Abstract
The present study describes the water quality scenario of some freshwater springs of South Kashmir during the two-year period (2013–2015) because of rising pollution risks endangering water resources globally. The accessibility to quality drinking water has become a challenge and is receiving renewed attention. A total of 96 samples from twelve springs were collected and analyzed for major drinking water quality parameters. Piper trilinear and Durov diagram depicted dominance of Ca–Mg–HCO3 hydrochemical facies and simple dissolution and mixing process. Water quality was falling in very good to excellent class and well within the desirable limits of WHO thereby indicating huge potential for meeting rising drinking water demand. The principal component analysis (PCA) revealed the generation of three components (PC1, PC2, and PC3) with higher eigenvalues of 3 or more (3–6) explaining 40, 21, and 17% of the overall variance in water quality data sets, respectively. The components obtained from PCA indicate that the parameters responsible for variations are mainly related to discharge, temperature, and dissolved oxygen (natural), nutrients (agriculture), and cation and anions (lithology). The results suggest that the hydrochemistry of springs is jointly controlled by lithology and anthropogenic inputs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Springs have recently attained an extraordinary importance due to the role they play in meeting the increasing drinking water demands (Bhat et al. 2020). Water shortage because of climate change and rising water demand across the globe has increased (Wegener et al. 2010; Burek et al. 2016), and freshwater scarcity is increasingly perceived as a global systemic risk (Mujumdar and Tiwari 2019). Water, an increasingly stressed resource, is witnessing a worldwide increase in consumption, especially from the last four decades largely due to a blend of rising human population growth, socio-economic development, and changing consumer choices and trends (WWDR 2019). Water is very vital for human life and overall development and catches more attention globally as 20% of the population is experiencing high water stress and another about 40% is facing severe water scarcity. This stress is bound to increase as demand for water progresses and the impacts of projected climate change intensify. Safe drinking water and proper sanitation have been considered a basic right of an individual as they are essential for healthy living and maintaining the dignity of humans (WWDR 2019). But, at the same time, a substantial percentage of the human population does not have an access to safe water to meet basic hygiene and domestic requirements. The increasing demand for scarce water resources has further intensified the competition not only between humans and their environment but also between different socio-economic sectors (Kurian 2017). Anthropogenic factors in the form of rising human population growth and subsequent urbanization are prominent in contributing to a rapid surge in the demand for various uses like drinking, sanitation, agriculture, energy production, industry, and environmental protection. Water and its judicial use and management is considered as one of the great challenges of the future. Groundwater being valuable, but facing continuous depletion, has a major role in meeting the demands of various sectors besides overall economic development and food security. Spring water utilization offers an array of services to people but with some costs like reduction in water quality (Howell et al. 1995). Population growth, urbanization, and unplanned application of agrochemicals and discharge of untreated sewage water are directly or indirectly impacting the quality and quantity of surface and subsurface freshwater resources, and accordingly there is a high demand for safe water for drinking, sanitation, agriculture, and environmental protection (FAO 2011a, b; WWAP 2012, 2015). But it seems that the sustainability of freshwater supply is critically susceptible due to the widespread diminution of groundwater, pollution of surface water, and climate change impacts (IPCC 2007; Gleeson et al. 2012). Access to safe drinking water supply is one of the fundamental needs for human survival, which should be acceptable, affordable, and physically accessible (Sogbanmu et al. 2019; UN 2012). The main drivers responsible for water quality crisis globally are attributed to rising human population growth, urbanization, land-use change, industrialization, food production practices, increased living standards, and poor waste water management (UNEP 2016). Springs have proved to be indispensable in the livelihoods of mountain communities throughout the world, including people living along the Himalayan foothills (Tambe et al. 2011; Risko 2018; Bhat and Pandit 2018). Continuing water scarcity, climate change, and human contamination place these hidden reserves at high risk in terms of their sustainability (Griebler et al. 2010). Springs stand underrepresented in scientific literature and are legally overlooked despite the numerous benefits and services they offer thereby neglecting the necessity to look deep into the various stressors and threats of spring ecosystem health (Barquin and Scarsbrook 2008; Nelson 2008; Unmack and Minckley 2008).
Springs have attained recently extraordinary importance due to the recognition of the role they play in ecosystem services especially in the scenario of climate change threat predicted for the Himalayas besides meeting the drinking water demands (IPCC 2014; Gupta and Kulkarni 2017). Even though springs have a critical role in water security, their role in the upliftment of socioeconomic profile and overall development is rarely debated at the policy and governance levels, including lack of wider scientific recognition (Springer et al. 2008; Kreamer et al. 2014; Gupta and Kulkarni 2017). The destruction of the natural habitat of springs is also because they are insufficiently covered by protective legislation (Nelson 2008; Cantonati et al. 2012). The rapid pace of industrialization, urbanization, and agricultural development has resulted in over-exploitation and contamination of groundwater resources in many parts of India (Singh and Singh 2002; Murtaza et al. 2019; Ustaoglu et al. 2020).
Springs act as principal sources of drinking water for millions of rural and urban populations in the Hindu Kush Himalayas (HKH) besides agriculture and ecosystem services (Gupta and Kulkarni 2017). There is a growing indication that springs are desiccating or their discharge is dipping and the quality of spring water is worsening throughout the HKH (Tiwari 2000). As a result of this water stress, communities are facing unprecedented daily distress. The importance of freshwater springs in society also reflects its deep connection with the establishment and existence of human populations in various parts of the world very adjacent to the springs (Fatchen 2000; Sada and Sharpe 2004). In the Himalayan region, springs are the primary sources of water for millions of people for meeting their drinking, domestic, and agricultural needs. Springs in the region have been reportedly drying up leading to water stress to local communities (Tiwari 2000; Jeelani et al. 2014). A lot of emphasis has been attached to springs from hydrogeology, ecology, hydrobiology, socio-culturally, and spiritually, but very little about ecological economics (Nabhan 2008; Phillips et al. 2009) despite the fact that they contribute markedly to urban water supply around the world (Petric 2010). Springs ecosystems in valleys are facing various types of threats dominated largely by encroachments and are imploring detailed exploration as they remain data-deficient systems throughout the Himalaya (Kumar et al. 2017; Bhat and Pandit 2018; Mishra and Kumar 2020). The magnitude of this problem is not precisely known given that there is a dearth of scientific studies at a large scale (Hoffsten and Malmqvist 2000; Bhat and Pandit 2018). It is now extensively endorsed that human activities may threaten the quality and quantity of spring waters at a massive scale ahead.
Water quality index (WQI) is considered as an effective grading technique for measuring water quality for human consumption (Akter et al. 2016; Ustaoglu et al. 2020). A variety of water quality parameters are included in a mathematical equation to get a distinct number to demonstrate the overall water quality, determining its suitability for human consumption (Tyagi et al. 2013; Ochuko et al. 2014). Water quality index involves selection of parameters, quality function, and significance of parameter and combination through a mathematical equation (Tyagi et al. 2013). Principal component analysis (PCA), a multivariate statistical technique, allows complete assessment of the potential sources responsible for the differences in water quality and environmental status of the study area by dealing with large and complex multivariate data sets (Muangthong and Shrestha 2015; Ustaoglu and Tepe 2019). Principal component analysis reduces the dimensionality of the large and complex data sets without losing the possible variation present in the data set (Paliy and Shankar 2016).
The population of Kashmir Himalaya, like other parts of India, has been witnessing enormous growth, and the supply of drinking water poses a great challenge due to the limited water infrastructure of mountain settlements (Wescoat Jr et al. 2016). While the springs used to suffice the water demand in the recent past, people have started to face increasing water shortages of water since the early 2000s, although the government has improved the water distribution network. Because of the diminishing accessibility of freshwater in various regions of the world, a global water crisis is predicted if suitable monitoring, water conservation, and adaptation measures are not undertaken appropriately and adequately. Springs being highly individualistic and relatively self-contained are globally threatened by human activities despite their hydrogeological and ecological relevance, and the aquifers that support springs (Cantonati et al. 2015).
Therefore, one of the most pressing issues the world faces is the management of freshwater resources, among which springs have become a focal point because of their geological, ecological, scientific, cultural, and societal importance besides burgeoning demands for drinking, irrigation, industrial, fishery, and recreational purposes in different parts of the world, including the Kashmir Valley (Glazier 2014; Bhat and Pandit 2018). Despite having such importance, they are also insufficiently covered by protective legislation and policy framework, often ensuing destruction and degradation of their natural settings (Cantonati et al. 2012). Keeping in view the above-noted scenario, hydrochemical assessment of twelve freshwater springs in four districts of South Kashmir, India, was carried out for suitability and appropriateness of drinking water quality standards for life-supporting demand of humankind.
Material and methods
Study area
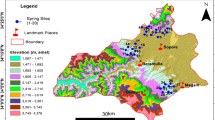
The high-altitude valley of Kashmir is an intermountain depression existing between lesser and greater Himalaya. It is of tectonic origin that covers a total area of about 4920 km2. The valley of Kashmir is 135 km long and 45 km broad at its middle, lying as an oval bowl between the Zanskar range to the north and Pir Panjal Range to the south (Raza et al. 1978). The valley is surrounded by high mountains on all sides which remain snow-clad for most of the year. The valley abounds in snow-fed, spring-fed streams, rivers with a network of their tributaries, and numerous freshwater lakes. The Kashmir valley harbors hundreds of springs of different magnitudes that attract the tourists and thereby play an important role in the socio-economic upliftment of the local population. The valley of Kashmir is gifted with plentiful lovely lakes, swamps, springs, and streams. Hydrogeologically, both porous and fissured formations occur in the Jhelum basin. Groundwater arises underwater table and confined environments in unconsolidated alluvial and Karewa formations in the valley. These formations form multi-layered aquifers and have prolific yields. Groundwater occurs in perched condition and gives rise to springs in the phreatic zone water table. Occurrence and movement of groundwater is largely controlled by principal inter-granular porosity in the yielding sedimentary quaternary alluvium and Karewa formations (CGWB 2009). Among these freshwater bodies, springs locally called “Nag” have either historical background or fascinating myths after them. They have broad distribution and emerge both in plain areas and at high altitudes. The water in the majority of the springs is cold in summer and warm in winter and as such are regarded as sacred. In addition to their religious significance, these freshwater ecosystems offer water for drinking and irrigation, apart from other desired uses. A total of 12 perennial springs from four districts representing South Kashmir (Anantnag, Kulgam, Shopian, and Pulwama) were selected for the present study (Fig. 1). A total of three springs were selected from each district, which in most of the springs are surrounded by large tracts of agricultural and horticulture fields with large trees comprising of Populus, Ulmus, Acacia, Juglans, and Platanus. The characteristic features of the selected springs along with their names are summarized in Table 1. The springs under investigation show marked seasonal variations in discharge with the change in seasons. The springs show peak discharge in spring-summer seasons and lean discharge in autumn-winter seasons. The substantial variation in discharge and temperature plays an important role in mineralization of underlying rock, which in turn profoundly influences the water quality.
General hydrogeology of the study area
Geologically, the study area is occupied by rocks of underlying Agglomerate Slates and Panjal Trap Formation of Permo-Carboniferous age overlying Triassic Limestone Formation (Tyagi et al. 1964; Bhusan et al. 1972). These rock types are distributed widely in the valley floor and are exposed in peripheral elevated parts. The rocks of the Agglomerate Slates and Panjal Trap formation are observed in Dubjan-Aharbal Section Shopian, Dhamhal-Chimar Section Kulgam, and in lower sections of the peripheral parts of Anantnag apart from the occurrence of Triassic Limestone. These older rock types in the study area are overlain by Karewa Sediments of Quaternary age. The Karewa sediments are fluvial-glacial in nature and are widely distributed on the valley floor. Lithologically, Lower Karewa deposits are gently inclined and better exposed to the Pir Panjal flank and have an unconformable contact with the almost horizontal Upper Karewas, which are better exposed to the Himalayan flank. The best exposed Lower Karewa sections are along the River Rembiara between Dubjan–Hirpora–Krachipatra and along River Romushi between Ichhagoz and Romu (Bhat and Bhat 2014). Lower Karewas are further characterized by mudstones, unconsolidated sandstones, lignite beds, and conglomerate horizons. Upper Karewas are laminated clay-stones, sandstones, and some conglomeratic layers but without lignite beds. The Karewa sediments as a whole are capped by loess deposits associated with dark humid layers commonly known as “Palaeosols” famous for saffron cultivation. The Upper Karewa (greenish sand and gray clay with calcareous laminae), Lower Karewa (bluish gray clay and conglomerates with coarse to fine sand and alternate gray sand clays), fluvio-glacial boulder bed and Alluvium (clay, silt and sand) of Quaternary and Tertiary age (Plie-Pleistocene) age lie beneath the valley floor with alternate bands of sand, silt, gravel, and clay interspersed by glacial boulder beds. This formation is essential from the groundwater point of view and sustains the water supply in the area (Wadia 1975). Groundwater occurrence and movement is primarily controlled by the predominant inter-granular porosity of the soft sedimentary quaternary alluvium formations and the Karewa formations. This unconsolidated sedimentary deposit is the largest multi-layer aquifer system. Unconsolidated sediments consisting of fluvio-glacial, Karewas (lacustrine deposits), the recent alluvium, terrace deposits, and alluvial fan deposits constitute the porous aquifer system. A prolific aquifer system is formed by sediments that consist of sand, gravel, cobbles, pebbles, and boulders interlaced with thick clay beds (CGWB 2009).
Sample collection and analysis
Spring water samples were obtained in high-density polyethylene (HDPE) bottles on a seasonal basis for two years from March 2013 to February 2015 from the twelve springs (Table 1 and Fig. 1), three from each of four districts, namely Anantnag district (SP1, SP2, and SP3), Kulgam district (SP4, SP5, and SP6), Shopian district (SP7, SP8, and SP9), and Pulwama district (SP10, SP11, and SP12). The samples thus obtained were subjected to analysis in the laboratory using standard methods and following standard procedures to ensure data quality consistency (Wetzel and Likens 2000; APHA (American Public Health Association) 2012). Parameters, like water temperature and discharge, were determined on the spot at sampling sites. The dissolved oxygen (DO), total alkalinity, free carbon dioxide (CO2), total hardness (TH), magnesium hardness, and chloride were estimated by the titrimetric method in the laboratory. Moreover, ammonical nitrogen, NO3−, SO42−, PO43−, total phosphorus, and dissolved silica were examined using the standard methods recommended by the American Public Health Association (APHA 2012). The concentrations of the cations (i.e., Na and K) were measured with the aid of a flame photometer. Furthermore, water quality index (WQI) had been calculated to evaluate the suitability of spring water quality for drinking purposes (Gebrehiwot et al. 2011). All statistical studies were done employing a computer program of Origin 8.1 and RockWare Aq-Qa (1.5). Geographic Information System (GIS)–based water quality mapping in the form of visually communicating contour maps that were developed using Arc GIS version 9.0 software (Fig. 2a–q) to delineate the spatial distribution of water quality parameters (Hoseinzadeh et al. 2016).
Results and discussion
Water quality degradation is a problem growing in complexity as prosperity expands and new contaminants emerge (Damania et al. 2019). The relative constancy of water temperature across the springs indicates the thermal stability owing to the lesser amount of solar radiation reaching the water due to shade and thermally buffered water emanating from the underlying rock (van der Kamp 1995; Grasby et al. 2000; Glazier 2014). Based on water temperature, springs under investigation fall under cold water springs (Nathenson et al. 2003; Bhat and Pandit 2010a; Glazier 2014). The variation in annual air temperatures seems behind the sinusoidal pattern in spring water temperatures with maxima in summers and minima in winters (Liu et al. 2007). Spatio-temporal variability in pH shows a characteristic relation between precipitation, rock water interaction, biological activity, and organic matter decomposition (Hem 1992; Wetzel 2001; Ojha and Mandloi 2004; Oki and Akana 2016). The pH of springs, which approached towards neutrality during spring and summer seasons, is due to an increase in flow owing to rapid snowmelt in the upper areas that do not get enough time to percolate through the soil before reaching the spring and followed by increasing photosynthetic activity (Clayton 1998). The electrical conductivity (EC) values of the spring water varied from 185 to 698 μS/cm (Table 2) and fell well within the desirable limits of the WHO (Table 3). The spring waters were alkaline with moderate to high electrical conductivity, which is typical for water on carbonate bedrock (Jeelani 2008). Lower electrical conductivity during the summer season is attributed to the dilution effect caused by the increased discharge due to snowmelt and rainfall runoff (Jeelani 2008; Moore et al. 2008). The DO values show variation within almost anoxic conditions at SP4 to a maximum of 9.3 mg/L (Table 2). A total absence of DO from the Nagbalnag spring is possibly due to the effect of mineral turbidity caused by the large amount of clay particles stirring up the underlying rock, the biogeochemical process including the precipitation of calcite and sulfate flowing over carbonate karst terrain, and higher ammonical-nitrogen interfering with the solubility of oxygen (Constable et al. 2003). Lower DO concentration recorded during summer is due to temperature increase that has a stronger effect on organic matter decomposition and dominates over the photosynthetic activity besides that warm water relatively hold less oxygen than cold water (Goldman and Horne 1983; Metcalf and Eddy 1979; Kumar et al. 1996; Joshi and Kothyari 2003). Total hardness ranged between 75 and 208 mg/L, which showed that the concentration lies below the permissible limit. The major source of Ca2+ and Mg2+ in valley groundwater might be lacustrine deposits as sedimentary rocks like limestone, gypsum, and dolomite are in rich sources of calcium in the spring water (Wadia 1975; Barakat et al. 2018). Results show that almost all the samples fall in the category of hard to very hard. The springs were characterized by low chloride concentration ranging from 6.9 to 50.4 mg/L, which is also within the desirable limit recommended by the WHO (250 mg/L) (Table 3). The concentration of NH4-N in the samples ranged from 46.5 to 896 μg/L (Table 2) and was below the limit prescribed by the WHO (Table 3). The presence of ammonia is normally indicative of slight sewage and animal waste pollution (Paramsivam and Srinivasan 1981; Bhat and Pandit 2018). Chloride usually exists as chloride salts (NaCl, CaCl2, MgCl2) and is typically connected with leaching from sedimentary rocks and other evaporative minerals (Berzas et al. 2009; Pradhan and Pirasteh 2011). Natural sources include underlying rock-water interactions, seawater intrusion, and minor atmospheric contributions. Human activities–related sources include road salts, domestic sewage, and agricultural chemicals (EPA 1994; Rout and Sharma 2011). The relatively maximum concentration of chloride recorded at Astannag spring in both years of investigation is characterized by sluggish water movement which allows extended rock-water interaction that underlies the studied spring (Hem 1992). The previous studies on the limnological aspects of some important springs of South Kashmir have reported chloride concentration in the range of 11–51 mg/L, which corroborates with the results of the present study (Jeelani 2005; Bhat et al. 2010; Bhat and Pandit 2018). The concentration of NO3-N in the samples ranged from 292 to 3678 μg/L (Table 2) which in comparison to the WHO limit of 50 mg/L fell well below (Table 3). The chemistry of the water samples depicted the ionic ratio as Ca2+ ˃ Mg2+ ˃ Na+ ˃ K+ and HCO3− ˃ Cl− ˃ SO42− ˃ NO3−.
Spring water chemistry is controlled by several features like geology, chemical weathering of the diverse rock varieties, recharge water quality, and impact of allochthonous pollution whose resultant interaction leads to complex water quality (Singh and Singh 2002). Temperature is an important factor in the functioning and development of aquatic systems. The temperature of the majority of springs was recorded in a range of 13.3–17 °C. Among the springs, the SP1 spring was having the highest discharge throughout the study period with an average value of 1610 L/s because of its karstic nature (Jeelani 2008). The pH of most springs is basic (pH range: 7.4–7.8) which is due to limestone-rich lithology of the valley and sedimentary terrain thereby liberating Ca2+, Mg2+, and alumina-silicates into the solution (Coward et al. 1972; Jeelani 2008; Kurwadkar and Venkatramanan 2013; Barakat et al. 2018). Higher conductivity indicates the presence of dissolved minerals (WHO 2011). The lower concentration of DO oxygen might be due to a rise in biological activity, respiration of organisms, and the augmented rate of decay of organic matter (Joshi and Kothyari 2003). The absence of oxygen at SP4 is owing to a large amount of clay particles and a higher concentration of ammonia which might interfere with the solubility of oxygen (Lone et al. 2012). The nitrification process consumes a large amount of oxygen (Constable et al. 2003). The large concentration of free carbon dioxide at SP2 is a result of the decay of organic matter, which yields a high amount of CO2 (Verma et al. 2012). Dissolution of carbonic acid (H2CO3) is also the source of HCO3 ions (Ramesh and Jagadeeswari 2012). Except springs like SP7 (43.6 mg/L) and SP8 (50.4 mg/L) having a relatively higher concentration of chloride, the rest of the springs witnessed the very low concentration that were well within permissible limits and as reported earlier also (Bhat and Pandit 2018; Hameed et al. 2018). Higher concentrations were found to be caused due to some impurities added to springs by different anthropogenic activities like industrial, municipal wastes, and agricultural activities (Dinka et al. 2015) that are usually localized in nature. Sulfate in carbonate rocks may be derived from the dissolution of sulfate minerals (primarily gypsum and anhydrite) or oxidation of pyrite (Nikanorov and Brazhnikova 2012). The concentration of sulfate in the spring waters may be controlled by a study state dissolution process which perhaps gets influenced by biochemical processes (Herojeet et al. 2013). The reason for low sulfate values in the studied springs may be attributed to rock formation being impregnated with a low concentration of CaSO4 which is also reflected in the water chemistry especially when the water is issuing from underground sources (Cole 1983). Previous studies on hydrochemical characterization of spring water in Kashmir valley have reported sulfate concentration in the range of 4–86 mg/L (Jeelani 2010; Bhat and Pandit 2018; Hameed et al. 2018; Bhat and Pandit 2020). Lower sulfate concentration at Nagbalnag is due to the undersaturation of water for gypsum and anhydrite (Klimchouk 1996; Jeelani and Shah 2006).
The leaching of nitrate with the percolating water adds nitrate into springs. Sewage and wastes rich in nitrates cause pollution of springs (Sirajudeen and Mohamed 2013) and apart from the decomposition of organic matter and agriculture activities have been observed to contribute to the nitrate enrichment in spring waters (Elhatip 1997: Bhat and Pandit 2018). Nitrate is highly leachable and readily moves through the soil profile. In addition to fertilizers applied in agricultural and horticulture lands, nitrate becomes available from decaying plants and animals through microbial activity (Bremner 1965). The previously reported values on nitrate-nitrogen of some important freshwater springs of South Kashmir vary over a range of trace-8 mg/L (Jeelani 2010) and 0.01–3 mg/L (Bhat and Pandit 2018). In the study area, higher values of ammonical-nitrogen were recorded at SP4. The occurrence of ammonia at a high level than geogenic levels is an essential marker of fecal pollution or strata rich in humic substances (Li et al. 2011). Phosphate may occur in spring water as a result of dissolution from surrounding rocks, but most phosphorus finds its way through domestic sewage, detergents, and agricultural effluents with fertilizers (Murhekar 2011). Normally, the water of springs contains a meager amount of phosphorus concentration because of the low solubility of natural phosphate minerals and the competency of soils to hold phosphate (Devendra et al. 2014). The contour maps showed spatial distribution of spring water quality spread over four districts of Kashmir Valley (Fig. 2a–q). From the above-mentioned results, there is a comprehensive picture arising that by and large, the drinking water quality of springs is still fine. Therefore, in the interest of safeguarding the further deterioration of spring water quality, it is recommended that such springs having a huge potential for drinking water supply should receive the highest priority in the management of the springs.
Statistical analysis
Analysis of variance and Tukey’s box plot
Analysis of variance (ANOVA) and Tukey’s box plot were employed to see the significance level existing and operating between various parameters. The outcome inferred that some hydrochemical parameters have significant as well as non-significant variation. Results revealed that hydrochemical parameters interpreted in terms of box plot showed overall patterns of variation in water quality. The values were dispersed within quartiles in almost all the parameters with few outliers notably in case of water temperature, discharge, pH, conductivity, DO, bicarbonate alkalinity, chloride, total hardness, nitrate-nitrogen (Fig. 3). The analysis further observed F values having significance in observed variation for each parameter at a 5% level of significance.
Cluster analysis
Cluster analysis revealed SP4 (Nagbalnag) (cluster A) and SP12 (Khushednag) (cluster C) different from the rest of the springs but SP5 (Chilnagin) and SP6 (Hablishnag) (cluster B) exhibited a similarity of 75% while SP7 (Kharnag) and SP8 (Astannag) (cluster D) showed a similarity of 45%. SP3 (Patulehnag), SP9 (Batnagin), SP10 (Takibalnag), and SP11 (Batnag) (cluster F) depicted a least similarity of 18% while SP1 (Martandnag) and SP2 (Becharinag) (cluster E) showed a similarity of 30%. Besides, cluster analysis inferred that SP4 and SP12 were different from other selected springs based on discharge, dissolved oxygen, sulfate, ortho phosphorus, and total phosphorus (Fig. 4).
Principal component analysis
The principal component analysis (PCA) plot helped to visualize the water quality pattern of the twelve springs. The first two principal components (PC) are responsible for 61% of these variations suggesting the relative importance of the first two principal components in describing the variation in water quality parameters. The SP4 is distinct and unique from other springs with the highest overall ammonical-nitrogen, total, and ortho-phosphorus. Also notable is SP10 with higher concentration of hardness causing elements along with conductivity. SP5 and SP6 have a similar overall concentration of different water quality parameters from the rest of the springs. SP7and SP8 is similar in terms of chloride concentrations. Discharge and sulfate did not change much from the origin and their variation was minimal as compared to other parameters (Fig. 5). PC1, explaining 40% (eigenvalue, 6.0) of the total variance, has strong positive loadings on conductivity, dissolved oxygen, chloride, nitrate-nitrogen, and strong negative loading on water temperature, ammonical-nitrogen, total phosphorus, ortho-phosphorus and silica. The inverse relationship between temperature and dissolved oxygen is a natural process because warmer water holds less oxygen (Prathumratana et al. 2008). Positive loadings on conductivity, dissolved oxygen, chloride, nitrate-nitrogen at PC1 are chiefly due to seasonal changes in dilution, solubility, and oxidation (Shrestha and Kazama 2007). Furthermore, PC1 has strong negative loadings on water temperature, ammonical-nitrogen, total phosphorus, ortho-phosphorus, and dissolved silica indicating that wherever there is low water temperature there would be less concentration of these variables. This factor also represents the contribution of non-point source pollution like agricultural activities and washing of clothes. PC2, explaining 21% (eigenvalue, 4.0) of the total variance, has moderate positive loadings on free carbon, total alkalinity, and pH. This factor represents the natural relation between free carbon dioxide, alkalinity, and pH (Kazama and Yoneyama 2002; Hill and Neal 1997). Furthermore, PC2 has a strong positive loading on total hardness, calcium, and magnesium hardness. This factor explains the weathering of limestone (dolomite) in the underlying aquifer. The principal source of hardness causing constituents are present in many sedimentary rocks, the most common being limestone and chalk (Jeelani et al. 2011). The observations from PCA analysis revealed that water temperature, DO, ammonical-nitrogen, total phosphorus, ortho-phosphorus, and silica were predominantly contributing to water quality variations of springs (Fig. 5).
PCA scatter plot of water quality parameters of twelve springs based on the first two principal components. Sampling points indicated as red color circles. TH, total hardness; TA, total alkalinity; CH, calcium hardness; MH, magnesium hardness; CL, chloride; NN, nitrate nitrogen; DO, dissolved oxygen; FC, free carbon dioxide; Dis, discharge; TP, total phosphate; OP, orthophosphate; Wt., water temperature; Dsil, dissolved silica; AN, ammonical nitrogen; Sul, sulfate
Water quality evaluation
The WQI categorized springs understudy into four classes viz., marginal, fair, good, and excellent for drinking water purposes (Fig. 6). Springs like SP1 (Martandnag), SP2 (Becharinag), SP3 (Patulehnag), SP11 (Batnag), and SP9 (Batnagin) fall in good water quality while SP7 (Kharnag), SP8 (Astannag), and SP10 (Takibalnag) are in the excellent water class. Furthermore, SP5 (Chilnagin), SP6 (Hablishnag), and SP12 (Khushednag) showed fair water quality. SP4 (Nagbalnag) is the only spring that has marginal water quality (Fig. 6).
The categorization of springs into four groups from marginal to excellent drinking water quality through WQI reflects that almost all the springs are fit for drinking purposes except SP4 which has marginal water quality due to presence of a huge load of ammonia, phosphates and slit which makes its color milky (Fig. 7). Similar observations have been almost reported on various springs of Kashmir (Jeelani 2010; Bhat and Pandit 2010b; Bhat et al. 2010; Bhat and Pandit 2018; Hameed et al. 2018). The samples analyzed from the spring waters were observed to be well within the WHO (2011) limits. Furthermore, this study concludes that the water of studied springs is good for drinking purposes based on chemical parameters. However, to draw a concrete conclusion on the potability of spring waters, there is a need to have further studies on the identification and impact of pesticides, fertilizers, and discarded drugs on the water quality which at this point is beyond the scope of the study.
Piper trilinear diagram
To have some insights on the types of water prevalent in the study area of springs, the analytical data obtained from the water samples of the springs were plotted on Piper (1944) tri-linear diagram. Hydrochemical signatures thus obtained provided the information on overall ionic composition to comprehend rock water interaction. As is evident from the Piper trilinear diagram (Fig. 7), ions got concentrated on the left quadrant of the diamond plot of Piper diagram thereby indicating the dominance of Ca- HCO3 dissolution (Piper 1953; Langguth 1966) with following ionic sequence: Ca2+ > Mg2+ > Na+ > K+ and HCO3− > Cl− > SO42− > NO3− > PO43−. The ionic chemistry of water samples further revealed that the water chemistry originates from dissolution of carbonate rocks with shallow aquifers (Jeelani 2008).
Water types and processes by Durov diagram
The plotted points in the expanded diagram (Fig. 8) fall into two fields (Durov 1948), suggesting CaMg-HCO3 dominance along the simple dissolution or mixing hydrochemical process. Due to the dominance of bicarbonate alkalinity, the pH of all springs is on the alkaline side which dilutes the impact of various acids being released while the decomposition of organic matter in springs and immediate catchment occurs. The electrical conductivity of most of springs lies in the range of drinking water standards (Fig. 8). SP7 and SP8 springs lying on the extreme right of Durov Diagram fall in the TDS range of 400–500 mg/L. Globally, groundwater is the source of drinking water to 50% of the global population and 43% of all water consumed for irrigation (FAO 2011a, b). Throughout the world, 2.5 billion populations solely depend on groundwater for their basic requirements (UNESCO 2012). In the Kashmir Himalayan region, natural springs are the major source of water for drinking purposes in hilly areas and secondary sources in plain areas. However, several studies have indicated that the changing climate in the Himalayas will increase water scarcity in the future (Birch 2014). Summing up the highlights of the study, it is evident that the quality aspects of the water from the spring are of excellent and good quality. But, it is equally important to have further studies regarding coliform, metal, and pesticide pollution to reach at better and holistic understanding of the water quality of the springs. Therefore, a good database on various aspects of the Kashmir Himalayan springs can be a better supplement for policymakers and conservationists in the State of Jammu and Kashmir.
Durov expanded plot depicting hydro chemical processes involved in springs under study (Lloyd and Heathcoat 1985)
Conclusion
From our findings, it is observed that springs in the Kashmir offer a fair to excellent water quality class besides other desired uses. However, historical lack of effective management practices may further jeopardize their ecological health unless extensively studied, monitored, and appropriately managed, which have the potential to offer the solution to water crises for the burgeoning population. These springs can be very helpful in fulfilling the rising demand for access to safe drinking water and hygiene because of rising pollution scenario and threats operating at various levels of surface water resources. Future explorations on springs must look at risks operating at various scales to safeguard the sustainability of these systems closely tied to continued human existence and cultural wellbeing. The present study was restricted to water quality evaluation and with only few springs in light of some important of physicochemical characteristics, but there is an urgent need to have a thorough study on how fertilizers, pesticides, and heavy metals have impacted the spring water quality. Furthermore, studies on coliform bacteria involving an adequate number of springs spread throughout the valley of Kashmir merit an attention to offer some useful insights on the overall quality of water from the springs.
References
Akter T, Jhohura FT, Akter F, Chowdhury TR, Mistry SK, Dey D, Barua MK, Islam MA, Rahman M (2016) Water quality index for measuring drinking water quality in rural Bangladesh: a cross-sectional study. J Health Popul Nutr 35:4. https://doi.org/10.1186/s41043-016-0041-5
APHA (2012) Standard methods for the examination of water and waste water, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation
Barakat A, Meddah R, Afdali M, Touhami F (2018) Physicochemical and microbial assessment of spring water quality for drinking supply in Piedmont of Béni-Mellal Atlas (Morocco). Phys Chem Earth 104:39–46. https://doi.org/10.1016/j.pce.2018.01.006
Barquin J, Scarsbrook M (2008) Management and conservation strategies for Coldwater Springs. Aquat Conserv Mar Freshwat Ecosyst 18:580–591. https://doi.org/10.1002/aqc.884
Berzas NJJ, Rodriguez MRC, Moreno MJ (2009) Mercury speciation in the Valdeazogues River–La Serena reservoir system: influence of Almadén (Spain) historic mining activities. Sci Total Environ 407:72–82. https://doi.org/10.1016/j.scitotenv.2008.12.006
Bhat AA, Bhat NA (2014) Geochemical mapping of Kashmir Nappe and Karewa group of rocks covering parts of Anantnag, Baramulla and Srinagar districts, J&K in toposheet nos. 43K/13 and 43K/14. Geological Survey of India (Field Season: 2013–14). file:///C:/Users/AADIL%20HAMID/Downloads/GEOCHEMICAL_MAPPING_OF_KASHMIR_NAPPE_AND.pdf
Bhat SU, Pandit AK (2010a) Comparative ecology of freshwater springs of Kashmir Himalaya. Ph.D. Thesis University of Kashmir Srinagar
Bhat SU, Pandit AK (2010b) Limnochemistry of three fresh water springs of Kashmir Himalaya. Hydro Nepal- J Wat Ener Environ 7:54–59. https://doi.org/10.3126/hn.v7i0.4237
Bhat SU, Pandit AK (2018) Hydrochemical characteristics of some typical freshwater springs—a case study of Kashmir Valley Springs. Int J Water Res Arid Environ 7(1):90–100 https://psipw.org/attachments/article/407/8e.pdf
Bhat SU, Pandit AK (2020) Water quality assessment and monitoring of Kashmir Himalayan freshwater springs—a case study. J Aquat Ecosyst Manag Health. Accepted
Bhat SU, Pandit AK, Mudathir R (2010) Limnological investigation of three freshwater springs of Pulwama District-Kashmir Valley. Recent Res Sci Technol 2(2):88–94. https://updatepublishing.com/journal/index.php/rrst/article/view/381
Bhat SU, Mushtaq S, Qayoom U, Sabha I (2020) Water quality scenario of Kashmir Himalayan Springs—a case study of Baramulla District, Kashmir Valley. Water Air Soil Pollut. https://doi.org/10.1007/s11270-020-04796-4
Bhusan B, Raina KB, Maiti RP, Pathak SC (1972) Geological mapping of part of the Pir Panjal Range in Kulgam Tehsil, Anantnag District, Jammu and Kashmir State. Geological Survey of India (Field Season 1971–72)
Birch EL (2014) A review of climate change 2014: impacts, adaptation, and vulnerability and climate change 2014: mitigation of climate change. J Am Plan Assoc 80(2):184–185. https://doi.org/10.1080/01944363.2014.954464
Bremner JM (1965) Inorganic forms of nitrogen. In: Norman AG (ed) Methods of soil analysis, part 2, agronomy monograph no. 9. ASA and SSSA, Madison, pp 1179–1237. https://doi.org/10.2134/agronmonogr9.2.c33
Burek P, Satoh Y, Fischer G, Kahil, MT, Scherzer A, Tramberend S. et al (2016) Water futures and solution: fast track initiative (final report). IIASA working paper. Laxenburg, Austria, International Institute for Applied Systems Analysis (IIASA). http://pure.iiasa.ac.at/id/eprint/13008/
Cantonati M, Fureder L, Gerecke R, Juttner I, Cox EJ (2012) Crenic habitats, hotspots for freshwater biodiversity conservation: toward an understanding of their ecology. Freshw Sci 31:463–480. https://doi.org/10.1899/11-111.1
Cantonati M, Komarek J, Montejano G (2015) Cyanobacteria in ambient springs. Biodivers Conserv 24:865–888. https://doi.org/10.1007/s10531-015-0884-x
CGWB (Central Ground Water Board North Western Himalayan Region Ministry of Water Resources, Jammu) (2009) Dynamic ground water resources of Jammu and Kashmir (as on 31st March 2009). Case study from central Himalayas, India. Environmental Geology:572–578 http://cgwb.gov.in/Documents/Dynamic-GW-Resources-2009.pdf
Clayton JL (1998) Alkalinity generation in snowmelt and rain runoff during short distance flow over rock. Res. Pap. RMRS-RP-12. Ogden, UT: U.S. Department of Agriculture, Rocky Mountain Research Station. pp 7. https://doi.org/10.2737/RMRS-RP-12
Cole GA (1983) Text book of limnology, 3rd edn. C.V. Mosby, St. Louis
Constable M, Charlton M, Jensen F, McDonald K, Craig G, Taylor KW (2003) An ecological risk assessment of ammonia in the aquatic environment. Hum Ecol Risk Assess 9(2):527–548. https://doi.org/10.1080/713609921
Coward JMH, Waltham AC, Bowser RJ (1972) Karst springs in the Vale of Kashmir. J Hydrol 16:213–223. https://doi.org/10.1016/0022-1694(72)90053-4
Damania R, Desbureaux S, Rodella AS, Russ J, Zaveri E (2019) Quality unknown: the invisible water crisis. The World Bank, Washington, DC https://openknowledge.worldbank.org/handle/10986/32245
Devendra D, Shriram D, Atul K (2014) Analysis of ground water quality parameters: a review. Res J Eng Sci 3(5):26–31 http://www.isca.in/IJES/Archive/v3/i5/3.ISCA-RJEngS-2014-24.pdf
Dinka MO, Loiskandl W, Ndambuki JM (2015) Hydrochemical characterization of various surface water and groundwater resources available in Matahara areas, Fantalle Woreda of Oromiya region. J Hydrol Reg Stud 3:444–456. https://doi.org/10.1016/j.ejrh.2015.02.007
Durov SA (1948) Natural waters and graphic representation of their composition. Dokl Akad Nauk SSSR 59:87–90
Elhatip H (1997) The influence of karst features on environmental studies in Turkey. Environ Geol 31:27–33. https://doi.org/10.1007/s002540050160
EPA (Environmental Protection Agency) (1994) Reach file version 3.0 alpha release (RF3-Alpha) technical reference. EPA, Washington D.C
FAO (2011a) Proposed national standard for treated domestic wastewater reuse for irrigation. Special report no-4, Project No.: 2008.2162.9 Rome, United Nations—Food and Agricultural Organization (FAO). https://www.bgr.bund.de/EN/Themen/Wasser/Projekte/abgeschlossen/TZ/Libanon/spec_rep_4.pdf?__blob=publicationFile&v=4
FAO (2011b) Climate change, water and food security. Food and Agriculture Organization of the United Nations. http://www.fao.org/3/i2096e/i2096e.pdf
Fatchen TJ (2000) Mound springs management planning: management issues, strategies, and prescriptions for mound springs in far north South Australia. South Australian Department for Environment and Heritage, Adelaide
Gebrehiwot AB, Tadesse N, Jigar E (2011) Application of water quality index to assess suitability of groundwater quality for drinking purposes in Hantebet watershed, Tigray, Northern Ethiopia. ISABB Journal of Food and Agricultural Sciences 1 (1): 22–30 https://academicjournals.org/journal/ISABB-JFAS/article-full-text-pdf/51BA97E44852
Glazier DS (2014) Is metabolic rate a universal ‘pacemaker’ for biological processes? Search results. Biol Rev 90:377–407. https://doi.org/10.1111/brv.12115
Gleeson T, Wada Y, Bierkens MFP, van Beek LPH (2012) Water balance of global aquifers revealed by groundwater footprint. Nature 488(7410):197–200. https://doi.org/10.1038/nature11295
Goldman RC, Horne AJ (1983) Limnology. McGraw-Hill Book Company, New York
Grasby SE, Hutcheon I, Krouse HR (2000) The influence of water-rock interaction on the chemistry of thermal springs in Western Canada. Appl Geochem 15:439–454. https://doi.org/10.1016/S0883-2927(99)00066-9
Griebler C, Stein H, Kellermann C, Berkhoff S, Brielmann H, Schmidt SI, Selesi D, Steube C, Fuchs A, Hahn HJ (2010) Ecological assessment of groundwater ecosystems–vision or illusion? Ecol Eng 36:1174–1190. https://doi.org/10.1016/j.ecoleng.2010.01.010
Gupta A, Kulkarni H (2017) Inventory and revival of springs in Himalayas for water security. Report of the NITI Aayog Working Group as part of initiatives on Sustainable Development of Mountains of Indian Himalayan Region https://niti.gov.in/writereaddata/files/document_publication/doc1.pdf
Hameed A, Bhat SU, Sabha I, Lone SA (2018) Water quality monitoring of some freshwater springs in Hazratbal Tehsil, Srinagar, Kashmir Himalaya. J Himalayan Ecol Sustain Dev 13:61–74 https://www.researchgate.net/publication/331928217_Water_Quality_Monitoring_of_Some_Freshwater_Springs_in_Hazratbal_Tehsil_Srinagar_Kashmir_Himalaya
Hem JD (1992) Study and interpretation of chemical characteristics of natural water (3rd ed.), USGS water-supply paper, pp 2254 https://pubs.usgs.gov/wsp/wsp2254/pdf/wsp2254a.pdf
Herojeet RK, Rishi SM, Sidhu N (2013) Hydrochemical characterization, classification and evaluation of ground water regime in SirsaWatershed, Nalagarh Valley, Himachal Pradesh, India. Civil and Environmental Research 3(7):47–57 https://iiste.org/Journals/index.php/CER/article/view/6113
Hill T, Neal C (1997) Spatial and temporal variation in pH, alkalinity and conductivity in surface runoff and groundwater for the Upper River Severn catchment. Hydrol Earth Syst Sci 1(3):697–715. https://doi.org/10.5194/hess-1-697-1997
Hoffsten PO, Malmqvist B (2000) The macroinvertebrate fauna and hydrogeology of springs in central Sweden. Hydrobiologia 436:91–104. https://doi.org/10.1023/A:1026550207764
Hoseinzadeh E, Wei C, Chavoshi E, Faghih MA (2016) Groundwater quality and nitrate pollution modeling: an integrated study of contour mapping and geographic information system. Desalin Water Treat 57(52):24882–24893. https://doi.org/10.1080/19443994.2016.1150886
Howell JM, Coyne MS, Cornelius PL (1995) Faecal bacteria in agricultural waters of the blue grass region of Kentucky. J Environ Qual 24:411–419. https://doi.org/10.2134/jeq1995.00472425002400030003x
IPCC (2007) The physical science basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, p 996 https://www.ipcc.ch/site/assets/uploads/2018/05/ar4_wg1_full_report-1.pdf
IPCC (2014) Climate change 2014: synthesis report. In: Core Writing Team, Pachauri RK, Meyer LA (eds) Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, Switzerland, p 151 https://epic.awi.de/id/eprint/37530/1/IPCC_AR5_SYR_Final.pdf
Jeelani G (2005) Chemical quality of the spring waters of Anantnag, Kashmir. Geological Society of India 66(4):453–462 http://www.geosocindia.org/index.php/jgsi/article/view/82173
Jeelani G (2008) Hydrogeology of hard rock aquifer in Kashmir Valley: complexities and uncertainties. In: Ahmed S, Jayakumar R, Salih A (eds) Groundwater dynamics in hard rock aquifers. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-6540-8_19
Jeelani G (2010) Chemical and microbial contamination of Anantnag springs, Kashmir Valley. J Himalayan Ecol Sustain Dev 5:1–10 (offline)
Jeelani G, Shah AQ (2006) Geochemical characteristics of water and sediment from the Dal Lake, Kashmir Himalaya: constraints on weathering and anthropogenic activity. Environ Geol 50(1):12–23. https://doi.org/10.1007/s00254-005-0168-y
Jeelani G, Bhat NA, Shivanna K (2011) Geochemical characterization of surface water and spring water in SE Kashmir Valley, western Himalaya: implications to water–rock interaction. J Earth Syst Sci 120:921–932. https://doi.org/10.1007/s12040-011-0107-0
Jeelani G, Shah R, Hussain A (2014) Hydrogeochemical assessment of groundwater in Kashmir Valley, India. J Earth Syst Sci 123:1031–1043. https://doi.org/10.1007/s12040-014-0446-8
Joshi BK, Kothyari BP (2003) Chemistry of perennial springs of Bhetagad watershed: a case study from central Himalaya. Environ Geol 44:572–578. https://doi.org/10.1007/s00254-003-0793-2
Kazama F, Yoneyama M (2002) Nitrogen generation in the Yamanashi prefecture and its effects on the groundwater pollution. Environ Sci 15:293–298. https://doi.org/10.11353/sesj1988.15.293
Klimchouk A (1996) The dissolution and conversion of gypsum and anhydrite. Did you mean. Int J Speleol 25:3–4 https://pdfs.semanticscholar.org/2ebd/fdc0cc6e5dbaa5261b23530062b7f40d0d95.pdf
Kreamer DK, Stevens LE, Ledbetter JD (2014) Groundwater dependent ecosystems—science, challenges, and policy: In Adelana SM, (eds) Groundwater. Nova Science Publishers, Inc., Hauppauge, NY. pp. 205–230
Kumar K, Rawat DS, Joshi R (1996) Chemistry of spring water in Almora, Central Himalaya, India. Environ Geol 28(2):1–7. https://doi.org/10.1007/s002540050174
Kumar A, Sharma MP, Rai SP (2017) A novel approach for river health assessment of Chambal using fuzzy modeling, India. Desalin Water Treat 58:72–79. https://doi.org/10.5004/DWT.2017.0144
Kurian M (2017) The water-energy-food nexus trade-offs, thresholds and transdisciplinary approaches to sustainable development. Environ Sci Policy 68:97–106. https://doi.org/10.1016/j.envsci.2016.11.006
Kurwadkar S, Venkatramanan K (2013) Reconnaissance of groundwater quality impact. Water Environ Res 85:1700–1714. https://doi.org/10.2175/106143013X13698672322787
Langguth HR (1966) Die Grundwasserverhaltnisse Bereich des Velberter Sattels Rheinisches Schiefergeberge, der Minister fur Ernahrung. Landwirtschaft and Forsten, NRW, Duseldorf
Li M, Zhu X, Zhu F, Ren G, Cao G, Song L (2011) Application of modified zeolite for ammonium removal from drinking water. Desalination 271:295–300. https://doi.org/10.1016/j.desal.2010.12.047
Liu ZQ, Li HS, Wang J (2007) Seasonal, diurnal and storm-scale hydrochemical variations of typical epikarst springs in subtropical karst areas of SW China: soil CO2 and dilution effects. J Hydrol 337:207–223. https://doi.org/10.1016/j.jhydrol.2007.01.034
Lloyd JW, Heathcoat JA (1985) Natural inorganic chemistry in relation to groundwater. Clarendon Press, Oxford
Lone SA, Pandit AK, Bhat SU (2012) Dynamics of periphytic algae in some crenic habitats of district Anantnag, Kashmir. J Himalayan Ecol Sustain Dev 7:28–34 https://d1wqtxts1xzle7.cloudfront.net/35758414/periphyton_published.pdf?
Metcalf, Eddy (1979) Wastewater engineering: treatment, disposal, and reuse, 2nd edn. McGraw-Hill
Mishra S, Kumar A (2020) Estimation of physicochemical characteristics and associated metal contamination risk in river Narmada, India. Environ Eng Res 26(1). https://doi.org/10.4491/eer.2019.521
Moore RD, Richards G, Story A (2008) Electrical conductivity as an indicator of water chemistry and hydrologic process. Streamline Water Mangt Bull 2:25–29 https://pdfs.semanticscholar.org/d133/9c3f92cb6fda50b4b3f9a5b02df28bee9b72.pdf
Muangthong S, Shrestha S (2015) Assessment of surface water quality using multivariate statistical techniques: case study of the Nampong River and Songkhram River, Thailand. Environ Monit Assess 187(9). https://doi.org/10.1007/s10661-015-4774-1
Mujumdar PP, Tiwari VM (eds) (2019) Water futures of India: status of science and technology. IISc/INSA, India
Murhekar GH (2011) Determination of physico-chemical parameters of surface water samples in and round AkotCity. Int J Res Chem Environ 1(2):183–187 http://ijrce.org/download.php?file=18
Murtaza O, Romshoo SA, Rashid I, Shah W (2019) Geospatial assessment of groundwater quality in Udhampur District, Jammu and Kashmir, India. P Natl A Sci India A. https://doi.org/10.1007/s40010-019-00630-7
Nabhan GH (2008) Plant diversity influenced by indigenous management of freshwater springs: flora of Quitovac, Sonora, Mexico. In: Stevens LE, Meretsky VJ (eds) Aridland springs of North America: ecology and conservation. University of Arizona Press, Tucson, pp 244–267
Nathenson M, Thompson JM, White LD (2003) Slightly thermal springs and non-thermal springs at Mount Shasta, California: chemistry and recharge elevations. J Volcanol Geotherm Res 121:137–153. https://doi.org/10.1016/S0377-0273(02)00426-2
Nelson N (2008) Between the cracks: water law and springs conservation in Arizona. In: Aridland springs of North America: ecology and conservation. University of Arizona Press, Tucson, pp 318–331
Nikanorov AM, Brazhnikova LV (2012) Types and properties of water II: water chemical composition of rivers, lakes and wetlands. https://www.eolss.net/Sample-Chapters/C07/E2-03-04-02.pdf
Ochuko U, Thaddeus O, Oghenero OA, John EE (2014) A comparative assessment of water quality index (WQI) and suitability of river Ase for domestic water supply in urban and rural communities in Southern Nigeria. Int J Humanit Soc Sci 4(1):234–245 http://www.ijhssnet.com/journals/Vol_4_No_1_January_2014/27.pdf
Ojha P, Mandloi AK (2004) Diurnal variation of pH in freshwater fish culture pond. Ecol Environ Conserv 10:85–86 http://www.envirobiotechjournals.com/article_abstract.php?aid=3263&iid=118&jid=3
Oki AO, Akana TS (2016) Quality assessment of groundwater in Yenagoa, Niger Delta, Nigeria. Geosciences 6(1):1–12. https://doi.org/10.5923/j.geo.20160601.01
Paliy O, Shankar V (2016) Application of multivariate statistical techniques in microbial ecology. Mol Ecol 25(5):1032–1057. https://doi.org/10.1111/mec.13536
Paramsivam M, Srinivasan A (1981) Changes in algal flora due to pollution in Cavery River. Indian J Environ Health 23(3):222–238
Petric M (2010) Characterization, exploitation, and protection of the Malenscica karst spring, Slovenia. In: Kresic N, Stevanovic Z (eds) Groundwater hydrology of springs. Engineering, theory, management, and sustainability. Butterworth-Heinemann, Oxford, pp 428–441
Phillips OL, Aragao LE, Lewis SL, Fisher JB, Lloyd J, Lopez-González G (2009) Drought sensitivity of the Amazon rainforest. Science 323:1344–1347. https://doi.org/10.1126/science.1164033
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–928. https://doi.org/10.1029/TR025i006p00914
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analyses [M]. U.S. Geological Survey, groundwater note, vol 12, p 63
Pradhan B, Pirasteh S (2011) Hydro-chemical analysis of the ground water of the basaltic catchments: upper Bhatsai region, Maharashtra. Open Hydrol J 5:51–57. https://doi.org/10.2174/1874378101105010051
Prathumratana L, Sthiannopkao S, Kim KW (2008) The relationship of climatic and hydrological parameters to surface water quality in the lower Mekong River. Environ Int 34:860–866. https://doi.org/10.1016/j.envint.2007.10.011
Ramesh K, Jagadeeswari BP (2012) Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Periyakulam taluk of Theni district, Tamil Nadu. Int Res J Environ Sci 1(1):19–27 http://www.isca.in/IJENS/Archive/v1/i1/4.ISCA-IRJEvsS-2012-007.php
Raza M, Ahmad A, Mohammad A (1978) The valley of Kashmir, a geographical interpretation. Vikas Publication House, New Delhi
Risko J (2018) Sacred springs: perceptions of religion and water in village communities of Uttarakhand. Independent Study Project (ISP) Collection. pp. 2852 https://digitalcollections.sit.edu/isp_collection/2852
Rout C, Sharma A (2011) Assessment of drinking water quality: a case study of Ambala cantonment area, Haryana, India. Int J Environ Sci 2(2):933–945 https://scinapse.io/papers/1490641158
Sada DW, Sharpe SE (2004) Conference proceedings, spring-fed wetlands: important scientific and cultural resources of the intermountain region, 7–9 May 2002, Las Vegas, NV, DHS Publication No. 41210
Shrestha S, Kazama F (2007) Assessment of sur-face water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22:464–475. https://doi.org/10.1016/j.envsoft.2006.02.001
Singh DK, Singh AK (2002) Groundwater situation in India: problems and perspectives. Int J Water Resour Dev 18(4):563–580. https://doi.org/10.1080/0790062022000017400
Sirajudeen J, Mohamed M (2013) Statistical approach and assessment of physico-chemical status of ground water in near proximity of South Bank Canal, Tamil Nadu, India. Arch Appl Sci Res 5(2):25 https://www.scholarsresearchlibrary.com/articles/statistical-approach-and-assessment-of-physicochemical-status-of-ground-water-in-near-proximity-of-south-bank-canal-tami.pdf
Sogbanmu TO, Aitsegame SO, Otubanjo OA, Odiyo JO (2019) Drinking water quality and human health risk evaluations in rural and urban areas of Ibeju-Lekki and Epe local government areas, Lagos, Nigeria. Hum Ecol Risk Assess 26:1062–1075. https://doi.org/10.1080/10807039.2018.1554428
Springer AE, Stevens LE, Anderson DE, Parnell RA, Kreamer DK, Levin LA, Flora SP (2008) A comprehensive springs classification system. In: Aridland springs in North America: ecology and conservation. University of Arizona Press and Arizona–Sonora Desert Museum, Tucson, pp 49–75
Tambe S, Arrawatia ML, Bhutia NT, Swaroop B (2011) Rapid, cost effective and high resolution assessment of 350 climate-related vulnerability of rural communities of Sikkim Himalaya, India. Curr Sci 101(2):165–173 http://www.sikkimforest.gov.in/climate-change-in-sikkim/15-Chapter-Rapid,%20Cost%20Effective%20and%20High%20Resolution%20Assesment%20of%20Climate.pdf
Tiwari P (2000) Land-use changes in Himalaya and their impact on the plains ecosystem: need for sustainable land use. Land Use Policy 17(2):101–111. https://doi.org/10.1016/S0264-8377(00)00002-8
Tyagi RC, Mallikarjuna C, Rastogi SP (1964). Systematic geological mapping of Liddar and Sind Valleys, Anantnag District, Jammu and Kashmir State. Geological Survey of India. (Field Season 1963–64)
Tyagi S, Sharma B, Singh P, Dobhal R (2013) Water quality assessment in terms of water quality index. Am J Water Resour 1(3):34–38 http://article.journalofwaterresources.com/pdf/ajwr-1-3-3.pdf
UN (United Nations) (2012) Millennium development goals report 2012. New York, pp 1–72
UNEP (2016) UNEP Frontiers report: emerging issues of environmental concern. United Nations Environment Programme, Nairobi https://environmentlive.unep.org/media/docs/assessments/UNEP_Frontiers_2016_report_emerging_issues_of_environmental_concern.pdf
UNESCO (2012) The United Nations world water development report 4: managing water under uncertainty and risk. World Water Development Programme (WWAP). Paris, France http://www.unesco.org/new/fileadmin/MULTIMEDIA/HQ/SC/pdf/WWDR4%20Volume%201-Managing%20Water%20under%20Uncertainty%20and%20Risk.pdf
Unmack PJ, Minckley WL (2008) The demise of desert springs. In: Stevens LE, Meretsky VI (eds) Aridland springs in North America: ecology and conservation. University of Arizona Press, Tucson
Ustaoglu F, Tepe Y (2019) Water quality and sediment contamination assessment of Pazarsuyu Stream, Turkey using multivariate statistical methods and pollution indicators. Int Soil Water Conserv Res 7(1):47–56. https://doi.org/10.1016/j.iswcr.2018.09.001
Ustaoglu F, Tepe Y, Taş B (2020) Assessment of stream quality and health risk in a subtropical Turkey river system: a combined approach using statistical analysis and quality index. Ecol Indic 113:105815. https://doi.org/10.1016/j.ecolind.2019.105815
Van der Kamp G (1995) The hydrogeology of springs in relation to the biodiversity of spring fauna: a review. In Ferrington LC Jr (eds) Biodiversity of aquatic insects and other invertebrates in springs. Special publication no. 1 of the Journal of the Kansas Entomological Society 4–17
Verma P, Chandawat D, Gupta U, Solanki H (2012) Water quality analysis of an organically polluted lake by investigating different physical and chemical parameters. Int J Res Chem Environ 2(1):105–111 file:///C:/Users/AADIL%20HAMID/Downloads/Chandolalake.pdf
Wadia DN (1975) Geology of India. Tata McGraw Hill, New Delhi, p 560
Wegener T, Sivapalan M, Troch PA, McGlynn BL, Harman CJ, Gupta HV et al (2010) The future of hydrology: an evolving science for a changing world. Water Resour Res 46(5):W05301. https://doi.org/10.1029/2009WR008906
Wescoat JL Jr, Fletcher S, Novellino M (2016) National rural drinking water monitoring: progress and challenges with India’s IMIS database. Water Policy 18(4):1015–1032. https://doi.org/10.2166/wp.2016.158
Wetzel RG (2001) Structure and productivity of aquatic ecosystems. In: Limnology. Academic Press, San Diego, pp 132–133
Wetzel RG, Likens GE (2000) Limnological analyses, 3rd edn. Springer–Verlag, Inc., New York, p 429
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva https://www.indiawaterportal.org/sites/indiawaterportal.org/files/Updated_edition_of_guidelines_for_drinking_water_quality_World_Health_Organisation_2011.pdf
WWAP (United Nations World Water Assessment Programme) (2012) The United Nations world water development report 4: managing water under uncertainty and risk. UNESCO, Paris http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/wwdr/wwdr4-2012/
WWAP (United Nations World Water Assessment Programme) (2015) The United Nations world water development report. Water for a sustainable world. Paris, UNESCO http://www.unesco.org/new/en/natural-sciences/environment/water/wwap/wwdr/2015-water-for-a-sustainable-world/
WWDR (The United Nations World Water Development) (2019) The United Nations World Water Development report; leaving no one behind. https://unesdoc.unesco.org/ark:/48223/pf0000367306
Acknowledgments
The authors of this work would like to thank the Head Department of Environmental Science, University of Kashmir, for providing laboratory facilities. Dr. N.C. Mondal from the National Geophysical Research Institute (NGRI) Hyderabad is highly acknowledged for his support in preparation of the contour maps. This work was supported by the University of Kashmir through scholarship grant in favor of the first author. We would also like to thank wholeheartedly the four anonymous reviewers whose in-depth review, detailed, useful comments, and critical analysis have substantially improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lone, S.A., Bhat, S.U., Hamid, A. et al. Quality assessment of springs for drinking water in the Himalaya of South Kashmir, India. Environ Sci Pollut Res 28, 2279–2300 (2021). https://doi.org/10.1007/s11356-020-10513-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10513-9