Abstract
In this study, the application of novel biocarrier Orchis mascula plant for immobilization of non-adapted mixed cells biodegrade reactive azo dyes in aqueous solution was investigated via a sequential anaerobic-aerobic process. Three reactive azo dyes including red (RR2), blue (RB4), and yellow (RY15) were individually used as the target pollutants. Results revealed that the immobilized biocatalysts had a potential degrading efficiency, targeting the initial structure and the formed aromatic/aliphatic compounds, respectively. Complete decolorization (100%) of RR2, RB4, and RY15 was observed in the anaerobic phase with significant COD removal which has been enhanced during the subsequent aerobic phase. Results demonstrated that COD removals at 10, 20, and 40 mg/L were 100 ± 0.13%, 95 ± 0.05%, and 90 ± 0.03% for RR2; 90 ± 0.11%, 85 ± 0.9%, and 81 ± 0.18% for RB4; and 100 ± 0.08%, 92 ± 0.16%, and 86 ± 0.06% for RY15, respectively. Immobilized cells exhibited better performance compared with free cells, particularly for high dye concentration up to 100 mg/L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The application of reactive azo dyes, the most commonly used type of azo dyes, is linked to the discharge of a large amount of unfixed dyes. The effluent might contain as much as 50% of the initial dye load (Fitzgerald and Bishop 1995). A normal-sized textile mill consumes around 1.6 million liter of water every day, out of which 16% is used for dyeing and about 8% in printing (Chen et al. 2003). Bioremediation is a promising alternative or supplement for the current dye treatment processes. The wide diversity of microorganisms offers a spectrum of genetic resources for cleaning the environment. Many biotreatment approaches using encapsulation are on top of the list for having a promising degrading potential for pollutants in an immobilized matrix (Keskin et al. 2018). When bacteria are immobilized, they are usually trapped inside a material, often a gel matrix which inhibits their flow. Immobilizing bacteria increases bacterial density which can be reflected in an increased pollutant degradation rate (Chen et al. 2005). Biocarriers for microorganism immobilization must be non-toxic with a rough irregular surface. The matrix must be porous and hydrophilic to promote the proliferation and adherence. High concentration of chemical-degrading microorganisms colonizes the biocarriers; their attachment is promoted by the porous structure and the surface texture provided by the used biocarrier (Kuroda et al. 1988; Kindzierski et al. 1992; Durham et al. 1994).
The choice of the carrier for immobilization is the most important. It is of significant relevance to find an immobilized carrier with stable performance, good mass transfer, high intensity, long lifespan, and low price. Most of the reported immobilized materials belong to chemicals which can only be obtained through industrial processing so the costs are always high. The global attention has directed toward natural biocarriers which has the advantages of lower cost, easier obtainment, and no complicated experimental preparation at earlier stage, which can be widely used in production (Zhang et al. 2019; He et al. 2019). Organic natural carriers that can be used as support include alginate, carrageenan, agar, agarose, chitosan, and chitin. Alginates (polymers made of different proportions and sequences of mannuronic and guluronic acids extracted from brown algae) are the polymers of choice in most systems of immobilization because they are easy to handle; non-toxic to humans, the environment, and the entrapped microorganisms; legally safe for human use; available in large quantities; and inexpensive (Bayat et al. 2015). In the current work, the validity of using another natural organic carrier has been tested, and its efficiency in supporting the biodegradation system of three organic dyes has been investigated. Several studies have been reported on using free cells for biodegradation of reactive dyes. However, limited studies were performed for azo dyes biodegradation using immobilized cells by different types of biocarriers, mainly the conventional calcium/sodium alginate. Prasad and Aikat (2014) evaluated the potentiality of calcium-alginate-immobilized newly identified bacterium, isolated from the petroleum-polluted soil samples, Enterobacter sp. SXCR to decolorize textile dyes, in particular azo dye Congo red. Lade et al. (2015) examined the capacity of an immobilized consortium that is capable of growing in wheat bran for the decolorization of Congo red. Polyurethane foam was used for the immobilization of the used microbial consortium. Suganya and Revathi (2016) tested the validity of using polyacrylamide and sodium alginate for the immobilization of Pseudomonas putida and Bacillus licheniformis cells to decolorize the reactive dyes (RR 195, RO 72, RY 17, RB 36). Frindt et al. (2017) studied the immobilization of cells in polyurethane foamed carriers coated with activated carbon for biodegradation of sulfonated azo dyes, reactive orange 107. Hameed and Ismail (2018) investigated the decolorization and biodegradation of reactive red dye using non-adapted mixed cells immobilized in starch as a non-conventional biocarrier.
This study aimed to investigate the potentiality of using a novel biocarrier, Orchis mascula plant (commercially known as Salepi), for immobilization of mixed cells to decolorize and biodegrade three different reactive azo dyes.
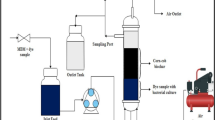
Materials and methods
Inoculum
The source of the mixed cells is a local plant in Baghdad, Iraq, which is specialized in sewage treatment. They were either immobilized or used directly as free cells, without any prior adaptation for the anaerobic/aerobic sequential treatment phases. The stock cultures were stored at 4 °C.
Mineral salt medium solutions
In the current study, two types of mineral salt medium were used (based on biotreatment conditions). The anaerobic mineral salt medium (MSM) solution was prepared according to Ghangrekar et al. (2005) by dissolving (in grams): MgSO4.7H2O, 0.2; CaCl2, 0.015; FeCl3.6H2O, 0.001; MnSO4.H2O, 0.02; NaH2PO4, 1.825; KH2PO4, 0.35; NaHCO3, 0.42, and various YE doses in liter distilled water. The aerobic MSM was prepared according to Marrot et al. (2006) by dissolving (in grams): NH4Cl, 0.2; K2HPO4, 0.029; KH2PO4, 0.023; MgSO4, 0.013; CaCl2, 0.007; and YE, 2.0. The MSM solutions were adjusted to pH 7, autoclaved for 20 min at 121 °C, and then, cooled at room temperature.
Azo dyes
Three commercial reactive azo dyes, reactive red (RR2), reactive blue (RB4), and reactive yellow (RR15), were alternatively used in this study. The three reactive dyes were obtained from the department of dying and printing at a local textile manufacturing plant, Baghdad. Chemical structure and specifications of these azo dyes are given in Table S1.
Biocarrier
The natural polymer Orchis mascula commercially known as Salepi (Sal) powder was used as a new type of biocarrier for cell immobilization. This natural polymeric biocarriers, as compared with synthetic polymers, are environmentally friendly and abundantly available but are characterized as having less stability in water (Stolarzewicz et al. 2011). In order to increase the mechanical stability of this natural polymer, it was cross-linked with polyvinyl alcohol (PVA).
Analytical methods
The concentration of the three reactive azo dyes (RR2, RB4, RY15) was determined using a spectrophotometer (type: Advanced Microprocessor UV-VIS Spectrophotometer Single-Beam LI-295) at 420, 540, and 580 nm, respectively. Residual chemical oxygen demand (COD) concentrations in aqueous samples were measured based on the procedure outlined in the standard method (APHA 2005) using the COD analyzer.
Free cell concentrations were measured as volatile suspended solid (VSS) measurement according to the procedure reported in the Standard Method (APHA, 2005). Counting of immobilized cells in each experiment involves destructing the sample and the removal of cells from the immobilized matrix. The procedure reported by Cruz et al. (2013) was followed in this study. The beads were dissolved in 4% NaHCO3 solution for 30 min, and the concentration of biomass within the beads was determined by the approach of volatile suspended solids. A scanning electron microscopy (SEM) test was done to compare the morphology of the bead surface and internal cells before and after biodegradation. The procedure of preparing the samples for analysis involved treating the gel beads with 2.5% glutaraldehyde for 3 h, and then dehydrated with 50–100% acetone for 30 min. For an enhancement of the produced image quality, gel beads were coated with gold by sputtering.
Immobilization protocol
Salepi-PVA matrix was prepared by dissolving 2 g of Salepi in 100 ml distilled water. PVA solution was heated to 60 °C to be completely dissolved. A solution of 3.5 ml of Salepi was prepared by gentle mixing for 30 min, and then added to the PVA solution and left at room temperature to cool down. The mixture was then inoculated with 5 ml of mixed cells and was well-mixed. The obtained solution was poured into sterile microplates and frozen for 12 h, and then thawed. Improving bead stability requires repeating that process. The samples were exposed to three to seven cycles of freezing for 8 h at − 20 °C and thawing for 4 h at 25 °C (Hassan and Peppas 2000).
Phytotoxicity test
Plant growth bioassays and seed germination are the most commonly used techniques for evaluating the phytotoxicity (Kapanen and Itavaara 2001). In this study, the phytotoxicity assays were conducted with Triticum aestivum at room temperature (Gomare and Govindwar 2009; Zhao et al. 2014). Inoculation of Triticum aestivum crop seeds occurred using four filter paper covered petri plates. Seeds’ sterilization was accomplished using 3% H2O2. They were then repeatedly washed using distilled water before their inoculation. Ten healthy seeds were placed in each of the four petri plates, and a daily 5-ml plant watering was carried out using tap water, untreated colored solution, the solution after the anaerobic treatment (first stage), and in order to determine the effect of aerobic treatment, the solution after the second stage (aerobic treatment) was also used.
Results and discussion
UV-vis spectra
The decolorization potential for RR2, RB4, and RY15 using mixed microbial cultures was initially detected in alternative anaerobic and aerobic conditions. The results demonstrated very limited color removal during the aerobic conditions (data not shown) in comparison with promising decolorization results in the anaerobic conditions. According to Dos Santos et al. (2007), this observation can be justified by the adverse effect of oxygen on the activity of azo reductase enzyme, not to mention that the oxygen molecule is a more effective electron acceptor than azo dyes. Figure 1 shows the UV-vis spectrum and absorbance peaks for each of the three reactive dyes, RR2, RB4, and RY15, presenting maximum values at 540, 580, and 420 nm, respectively. The maximum peaks are related to the chromophore group associated with each azo dye structure. After the occurence of complete decolorization at the anaerobic phase, the UV-scan curves displayed no peak in the visible region, evidently due to the breakage of the chromophore in all the three tested dyes, in contrary to the scan results of the dye-laden solutions. At 280 nm, a sharp peak can clearly be noticed as well. It has been presented by Franciscon et al. (2012) that a band range between 280 and 350 is consistent with a substituted benzene and naphthalene compounds. Therefore, the formation of aromatic compounds necessitates adding an aerobic phase following the anaerobic one to accomplish the oxidation of the formed amine compounds (Jonstrup et al. 2011). RR2 and RY15 showed approximately similar behavior which was reflected in the UV-scan after the anaerobic phase when the peak at 280 nm was shifted to a lower wavelength at 260 nm with a significantly lower absorbance value indicating the modification of the degradation products. The peak at 260 nm can be associated with the presence of phenolic compounds (Mielgo et al. 2001; Hsueh and Chen 2008). The UV-scan of RB4 at the aerobic phase showed a higher shift of the curve compared with that of the anaerobic curve. This could be attributed to a greater rate of additional transformation to the intermediate products resulted from their accumulation at a greater rate within the same wavelength range, a trend which has also been noticed by Franciscon et al. (2012).
Dominant mechanism for dye removal
The results presented in this section are clearly displayed by the UV-vis spectra for RR2, RB4, and RY15 at different operational conditions concerning the bacterial activity as given in Fig. 2. This figure presents the UV-scan for the reactive dye–loaded aqueous solutions, which were anaerobically biotreated by using alternatively (1) inactive mixed cells (autoclaved cells), and (2) active mixed cells. Both cases were compared with the UV-scan of the dyes in their aqueous solutions at initial conditions before exposing them to any biotreatment.
Results of the UV-vis spectra with a wavelength scan starting from 200 to 1100 nm exhibited peaks at 540, 580, and 420 nm in the visible region for RR2, RB4, and RY15, respectively, along with traces of detected amines in the UV region. As given in Fig. 2, the spectrum of each dye after the anaerobic biodegradation showed a disappearance of visible region peaks pointing to the cleavage of the azo bond as a result to the biodegradation process. It was also associated with a formation of a new peak at 280 nm indicating the destruction of the parent structure of the reactive azo dyes and the formation of a new aromatic structure (Franciscon et al. 2012). On the other hand, the results represented by the dye UV spectra strongly pointed out the extremely limited contribution of biosorption to the dye removal process. Cheng et al. (2012) observed similar results. In conclusion, the UV-vis spectra confirmed and assured that the dominant mechanism is inevitably biodegradation, most likely, the sole mechanism.
Effect of initial dye concentration on decolorization
The variation of initial concentrations of RR2, RB4 and RY15 from 10 to 20, and then up to 40 mg/L using free mixed cells, as shown in Fig. 3, resulted in the reduction of color removal rate but maintained the total complete removal efficiency intact for RR2 and RB4. The decolorization of both RR2 and RB4 was in 20, 30, and 40 h, in treating 10, 20, and 40 mg/l solutions, respectively. The previously mentioned findings could be reclined to the fact that higher dye concentration poses potentially higher toxic effect. Cui et al. (2014) showed similar results; a decolorization efficiency of 95% for methyl red treated with Klebsiella sp. did not change as the initial concentration increased from 200 to 800 mg/l, but it required longer treatment duration. However, the results were in disagreement with the findings outlined by Vaigan et al. (2009), revealing a noticeable reduction of reactive blue (Brill Blu) from 57 to 31% as the initial concentration increased from 20 to 40 mg/l. For RY15, the change in the initial concentration did not affect the decolorization rate. All the three initial concentrations 10, 20, and 40 mg/l were decolorized in 25 h. The result might be due to the difference in the structure of the used reactive dyes holding different toxic effects in respect with the change in their concentration.
Effect of inoculum size
The MSM was inoculated with 3%, 5%, 7%, and 10% (v/v) mixed cells in order to determine the contribution of inoculum size on the decolorization process of RR2, RB4, and RY15. Figure 4 clearly shows that for RR, and within 30 h, the use of 3% and 5% inoculum sizes resulted in decolorization efficiencies of 88% and 84% during the anaerobic treatment. The use of higher inoculum size (7%) not only resulted in a complete color RR removal but also reduced the required time to 20 h. That can be explained in terms of the availability of more active biomass targeting the degradation of the dye molecules. Inoculum size of 10% did not show a noticeable difference in decolorization process in comparison with that of 7%. Sani and Banerjee (1999) also present that higher dye removal can be attributed to a higher inoculum size up until 10% (v/v), when further increase in inoculum size of Kurthia sp. has not enhanced the decolorization efficiency. However, RB4 and RY15 exhibited similar trends in terms of decolorization rate and duration (Fig. 4). The decolorization rates of 94% and 100% were the results of using 7% and 10% inoculum sizes, respectively. Both 3% and 5% resulted in 83% and 82% decolorization with RB and RY after 25 h, respectively, with no further increase indicating insufficient culture concentration and necessitates the importance of increasing the sludge concentration.
Yeast extract concentration effect on decolorization process
At 10 mg/l, the effect of adding different YE concentrations using free cells on the decolorization of RR2, RB4, and RY15 can be depicted in Fig. 5. For RR2, upon an increase in YE concentration from 0.1 to 0.7 g/l and in 30 h, a remarkable increase in decolorization rate was observed. Similar trend was noticed with RB4, as the used concentration of YE increased from 0.1 to 1 g/l within 35 h. As a result of further increasing YE for both RR2 and RB4, the removal efficiency was unexpectedly reduced. This might be attributed to the saturation of the active enzymes at 0.7, and 1 g/l of YE, respectively. Imran et al. (2016) supported the previously mentioned explanation by reporting that a slower enzyme response can occur upon increasing the YE over a certain limit. Bacterial cultures might also favor consuming YE as a nitrogen source, at the expense of following the complex route of destruction the azo bonds in the dye, leading to a lower decolorization rate as a result of increasing YE upon a certain limit.
As for RY2, it has been noticed that an increase in YE over 1 g/l did not show any change in decolorization rate. According to Yatome et al. (1981) the difference between RR2, RB4, and RY15 regarding their response to YE might be influenced by the permeability of cell membrane to compounds of different molecular weights and intramolecular hydrogen bonds between the hydroxyl groups of azo dyes.
Effect of dye initial concentration on decolorization with free versus immobilized cells
Figures 6 and 7 show that an increase in the initial concentration of RR2 and RB4 using free mixed cells reduced the decolorization rate as the initial concentration increased leading to lower decolorization rate. However, Fig. 8 shows that RY15 decolorization rate was not affected by the concentration change. On the other hand, culture immobilization using, PVA-Sal as biocarriers extended the time duration for RR2, RB4, and RY15 decolorization up to 30, 50, and 60 h at initial concentrations of 10, 20, and 40 mg/l, respectively. For RR2, RB4, and RY15 decolorization, the activity of free cells ceased as the dyes’ concentration increased up to 100 mg/L, in contrary to the long-lasting activity of immobilized cells (data not shown). The used gel for immobilization showed a potential ability to host and protect the cells resulting in a complete decolorization of the three used reactive dyes. In comparison with free cells, the use of immobilized cells showed to a certain extent lower decolorization rate, by far due to mass transfer effect. The results are in concurrence with those reported by Saratale et al. (2011), where free Proteus vulgaris required about 5 h for complete decolorization of reactive blue 172, while PVA-immobilized beads achieved only 8% decolorization at the same period. However, the results in this study were in disagreement with the findings outlined by Cheng et al. (2012), when 90% decolorization was achieved using immobilized cells of Burkholderia vietnamiensis compared with 85% color removal by using free cells.
Phytotoxicity
The results of using the loaded solutions of RR2, RB4, and RY15 as an irrigation source for Triticum aestivum seeds are presented in Fig. 9 and Table 1. The germination rates were 30%, 20%, and 0%, which were remarkably low in comparison with 100% resulting by tap water. Plant watering with the anaerobically treated solutions of RR2, RB4, and RY15 revealed that the germination rate increased to 60%, 30%, and 40%, respectively.
The aerobic treatment showed a noticeable shift to the solution quality leading to a 100% germination rate for all the three reactive dyes. Plumule and radicle lengths are considered very important signs in determining the quality and level of toxicity of the irrigation water. For RR2, RB4, and RY15, applying the anaerobically treated solutions resulted in 1.32 and 0.60 cm, 0.68 and 0.50 cm, and 0.74 and 0.64 plumule and radicle lengths, respectively. These results are considerably propitious compared with 0.5 and 0.2 cm, 0.4 and 0.26 cm, and 0.31 and 0.37 cm of plumule and radicle lengths resulted from the dye-loaded solutions, respectively. For plumule and radicle lengths, in comparison with 1.1 and 0.5 cm, the use of the resulted solution after the aerobic treatment of RR solution carried out unprecedented results of 2.4 and 1.8 cm, respectively.
Those findings could be explained in terms of the mineral content of the MSM which aids plant growth. As for RB4 and RR2, plumule and radicle lengths of 1.06 and 0.51 cm and 1.10 and 0.80 cm showed similar results using tap water, indicating the potential effect of the suggested treatment.
Effect of dye initial concentration on COD removal
Figures 10, 11, and 12 present the profiles of COD and dye reduction by the sequential anaerobic and aerobic phases for RR2, RB4, and RY15, respectively, using the immobilized PVA-Sal beads. As can be noted from Table 2, the removal of COD was during the anaerobic phase, with an increase in the initial concentration from 10 to 40 mg/l; the anaerobic phase contribution to the COD removal increased for each reactive dye. The results could be explained by the possible formation of some aromatic amines that can be mineralized under anaerobic conditions. The higher the initial concentration, the higher availability of those amines, which can be utilized as the sole carbon source and sulfur source by bacterial cultures under anaerobic conditions (Nachiyar and Rajkumar 2004).
The aerobic phase shifted the COD removal for the three reactive dyes during a full treatment duration. Most of the readily biodegradable compounds are consumed during the anaerobic phase which is reflected in relatively higher COD removal rate compared with the aerobic phase. The same trend has been published by Kapdan and Oztekin (2006), who tested the validity of the sequential anaerobic-aerobic reactors for Remazol Red containing wastewater. The anaerobic phase contribution to COD removal was 50%, while the aerobic phase enhanced the overall reduction to reach 80%. The applicability of (Sal) as an efficient biocarrier is supported by two of our previous works (Hameed and Ismail 2019; Hameed and Ismail 2018) showing comparable results in terms of total COD removal percentage using the commonly used carrier (sodium alginate) after sequential anaerobic and aerobic treatment stages. In the first research, the total COD removal percentage of reactive blue azo dye by using alginate-immobilized mixed cultures were 88, 87, and 87% with 10, 20, and 40 mg/l of the dye, while the second work resulted in COD removal percentage of 96, 93, and 82% with 10, 20, and 40 mg/l of reactive red, using alginate-immobilized mixed cells after the sequential treatment stages.
Immobilized cell recycling
Bead recycling is a crucial advantage that is offered by immobilized cells over free cells.
The use of PVA-Sal immobilized cells with the three tested reactive dyes, as shown in Fig. 13, did not result in living cell weariness. During the second cycle in RR2 treatment, insignificant reduction in COD removal occurred during the anaerobic phase.
This might be explained in terms of the relatively partial pore blockage inside the beads resulting from the dye biodegradation which limits the access of dye particles toward the active sites. Daassi et al. (2014) suggested that the slightly reduced activity might be attributed to bead washing causing enzyme leakage. Recycled beads with RB4 were capable of maintaining their activity during the anaerobic phase, while it showed higher COD removal during the aerobic phase which resulted in complete mineralization. For RY15, COD removal rate reduced during the anaerobic phase, whereas a noticeable increase in the COD removal occurred throughout the aerobic phase. That might be due to the cultures’ capacity to produce aerobic enzymes (which are mainly responsible for the cleavage of aromatic ring). Yaşar et al. (2012) have mentioned that the aerobic enzyme (catechol 2, 3-dioxygenase (C23DO)), which has been extensively used as an indicator for aromatic amine aerobic biodegradation, has increased along with an increase in the aerobic period, which is the case when the same cultures used as recycled beads during the subsequent treatment cycles.
Scanning electron microscopy
The characterization of the PVA-Sal structure and morphology along with the development and distribution of the microbial population was accomplished by SEM. Figure S1 (a) shows the wrinkled texture of the bead, a heliciform embossment with sunken troughs. Magnified images (b) and (c) clearly show the honeycomb divergent of the bead. According to Fei et al. (2015), the beads’ significantly high level of mechanical capacity is attributed to the honeycomb structure, one of the most remarkably fixed constructions.
This magnified image shows the highly porous structure with pore diameters that are significantly larger than walls’ thickness. The importance of the highly porous structure lays in allowing prominent bacterial growth and facilitating substrate diffusion. Images (d), (e), and (f) show the immobilized culture growth within bead pores, evidencing that the immobilization technique promoted the bioactivity of the cultures, allowing them to propagate and grow.
Conclusion
The cross-linking of Salepi, the non-conventional, natural, and non-toxic polymer, with polyvinyl alcohol (PVA), was done to enhance the strength of the matrix along with the restriction of bacterial release. The anaerobic treatment using PVA-Sal resulted in COD removal of 38%, 44%, and 74% for RR2; 47%, 50%, and 75% for RB4; and 55%, 59%, and 78% for RY15, for 10, 20, and 40 mg/L in 30, 50, and 60 h. The aerobic treatment using PVA-Sal shifted the COD removal to a total of 100%, 95%, and 90% for RR2; 90%, 85%, and 81% for RB4; and 100%, 92%, and 86% for RY15, for 10, 20, and 40 mg/L of initial dye concentration in 70, 90, and 100 h of total treatment duration. The immobilized cells exhibited better biodegradation performance, during both the anaerobic/aerobic phases in response to an increase in the initial concentration of the three reactive dyes under the study, up to 100 mg/L, unlike the free cells which were inhibited, correspondingly. The recyclability of PVA-Sal with RR2, RB4, and RY15 with the anaerobic and aerobic phases separately showed results with a high approximation in the second cycle to those of the first cycle, indicating the validity of the recycling approach.
References
American Public Health Association (APHA), (2005) Standard methods for examination of water and wastewater. American Public Health Association Inc., Washington DC 20th Edition
Bayat Z, Hassanshahian M, Cappello S (2015) Immobilization of microbes for bioremediation of crude oil polluted environments: a mini review. Open Microbial J 9:48–54
Chen KC, Wu JY, Liou DJ, Huang SCJ (2003) Decolorization of the textile dyes by newly isolated bacterial strains. J Biotechnol 101:57–68
Chen W, Mulchandani A, Deshusses MA (2005) Environmental biotechnology: challenges and opportunities for chemical engineers. AICHE J 51:690–695
Cheng Y, Lin H, Chen Z, Megharaj M, Naidu R (2012) Biodegradation of crystal violet using Burkholderia vietnamiensis C09V immobilized on PVA–sodium alginate–kaolin gel beads. Ecotox Environ Safe 83:108–114
Cruz I, Bashan Y, Carmona G, Bashan L (2013) Biological deterioration of alginate beads containing immobilized microalgae and bacteria during tertiary wastewater treatment. Appl Microbiol Biotechnol 97:9847–9858
Cui D, Li G, Zhao M, Han S (2014) Decolourization of azo dyes by a newly isolated Klebsiella sp. strain Y3, and effects of various factors on biodegradation. Biotechnol Biotec Eq 28:478–486
Daassi D, Rodriguez-Couto S, Nasri M, Mechichi T (2014) Biodegradation of textile dyes by immobilized laccase from Coriolopsis gallica into Ca-alginate beads. Int Biodeterior Biodegrad 90:71–78
Dos Santos AB, Cervantes FJ, van Lier JB (2007) Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour Technol 98:2369–2385
Durham DR, Marshall LC, Miller JG, Chmurny AB (1994) Characterization of inorganic biocarriers that moderate system upsets during fixed–film biotreatment processes. Appl Environ Microbiol 60:3329–3335
Fei L, Wen-juan M, Xue L, Xiao-yu W, Zhi-hua X, Yao-yu Z, Guang-ming Z (2015) Characterization of Microcystis Aeruginosa immobilized in complex of PVA and sodium alginate and its application on phosphorous removal in wastewater. J Cent South Univ 22:95–102
Fitzgerald SW, Bishop PL (1995) Two-stage anaerobic/aerobic treatment of sulfonated azo dyes. J Environ Sci Health 30:1251–1276
Franciscon, E., Grossman, M.J., Paschoal, J.A.R., Reyes, F.G.R., Durrant, L.R., (2012) Decolorization and biodegradation of reactive sulfonated azo dyes by a newly isolated Brevibacterium sp. strain VN-15. Springerplus. 1, 37
Frindt B, Mattusch J, Reemtsma T, Griesbeek AG, Rehorek A (2017) Multidimensional monitoring of anaerobic/aerobic azo dye based wastewater treatments by hyphenated UPLC–ICP–MS/ESI–Q–TOF–MS techniques. Environ. Sci. Pollut. Res. Int. 24m:10929–10938
Ghangrekar MM, Joshi SG, Asolekar SR (2005) Characteristics of sludge developed under different loading conditions during UASB reactor start-up. Water Res 39:1123–1133
Gomare SS, Govindwar SP (2009) Brevibacillus laterosporus MTCC 2298: a potential azo dye degrader. J Appl Microbiol 106:993–1004
Hameed BB, Ismail ZZ (2018) Decolorization, biodegradation and detoxification of reactive red azo dye using non-adapted immobilized mixed cells. Biochem Eng J 137:71–77
Hameed BB, Ismail ZZ (2019) Decolorization, biodegradation and detoxification of reactive blue azo dye using immobilized mixed cells. J Eng 25:53–66
Hassan CM, Peppas NA (2000) Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 33:2472–2479
He, J., Zhang, W., Ren, X., Xing, L., Chen, S., Wang, C., (2019) Preparation of different activated sludge immobilized carriers and their organic wastewater treatment performance by microbial community. Env. Eng, Sci. 36, No 5
Hsueh CC, Chen BY (2008) Exploring effects of chemical structure on azo dye decolorization characteristics by pseudomonas luteola. J Hazard Mater 154:703–710
Imran M, Arshad M, Negm F, Khalid A, Shaharoon B, Hussain S, Nadeem SM, Crowley DE (2016) Yeast extract promotes decolorization of azo dyes by stimulating azo reductase activity in Shewanella sp. strainIFN4. Ecotoxicol Environ Saf 124:42–49
Jonstrup M, Kumar N, Murto M, Mattiasson B (2011) Sequential anaerobic–aerobic treatment of azo dyes: decolourisation and amine degradability. Desalination. 280:339–346
Kapanen A, Itavaara M (2001) Ecotoxicity tests for compost applications. Ecotoxicol Environ Saf 49:1–16
Kapdan KI, Oztekin R (2006) The effect of hydraulic residence time and initial COD concentration on color and COD removal performance of the anaerobic–aerobic SBR system. J Hazard Mater 136:896–901
Keskin NOS, Celebioglu A, Sarioglu OF, Uyar T, Tekinay T (2018) Encapsulation of living bacteria in electrospun cyclodextrin ultrathin fibers for bioremediation of heavy metals and reactive dye from wastewater. Colloid Surface B 161:169–176
Kindzierski WB, Gray MR, Fedorak PM, Hrudey SE (1992) Activated carbon and synthetic resins as support material for methanogenic phenol–degrading consortia—comparison of surface characteristics and initial colonization. Water Environ Res 64:766–775
Kuroda M, Yuzawa M, Sakakibara Y, Okamra M (1988) Methanogenic bacteria adhered to solid supports. Water Res 22:653–656
Lade H, Kadam A, Paul D, Govindwar S (2015) A low–cost wheat bran medium for biodegradation of the benzidine–based carcinogenic dye trypan blue using a microbial consortium. Int J Environ Res Public Health 12:3480–3505
Marrot B, Martinez A, Moulin P, Roche N (2006) Biodegradation of high phenol concentration by activated sludge in an immersed membrane bioreactor. Biochem Eng J 30:174–183
Mielgo I, Moreira MT, Feijoo G, Lema JM (2001) A packed-bed fungal bioreactor for continuous decolourisation of azo-dyes (Orange II). J Biotechnol 89:99–106
Nachiyar CV, Rajkumar GS (2004) Mechanism of navitan fast blue S5R degradation by pseudomonas aeruginosa. Chemosphere. 57:165–169
Prasad SS, Aikat K (2014) Study of bio–degradation and bio–decolourization of azo dye by Enterobacter sp. SXCR. Environ Technol 35:956–965
Sani RK, Banerjee UC (1999) Decolorization of triphenylmethane dyes and textile and dye-stuff effluent by Kurthia sp. Enzym Microb Technol 24:433–437
Saratale GD, Saratale RG, Chang JS, Govindwar SP (2011) Fixed–bed decolorization of reactive blue 172 by Proteus vulgaris NCIM–2027 immobilized on Luffa cylindrica sponge. Int Biodeterior Biodegrad 65:494–503
Stolarzewicz I, Bialecka-Florjañczyk E, Majewska E, Krzyczkowska J (2011) Immobilization of yeast on polymeric supports. Chem Biochem Eng Quart 25:135–144
Suganya K, Revathi K (2016) Decolorization of reactive dyes by immobilized bacterial cells from textile effluents. Int J Curr Microbiol Appl Sci 5:528–532
Vaigan AA, Moghaddam MRA, Hashemi H (2009) Effect of dye concentration on sequencing batch reactor performance. J Environ Health Sci Eng 6:11–16
Yaşar S, Cirik K, Cinar O (2012) The effect of cyclic anaerobic–aerobic conditions on biodegradation of azo dyes. Bioprocess Biosyst Eng 35:449–457
Yatome C, Ogawa T, Koda D, Ikeda E (1981) Biodegradability of azo and triphenylmethane dyes by pseudomonas pseudomallei 13NA. J Soc Dyes Colour 97:166–169
Zhang W, Ren X, He J, Zhang Q, Qiu C, Fan B (2019) Application of natural mixed bacteria immobilized carriers to different kinds of organic wastewater treatment and microbial community comparison. J Hazard Mater 377:113–123
Zhao M, Sun PF, Du LN, Wang G, Jia XM, Zhao YH (2014) Biodegradation of methyl red by Bacillus sp strain UN2: decolorization capacity, metabolites characterization, and enzyme analysis. Environ Sci Pollut Res 21:6136–6145
Acknowledgments
The technical support provided by the staff of the State Company for Textile Industry, Ministry of Industry in Iraq is greatly appreciated by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1743 kb)
Rights and permissions
About this article
Cite this article
Hameed, B.B., Ismail, Z.Z. New application of Orchis mascula as a biocarrier for immobilization of mixed cells for biodegradation and detoxification of reactive azo dyes. Environ Sci Pollut Res 27, 38732–38744 (2020). https://doi.org/10.1007/s11356-020-09984-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09984-7