Abstract
A large number of iron ore tailings (IOTs) are produced in steel industry, posing threat to the environment during its storage and disposal. To effectively reutilize Fe in IOTs, we propose a comprehensive utilization scheme: (1) most Fe in IOTs is extracted by concentrated hydrochloric acid to form FeCl3 flocculants; (2) after separation from the FeCl3 flocculants, a small amount of Fe is absorbed on the residue solids, which is further washed out to synthesize micron Fe3O4 as magnetic seeds. Results show that the as-synthetic FeCl3 flocculants meet the product standard for FeCl3 flocculants in China (GB/T 4482-2018) after a series of treatments including rotary evaporation, neutralization, and dilution and have comparable performance with commercial polyaluminum chloride (PAC) and polyaluminum ferric chloride (PAFC). Moreover, the addition of synthetic superparamagnetic Fe3O4 (as magnetic seeds) doubled the flocculation rate compared with as-synthetic FeCl3 flocculants alone. Finally, the reutilization of Fe in IOTs can create a direct economic value of ¥ 1.27/kg IOTs, and produce 745 g high-silicon residues for further reutilization, which indicates that our comprehensive utilization scheme is of great application potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The steel industry plays an important role in developing countries such as China. Despite its benefits, large quantities of iron ore tailings (IOTs) are generated as byproducts during the processing of steel (Yao et al. 2015). In China, the total accumulation of IOTs has exceeded 7.5 billion tons (Chen et al. 2012), and the annual increment is about 500 million tons (Tang et al. 2019a). Large amounts of IOTs are piled up in tailing dams or landfilled in situ, the problems of which attract wide attention. Tailing dams have high risk of dam break under poor management or extreme weather conditions (Fontes et al. 2019). For example, a break of IOT dam in Anshan (in Liaoning Province, China) caused 13 casualties in 2007 (Yao et al. 2016). The landfill of IOTs would cause damages to surrounding soil and groundwater and impact flora and fauna, leading to long-term deterioration of the environment (Wong 1981; Wu et al. 2019). Therefore, comprehensive utilization of IOTs is required.
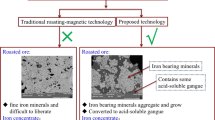
The easiest and the most wide utilization of IOTs is as a raw material of building materials, such as replacing the fine aggregate materials in cement concrete (Ghosh et al. 2011; Zhao et al. 2014). However, this approach has low economic value. Previous studies showed that iron (Fe) content of IOTs is in average of 8–12 wt%, occasionally as high as 27 wt% (Ajaka 2009; Tang et al. 2019a). Due to such high Fe amounts, separation and reutilization of Fe is a promising way for IOTs recycling. Reductive roasting and magnetic separation are commonly used to improve the Fe content of IOTs and recycled as iron ores. However, the procedure of reductive roasting requires 800 °C, which consumes a lot of energy. In addition, recycled iron ores still requires a series of treatments when utilized in steel and chemical industry (Li et al. 2014). Therefore, an economic method with low-energy consumption is required for comprehensive utilization of IOTs.
Herein, we propose a comprehensive utilization scheme of IOTs. Firstly, concentrated hydrochloric acid (HCl) is used to extract Fe in IOTs, and then the solution is separated from the residue solids to prepare FeCl3 flocculants. Due to the incomplete separation, a small amount of Fe3+ remains on the residue solids. Thus, secondly, this part of Fe is washed out by deionized water and then used to synthesize micron Fe3O4 as magnetic seeds for magnetic flocculation with as-synthetic FeCl3 flocculants (Chen et al. 2020; Tang et al. 2019b). Finally, the residue solids with high silicon amount can be used to produce high-economic-value glass ceramics (Chen et al. 2014; Wang et al. 2015).
In this paper, acid extraction, rotary evaporation, neutralization, and dilution were conducted stepwise to produce FeCl3 flocculants meeting the product standard in China (GB/T 4482-2018). Then, the synthetic flocculants were systematically assessed by jar test, optical microscope, and Fourier transform infrared spectroscopy (FTIR). Furthermore, Fe2+–Fe3+ co-precipitation method was used to synthesize magnetic seeds (micron Fe3O4). The characterization of synthetic magnetic seeds was investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), and vibrating sample magnetometer (VSM). The residue solids were also analyzed by XRD, SEM, X-ray fluorescence (XRF), and Brunner–Emmet–Teller (BET) to show its feasibility to produce glass ceramics. Finally, economic benefits of IOTs’ comprehensive utilization were analyzed to show its application prospect.
Materials and methods
Materials
Chemicals
All chemicals used in the experiments were of analytical grade except kaolin. Hydrochloric acid (HCl), sodium hydroxide (NaOH), calcium oxide (CaO), ferrous sulfate heptahydrate (FeSO4·7H2O), ammonium hydroxide (NH3·H2O), and kaolin were obtained from Sinopharm Chemical Reagent Co., Ltd, China. For comparison, three commercially available flocculants, polyaluminum chloride (PAC), polyferric sulfate (PFS), and polyaluminum ferric chloride (PAFC), were obtained from Xianglingde chemical company (Nanjing, China).
Iron ore tailings
IOTs used in this study were collected from the tailing dams of Fengshuigou in Anshan, Liaoning Province, China. The collected samples were dried naturally and stored in sample bags. XRD, SEM, XRF, and BET measurements were used to determine the crystal structure, composition, and surface areas of the IOTs, respectively (details could be found in Supplementary Information).
The XRD pattern for IOTs is shown in Fig. S1. The main phase of IOTs is quartz (SiO2, JCPDS: 46-1045) (Karamberi et al. 2007). Besides, hematite (Fe2O3, JCPDS: 33-0664) (Batin and Popescu 2011) and clay minerals such as cordierite (JCPDS: 84-1219) (Zhang and Li 2018) and chlorite-serpentine (JCPDS: 52-1044) (Tang et al. 2019a) are also found in IOTs. Based on XRF characterization (Table S1), the main chemical components of IOTs are SiO2 (70%) and Fe2O3 (23%). In addition, IOTs contain small amounts of Al2O3 (2.60%), MgO (1.60%), K2O (0.5%), and Na2O (0.31%), which is attributed to the clay minerals. This is also confirmed by the results of SEM-EDS mapping (Fig. S2). In addition, the total Fe content of IOTs is 16.3% (according to the chemical methods GB/T6730.5-2007), the moisture content is 0.250%, and the surface area is 2.45 m2/g (Table S2). Details of the particle size distribution is shown in Table S3.
IOT reutilization procedure
The reutilization experiments of Fe in IOTs were primarily divided into two processes: flocculant preparation and magnetic seed synthesis. In the flocculant preparation process, concentrated hydrochloric acid (HCl) was used to extract iron from IOTs, and then the extracted solution was rotary evaporated, neutralized, and diluted stepwise to prepare FeCl3 flocculants. The magnetic seed synthesis process was conducted to further recycle Fe absorbed on the extraction residues. FeCl3 was soaked and washed away from the residues and then co-precipitated with FeSO4 and NH3·H2O to form Fe3O4 magnetic seeds. After that, the synthetic flocculants and magnetic seeds were used together in a magnetic flocculation system. Details of this comprehensive reutilization flow chart are presented in Fig. 1.
Preparation of FeCl3 flocculants
As shown in Fig. 1, preparation of FeCl3 flocculants comprised the following three steps: Step 1 was to add a predetermined amount of IOTs and HCl to a round-bottom flask with a reflux condensation device and react at 20 °C, 40 °C, 60 °C, 80 °C, and the boiling temperature, respectively. Thus, most Fe in the IOTs could be extracted into solution. The effects of reaction temperature (°C), liquid-to-solid ratio (mL/g) between HCl solution and IOTs, initial HCl content (w/w), and reaction time (min) on Fe3+ content and recovery rate of iron were analyzed. Then, the extracted solution (mainly comprised of FeCl3 and HCl) was separated from the residual solids. Thus, in step 2, rotary evaporation of the solution was conducted to increase Fe content, reduce HCl content, and recycle excess HCl. The effects of temperature and time on Fe3+ and HCl content in the solution were determined. Step 3 was to add CaO to further reduce the free acid to less than 0.4%, as required by the product standard for FeCl3 flocculants in China (GB/T 4482-2018), and then dilute the solution to reduce the Fe3+ concentration to product concentration (to increase the yield and profitability). Fourier transform infrared spectroscopy (FTIR) spectra of FeCl3 solution after each step were recorded (details could be found in Supplementary Information).
Preparation of Fe3O4 magnetic seeds
After acid extraction and solid-liquid separation in step 1 in “Preparation of FeCl3 flocculants,” a small amount of Fe3+ is still absorbed on the residual solids. This part of Fe could be further utilized. Thus, the separated solids were soaked and washed with deionized water at a ratio of 0.5 mL/g, and then solids were again separated from liquids. The residual Fe3+ on the SiO2 would be secondly extracted into the washed solution and further used to synthesize Fe3O4 magnetic seeds. The synthesis of Fe3O4 was based on a co-precipitation method (Zhang et al. 2011) using FeSO4·7H2O with a molar ratio of Fe3+/Fe2+ of 2:1 and NH3·H2O under the protection of nitrogen. Finally, the Fe3O4 magnetic seeds were dried at 105 °C. The crystal state and morphology of these seeds were characterized by XRD and SEM, and magnetization curves were determined using a vibrating sample magnetometer (VSM) (details could be found in Supplementary Information).
IOTs utilization effects
To evaluate the practical effects of the synthetic flocculants and Fe3O4 magnetic seeds prepared from IOTs, both traditional and magnetic flocculation experiments were performed with the synthetic flocculants alone and in combination with magnetic seeds, respectively. The effect of reusing Fe3O4 magnetic seeds was also studied.
Traditional flocculation experiments
One liter high-turbidity wastewater was prepared with 0.5 g kaolin powder (Ma et al. 2018), aged for 24 h, with a final turbidity of 750.0 ± 84.6 NTU. A series of flocculation experiments were carried out in a jar experiment using a flocculating and agitating machine. The procedure was set as follows: a predetermined amount of synthetic flocculants was added to the high-turbidity wastewater, and then the mixture was stirred at 350 rpm for 2.5 min, at 60 rpm for 8 min and settling for 20 min, step by step. The turbidity of the supernatant (collected at one-half the height of the liquid) was measured after 20 min of settling to determine the optimum flocculant dose.
The effect of pH on flocculation was evaluated by adjusting the pH value of high-turbidity wastewater with NaOH and HCl. Furthermore, the flocculation effect of synthetic flocculants was compared with three commercial flocculants, namely, polyaluminum chloride (PAC), polyferric sulfate (PFS), and polyaluminum ferric chloride (PAFC), at the optimal dosage and pH.
Magnetic flocculation experiments
The synthetic flocculants and magnetic seeds were added at desired amounts into synthetic wastewater, with three-stage dosing process: firstly, magnetic seeds were added and stirred at 350 rpm for 2 min; secondly, the synthesized flocculants were added and stirred at 350 rpm for 2.5 min; and thirdly, the stirring speed was reduced to 60 rpm for 8 min. Finally, a strong magnet was placed at the bottom of the beaker, and the solution was allowed to settle for 5 min to measure its residual turbidity at one-half the height of the liquid. The flocculation effects in the presence and absence of magnetic seeds were compared at the optimum dose and different settling time. In addition, images of flocs generated with and without magnetic seeds were obtained using an optical microscope (Olympus BX53).
Recycling procedure
The reusability of magnetic seeds was studied in five consecutive recycle experiments. After each cycle, magnetic seeds were collected by magnetic separation, washed three times with deionized water, and dried at 105 °C for 30 min for the next cycle.
Results and discussion
Fe extraction and flocculant preparation
Acid extraction of Fe
As one of the main components of IOTs, Fe2O3 reacts quickly with concentrated HCl and can be extracted from IOTs to form FeCl3. To determine the optimal extraction conditions, the effects of temperature, liquid-to-solid (L/S) ratio between IOTs and HCl solution, initial HCl content, and reaction time were assessed.
In order to study the influence of reaction temperature on acid extraction, Fe3+ content and iron recovery rate were tested at different temperature (from 20 °C to boiling temperature of extracted solution), while the other three factors were considered as invariant parameters (a L/S ratio of 1 g/ml, an initial HCl content of 31% (w/w), and a reaction time of 40 min). As shown in Fig. 2a, the iron recovery rate and Fe3+ content in the extracted solution increase gradually from 20 to 80 °C and sharply from 80 °C to boiling temperature. At boiling temperature, the Fe3+ content and iron recovery rate reach the maximum of 11.0% and 75.2%, respectively. This indicates high temperature is beneficial to the reaction between H+ and hematite in IOTs (Lavasani et al. 2019).
The liquid-to-solid (L/S) ratio has a considerable effect on Fe3+ content and iron recovery rate. To study the effect of L/S ratio, HCl acid content was kept constant at 31% (w/w), the extraction temperature was kept constant at boiling temperature, and reaction time was kept constant of 40 min. As shown in Fig. 2b, the iron recovery rate gradually increases while the Fe3+ content gradually decreases with an increase in L/S ratio. Higher L/S ratio means larger HCl acid dosage, which leads to more effective reaction between HCl acid and hematite and higher iron recovery rate. However, the increase of the extracted Fe amount is less than the increase of the volume. Thus, the Fe3+ content decreases with the increase of L/S ratio. In consideration of both Fe3+ content and iron recovery rate, 1 mL HCl acid/1 g IOTs is the optimum L/S ratio.
In addition, the influence of reaction time of IOTs and HCl acid was examined within 10 to 60 min at boiling temperature, a L/S ratio of 1 mL/g, and an initial HCl content of 31% (Fig. 2c). The increase of Fe3+ content and iron recovery rate are significant in the first 40 min, but unobvious in the last 20 min. In consideration of the energy consumption, 40 min is the optimum reaction time.
In order to study the effect of HCl acid concentration, the initial content of HCl acid was set as 18%, 26%, 31%, and 37% (w/w) and other parameters were constant with a L/S ratio of 1 mL/g, a reaction time of 40 min, and a reaction temperature of boiling temperature. With the increase of initial content of HCl acid from 18 to 31% (w/w), Fe3+ content significantly increases from 6.30 to 11.0%, while iron recovery rate increases from 39.7 to 75.2% and residual H+ content increases from 7.14 to 12.3% (Fig. 2d). Then, the increment retards when initial content of HCl acid increases from 31 to 37%. Therefore, 31% (w/w) was determined to be the optimal initial HCl content.
In conclusion, it is optimum to use initial HCl content of 31% (w/w) with an L/S ratio of 1 mL/g to extract iron for 40 min at boiling temperature. Under these conditions, the iron recovery rate was 75.2% and the Fe3+ content was 11.0% (w/w).
Rotary evaporation
After the acid extraction by HCl under optimal conditions, the Fe content (11.0%) was less than the requirement of FeCl3 flocculant standard in China (≥ 14.0%, GB/T 4482-2018). In addition, the residual HCl content in the extracted solution was 12.3%, much more than the requirement (≤ 0.4%). Thus, rotary evaporation was used to concentrate FeCl3 solution and simultaneously reduce and recycle HCl in extracted solution.
Figure 3 shows the effects of rotary evaporation temperature and time on HCl and Fe3+ content. When the rotary evaporation duration is fixed to 10 min, Fe3+ content gradually increases from 11.1 to 16.7% with the increase of evaporation temperature from 20 to 60 °C, while the HCl content gradually decreases from 12.3 to 5.53%. The further increase of temperature (from 60 to 70 °C) has little effect on the HCl content. This is probably because HCl and H2O form an azeotrope when the temperature reaches 60 °C, making the HCl/H2O ratio in vapor the same as that in solution (Kalla et al. 2019; Othmer and Eyck 1949). For the consideration of energy conservation, 60 °C is selected as the optimal temperature.
When the rotary evaporation temperature is 60 °C, the effect of reaction time is evaluated (Fig. 3b). With the increase of reaction time from 5 to 20 min, HCl content of solution decreases and Fe3+ content increases. However, when the evaporation time is more than 10 min, Fe partially precipitates. The precipitation of Fe is not conducive for subsequent treatment. Therefore, 10 min is considered the optimum reaction time for rotary evaporation.
In conclusion, the optimal temperature and time for rotary evaporation should be set at 60 °C and 10 min, respectively. Under these conditions, Fe3+ content in the FeCl3 solution increases to 16.7% and the HCl content decreases to 5.53%. Based on product standard of China (Fe3+ content ≥ 14% and HCl content ≤ 0.4%), further treatment of the solution is required.
Neutralization and dilution
Neutralization was conducted to further reduce the HCl content in the FeCl3 solution. The acidity of the mixture was gradually neutralized by adding CaO (30.3 g/1000 g solution), and the basicity of the mixture increased gradually. Then, moderate amounts of water was added to the FeCl3 solution with Fe3+ content decreasing to 14.2%, to maximize economic benefits.
Characterization of FeCl3 flocculants
FTIR spectra of the FeCl3 solutions after acid extraction, rotary evaporation, and neutralization are presented in Fig. 4a to identify possible chemical bands of flocculants. Two peaks at 3390 and 1636 cm−1 are assigned to the stretching vibration of –OH group and bending vibration of –OH group in the water molecule, respectively, indicating FeCl3 solutions after each step may contain structural and adsorbed water (Jia et al. 2017; Wang et al. 2017). A characteristic absorbing peak at 600 cm–1 is assigned to the stretching vibration of Fe–OH (Chen et al. 2015; Ying et al. 2007; Zhang et al. 2015). Particularly, small absorbing peaks at 1075 and 878 cm−1 are existed in FeCl3 solutions after acid extraction, which is the bending vibration of Fe–O–Fe or Fe–OH–Fe (Chen et al. 2015; Ying et al. 2007). This may be attributed to the incomplete hydrolysis of iron during the acid extraction. When the extraction solution is rotary evaporated, the peaks at 1075 and 878 cm−1 vanish, so does to the FeCl3 solutions after neutralization, indicating that the Fe–O–Fe and Fe–OH–Fe structures are further hydrolyzed during the procedure of rotary evaporation (Wang et al. 2017). Moreover, the continuous reinforcement of spectral absorption of Fe–OH at 600 cm–1 after each step also confirms the deduction of Fe morphologic variation. Therefore, the final products are FeCl3 flocculants.
In addition, Fe3+ and HCl contents of the final products were determined to be 14.2 ± 0.1% and ≤ 0.10%, respectively, which met the product standard of China (GB/T 4482-2018, Fe3+ content ≥ 14% and HCl content ≤ 0.4%). Details of the Fe3+ and HCl content after each step could be found in Table S4. Furthermore, heavy metals (Zn, As, Pb, Hg, Cd, Cr) in the synthetic flocculants also met the standards (Table S4), meaning that the prepared FeCl3 flocculants could be used for wastewater treatment. Besides, 2.04% Al was found in the FeCl3 flocculants, which may participate in flocculation. The FeCl3 flocculants prepared from IOTs is hereafter referred as syn-FeCl3.
Flocculation effect of syn-FeCl3
To evaluate the flocculation effects of syn-FeCl3, the ability to purify high turbidity wastewater with different dosages and pH values was evaluated, and its performance was compared with three commercially available flocculants: polyaluminum chloride (PAC), polyferric sulfate (PFS), and polyaluminum ferric chloride (PAFC).
The effect of syn-FeCl3 dosage on turbidity after flocculation process was evaluated at a raw water pH value of 8.3 through jar testing (Fig. 4b). With the increase of syn-FeCl3 doses from 0 to 46 mg/L, the residual turbidity of wastewater decreases sharply from 750.0 NTU to 3.61 NTU. Further decrease in turbidity is insignificant with the increase of the syn-FeCl3 doses from 46 to 93 mg/L, and thus, the optimal syn-FeCl3 dosage was 46 mg/L.
The effect of pH of raw wastewater at the optimum syn-FeCl3 dosage was assessed, and the results are shown in Fig. 4c. In general, when the pH ranged between 4.3 and 10.3, the residual turbidity shows little change within the range of 3.41–7.37 NTU. This indicates syn-FeCl3 is effective in a wide range of pH values.
Interestingly, with an increase in pH, the flocculation efficiency firstly decreases from pH 5.3 to 7.3, then suddenly increases at pH 8.3, and decreases again in the pH range between 8.3 and 10.3. The reason for this trend may be related to the change of form and charge of Fe under different pH. From pH 4.3 to 8.0, the iron form slowly changes from Fe3+ to Fe(OH)2+ and then to Fe(OH)3 with a reduction of positive charge. In the pH range of 8.0–8.5, Fe(OH)3 precipitated, and then it changes to negative ion as Fe(OH)4– at a higher pH (Ching et al. 1994; Duan and Gregory 2003; Li et al. 2016). Meanwhile, kaolin in this system is negative charged in the pH range 4.3–10.3 due to its low isoelectric point (pH 2.3) (Teh et al. 2009). Therefore, the flocculation efficiency decreases from 5.3 to 7.3 due to the reduction of positive charge of iron; at pH 8.3, Fe(OH)3 precipitation promotes flocs settling which shows a sudden increase of flocculation efficiency; in the pH range of 8.3–10.3, the flocculation efficiency decreases again because of the increase of negative charge of iron and kaolin. Thus, syn-FeCl3 shows greater flocculation at pH 4.3, 5.3, and 8.3. For the convenience of experiments, 8.3 (the pH of raw water) was chosen to be the pH condition to investigate other parameters.
In addition, the flocculation effects of syn-FeCl3 and other three commercial flocculants were compared at pH 8.3 (Fig. 4d). After flocculation, the residual turbidity of wastewater with syn-FeCl3 is 3.61 NTU, which is lower than that of wastewater treated by polyferric sulfate (PFS, 11.2 NTU), and similar to that of wastewater flocculated by polyaluminum ferric chloride (PAFC) and polyaluminum chloride (PAC) of 2.41 and 2.92 NTU, respectively. This means that the flocculation ability of syn-FeCl3 prepared from IOTs is comparable to that of commercial PAFC and PAC, and better than that of PFS.
Characterization of magnetic seeds
Some Fe3+ absorbed on the residual solids after acid extraction and separation, reducing the SiO2 grade and impeding subsequent recycling of SiO2. Thus, soaking and washing were used to clean SiO2 and simultaneously generate a dilute Fe3+ solution (33.9 g/L). The dilute Fe3+ solution was further utilized to synthesize Fe3O4 magnetic seeds by co-precipitation with FeSO4·7H2O in NH3·H2O solution. The main mineral phase, magnetic characteristics, and morphology of magnetic seeds were studied by X-ray diffraction (XRD), vibrating sample magnetometer (VSM), and scanning electron microscopy (SEM). The XRD pattern (Fig. 5a) of synthetic magnetic seeds shows six characteristic diffraction peaks at 2θ = 30.095, 35.422, 43.052, 53.391, 56.942, and 62.515°, which correspond to (220), (311), (400), (422), (511), and (440) lattice planes of magnetite (JCPDS: 19-0629) (Chen et al. 2009). In addition, no crystal phase of other substances is found, indicating that the prepared seeds are Fe3O4 magnetic particles and has high purity.
The magnetization curves of Fe3O4 particles show that magnetization increases with an increase in external magnetic field intensity, and the saturation magnetization of synthetic Fe3O4 magnetic particles is 58.82 emu/g (Fig. 5b). In addition, the hysteresis loop is a single curve passing through the origin, and no coercivity and remanent magnetization appeared during this process, indicating that the sample is superparamagnetized. In the magnetic flocculation, the superparamagnetism of Fe3O4 seeds is beneficial for homogeneous distribution on flocs in the absence of magnetic field, while fast flocs settle in the presence of magnetic field. In addition, the superparamagnetism is also conducive to the recycle and reuse of Fe3O4 seeds (Ma et al. 2018; Omidvar-Hosseini and Moeinpour 2016; Wang et al. 2012).
SEM images of Fe3O4 magnetic particles are shown in Fig. 5c and d. The shape of synthetic Fe3O4 is irregular (Fig. 5c), and the particle size is about 1–5 μm. Zeng et al. (Zeng et al. 2015) showed that Fe3O4 can achieve better magnetic flocculation effect when its particle size is in the range of 1–10 μm. The enlarged image (Fig. 5d) shows that Fe3O4 particles are agglomerated by many nanometer-grade grains.
Magnetic flocculation effects of magnetic seeds
Magnetic flocculation is a separation method based on magnetic particles. By adding magnetic seeds, non-magnetic flocs become magnetized, and then under an external magnetic field, the sedimentation and separation of magnetized flocs can be accelerated to remove pollutants more effectively (Qiu et al. 2017). It means that, compared with traditional flocculation using only flocculants, the sedimentation rate of magnetic flocculation is faster.
The effects of magnetic seeds at different doses on flocculation were examined when the settling time was 5 min. As shown in Fig. 6a, when the dose of magnetic seeds increases from 0 to 300 mg/L, the residual turbidity decreases from 8.59 to 3.82 NTU. Subsequently, with an increase in dose of magnetic seeds from 300 to 500 mg/L, the residual turbidity of wastewater increases. When the dose of magnetic seeds is less than 300 mg/L, some of the flocs are not converted into magnetic flocs because of the lack of magnetic seeds. At higher doses (> 300 mg/L), excessive magnetic seeds cause damages to flocs, also reducing the magnetic flocculation effect (Chen et al. 2016; Qiu et al. 2017). Therefore, 300 mg/L is selected as the optimum magnetic seed dose in this experiment.
Effects of magnetic seeds on flocculation performance (pH = 8.3, T = 25 ± 2 °C). a Flocculation performance under different dosage of magnetic seeds with syn-FeCl3 as a flocculants (46 mg/L). b The residual turbidity of flocculation without magnetic seeds and of flocculation with magnetic seeds in five consecutive cycles. Floc images in the c absence and d presence of magnetic seeds under an optical microscope. Error bars in a and b represent standard deviations from triplicate experiments (n = 3)
In addition, the flocculation of syn-FeCl3 with and without the addition of magnetic seeds was compared under optimal flocculation conditions, where the doses of syn-FeCl3 and magnetic seeds were 46 mg/L and 300 mg/L, respectively. Turbidity was measured by settling in the presence of a magnetic field at different times (1, 2, 3, 4, 5, 10, 15, and 20 min). As shown in Fig. 6b, the turbidity of wastewater with magnetic seeds rapidly decreases with an increase in settling time, and the residual turbidity reaches 3.82 NUT at 5 min. After that, with an increase in settling time, the residual turbidity decreases very little. Therefore, 5 min is regarded as the optimum settling time of magnetic flocculation. During flocculation without magnetic seeds, residual turbidity also decreases. However, it is slower than the flocculation with magnetic seeds, and residual turbidity reaches 3.96 NTU after 10 min. This shows that the addition of magnetic seeds accelerates the sedimentation of flocs and shortens the settling time of flocculation by half. (Detailed images of the flocculation settling process are shown in Fig. S3). In addition, flocs formed in the absence and presence of magnetic seeds are shown in Fig 6c and d respectively to illustrate the morphology of floc and magnetic seeds. When magnetic seeds were added, it was obvious that many Fe3O4 magnetic particles were evenly distributed among the flocs, which help the magnetic flocs form and accelerated settling of nonmagnetic contaminants in the presence of a magnetic field (Qiu et al. 2017).
Recycling of magnetic seeds
One of the remarkable merits of Fe3O4 magnetic seeds is their ability to be easily recycled and reused after flocculation (Tang et al. 2019b). In this study, reuse of Fe3O4 seeds was investigated, and the results are shown in Fig. 6b.
When the magnetic seeds were reused, the performance of magnetic flocculation was slightly worse than the first use. This is due to that partial contaminants bound on the magnetic seeds during each time of recycling. Moreover, recycled Fe3O4 seeds were agglomerated during the heat to 105 °C, decreasing the dispersibility of seeds in flocs (Tang et al. 2019b; Wang et al. 2013). Therefore, the recycled Fe3O4 seeds performed worse than when they were first used. However, despite the reduction in the magnetic flocculation efficiency, magnetic flocculation with recycled magnetic seeds still performed much better than traditional flocculation without magnetic seeds. When the settling time for magnetic flocculation was 5 min, the residual turbidity in five consecutive cycles was 3.82, 4.71, 6.24, 5.76, and 4.94 NTU respectively, all of which were lower than the residual turbidity of 8.59 NTU without magnetic seeds. In summary, Fe3O4 seeds can be recycled for subsequent flocculation. After five recycles, the seeds still showed satisfactory removal with water washing.
Characterization of residue
After the recovery and utilization of iron, the physical properties of IOT residues were characterized by XRD, XRF, SEM, and BET. As shown in Fig. S1, the main mineral phase of residue is quartz and that a small amount of hematite and pargasite (JCPDS: 85-2161) are also present. The XRF results (Table S1) also show that the main components of IOT residues were SiO2, representing 92% of residues. Fe2O3 content decreased from 23 to 4.6%, which indicated that most of the iron was extracted in the IOT reutilization process. At the same time, the content of other metals reduced to less than 1%, showing great application potential to produce glass-ceramics (Ye et al. 2015). In addition, the BET results (Table S2) show that the surface area of IOT residues was 3.35 m2/g, slightly larger than that of IOTs, which may be attributed to the extraction of iron and other metals. Therefore, the SiO2 grade and the purity of IOT residue were greatly improved, increasing its availability, such as the application for glass-ceramics.
Mass balance and cost-benefit analyses of Fe comprehensive reutilization
As shown in Fig.1, Fe in IOTs flows into three products: 75.2% flows into the Fe flocculants, 8.6% flows into the Fe3O4 magnetic seeds, and 12.8% remains in the high-silicon residues. Thus, the total recovery rate of Fe is 96.6%. In addition, Si mainly remains in the residues, and its total recovery rate is 97.8%. (Details of the calculation of mass balance could be found in Supplementary Information.)
A simplified cost-benefit analyses based on Chinese conditions are calculated in Supplementary Information. As shown in Table S5, to reutilize 1 kg IOTs, the direct costs of chemicals are ¥ 0.23 and the energy costs are less than ¥ 0.39, except for equipment, labor, and site-use costs. Meanwhile, 1 kg IOTs is able to produce 863 g FeCl3 flocculants (¥ 1.60), 29 g Fe3O4 magnetic seeds (¥ 0.29) and also 745 g high-silicon residue (which could be further used to produce glass-ceramics). In addition, the 25.4% HCl acid collected in the fraction of rotary evaporation is recycled and reused in the acid extraction process (Fig. 1) and further reduces the cost. Therefore, regardless of the equipment, labor, and site-use costs, the comprehensive reutilization of IOTs can create an economic value of ¥ 1.27/kg IOTs, which is of great application potential.
Conclusions
The present study proposed a comprehensive reutilization procedure of iron in IOTs and verified its technical and economic feasibility. Specifically:
-
1.
Syn-FeCl3 flocculants produced after acid extraction, rotary evaporation, neutralization, and dilution step by step met the product standard of China (GB/T 4482-2018), showing comparable performance with commercial PAC and PAFC, and better than commercial PFS.
-
2.
Fe remaining on the surface of residues was washed out to synthesize Fe3O4 magnetic seeds, which shortened the settling time of flocculation by half and showed satisfactory efficiency after five recycles.
-
3.
According to the calculation of Fe mass balance, the total recovery rate of Fe is 96.6%. In addition, regardless of the equipment, labor, and site-use costs, the comprehensive reutilization of IOTs can create an economic value of ¥ 1.27/kg IOTs, which is of great application potential.
References
Ajaka E (2009) Recovering fine iron minerals from Itakpe iron ore process tailing. ARPN Journal of Engineering and Applied Sciences 4(9):17–28
Batin MN, Popescu V (2011) Synthesis and characterization of iron oxide powders. Power Metall 11(3-4):201–205
Chen J, Huang K, Liu S (2009) Hydrothermal preparation of octadecahedron Fe3O4 thin film for use in an electrochemical supercapacitor. Electrochim Acta 55(1):1–5
Chen H, Wu YW, Zhang H, Li ZC (2014) Phase, magnetism and thermal conductivity of glass ceramics from iron ore tailings. J Cent South Univ 21(9):3456–3462
Chen W, Zheng H, Zhai J, Wang Y, Xue W, Tang X, Zhang Z, Sun Y (2015) Characterization and coagulation–flocculation performance of a composite coagulant: poly-ferric-aluminum-silicate-sulfate. Desalination and Water Treatment 56(7):1776–1786
Chen Y, Luo M, Cai W (2016) Influence of operating parameters on the performance of magnetic seeding flocculation. Environ Sci Pollut Res Int 23(3):2873–2881
Chen H, Shen WG, Shan L, Xiong CB, Su YQ, Liu B, Rao JL (2012) Situation of discharge and comprehensive utilization of iron tailings domestic and abroad. Hunningtu(Concrete) (2):88–92
Chen T, Wang Q, Lyu J, Bai P, Guo X (2020) Boron removal and reclamation by magnetic magnetite (Fe3O4) nanoparticle: an adsorption and isotopic separation study. Sep Purif Technol 231:115930
Ching HW, Tanaka TS, Elimelech M (1994) Dynamics of coagulation of kaolin particles with ferric chloride. Water Res 28(3):559–569
Duan J, Gregory J (2003) Coagulation by hydrolysing metal salts. Adv Colloid Interface Sci 100-102:475–502
Fontes WC, Franco de Carvalho JM, Andrade LCR, Segadães AM, Peixoto RAF (2019) Assessment of the use potential of iron ore tailings in the manufacture of ceramic tiles: from tailings-dams to “brown porcelain”. Constr Build Mater 206:111–121
Ghosh I, Mondal AK, Singh N, Das SK (2011) Evaluation of iron ore tailings for the production of building materials. Industrial Ceramics 31(2)
Jia D, Li M, Liu G, Wu P, Yang J, Li Y, Zhong S, Xu W (2017) Effect of basicity and sodium ions on stability of polymeric ferric sulfate as coagulants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 512:111–117
Kalla S, Upadhyaya S, Singh K, Baghel R (2019) Experimental and mathematical study of air gap membrane distillation for aqueous HCl azeotropic separation. J Chem Technol Biotechnol 94(1):63–78
Karamberi A, Orkopoulos K, Moutsatsou A (2007) Synthesis of glass-ceramics using glass cullet and vitrified industrial by-products. J Eur Ceram Soc 27(2-3):629–636
Lavasani SH, Azimi E, Sarvi MN (2019) The dissolution of Fe in HCl from the ilmenite concentrate; evaluating the effect of operating parameters and mutual interactions. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science 50(6):2586–2595
Li Y-Y, Liang J-L, Liu Y-S (2014) Reclaiming iron powder by magnetization and magnetic separation from iron ore tailings. Environmental Engineering 1(32):634-638+660 (in Chinese)
Li YY, Liang JL, He X, Zhang L, Liu YS (2016) Kinetics and mechanisms of amorphous FeS2 induced Cr (VI) reduction. J Hazard Mater 320:216–225
Ma J, Fu X, Jiang L, Zhu G, Shi J (2018) Magnetic flocculants synthesized by Fe3O4 coated with cationic polyacrylamide for high turbid water flocculation. Environ Sci Pollut Res Int 25(26):25955–25966
Omidvar-Hosseini F, Moeinpour F (2016) Removal of Pb(II) from aqueous solutions using acacia nilotica seed shell ash supported Ni0.5Zn0.5Fe2O4 magnetic nanoparticles. J Water Reuse Desalin 6(4):562–573
Othmer DF, Eyck EHT (1949) Correlating azeotropic data. Industrial & Engineering Chemistry 41(12):2897–2900
Qiu Y, Xiao X, Ye Z, Guan Z, Sun S, Ren J, Yan P (2017) Research on magnetic separation for complex nickel deep removal and magnetic seed recycling. Environ Sci Pollut Res Int 24(10):9294–9304
Tang C, Li K, Ni W, Fan D (2019a) Recovering iron from iron ore tailings and preparing concrete composite admixtures. Minerals 9(4):232
Tang J, Wang J, Jia H, Wen H, Li J, Liu W, Li J (2019b) The investigation on Fe3O4 magnetic flocculation for high efficiency treatment of oily micro-polluted water. J Environ Manage 244:399–407
Teh EJ, Leong YK, Liu Y, Fourie AB, Fahey M (2009) Differences in the rheology and surface chemistry of kaolin clay slurries: the source of the variations. Chem Eng Sci 64(17):3817–3825
Wang Y, Zou B, Gao T, Wu X, Lou S, Zhou S (2012) Synthesis of orange-like Fe3O4/PPy composite microspheres and their excellent Cr(VI) ion removal properties. J Mater Chem 22(18):9034
Wang J, Wj L, Jia H, Zhang H (2013) Effects of recycling flocculation membrane filtration on drinking water treatment. J Water Supply Res Technol Aqua 62(7):433–441
Wang CL, Zheng YC, Liu SC, Yang J (2015) Microstructure and mechanical properties of glass-ceramics prepared with coal gangue and iron ore tailings. Rare Metal Materials and Engineering 44:234–238
Wang B, Shui Y, Liu P, He M (2017) Preparation, characterization and flocculation performance of the inorganic–organic composite coagulant polyferric chloride and polydimethyldiallylammonium chloride. J Chem Technol Biotechnol 92(4):884–892
Wong MH (1981) Environmental impacts of iron ore tailings—the case of Tolo Harbour, Hong Kong. Environ Manage 5(2):135–145
Wu S, Liu Y, Southam G, Robertson L, Chiu TH, Cross AT, Dixon KW, Stevens JC, Zhong H, Chan TS, Lu YJ, Huang L (2019) Geochemical and mineralogical constraints in iron ore tailings limit soil formation for direct phytostabilization. Sci Total Environ 651(1):192–202
Yao R, Liao S, Dai C, Liu Y, Chen X, Zheng F (2015) Preparation and characterization of novel glass–ceramic tile with microwave absorption properties from iron ore tailings. J Magn Magn Mater 378:367–375
Yao R, Liao S, Chen X, Tang G, Wang G, Zheng F (2016) Effects of ZnO and NiO on material properties of microwave absorptive glass-ceramic tile derived from iron ore tailings. Ceram Int 42(7):8179–8189
Ye C, He F, Shu H, Qi H, Zhang Q, Song P, Xie J (2015) Preparation and properties of sintered glass–ceramics containing Au–Cu tailing waste. Materials & Design 86:782–787
Ying FU, Shuili YU, Yanzhen YU, Qiu L, Hui B (2007) Reaction mode between Si and Fe and evaluation of optimal species in poly-silicic-ferric coagulant. Journal of Environmental Sciences-china 19(6):678–688
Zeng H, Li YR, Xu FY, Jiang H, Zhang WM (2015) Feasibility of turbidity removal by high-gradient superconducting magnetic separation. Environ Technol 36(19):2495–2501
Zhang C, Li S (2018) Utilization of iron ore tailing for the synthesis of zeolite A by hydrothermal method. Journal of Material Cycles and Waste Management 20(3):1605–1614
Zhang H, Zhao Z, Xu X, Li L (2011) Study on industrial wastewater treatment using superconducting magnetic separation. Cryogenics 51(6):225–228
Zhang Y, Li S, Wang X, Ma X, Wang W, Li X (2015) Synthesis, purification and characterization of polyaluminum ferric chloride (PAFC) with high (Al + Fe)b content. Sep Purif Technol 146:311–316
Zhao SJ, Fan JJ, Sun W (2014) Utilization of iron ore tailings as fine aggregate in ultra-high performance concrete. Constr Build Mater 50:540–548
Funding
This work was supported by the National Natural Science Foundation of China (No. 41907107), Natural Science Foundation Project of Chongqing Science and Technology Commission (cstc2018jcyjAX0320 and cstc2018jcyjAX0785), and Large Instruments Open Foundation of Chongqing University (202003150089).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 13630 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Tian, X., He, X. et al. Comprehensive reutilization of iron in iron ore tailings: preparation and characterization of magnetic flocculants. Environ Sci Pollut Res 27, 37011–37021 (2020). https://doi.org/10.1007/s11356-020-09742-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09742-9