Abstract
In the present study, magnetic seeding flocculation was applied to remove copper (200 mg/L) and turbidity (180 mg/L) from simulated microetch copper waste. Fe3O4 particles (40 to 1600 mesh) were used as magnetic seeds. Poly-aluminum chloride (PAC) and anionic polyacrylamide (PAM) were added as coagulant and flocculant, respectively. The effect of operating factors, such as the dosages of the coagulant and flocculant, initial pH of the wastewater, and dosage and size of the magnetic seeds, on copper and turbidity removal was systematically investigated. In addition, settling speed, floc-size distribution, and volume of sludge were measured with and without the addition of magnetic seeds to compare the efficiency of magnetic seeding to that of traditional flocculation. The results indicated that the highest settling speed, the largest floc size, and the smallest volume of sludge were obtained simultaneously when the dosage and size of magnetic seeds were 2.0 g/L and 300–400 mesh, respectively. High removal efficiencies of 98.53 and 94.72 % for copper and turbidity, respectively, were also achieved under this condition; values that are 4.11 and 0.61 % higher, respectively, than those found in traditional flocculation. The high performance might be attributed to efficient collision of particles and slightly moderate vortex centrifugal force of inertia among the magnetic seeds, which could produce larger magnetic flocs with lower moisture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of batteries, mining, and electroplating industries, large amounts of copper, lead, and mercury waste are released into the natural environment, which has become one of the most serious environmental problems (Agrawal et al. 2009; Ma et al. 2014; Paulino et al. 2006). Recently, there has been a growing concern about the removal of toxic heavy metals from aqueous environments with increased efficiency (Soares and Soares 2012; Wan et al. 2014; Xu et al. 2013).
Traditional approaches of removing heavy metals from industrial effluents include chemical precipitation (Mirbagheri and Hosseini 2003), ion exchange (Doula 2009), adsorption (Kongsuwan et al. 2009; Li et al. 2010a; Yalçın et al. 2012), membrane filtration (Samper et al. 2009), flotation (Polat and Erdogan 2007; Yuan et al. 2008), electrochemical treatment (Heidmann and Calmano 2008), etc., but these methods still present important drawbacks, and heavy metal removal is not satisfactory. For example, chemical precipitation generates large volumes of relatively low-density sludge, which can induce dewatering and disposal problems (Fu and Wang 2011). Electrochemical treatment involves a relatively large capital investment and an expensive electricity supply, limiting the scaling up of this process (Paulino et al. 2006). Compared with these traditional methods, flocculation improves the removal efficiency of wastewater particulates by charge neutralization and enmeshment (Heredia and Martin 2009; Ma 2011); however, it has a large footprint and slow sedimentation velocity, which restricts the utilization of flocculation technology. Thus, it is urgent to improve treatment efficiencies during the flocculation process (Zagklis et al. 2012).
Magnetic seeding removes non-magnetic water pollutants (Anand et al. 1985; Broomberg et al. 1999; Karapinar 2003). It was also proved that heavy metals can be removed from wastewater by means of hydroxide flocs (Nah et al. 2006; Terashima et al. 1986). The magnetic seeding technique has been used for heavy metal ion removal from wastewater since the 1970s (de Latour 1976; Feng et al. 2000; Franzreb and Watson 2001). Li et al. (2010b) investigated arsenic removal by combining magnetic seeding flocculation with open- or high-gradient superconducting magnetic separation. The combination of polymeric ferric sulfate (PFS) and magnetite had a significant effect on arsenic removal. The grain-size distribution, structure, and zeta potential of flocs were also studied to provide a complete picture of the magnetic seeding flocculation process.
Recently, an efficient technology combining traditional flocculation and the magnetic seeding technique was developed for wastewater treatment, and it was found to exhibit superior features (Li et al. 2010a; Stolarski et al. 2007). However, there are few studies involving the influence of the operating parameters and performance analysis of magnetic seeding flocculation. Thus, in this study, the effects of various factors on copper and turbidity removal from simulated microetch copper waste by magnetic seeding flocculation were systematically investigated.
Experimental
Materials
Poly-aluminum chloride (PAC) (effective aluminum oxide concentration of 28 % and basicity of 70–75 %), as a coagulant, was provided by Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Anionic polyacrylamide (PAM) (molecular weight of five million or more and high charge) as the flocculant was provided by Tianjin Kermel Chemical Reagent Co., Ltd (Tianjin, China). Fe3O4 used as the magnetic seeds was obtained from Beijing Jing Rui Kemai Water Purification Technology Co., Ltd (Beijing, China). Magnetic seeds with different ranges of particle sizes were prepared by sieving with different combinations of standard sieves. The physical characteristics of the magnetic seeds are presented in Table 1.
Wastewater sample
In the printed circuit board industry, a typical microetch copper waste usually contains a large amount of copper, a small amount of ammonia, and COD. In this study, a synthetic microetch copper waste was used. First, a copper solution with a Cu2+ concentration of 200 mg/L was prepared by dissolving analytical CuSO4 · 5H2O in distilled water. Then, analytical (NH4)2SO4 powder was added to the copper solution by stirring at room temperature to reach the final ammonia concentration of 100 mg/L. Finally, at 90 °C, soluble starch was added to the above solution with stirring to adjust the COD to 800 mg/L. The characteristics of the wastewater sample are presented in Table 2.
To detect the main form of the copper contaminants, analysis of the copper waste was performed as follows:
Depending on the actual particle system, the material balance is given as
Insoluble Cu(OH)2 precipitates exist at pH > 5.64 and remain in equilibrium with Cu2+. The precipitation dissolution equilibrium equation can be written as
In the presence of NH3, free Cu2+ ions are transformed to complex ions [Cu(NH3)4]2+.
The ionization equation of the weak electrolyte NH3 · H2O can be written as follows:
where K sp, K, and K b are, respectively, the solubility product of copper hydroxide [2.2 × 10−20 (mol/L)2], the stability constant of tetraammine copper [2.09 × 1013 (mol/L)2], and the ionization equilibrium constant of ammonia [1.8 × 10−5 (mol/L)2] (Gu et al. 2004).
The concentrations of different components were calculated with Eqs. (1), (2), (3), (4), and (5). For pH = 7.0, 9.0, and 11.0, the obtained values are given in Table 3, showing that Cu(OH)2 was the main contaminant of the simulated microetch copper waste in the range of pH values studied.
Magnetic seeding flocculation experiments
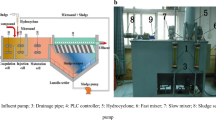
The magnetic seeding flocculation experiments were performed in a program-controlled jar test apparatus (ZR4-6, Zhongrun Water Industry Technology Development Co. Ltd., China) at room temperature. Test wastewater (600 mL) was transferred into a 1.0-L beaker, and the initial pH was adjusted to a set value (6–12) using 1.0 mol/L HCl or NaOH solutions. Under rapid stirring at 300 rpm (5 s−1), pre-determined amounts of PAC and magnetic seeds with different ranges of particle sizes were dosed together; after 1 min, the stirring speed was reduced to 140 rpm (5 s−1) for 2 min. A pre-determined amount of PAM was then dosed and slow stirring continued at 70 rpm (5 s−1) for 20 min. The clarified wastewater was then extracted from the beaker to measure copper and turbidity. All experimental runs were performed in triplicates, and the averaged values were applied.
Copper was analyzed using atomic absorption spectrophotometer (AA6000, Shanghai Tianmei Scientific Instrument Co., Ltd., China). The turbidity of the supernatant was measured using a portable turbidity meter (2100Q and 2100Q IS, Hach Company, USA).
Performance analysis
Based on the experimental conditions of traditional flocculation, the settling speed, floc-size distribution, and volume of sludge were measured in order to compare the efficiency of magnetic seeding to that of traditional flocculation.
First, to evaluate the settling speed of magnetic flocs, the height of the sludge interface at a settling time of 30 min was measured.
To measure the magnetic floc-size distribution, a MaiKeJi laser particle-size analyzer (MICROTRACS3500, USA) was used upon extension of the slow stirring stage described in Magnetic seeding flocculation experiments to 20 min. The magnetic floc samples were taken from the same position of the beaker every 2 min during this stage.
Finally, the amount of sludge was determined by the volumetric method using the Imhoff cone as per the standard method of the American Public Health Association (APHA 1995).
Results and discussion
Factors affecting traditional flocculation
In this section, the effect of operating factors such as coagulant dosage, flocculant dosage, and wastewater initial pH on the removal efficiency of traditional flocculation was investigated.
Effect of coagulant dosage
The variations of copper and turbidity removal against coagulant dosage from 0.30 to 3.30 g/L are shown in Fig. 1. The copper reduction was found to increase from 18.71 to 90.68 % with increasing coagulant dosage from 0.30 to 1.80 g/L, whereas a decrease is observed upon further increasing the coagulant dosage to 3.30 g/L. Similar trends were found for the removal of turbidity with increasing coagulant dosage from 0.30 to 1.80 g/L. However, no apparent variations in turbidity removal were observed with a further increase in the coagulant dosage.
Effect of flocculant dosage
Figure 2 illustrates the effect of flocculant dosage in the range of 0–0.035 g/L on copper and turbidity removal. The results indicate that the copper removal efficiency increased significantly with increasing flocculant dosage from 0 to 0.015 g/L. In this case, the flocs in the flocculation processes were effectively formed and the removal efficiency increased. However, a slight reduction of copper removal efficiency at higher flocculant dosages can be observed. Similar results are observed for the turbidity removal efficiency. The results show that the turbidity removal efficiency increased with increasing the dosage from 0.005 to 0.015 g/L, but at higher flocculant dosages, a gradual decrease in the efficiency is observed. Therefore, the increase in copper and turbidity removal was only marginal when the flocculant was added: the removal efficiency increased from 90.72 to 93.32 % for copper and from 83.1 to 94.10 % for turbidity. It is reasonable to use magnetic seeding flocculation at an optimum flocculation dosage of 15 mg/L.
Effect of wastewater initial pH
The initial pH of the wastewater plays an important role in the flocculation efficiency. In this experiment, the initial pH was adjusted using NaOH or HCl solutions at a 1.0-mol/L concentration. Figure 3 displays the effect of wastewater initial pH on traditional flocculation efficiency. The results demonstrate that the initial pH largely influenced both the copper and turbidity reduction. The removal rates were found to increase sharply with increasing the pH value from 6.0 to 9.5, whereas a slight decline of removal efficiency was observed upon further increasing the initial pH to 12.0. Aluminum in PAC has been found to exist in the forms of Al3+, Al(OH)2+, Al2(OH)4 2+, Al(H2O)6 3+, and Al(13) or higher polymers (Chin et al. 2006). In an acid environment, aluminum mainly exists in the form of Al3+, which is not conducive for the adsorption of colloids or for adhesion, bridges, and cross-linking, and thus PAC reduces the flocculation efficiency. Oppositely, in an alkaline environment, poly-nuclear (polymeric) species can be adsorbed onto the surface of colloidal particles, which promotes colloid aggregation. Nevertheless, when the PAC content exceeds a certain value, the Al(OH)3(s) generated will have a negative effect on the flocculation efficiency (Zheng et al. 2011). Because PAC is going to consume alkalinity from the system to produce the active coagulant species and because the dosage is high (1.8 g/L), there will be a large decrease in pH after the treatment. Therefore, the optimum pH for copper and turbidity reduction was 9.50, the value at which the reduction was 94.42 and 94.11 %, respectively.
Effect of magnetic seed dosage and size
Effect of magnetic seed dosage
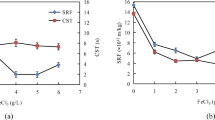
A series of experiments were performed to determine the optimal magnetic seed dosage in the magnetic seeding flocculation process. The dosage of Fe3O4 magnetic seeds was varied from 0 to 5.0 g/L. Figure 4 shows peaks in the copper and turbidity removal curves at a dosage of 2.0 g/L. In this case, the experimental copper and turbidity removal efficiencies reached 98.53 and 94.72 %, respectively, values that are greater than those in traditional flocculation. The optimum magnetic seed dosage needed to maximize the removal efficiency was therefore 2.0 g/L. However, at higher dosages, excessive magnetic seeds caused serious damage to magnetic flocs, resulting in a reduction in the copper and turbidity removal efficiency.
Effect of magnetic seed size
For magnetic seeding flocculation, the magnetic seed size plays an important role in determining the flocculation efficiency. Figure 5 shows the variation of copper and turbidity removal with magnetic seed size. The removal efficiencies increased significantly with decreasing the magnetic seed size until reaching a size of 300–400 mesh, at which point, reduction efficiencies of 98.53 and 94.72 % for copper and turbidity, respectively, were achieved. For the same dosage and a mesh size of 300–400, more particles are added to the wastewater. This increase in the concentration of particulate matter could lead to more collisions between the Fe3O4 particles and flocs, thus improving the magnetic seeding flocculation efficiency (Lipus et al. 2001; Stolarski et al. 2007). However, when the size exceeded 300–400 mesh, the efficiency began to decrease. In this case, the slight vortex centrifugal force of inertia generated between those magnetic seeds was too great, thus destroying the structure of flocs formed. Magnetic seeds with smaller particle sizes had little effect on the efficiency. Thus, the best reduction efficiency was obtained when the magnetic seed size was in the range of 300–400 mesh.
Optimization experiments of coagulant and flocculant dosage
Since different optimum dosages might be received for the coagulant and flocculant, the following experiments were conducted: (1) The effect of flocculant on copper and turbidity removal without coagulant and magnetic seeds and (2) the effect of flocculant on copper and turbidity removal without coagulant but with magnetic seeds. Table 4 shows the variations of copper and turbidity removal against flocculant dosage from 0.016 to 0.019 g/L with and without magnetic seeds. Maximum copper and turbidity removal rates of 93.64 and 93.25 %, respectively, were achieved at a flocculant dosage of 0.017 g/L (with magnetic seeds), whereas the rates were 91.11 and 92.32 %, respectively, at a flocculant dosage of 0.018 g/L (without magnetic seeds). These values were lower than the copper and turbidity removal rates of 98.53 and 94.72 %, respectively, at 0.015 g/L with coagulant, flocculant, and magnetic seeds. Treatment costs would be higher with a high coagulant dosage of 1.8 g/L. Therefore, despite lower copper and turbidity removal efficiencies, it is better to decrease chemical costs by using flocculant and magnetic seeds without coagulant.
Comparison of magnetic seeding and traditional flocculation
Settling speed analysis
Figure 6 shows the height of the sludge interface against settling time. With increasing magnetic seed dosage as well as decrease of seed size, the settling speed continuously increased until a dosage and size of 2.0 g/L and 300–400 mesh, respectively. However, a slight decrease in the settling speed is observed with further increases of magnetic seed dosage and decreases of magnetic seed size. Furthermore, the settling speed at a settling time of 1 min was 5 cm/min under optimum conditions, which is approximately 3.64 times that of traditional flocculation. Similar results were also reported by Bolto (1995).
Impact of magnetic seeds on the settling speed (wastewater initial pH 9.50, settling time 8 min, coagulant and flocculant 1.8 and 0.015 g/L, respectively). a Settling speed versus magnetic seed dosage (magnetic seed size 300–400 mesh). b Settling speed versus magnetic seed size (magnetic seed dosage 2.0 g/L)
Copper can be purified from wastewater by a flocculation and co-precipitation process in traditional wastewater treatment. This process requires large amounts of time and land, resulting in low copper treatment capacity. By contrast, the addition of magnetic seeds could favor agglomeration of the flocs; the specific gravity of those magnetic flocs combined with Fe3O4 is much larger than that of traditional flocs. Therefore, the settling speed and copper treatment capacity of the wastewater can be significantly improved (Li et al. 2010a; Yavuz and Celebi 2000.
Floc-size distribution analysis
The growth conditions of magnetic flocs were studied by comparing the particle-size distribution upon the addition of flocculant during the magnetic seeding flocculation process. The size distribution was determined with magnetic seeds using the optimal conditions for traditional flocculation.
Figure 7 displays the variation of magnetic flocs size, d50, during the slow mixing period of flocculation from 0 to 20 min. The size of microflocs formed by PAC, PAM, and magnetic seeds quickly increased at the beginning of the slow mixing process (0–8 min, step 1) and stabilized at a fixed value (8–18 min, step 2). Finally, the size decreased slightly at the end of this process (18–20 min, step 3).
Growth rates of magnetic flocs formed during slow stirring process (wastewater initial pH 9.50, coagulant and flocculant 1.8 g/L and 0.015 g/L, respectively). a Growth rate versus magnetic seed dosage (magnetic seed size 300–400 mesh). b Growth rate versus magnetic seed size (magnetic seed dosage 2.0 g/L)
With the addition of magnetic seeds, the initial rate of magnetic seeding flocculation increased (0–8 min, step 1). The total number of particles in the flocculation system increased when magnetic seeds were added, and the magnetic seeding flocculation rate was thus enhanced (Li et al. 2010a). Moreover, based on the variation curves, it is reasonable to assume that the different dosages and sizes of magnetic seeds resulted in different magnetic floc sizes and growth rates. The growth stage (step 1) indicated that the rate of magnetic seeding flocculation accelerated in the presence of magnetic seeds. However, when the dosage exceeded a certain value, the growth rates of the flocs began to decrease. Also, the magnetic flocs formed by 2.0 g/L of magnetic seeds exhibited a much higher growth rate as compared to other magnetic flocs. In the case of magnetic seed size, magnetic flocs formed from 300 to 400 mesh particles grew more rapidly than other flocs and reached a peak floc size after 8 min. The steady-state phase (step 2) exhibited trends similar to those of the growth stage (step 1).
To better understand the magnetic seeding flocculation mechanism, the variations of magnetic floc-size distribution under different experimental conditions were investigated. Magnetic flocs at slow mixing times of 8 and 18 min were chosen as the representative flocs of the floc growth stage (step 1) and steady-state phase (step 2), respectively.
The magnetic floc-size distributions for steps 1 and 2 are shown in Figs. 8 and 9, respectively. In both cases, the major peak of the floc-size shifted to higher values with the addition of magnetic seeds. There was also a considerable decrease in the smaller peak for magnetic floc-size between 10 and 20 μm. The magnetic floc-size distribution suggests that the smaller peak was reduced with a shift to the right of the major peak after the completion of floc growth. These results indicate that the microflocs were adsorbed by large magnetic flocs during the floc growth stage, which brought about the continuous growth of magnetic flocs. The steady-state size of magnetic flocs is achieved by the balance between weak forces, which facilitate efficient collisions, and the breakup due to mixing-induced shear conditions (Yu et al. 2006). Compared to other variables, the combination of the 2.0 g/L dosage and the 300–400 mesh particle size gave a higher floc growth rate. This is presumably attributed to efficient collisions occurring between particles and the slightly moderate vortex centrifugal force of inertia generated between magnetic seeds. The distinction in the above effects resulted in a complicated magnetic floc aggregation process, generating magnetic flocs with larger size and lower moisture.
Particle-size distribution for magnetic flocs during the floc growth stage (step 1) (wastewater initial pH 9.50, coagulant and flocculant 1.8 and 0.015 g/L, respectively). a Floc-size distribution versus magnetic seed dosage (magnetic seed size 300–400 mesh). b Floc-size distribution versus magnetic seed size (magnetic seed dosage 2.0 g/L)
Particle-size distribution for magnetic flocs during the floc steady-state phase (step 2) (wastewater initial pH 9.50, coagulant and flocculant 1.8 and 0.015 g/L, respectively). a Floc-size distribution versus magnetic seed dosage (magnetic seed size 300–400 mesh). b Floc-size distribution versus magnetic seed size (magnetic seed dosage 2.0 g/L)
Volume of sludge analysis
The management of flocculation sludge, now often referred to as dewatering and recovery of copper by electrolysis, accounts for a major portion of the cost of the wastewater treatment process. The amount of the sludge is an important index to evaluate the magnetic seeding flocculation efficiency (Aguilar et al. 2005). Figure 10 displays the variations of sludge volume with the size and dosage of magnetic seeds. The results show that the volume of sludge decreased from 38.46 to 20.21 % with increasing magnetic seed dosages from 0 to 2.0 g/L, whereas a slight increase is observed upon further increasing the magnetic seed dosage to 3.0 g/L. The size of the magnetic seeds gave similar variation; the lowest volume of sludge was obtained at 300–400 mesh. Therefore, under the experimental conditions investigated, the smallest volume of sludge (20.21 %) was achieved at a magnetic seed dosage of 2.0 g/L and size of 300–400 mesh. This sludge volume is 18.25 % lower than that achieved with traditional flocculation. These results illustrate the advantages attributed to magnetic seeds, namely, that the addition of Fe3O4 particles improved the ability to dewater sludge and recover copper as compared to traditional flocculation.
Volume of sludge produced by the different variables (wastewater initial pH 9.50, settling time 30 min, coagulant and flocculant 1.8 and 0.015 g/L, respectively). a Volume of sludge versus magnetic seed dosage (magnetic seed size 300–400 mesh). b Volume of sludge versus magnetic seed size (magnetic seed dosage 2.0 g/L)
Conclusions
The addition of magnetic seeds increases the settling speed and floc size, as well as decreases sludge production. For copper and turbidity, the highest removal efficiencies of 98.53 and 94.72 %, respectively, were achieved under the following conditions: coagulant and flocculant dosages of 1.8 g/L and 0.015 g/L, respectively, wastewater initial pH value of 9.50, magnetic seed dosage of 2.0 g/L, and magnetic seed size of 300–400 mesh.
The highest settling speed, the largest floc size, and the smallest volume of sludge were achieved simultaneously at the maximum removal efficiencies of copper and turbidity. A possible explanation for this phenomenon is that magnetic seeds with a dosage of 2.0 g/L and 300–400 mesh particle size can generate efficient collisions of particles and slightly moderate vortex centrifugal force of inertia among seeds. Thus, magnetic flocs with larger size and lower moisture were produced, improving the removal efficiency of copper and turbidity, accelerating the floc sedimentation rate, and reducing the volume of the sludge, all of them needs further study.
References
Agrawal A, Kumari S, Sahu KK (2009) Iron and copper recovery/removal from industrial wastes: a review. Ind Eng Chem Res 48:6145–6161
Aguilar MI, Saez J, Llorens M, Soler A, Ortuno JF, Meseguer V, Fuentes A (2005) Improvement of coagulation–flocculation process using anionic polyacrylamide as coagulant aid. Chemosphere 58:47–56
Anand P, Etzel J, Friedlaender F (1985) Heavy metals removal by high gradient magnetic separation. IEEE Trans Magn 21:2062–2064
APHA (1995) Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, DC
Bolto BA (1995) Soluble polymers in water purification. Prog Polym Sci 20:987–1041
Broomberg J, Gelinas S, Finch JA, Xu Z (1999) Review of magnetic carrier technologies for metal ion removal. Magnetic and Electrical Separation 9:169–188
Chin CJM, Chen PW, Wang LJ (2006) Removal of nanoparticles from CMP wastewater by magnetic seeding aggregation. Chemosphere 63:1809–1813
De Latour C (1976) Seeding principles of high gradient magnetic separation. J Am Water Works Ass 68:443–446
Doula MK (2009) Simultaneous removal of Cu, Mn and Zn from drinking water with the use of clinoptilolite and its Fe-modified form. Water Res 43:3659–3672
Feng D, Aldrich C, Tan H (2000) Removal of heavy metal ions by carrier magnetic separation of adsorptive particulates. Hydrometallurgy 56:359–368
Franzreb M, Watson JHP (2001) Elimination of heavy metals from waste waters by magnetic technologies. Environmental separation of heavy metals: engineered processes. CRC Press, London, UK, pp 97–140
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418
Gu WL, Yuan AH, Lin SL (2004) Inorganic and analytical chemistry, 3rd edn. Harbin Institute Technol. Press, Heilongjiang
Heidmann I, Calmano W (2008) Removal of Zn (II), Cu (II), Ni (II), Ag (I) and Cr (VI) present in aqueous solutions by aluminium electrocoagulation. J Hazard Mater 152:934–941
Heredia JB, Martin JS (2009) Removing heavy metals from polluted surface water with a tannin-based flocculent agent. J Hazard Mater 165:1215–1218
Karapinar N (2003) Magnetic separation of ferrihydrite from wastewater by magnetic seeding and high-gradient magnetic separation. Int J Miner Process 71:45–54
Kongsuwan A, Patnukao P, Pavasant P (2009) Binary component sorption of Cu(II) and Pb(II) with activated carbon from Eucalyptus camaldulensis Dehn bark. J Ind Eng Chem 15:465–470
Li YH, Liu FQ, Xia B, Du QJ, Zhang P, Wang DC, Wang ZH, Xia YZ (2010a) Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites. J Hazard Mater 177:876–880
Li YR, Wang J, Zhao Y, Luan ZK (2010b) Research on magnetic seeding flocculation for arsenic removal by superconducting magnetic separation. Sep Purif Technol 73:264–270
Lipus LC, Krope J, Crepinsek L (2001) Dispersion destabilization in magnetic water treatment. J Colloid Interface Sci 236:60–66
Ma A, Hadi P, Barford J, Hui CW, McKay G (2014) Modified empty bed residence time model for copper removal. Ind Eng Chem Res 53:13773–13781
Ma M (2011) Enhancement of hematite flocculation in the hematite–starch–(low-molecular-weight) poly (acrylic acid) system. Ind Eng Chem Res 50:11950–11953
Mirbagheri SA, Hosseini SN (2003) Pilot plant investigation on petrochemical wastewater treatment for the removal of copper and chromium with the objective of reuse. Int J Environ A 28:369–374
Nah IW, Hwang KY, Jeon C, Choi HB (2006) Removal of Pb ion from water by magnetically modified zeolite. Miner Eng 19:1452–1455
Paulino AT, Minasse FAS, Guilherme MR, Reis AV, Muniz EC, Nozaki J (2006) Novel adsorbent based on silkworm chrysalides for removal of heavy metals from wastewaters. J Colloid Interface Sci 301:479–487
Polat H, Erdogan D (2007) Heavy metal removal from waste waters by ion flotation. J Hazard Mater 148:267–273
Samper E, Rodriguez M, De la Rubia MA, Prats D (2009) Removal of metal ions at low concentration by micellar-enhanced ultrafiltration (MEUF) using sodium dodecyl sulfate (SDS) and linear alkylbenzene sulfonate (LAS). Sep Purif Technol 65:337–342
Soares EV, Soares HMVM (2012) Bioremediation of industrial effluents containing heavy metals using brewing cells of Saccharomyces cerevisiae as a green technology: a review. Environ Sci Pollut R 19:1066–1083
Stolarski M, Eichholz C, Fuchs B, Nirschl H (2007) Sedimentation acceleration of remanent iron oxide by magnetic flocculation. China Part 5:145–150
Terashima Y, Ozaki H, Sekine M (1986) Removal of dissolved heavy metals by chemical coagulation, magnetic seeding and high gradient magnetic filtration. Water Res 20:537–545
Wan SL, Ma ZZ, Xue Y, Ma MH, Xu SY, Qian LP, Zhang QR (2014) Sorption of lead (II), cadmium (II), and copper (II) ions from aqueous solutions using tea waste. Ind Eng Chem Res 53:3629–3635
Xu X, Cao X, Zhao L et al (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut R 20:358–368
Yalçın S, Sezer S, Apak R (2012) Characterization and lead (II), cadmium (II), nickel (II) biosorption of dried marine brown macro algae Cystoseira barbata. Environ Sci Pollut R 19:3118–3125
Yavuz H, Celebi SS (2000) Effects of magnetic field on activity of activated sludge in wastewater treatment. Enzyme Microb Tech 26:22–27
Yuan XZ, Meng YT, Zeng GM, Fang YY, Shi JG (2008) Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloid Surf A-Physicochem Eng Asp 317:256–261
Yu JF, Wang DS, Ge XP, Yan MQ, Yang M (2006) Flocculation of kaolin particles by two typical polyelectrolytes: a comparative study on the kinetics and floc structures. Colloid Surf A-Physicochem Eng Asp 290:288–294
Zagklis DP, Koutsoukos PG, Paraskeva CA (2012) A combined coagulation/flocculation and membrane filtration process for the treatment of paint industry wastewaters. Ind Eng Chem Res 51:15456–15462
Zheng HL, Zhu GC, Jiang SJ, Tshukudu T, Xiang XY, Zhang P, He QA (2011) Investigations of coagulation–flocculation process by performance optimization, model prediction and fractal structure of flocs. Desalination 269:148–156
Acknowledgments
This research was funded by the Shenzhen Strategic New Industry Development Project (ZDSY20120619093952884) and Shenzhen Nanshan District Science and Technology R&D Funds (KC2013ZDZJ0005A).
Conflict of interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Chen, Y., Luo, M. & Cai, W. Influence of operating parameters on the performance of magnetic seeding flocculation. Environ Sci Pollut Res 23, 2873–2881 (2016). https://doi.org/10.1007/s11356-015-5601-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5601-5