, with prospective effects on important symbiotic connections in terrestrial ecosystems such as mycorrhizal groups (Hallett et al. 2009) and N-fixing organizations. Such prospective physical effects on the structure of soil and operation are of specific interest to the soil microbiome because the mechanistic knowledge of the loss of biodiversity and extinction in those ecosystems are not completely understood (Mattsson et al. 2015). Plastic hydrophobic surfaces and their eco-corona associate are also recognized with hydrophobic compounds (Barnes et al. 2009; Galloway et al. 2017; Zhan et al. 2016). Trophic impacts as well as other ecological effects were noted when adsorbed chemicals on microplastic were connected to intra- or interspecies interaction processes in the marine environment (Galloway et al. 2017). Many amphiphilic and hydrophobic compounds already control the communication of species and ecosystem processes in soils. For example, hydrophobins are ubiquitous amphiphilic protein in soil that is naturally produced by the fungus. These polypeptides, rich in cysteine, play significant functions like hydrophobicity and aggregate stabilization with immediate impacts on soil erosion and biogeochemical cycles. MPs were suggested to have separate sorption characteristics for inorganic soil components ( Hodson et al. 2017) and research laboratory findings indicate that hydrophobins play a function in protecting filamentous fungi from nanoplastic toxicity (Nomura et al. 2016). Highly appropriate biogeochemical modifications may happen when hydrophobic surfaces of microplastics interact significantly from natural hydrophobic soil particles or other soil structure chemical drivers. Therefore, further study is needed to explain to which level of microplastic pollution can impact soil composition, texture, composition and activity.
Impact of MPs/NPs on terrestrial and marine organisms
One influencing mechanism is acknowledged as significant for both designed nanomaterials and nano/microstructures, i.e., physical interactions between particles and organisms (Wagner et al. 2018). It includes interference and inflammation of energy balance induced by the adsorption and absorption of particles into the intestine, thereby restricting food consumption. Various types of designed nanomaterial along with nanoplastic were noted to attach to the microalgae surface, possibly triggering a cellular shading impact (Wright et al. 2013). Recent reviews of the physical effect of MPs on marine organisms are shown in Wright et al. (2013). Mechanisms mentioned as possibly appropriate include digestive blockage, tissue abrasion, blockage of invertebrate feeding appendages, tissue embedding, enzyme blockage manufacturing, nutrient dilution, reduced feeding stimulus, lower development rate, and lower steroid hormone concentrations and impaired breeding. MP effectiveness for causing these physical impacts on species relies on a variety of variables. MP effectiveness for causing these physical impacts on species relies on a variety of variables. Particles with large accumulation ability in organisms and translocation into tissues are anticipated to have a greater physical effect (Wright et al. 2013). As described below, this is tightly associated with particle size. The shape also serves a significant part as uneven, sharp pieces are more probable than round, soft particles to cause damage. Fibers in the digestive system are more probable to accumulate. The ability of individual species to consume microplastic is also considered to be vital due to the reason that this technique will find how quickly an organism is introduced to particle (Wright et al. 2013).
Several types of research have described that fish and other organisms, particularly in marine environment, can intake MPs owing to their smaller size and UN degradability (Ivar Do Sul and Costa 2014). In these studies, the MP toxicity to parameters include tissue distribution (Von Moos et al. 2012), rate of growth (Jeong et al. 2016), biological enzyme activity, reproduction, and oxidative damage (Yu et al. 2018), which are mainly evaluated. The presence of huge quantities of microplastics destroys a number of species, including plankton, vertebrates, and invertebrates, resulting in numerous unexpected effects (Frydkjær et al. 2017). Furthermore, MPs also have harmful impact on certain microorganisms like fungus and bacteria. For instance, polystyrene nanoparticles can have harmful impacts on yeast cells (Nomura et al. 2016). MPs can prevent their formation for marine bacteria Halomona salkaliphila and disrupt the environmental role (Lee et al. 2013).
In addition, MPs have a number of harmful impacts on marine animals, which include invertebrates like zooplankton and certain benthonic animals, vertebrates like fish, seabirds, and amphibians (Fig. 1). The impacts on zooplankton are triggered primarily by obstruction of the digestive system, decreased appetite, eating impact, malnutrition, slow development, and sometimes even death (Lee et al. 2013). MPs effect on marine benthic organisms including oysters and mussels is due primarily to their presence of sediments in the deep sea (Van et al. 2013) that mussels move MPs through endocytosis into the gastrointestinal system, This ultimately leads to inflammation and reduction in stability of lysosome membranes. MPs may increase mortality of oysters, slow growth, influence absorption of energy, and interferes with reproductive ability and progress of offspring. Furthermore, MPs already have a harmful impact on certain microorganisms such as fungus and bacteria. For instance, polystyrene (PS) nanoparticles can have harmful impacts on yeast cells (Nomura et al. 2016). MPs can prevent their formation for marine bacteria Halomonas alkaliphila and disrupt the environmental role (Sun et al. 2018).
The researches of MPs on terrestrial ecosystems are comparatively rare compared with the research of aquatic organisms. Scientists have started paying attention in the latest years to the MP effect on terrestrial organisms and ecosystems (Rillig 2012). In reality, contamination of MPs in the soil can be more severe than the aquatic environment because of the plastic agricultural film and fibers used in industrial manufacturing applications (Ramos et al. 2015). MPs cause a risk to terrestrial organisms and can also harm human health via the food supply chain and other routes (Sharma and Chatterjee 2017). Polystyrene MPs can prevent proliferation and even kill earthworms in the soil (Cao et al. 2017). These findings indicate that soil pollution from MPs has a negative impact on soil organisms, implying the environmental impact of MPs in terrestrial ecosystems. At the moment, MP evaluation of the toxicity of terrestrial mammals is comparatively inadequate.
Several aquatic species were used for NPs to show the hydrophobic organic polymer (POP) adsorption and the chemical leaching and POPs to illustrate their significant environmental biological and toxicological effects (Galloway 2015). Several studies are indicating negative impacts including ROI manufacturing and dysfunction of reproduction when NPs are exposed to aquatic organisms. The levels of orders of different magnitudes were greater than those expected to be essential for the environment, such as 1 pg/L–15 μg/L for NPs of approximately 50 nm (Lenz et al. 2016). Focusing on the above reality will assist in comprehending the effect of environmentally appropriate nanoplastic levels. There is also a lack of understanding about how NPs are transported to the food supply chain and how they accumulate and communicate with the environment especially with organisms.
Routes of human exposure
MPs as contaminants in the larger and more diverse environment poses risks to human health because it has been shown that they can be ingested via a wide range of aquatic organisms, both freshwater and marine and can therefore be accumulated via the food web. Aquatic species for which the ingestion of MPs has been reported in the discipline consist of fish, turtles, seabirds, worms, and crustaceans throughout the marine food web (Wright et al. 2013). Experimental trials have shown that several other organisms are capable of ingesting microplastic-containing zooplankton (Setala et al. 2014). Most experiments have reported microplastics in organism guts, an organ that is not normally immediately bumped off by humans now.
Exceptional cases to this are shellfish like clams and mussels, and a few other shrimps were consumed with their intestines or in total. The threat of ingestion of MPs in further tissues is based on the level to which uptake of microplastics and translocation and redistribution and retention happens within distinct bodily tissues. Moreover, in regard to human ingestion, this idea is discussed below in comparison with the possible consequences of ingestion owing to intestinal blockages and/or harm or reduced energy conversion (Wright et al. 2013). The huge floor vicinity of MPs is capable of continuously absorbing environmental pollutants on the surface of the particles, with the possibility of being moved to the tissues of the body once consumed. Microplastics are used for a wider spectrum of natural world species. The change in the adjacent environment to hydrophobic pollution tissues makes the reader conscious of outstanding recent reviews and various chapters in this problem (Engler 2012). With regard to this problem, no information are presently accessible to demonstrate the uptake or biological results of terrestrial or marine debris nanoplastics ingested through the food chain by individuals.
NP exposure could possibly occur through oral consumption/absorption, inhalation of plastic products through the skin or involuntarily (Figs. 3, 4, and 5). Inhalation is probably important in work advertising instances involving nanoplastic-constituting aerosols (Oriekhova and Stoll 2018), whereas achievable skin contact may occur through the use of contaminated air, water, and products like pores, skincare, and washing products that contain NPs. Particle ingestion of NPs is probably the primary route of intake, according to contemporary understanding, because particles of NPs can be ingested either through consumption of seafood or through the ingestion of contaminated water. In fact, nanoplastic consumption and accumulation have been verified under experimental circumstances as correctly as trophic nanoplastic change inside aquatic organisms; this increases the potential for NPs to accumulate in the food chain and consequently resulting in human toxicity (Mattsson et al. 2015). Microplastic particles have been recognized in many kinds of seafood, like bivalves, shrimps, and fish also in various foods including salt, beer, sugar, and honey (Li et al. 2015). Current investigations of the use of Fourier transform infrared spectroscopy also showed that MPs are also identified from groundwater sources, boiled water and tap water. In 159 samples of worldwide faucet water, the integration of microplastic particles were discovered to be around 81%, more often than not lower than 5 mm fibers with a prevalent implication of 5.45 particles/L (Kosuth et al. 2018). In a total of 11 personal water cans from 11 particular products and 27 outstanding lots, 93% reported presence of microplastic disease with a standard 10.4 particles/L (Kosuth et al. 2018). Floor water analysis from the northwest region of Germany disclosed that a normality of 0.7 MPs/m3 can be identified (Mintenig et al. 2019). All these investigations reiterate that the prevalence of NPs in a variety of meal merchandises cannot be excluded. Currently, there are no achievable movement approaches that allow NPs to be detected in food, and as a consequence, there are no statistics beyond the lookup activities listed previously in this thread.
It can also influence human health even though chemical additives are leached or switched from the plastic fabric itself. The chemicals including stabilizers, plasticizers, and pigments are supplied in the plastic-producing method to supply the closing product’s required characteristics, e.g., their stability, flexibility, and color. There are currently lots of chemicals used for these reasons, and it is known that some of these chemicals may pass out into the atmosphere at some point in the item life process, mainly to disrupt endocrine or acute toxicity when organism publicity occurs (Lithner et al. 2011). The same applies to the practice of the monomers (i.e., chemical structural blocks) used in the first area to generate the polymers (in which small quantities can stay in polymers), and products evolved through the degradation of chemical polymers. Bisphenol A (BPA) is used in polycarbonate and secure epoxy resins, it is also the best instance of a leaching monomer. BPA has been shown to cause harmful impacts in humans owing to its estrogenic activity (EA), including countless metabolic disorders as well as sexual and behavioral impacts (Lang et al. 2008; Ehrlich et al. 2012). Containers of polycarbonate used for newborns confirmed higher BPA leaching. Newborns are at a risk of greater danger than adolescents because of the fact that increased physical stress is expressed as blood or plasma consciousness, owing to enhanced absorption or reduced withdrawal relative to the internal physical burden of adults (Hengstler et al. 2011).
Impact on human health of micro/nanoplastics
Few marines and terrestrial organisms show the MP translocation across the gastrointestinal tract (Rodriguez-Seijo et al. 2017), but fewer reviews on mammals (Schmidt et al. 2013). A variety of forms and sizes of MPs (among 0.1 to 150 μm) have been created in research concerning individuals (0.2–150 μm) via the mammalian stomach in the lymphatic system (Hussain et al. 2001). PVC (5 to 110 m) appeared in the portal vein of dogs, which then reaches the liver (Volkheimer 1974). Uptake of 2 μm latex particles are shown in small intestines in rodents (0.04–0.3%) (Carr et al. 2012). Restrained intake of 0.2% poly(lactic-co-glycolic acid) microplastics of 3 μm in human colon mucosal tissue were evaluated in an in vitro study. The patient’s colon mucosal tissue with severe illness such as inflammatory bowel disease (IBD) revealed rapid transportation (0.45% in healthy controls relative to 0.2%) compared with greater intestinal permeability (Schmidt et al. 2013). Few studies have explored the feasible MP absorption system. Due to their small size, endocytosis or phagocytosis may be the preferable uptake path for MPs. Phagocytosis may also occur with particles > 0.5 μm by using macrophages in the intestinal epithelium (Yoo et al. 2011). The endothelial cells may also internalize a huge 5-μm particles by endocytosis (Gratton et al. 2008). Biodistribution information on microplastics is not available (Yoo et al. 2011). The capacity to enter cells and the gut epithelium is a unique problem of NPs. Oral consumption of polystyrene NPs has been researched for several centuries, and translocation of gastrointestinal digestion (in vivo as well as in vitro) of manufactured NPs (zinc oxide, titanium dioxide, silver) has been investigated (Brun et al. 2014). NPs can enter the circulation, following translocation through the gut barrier, based on their surface charge and size. The PS NP oral bioavailability (50 nm) was projected to differ among 0.2 and 2% in rodent (in vivo) and human (in vitro) research. The relationship between the composition, size, and uptake of NPs has not yet been established. Various polystyrene particle uptakes (50 to 500 nm) were evaluated in different intestinal models (in vitro) varying from 1.5 to 10%, with different NP sizes and surface composition (He et al. 2010). Interestingly, oral in vitro exposure to 50 nm PS particles has resulted in increased iron absorption, suggesting that NP exposure impacts the barrier characteristics of the intestinal epithelium (Mahler et al. 2012).
Toxicity of micro- and nanoplastics
MPs may also be harmful because of their intrinsic capacity to cause tissue obstruction (Pedà et al. 2016). According to researchers, damage reported after 90 days of advertising should be entirely liable for compromising intestinal activity. To date, it is possible to determine in vitro studies on the toxicity of MPs to human health. After advertising to 10 mg/L of polystyrene (PS) MPs (10 μm) and NPs (40 and 250 nm), the researchers evaluated oxidative stress in epithelial and cerebral human cells through reactive oxygen species (ROS) (Schirinzi et al. 2017). Polystyrene MPs have recently been investigating fitness danger particularly to tissue distribution, accumulation, and tissue in mice (Deng et al. 2017). Outcomes stated that MP ingestion (0.5 mg daily of 5 and 20 μm polystyrene) resulted in particle accumulation in the kidney, lungs, and intestine (Fig. 6). Therefore, the kinetics of accumulation of tissue and distribution samples was once correlated with the particle size of MPs. Furthermore, analysis of biochemical biomarkers and metabolomics characteristics in mice brain proposed that advertising from MPs precipitated changes in oxidative stress, lipid, and energy metabolism and also neurotoxicological effects. These impacts increase the cell toxicity scenario in the manner of human liver cells. MPs in the lumen can interfere with the fluid through adsorptive reactions backed by big surface area and charging. The surface of plastic particles can be adsorbed with large proteins, which may contribute to modifications in the immune system of the intestine and adjacent inflammation (Powell et al. 2007). This should also lead to the adoption of MPs through the gut (Handy et al. 2008).
Furthermore, toxicity can be associated with the nanoparticles’ surface area-to-volume ratio, which leads to change in their reactivity. Because NPs are completely inert substances, a starting variable should be the fate of inert nanoparticles such as gold (Au) in finding out how toxic NPs are. The earlier study assessed the intake of Au nanoparticles in vivo macrophages in spleen and liver and verified the stimulation of acute inflammation and apoptosis in the liver. These findings were considered size dependently and endorsed by the use of a differential expression of genes in terms of lipid metabolism, detoxification, and reactions to protection. NPs have one type of physicochemical habitats relative to nanoparticles, which could have accurate effects (Lambert et al. 2017). Although studies with these accurate steel nanoparticles should provide valuable insights into NP toxicity, new nanoparticle schemes should be regarded for future studies evaluating the feasible NP toxicity. The toxicity of micro- and nanoplastics are shown in Table 1.
Neurotoxicity
A few studies have discussed MPs/NPs neurotoxicity so far (Barboza et al. 2018; Zhao et al. 2017). The researcher’s first explored motor behavior includes modifications and different kinds of movement-correlated nematode neurons after MP/NP exposure. The findings indicated that polystyrene MP/NP exposure increased frequently in worm twisting and head thrashing. In addition, in nematodes exposed to 0.1 and 2.0 μm MPs/NPs, substantial rises in the speed of crawling were found. These suggested that polystyrene MPs/NPs might lead to exciting organism’s motor behavioral toxicity. The motor behaviors of Caenorhabditis elegans which are controlled by various neuronal subtypes, such as dopaminergic neurons, cholinergic, and GABAergic are normal. These cholinergic neurons are equally probable to cause subsequent rhythmogenesis during nematode forward locomotion (Fouad et al. 2018). Furthermore, unc-17 codes the vesicular acetylcholine transporter (VAChT) which sets up the flow of acetylcholine from presynapse to synapse and justifies its motor motion significance (Alfonso et al. 1993; Zhu et al. 2001). In the current research, changes of dat-1, unc-17, and unc-47 expression involving the motor modulation function of these neurons were examined. The analysis showed that micro- and nanosized polystyrene particles can substantially prompt down-regulated expression of these prospective neurodegeneration markers, which showed an important damage to GABAergic and cholinergic neurons, and there was no change in dopaminergic neurons. These results show the toxicity of polystyrene MPs/NPs’ selective neurodegeneration on organisms. The MP/NP excitation influences the locomotion of C. elegans. They conclude that the impacts on cholinergic neurons may be damaging. In addition, the observed disabilities of GABAergic inhibitory neurons may lead to an imbalance excito-inhibitory process and have exciting impacts on locomotive manners in C. elegans. Apart from contemplating the absence of blood–brain barrier (BBB) in C. elegans (Li et al. 2017; Xu et al. 2017), NPs may be more likely to interact with neurons and practice nematodes neurotoxicity. Moreover, more underlying neurotoxicity mechanisms require more studies.
Conclusion
An expanding information base has demonstrated the adverse impacts of MPs/NPs on aquatic and terrestrial species. But, apart from MPs, NPs in aquatic structures can reach intestinal tissue and thus the human food chain ends. Thereby, the investigation of the existence and fate of nanoplastics in the environment seems crucial. In comparison with research, the impacts of NPs on marine species affect humans by the risk of exposure to people through the food chain. The MPs/NPs accumulate throughout the entire environment and food chain in both animals and humans. It is vital to systematically assess the impact of MPs/NPs on living organisms, especially as they are capable of adsorbing potential toxicants including organic macromolecules, pollutants, and heavy metals that intermingle in the environment. It is obvious that our interpretation of the prospective toxicity of the human species by environmental MPs/NPs is in its early stages and has left many unanswered questions. Is there substantial bioaccumulation and trophic transfer in the environment for MPs/NPs? If it does, which species are at greatest risk? How the aging of plastics eventually affects the environment and animals?
Moreover, the long-term data of ingested NPs’ destiny in human and aquatic species are restricted, and several major problems remain for future studies, including what would be the concentration of water nanoplastics? Can this concentration influence the aquatic environment, and hence the food supply chain, leading to significant dangerous human impacts? In order to determine potential human exposure, can we verify the existence of nanoplastics in the human food chain? In view of these unresolved issues, new technological approaches to detect the particles of NPs in the environment and in humans are required. Future studies should shift towards an even lesser recognized terrestrial environment. Research including terrestrial but still freshwater ecosystems will support the general explanation of environment MP/NP pollution and its potential effects on human health.
References
Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB (1993) The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science 261(5121):617–619
Awet TT, Kohl Y, Meier F, Straskraba S, Grün AL, Ruf T, Jost C, Drexel R, Tunc E, Emmerling C (2018) Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ Sci Eur 30(1):11
Bakir A, Rowland SJ, Thompson RC (2012) Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar Pollut Bull 64(12):2782–2789
Barboza LGA, Vieira LR, Branco V, Figueiredo N, Carvalho F, Carvalho C, Guilhermino L (2018) Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol 195:49–57
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B Biol Sci 364(1526):1985–1998
Bergmann M, Gutow L, Klages M (2015) Marine anthropogenic litter. Marine Anthropogenic Litter
Bergmann J, Verbruggen E, Heinze J, Xiang D, Chen B, Joshi J, Rillig MC (2016) The interplay between soil structure, roots, and microbiota as a determinant of plant–soil feedback. Ecol Evol 6(21):7633–7644
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48(20):12336–12343
Bhargava S, Lee SSC, Ying LSM, Mei LN, Teo LM, Valiyaveettil S (2018) Fate of nanoplastics in marine larvae: a case study using barnacles, amphibalanus amphitrite. ACS Sustain Chem Eng 6:6932–6940
Bhattacharya P, Lin S, Turner JP, Ke PC (2010) Physical adsorption ofcharged plastic nanoparticles affects algal photosynthesis. J Phys Chem C 114:16556–16561
Bouwmeester H, Hollman PCH, Peters RJB (2015) Potential health impact of environmentally released micro- and nanoplastics in the human food production Chain: experiences from nanotoxicology. Environ Sci Technol 49(15):8932–8947
Brandts I, Teles M, Gonçalves AP, Barreto A, Franco-Martinez L, Tvarijonaviciute A, Martins MA, Soares AMVM, Tort L, Oliveira M (2018) Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci Total Environ 643:775–784
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42(13):5026–5031
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thaompson R (2011) Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ Sci Technol 45(21):9175–9179
Brun E, Barreau F, Veronesi G, Fayard B, Sorieul S, Chanéac C, Rabilloud T, Mabondzo A, Herlin-Boime N, Carrière M (2014) Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part Fibre Toxicol 11:13
Cai L, Wang J, Peng J, Wu Z, Tan X (2018) Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci Total Environ 628-629:740–747
Canesi L, Ciacci C, Fabbri R, Balbi T, Salis A, Damonte G, Cortese K, Caratto V, Monopoli MP, Dawson K (2016) Interactions of cationic polystyrene nanoparticles with marine bivalve hemocytes in a physiological environment: role of soluble hemolymph proteins. Environ Res 150:73–81
Cao D, Wang X, Luo X, Liu G, Zheng H (2017) Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. IOP Conf Ser Earth Environ Sci 61(1):012148
Carr KE, Smyth SH, McCullough MT, Morris JF, Moyes SM (2012) Morphological aspects of interactions between microparticles and mammalian cells: intestinal uptake and onward movement. Prog Histochem Cytochem 46(4):185–252
Chadwick SS (1988) Ullmann’s encyclopedia of industrial chemistry. Ref Serv Rev 16:31–34
Chen Q, Yin D, Jia Y, Schiwy S, Legradi J, Yang S, Hollert H (2017) Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci Total Environ 609:1312–1321
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Tamara SG (2013) Microplastic ingestion by zooplankton. Environ Sci Technol 47(12):6646–6655
De Sá LC, Oliveira M, Ribeiro F, Rocha TL, Futter MN (2018) Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci Total Environ 645:1029–1039
De Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC (2018) Microplastics as an emerging threat to terrestrial ecosystems. Glob Chang Biol 24:1405–1416
Dekiff JH, Remy D, Klasmeier J, Fries E (2014) Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ Pollut 186:248–256
Deng Y, Zhang Y, Lemos B, Ren H (2017) Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep 7:46687
Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852
Desforges JPW, Galbraith M, Dangerfield N, Ross PS (2014) Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar Pollut Bull 79(1-2):94–99
Ding J, Zhang S, Razanajatovo RM, Zou H, Zhu W (2018) Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ Pollut 238:1–9
Dubaish F, Liebezeit G (2013) Suspended microplastics and black carbon particles in the Jade system, southern North Sea. Water Air Soil Pollut 224:1352
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 75:63–82
Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, Petrozza JC, Wright D, Hauser R (2012) Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod 27(12):3583–3592
Engler RE (2012) The complex interaction between marine debris and toxic chemicals in the ocean. Environ Sci Technol 46(22):12302–12315
Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Francois G, Peter GR, Julia R (2014) Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One:1–15
Farrell P, Nelson K (2013) Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ Pollut 177:1–3
Fossi MC, Marsili L, Baini M, Giannetti M, Coppola D, Guerranti C, Caliani I, Minutoli R, Lauriano G, Finoia MG, Rubegni F, Panigada S, Bérubé M, Urbán Ramírez J, Panti C (2016) Fin whales and microplastics: the Mediterranean Sea and the Sea of Cortez scenarios. Environ Pollut 209:68–78
Fouad AD, Teng S, Mark JR, Liu A, Alvarez-Illera P, Ji H, Du A, Bhirgoo PD, Cornblath E, Guan SA, Fang-Yen C (2018) Dstributed rhythm generators underlie Caenorhabditis elegans forward locomotion. Elife 7:e29913
Frydkjær CK, Iversen N, Roslev P (2017) Ingestion and egestion of microplastics by the Cladoceran Daphnia magna: effects of regular and irregular shaped plastic and sorbed phenanthrene. Bull Environ Contam Toxicol 99(6):655–661
Galloway TS (2015) Micro- and nano-plastics and human health. Mar Anthropogenic Litter:343–366
Galloway TS, Cole M, Lewis C (2017) Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol 1(5):0116
Gewert B, Plassmann MM, Macleod M (2015) Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impacts 17(9):1513–1521
Gong JL, Wang B, Zeng GM, Yang CP, Niu CG, Niu QY, Zhou WJ, Yi L (2009) Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J Hazard Mater 164:1517–1522
Gonzalezfernandez C, Tallec K, Le NG, Lambert C, Soudant P, Huvet A, Suquet M, Berchel M, Paulpont I (2018) Cellular responses of Pacific oyster (Crassostrea gigas) gametes exposed in vitro to polystyrene nanoparticles. Chemosphere 208:764–772
Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM (2008) The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A 105(33):11613–11618
Hallett PD, Feeney DS, Bengough AG, Rillig MC, Scrimgeour CM, Young IM (2009) Disentangling the impact of AM fungi versus roots on soil structure and water transport. Plant Soil 314:183–196
Handy RD, Henry TB, Scown TM, Johnston BD, Tyler CR (2008) Manufactured nanoparticles: their uptake and effects on fish - a mechanistic analysis. Ecotoxicology 17(5):396–409
Hayman G, Derwent RD (1997) Atmospheric chemical reactivity and ozone-forming potentials of potential CFC replacements. Environ Sci Technol 31:327–336
He C, Hu Y, Yin L, Tang C, Yin C (2010) Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31(13):3657–3666
Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Völkel W, Wollin K-M, Gundert-Remy U (2011) Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol 41(4):263–291
Hidalgo-Ruz V, Thiel M (2013) Distribution and abundance of small plastic debris on beaches in the SE Pacific (Chile): a study supported by a citizen science project. Mar Environ Res 87-88:12–18
Hodson ME, Duffus-Hodson CA, Clark A, Prendergast-Miller MT, Thorpe KL (2017) Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol 51(8):4714–4721
Holm P, Schulz G, Athanasopulu K (2013) Meeresverschmutzung der neuen Art: Mikroplastik—ein unsichtbarer Störenfried. Biol Unserer Zeit 43(1):27–33
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C (2017) Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 586:127–141
Hussain N, Jaitley V, Florence AT (2001) Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev 50(1-2):107–142
Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C (2013) Contamination of beach sediments of a sub alpine lake with microplastic particles. Curr Biol 23:R867–R868
Ivar Do Sul JA, Costa MF (2014) The present and future of microplastic pollution in the marine environment. Environ Pollut 185:352–364
Jeong CB, Won EJ, Kang HM, Lee MC, Hwang DS, Hwang UK, Zhou B, Souissi S, Lee SJ, Lee JS (2016) Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ Sci Technol 50(16):8849–8857
Kanhai LDK, Gårdfeldt K, Lyashevska O, Hassellöv M, Thompson RC, O’Connor I (2018) Microplastics in sub-surface waters of the Arctic Central Basin. Mar Pollut Bull 130:8–18
Kosuth M, Mason SA, Wattenberg EV (2018) Anthropogenic contamination of tap water, beer, and sea salt. PLoS One 13(4):e0194970
Lagarde F, Olivier O, Zanella M, Daniel P, Hiard S, Caruso A (2016) Microplastic interactions with freshwater microalgae: Heteroaggregation and changes in plastic density appear strongly dependent on polymer type. Envtl. Pollu. 215:331–339
Lambert S, Wagner M (2016a) Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145:265–268
Lambert S, Wagner M (2016b) Formation of microscopic particles during the degradation of different polymers. Chemosphere 161:510–517
Lambert S, Sinclair CJ, Bradley EL, Boxall ABA (2013) Effects of environmental conditions on latex degradation in aquatic systems. Sci Total Environ 447:225–234
Lambert S, Scherer C, Wagner M (2017) Ecotoxicity testing of microplastics: Considering the heterogeneity of physicochemical properties. Integr Environ Assess Manag 13(3):470–475
Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D (2008) Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300(11):1303–1310
Lee KW, Shim WJ, Kwon OY, Kang JH (2013) Size-dependent effects of micro polystyrene particles in the marine copepod tigriopus japonicus. Environ Sci Technol 47(19):11278–11283
Lenz R, Enders K, Nielsen TG (2016) Microplastic exposure studies should be environmentally realistic. Proc Natl Acad Sci U S A 113(29):E4121–E4122
Li J, Yang D, Li L, Jabeen K, Shi H (2015) Microplastics in commercial bivalves from China. Environ Pollut:190–195
Li WC, Tse HF, Fok L (2016) Plastic waste in the marine environment: a review of sources, occurrence and effects. Sci Total Environ 566-567:333–349
Li P, Xu T, Wu S, Lei L, He D (2017) Chronic exposure to graphene-based nanomaterials induces behavioral deficits and neural damage in Caenorhabditis elegans. J Appl Toxicol 37(10):1140–1150
Li J, Liu H, Paul Chen J (2018) Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374
Lithner D, Larsson A, Dave G (2011) Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci Total Environ 409(18):3309–3324
Liu Z, Cai M, Yu P, Chen M, Wu D, Zhang M, Zhao Y (2018) Age-dependent survival, stress defense, and AMPK in Daphnia pulex after short-term exposureto a polystyrene nanoplastic. Aquat Toxicol 204:1–8
Ma Y, Huang A, Cao S, Sun F, Wang L, Guo H, Ji R (2016) Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ Pollut 219:166–173
Machado AAS, Valyi K, Rillig MC (2017) Potential environmental impacts of an “underground revolution”: a response to Bender et al. Trends Ecol Evol 32(1):8–10
Mahler GJ, Esch MB, Tako E, Southard TL, Archer SD, Glahn RP, Shuler ML (2012) Oral exposure to polystyrene nanoparticles affects iron absorption. Nat Nanotechnol 7(4):264–271
Mattsson K, Ekvall MT, Hansson LA, Linse S, Malmendal A, Cedervall T (2015) Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ Sci Technol 49(1):553–561
McCulloch A (2003) Fluorocarbons in the global environment: a review of the important interactions with atmospheric chemistry and physics. J Fluor Chem 123(1):21–29
Miao M, Yuan W, Zhu G, He X, Li DK (2011) In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod Toxicol 32(1):64–68
Mintenig SM, Löder MGJ, Primpke S, Gerdts G (2019) Low numbers of microplastics detected in drinking water from ground water sources. Sci Total Environ 648:631–635
Murphy F, Quinn B (2018) The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environ Pollut 234:487–494
Naik V, Jain AK, Patten KO, Wuebbles DJ (2000) Consistent sets of atmospheric lifetimes and radiative forcings on climate for CFC replacements: HCFCs and HFCs. J Geophys Res 105(D5):6904–6914
Neves D, Sobral P, Ferreira JL, Pereira T (2015) Ingestion of microplastics by commercial fish off the Portuguese coast. Mar Pollut Bull 101(1):119–126
Nomura T, Tani S, Yamamoto M, Nakagawa T, Toyoda S, Fujisawa E, Yasui A, Konishi Y (2016) Cytotoxicity and colloidal behavior of polystyrene latex nanoparticles toward filamentous fungi in isotonic solutions. Chemosphere 149:84–90
Obbard RW, Sadri S, Wong YQ, Khitun AA, Baker I, Thompson RC (2014) Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Futur 2:315–320
Ogonowski M, Schur C, Jarsen A, Gorokhova E (2016) The effects of natural and anthropogenic microparticles on individual fitness in Daphnia magna. PLoS One 11(5):e0155063
Oriekhova O, Stoll S (2018) Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter. Environ Sci Nano 5:792–799
Pedà C, Caccamo L, Fossi MC, Gai F, Andaloro F, Genovese L, Perdichizzi A, Romeo T, Maricchiolo G (2016) Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ Pollut 212:251–256
Peng G, Xu P, Zhu B, Bai M, Li D (2018) Microplastics in freshwater river sediments in Shanghai, China: a case study of risk assessment in mega-cities. Environ Pollut 234:448–456
Powell JJ, Thoree V, Pele LC (2007) Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract. Br J Nutr 98(Suppl 1):S59–S63
Ramos L, Berenstein G, Hughes EA, Zalts A, Montserrat JM (2015) Polyethylene film incorporation into the horticultural soil of small periurban production units in Argentina. Sci Total Environ 523:74–81
Rehse S, Kloas W, Zarfl C (2016) Short-term exposure with high concentrations of pristine microplastic particles leads to immobilization of Daphnia magna. Chemosphere 153:91–99
Reisser J, Shaw J, Hallegraeff G, Proietti M, Barnes DKA, Thums M, Chris W, Hardesty BD, Charitha P (2014) Millimeter-sized marine plastics: a new pelagic habitat for microorganisms and invertebrates. PLoS One 9(6):e100289
Revel M, Châtel A, Mouneyrac C (2018) Micro(nano)plastics: a threat to human health? Curr Opin Environ Sci Health 1:17–23
Rillig MC (2012) Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol 4(12):6453–6454
Rist S, Baun A, Hartmann NB (2017) Ingestion of micro- and nanoplastics in Daphnia magna - quantification of body burdens and assessment of feeding rates and reproduction. Environ Pollut 228:398–407
Rochman CM (2018) Microplastics research-from sink to source. Science 360:28–29
Rochman CM, Hoh E, Kurobe T, Teh SJ (2013) Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci Rep 3:3263
Rodriguez-Seijo A, Lourenço J, Rocha-Santos TAP, da Costa J, Duarte AC, Vala H, Pereira R (2017) Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ Pollut 220(Pt A):495–503
Rosenkranz P, Chaudhary Q, Stone V, Fernandes TF (2009) A comparison of nanoparticle and fine particle uptake by Daphnia magna. Environ Toxicol Chem 28:2142–2149
Schirinzi GF, Pérez-Pomeda I, Sanchís J, Rossini C, Farré M, Barceló D (2017) Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ Res 159:579–587
Schmidt C, Lautenschlaeger C, Collnot EM, Schumann M, Bojarski C, Schulzke JD, Lehr CM, Stallmach A (2013) Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa - a first in vivo study in human patients. J Control Release 165(2):139–145
Setala O, Fleming-Lehtinen V, Lehtiniemi M (2014) Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut 185:77–83
Sharma S, Chatterjee S (2017) Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ Sci Pollut Res 24(27):21530–21547
Sjollema SB, Redondo-Hasselerharm P, Leslie HA, Kraak MHS, Vethaak A (2016) Do plastic particles affect microalgal photosynthesis and growth? Aquat Toxicol 170:259–261
Somani A, Nandi TK, Pal SK, Majumder AK (2017) Pre-treatment of rocks prior to comminution – a critical review of present practices. Int J Min Sci Technol 54:202–211
Stephens B, Azimi P, El Orch Z, Ramos T (2013) Ultrafine particle emissions from desktop 3D printers. Atmos Environ 79:334–339
Sun X, Chen B, Li Q, Liu N, Xia B, Zhu L, Keming Q (2018) Toxicities of polystyrene nano- and microplastics toward marine bacterium Halomonas alkaliphila. Sci Total Environ 642:1378–1385
Suquet M, Berchel M, Paulpont I (2018) Cellular responses of Pacific oyster (Crassostrea gigas) gametes exposed in vitro to polystyrene nanoparticles. Chemosphere 208:764–772
Tagg AS, Sapp M, Harrison JP, Ojeda JJ (2015) Identification and quantification of microplastics in wastewater using focal plane array-based reflectance micro-FT-IR imaging. Anal Chem 87(12):6032–6040
Teuten EL, Rowland SJ, Galloway TS, Thompson RC (2007) Potential for plastics to transport hydrophobic contaminants. Environ Sci Technol 41(22):7759–7764
Van A, Rochman CM, Flores EM, Hill KL, Vargas E, Vargas SA, Euhna H (2012) Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 86:258–263
Van CL, Vanreusel A, Mees J, Janssen CR (2013) Microplastic pollution in deep-sea sediments. Environ Pollut 182:495–499
Veresoglou SD, Halley JM, Rillig MC (2015) Extinction risk of soil biota. Nat Commun 6:8862
Villarrubia-Gómez P, Cornell SE, Fabres J (2018) Marine plastic pollution as a planetary boundary threat – the drifting piece in the sustainability puzzle. Mar Policy 96:213–220
Volkheimer G (1974) Passage of Particles through the wall of of the gastrointestinal tract. Environ Health Perspect 9:215–225
Von Moos N, Burkhardt-Holm P, Köhler A (2012) Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol 46(20):11327–11335
Wang J, Peng J, Tan Z, Gao Y, Zhan Z, Chen Q, Liqi C (2017) Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere 171:248–258
Woodall LC, Sanchez-Vidal A, Canals M, Paterson GLJ, Coppock R, Sleight V, Antonio C, Alex DR, Bhavani EN, Richard CT (2014) The deep sea is a major sink for microplastic debris. R Soc Open Sci 1:140317
Wright SL, Kelly FJ (2017) Plastic and human health: a micro issue? Environ Sci Technol 51(12):6634–6647
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: a review. Environ Pollut 178:483–492
Xu T, Zhang M, Hu J, Li Z, Wu T, Bao J, Wu S, Lei L, He D (2017) Behavioral deficits and neural damage of Caenorhabditis elegans induced by three rare earth elements. Chemosphere 181:55–62
Yadav V, Sherly MA, Ranjan P, Tinoco RO, Boldrin A, Damgaard A, Laurent A (2020) Framework for quantifying environmental losses of plastics from landfills. Resour Conserv Recycl 161:104914
Yigit E (1976) Three mathematical comminution models based on strain energy. Int J Miner Process 365-374
Yoo JW, Doshi N, Mitragotri S (2011) Adaptive micro and nanoparticles: temporal control over carrier properties to facilitate drug delivery. Adv Drug Deliv Rev 63(14–15):1247–1256
Yu P, Liu Z, Wu D, Chen M, Lv W, Zhao Y (2018) Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol:28–36
Zarfl C, Matthies M (2010) Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar Pollut Bull 60(10):1810–1814
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47(13):7137–7146
Zhan Z, Wang J, Peng J, Xie Q, Huang Y, Gao Y (2016) Sorption of 3,3′,4,4′-tetrachlorobiphenyl by microplastics: a case study of polypropylene. Mar Pollut Bull 110:559–563
Zhao L, Qu M, Wong G, Wang D (2017) Transgenerational toxicity of nanopolystyrene particles in the range of μg L−1 in the nematode: Caenorhabditis elegans. Environ Sci Nano 4:2356–2366
Zhao J, Ran W, Teng J, Liu Y, Liu H, Yin X, Cao R, Wang Q (2018) Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China. Sci Total Environ 640-641:637–645
Zhu H, Duerr JS, Varoqui H, McManus JR, Rand JB, Erickson JD (2001) Analysis of point mutants in the Caenorhabditis elegans vesicular acetylcholine transporter reveals domains involved in substrate translocation. J Biol Chem 276(45):41580–41587
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Christian Gagnon
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sana, S.S., Dogiparthi, L.K., Gangadhar, L. et al. Effects of microplastics and nanoplastics on marine environment and human health. Environ Sci Pollut Res 27, 44743–44756 (2020). https://doi.org/10.1007/s11356-020-10573-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10573-x