Abstract
The present study evaluated the sequential biodiesel-bioethanol production from seaweeds. A total of 22 macroalgal species were collected at different seasons and screened based on lipid and carbohydrate contents as well as biomass production. The promising species was selected, based on the relative increase in energy compounds (REEC, %), for further energy conversion. Seasonal and annual biomass yields of the studied species showed significant variations. The rhodophyte Amphiroa compressa and the chlorophyte Ulva intestinalis showed the highest annual biomass yield of 75.2 and 61.5 g m−2 year−1, respectively. However, the highest annual carbohydrate productivity (ACP) and annual lipid productivity (ALP) were recorded for Ulva fasciata and Ulva intestinalis (17.0 and 3.0 g m−2 year−1, respectively). The later was selected for further studies because it showed 14.8% higher REEC value than Ulva fasciata. Saturated fatty acids (SAFs) showed 73.4%, with palmitic acid as a dominant fatty acid (43.8%). Therefore, biodiesel showed high saturation degree, with average degree of unsaturation (ADU) of 0.508. All the measured biodiesel characteristics complied the international standards. The first route of biodiesel production (R1) from Ulva intestinalis showed biodiesel recovery of 32.3 mg g−1 dw. The hydrolysate obtained after saccharification of the whole biomass (R2) and lipid-free biomass (R3) contained 1.22 and 1.15 g L−1, respectively, reducing sugars. However, bioethanol yield from R3 was 0.081 g g−1 dw, which represented 14.1% higher than that of R2. Therefore, application of sequential biofuel production using R3 resulted in gross energy output of 3.44 GJ ton−1 dw, which was 170.9% and 82.0% higher than R1 and R2, respectively. The present study recommended the naturally-grown Ulva intestinalis as a potential feedstock for enhanced energy recovery through sequential biodiesel-bioethanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, consumption of fossil fuels is estimated to represent about 84% of the global energy consumption (IEA 2010). The world energy demand is projected to increase from a current 12,300 Mtoe to 16,700 Mtoe by 2035 (Cozzi and Gould 2011). The liquid fuel consumption in the transportation sector represents about 30% of the global energy consumption, which is expected to increase over the period 2006–2030 by about 80% (EIA 2010; Gruenspecht 2010). This development in the global energy consumption threatens the world with an energy crisis due to expectation with exhaustion of the global fossil fuel reserves in less than 30 years (Abomohra et al. 2016). In addition, the continuous increase in atmospheric CO2 concentration makes it a necessity to introduce alternative clean energy feedstocks to replace the conventional fossil fuels. Therefore, utilization of sustainable and renewable energy is a strategic option to improve the energy self-sufficiency, guarantee energy security, decrease the CO2 emissions, and improve the economic stability (Sudhakar et al. 2014).
Biofuels are a type of energy that is produced from biomass, which is a carbon-neutral renewable feedstock with the potential to reduce the dependence on fossil fuels, extenuating the environmental issues such as global warming (Hu et al. 2018). Nowadays, bioethanol from sugarcane or corn and biodiesel from oil terrestrial plants are the most feasible and abundant biofuels for large scale production (Somma et al. 2010) due to the cost-effective oil extraction and the well-established farming practices (Wei et al. 2013). However, the recent development in biofuel production from food crops raised many ethical issues due to food-versus-fuel dispute. Utilization of lignocellulosic residues as a second generation non-edible biofuel feedstocks has been discussed as a possible alternative (John et al. 2011; Liu et al. 2018; Martínez-Ruano et al. 2018; Jiang et al. 2019; Elsayed et al. 2020). However, presence of lignin requires high-cost pretreatment which limits their industrialization (Elsayed et al. 2018; Ebaid et al. 2019; Novakovic et al. 2020). Therefore, algae have been discussed recently as a potential feedstock for biofuels that are able to encounter these issues (Abomohra et al. 2014; Tu et al. 2015; Mousavi et al. 2018; Wang et al. 2019b; Pandey et al. 2020). Compared with terrestrial plants such as soybean or rapeseed, marine algae are recognized as one of the best non-edible feedstocks for biofuel production (Abomohra et al. 2017; Chandra et al. 2017; Wang et al. 2019a; Hadizadeh et al. 2020). The precedence of marine algae is due to the high CO2 fixation rate and the higher photosynthetic efficiency compared with terrestrial plants (Aresta et al. 2005; Jung et al. 2013). Macroalgal biomass production does not require utilization of pesticides or chemical fertilizers (McKendry 2002; Wei et al. 2013; Abomohra et al. 2018b). Macroalgae have no lignin (Ven Den Hoeck et al. 1995; Wi et al. 2009) and, therefore, it is relatively easy to microbially digest or chemically convert the biomass without prior pretreatment (Østgaard et al. 1993; Vergara-Fernández et al. 2008). No need for freshwater or agricultural land to grow the macroalgae, which is beneficial for the countries that lack the arable land (Gao and McKinley 1994). Moreover, some macroalgal species have biomass productivity higher than the most productive tropical rainforests and land plants (Salisbury and Ross 1992; Gao and McKinley 1994). In contrast to microalgae, wild seaweeds can be manually or mechanically harvested, while microalgae harvesting and dewatering involves use of technical procedures, such as filtration, flotation, flocculation, centrifugation, which raise the production cost. In addition, seaweeds contain unique components including carrageenan, laminarin, alginate, agar, mannitol, fucoidin, ulvan, fucose, and uronic acid which give them more value by biorefinery. After biofuel recovery, the residual biomass can be used for many purposes such as application into the soil as organic fertilizers, heating by direct combustion, or as a feedstock to produce different kinds of biofuels (Vergara-Fernández et al. 2008; Ge et al. 2011; Abomohra et al. 2018b).
Currently, biodiesel is one of the most broadly used biofuels globally, while the feedstock was reported to represent 70–80% of the biodiesel production cost (Ma et al. 2016). Therefore, using vegetable oils as a biodiesel feedstock results in high production cost and requires vast arable land areas that are currently harnessed for human food. Therefore, exploring non-edible cost-effective feedstocks to decrease the cost of biodiesel production remains the main challenge for its commercialization. There are many naturally grown macroalgal species with relatively high lipid content that are promising feedstock for biodiesel production (Abomohra et al. 2018b). In addition, using seaweeds as bioethanol feedstock is receiving extensive interest due to the relatively high carbohydrate content (Devendra et al. 2016). After lipid extraction, a massive quantity of biomass rich in carbohydrates is thrown away as a waste byproduct. An alternative economic method is to use the residual seaweed biomass as biofertilizer for agricultural purpose. However, carbohydrates-rich residual biomass can be recycled as a potential substrate for bioethanol production (Sudhakar et al. 2016; Abomohra et al. 2018a; Xu et al. 2019).
Although many researchers confirmed the effectiveness of seaweeds as a biodiesel or bioethanol feedstock, biorefining of seaweeds through sequential biofuel recovery would enhance the energy yield and conversion efficiency. Therefore, this work aimed to assess the efficiency of local macroalgal species collected throughout the year from the coastal area of Mediterranean Sea at Alexandria, Egypt, for biodiesel and bioethanol production. After screening of macroalgae collected at different seasons, the species with high biomass yield and high carbohydrate and/or lipid productivity was chosen. Lipids of the selected species were converted by transesterification into fatty acid methyl esters (FAMEs), and the properties of the produced biodiesel were estimated. Moreover, the potential of the lipid-free biomass for bioethanol production was compared with that of the whole biomass.
Materials and methods
Study area and macroalgae collection
Seaweeds were collected seasonally during January, April, July, and October 2018 along the Abu Qir Bay (31° 19′ 20″ N and 30° 03′ 40″ E) Alexandria, Egypt (Fig. 1). Macroalgae were collected from an area of 6 m2 by quadrate technique using 1 m2 steel quadrate (Russell 1977). All algal thalli were washed carefully with the seawater to remove sand, rock debris, and epiphytes. They were sorted and transported separately in plastic bags under iced conditions to the laboratory. Seaweeds were washed thoroughly with tap water and distilled water to remove salt and any impurities. Samples of the collected seaweeds were identified morphologically and fixed on white sheets for herbarium preservation. In addition, all seaweeds were air-dried on absorbent papers at room temperature until constant weight. The dried samples were grounded and stored in sealed vacuumed bags until further use. Biomass production was measured for each individual species and represented as g dry weight (dw) per m2.
Physicochemical characteristics of seawater
Temperature, conductivity, pH, salinity, and turbidity of seawater at the collection site were measured during sampling process in the field. The pH and temperature were measured using a digital pH meter (JENCO 6173). Conductivity and salinity were measured by a conductivity meter (HANNA HI 2300). Turbidity was measured by a portable turbidity meter (HANNA HI 98703). Other water parameters were measured in seawater samples collected and transported to the laboratory in sterilized bottles. Chemical oxygen demand (COD), total dissolved solids (TDS), dissolved oxygen (DO), NO3–N, NO2–N, NH4–N, biological oxygen demand (BOD), total phosphate (TP), copper, zinc, and lead were measured following the protocol of the American Public Health Association standard methods (APHA 1998).
Estimation of proteins and carbohydrates
Algal cells (250 mg dw) were extracted according to Payne and Stewart (1988) by incubation with 10 mL 1 N NaOH for 2 h in a boiling water bath. Protein concentration was determined according to Bradford method (Bradford 1976). Briefly, 3 mL of Bradford reagent solution were added to 100 μL of the extract, mixed thoroughly and kept for 5 min at room temperature. The absorbance was measured by UV/visible spectrophotometer (UNICO UV-2000) at 595 nm against blank. Bovine serum albumin was used to construct a protein standard curve, where protein content was estimated as %dw. The concentration of the total carbohydrates was determined by phenol-sulfuric acid method (Kochert 1978). Briefly, 1 mL of phenol reagent (5%) was added to 0.5 mL of NaOH extract, then 5 mL of conc sulfuric acid were slowly mixed with the contents. It was kept at room temperature for 30 min, then the absorption was measured against the blank at 490 nm. Total carbohydrates were determined per dry weight using a standard curve of glucose.
Lipid determination
Lipid content was measured according to the modified method of Folch et al. (1957) using chloroform:methanol (2:1, v/v). About 500 mg of the dried seaweeds were homogenized with 20 mL of chloroform:methanol using Wise Tis® homogenizer, then 100 μL of 1 M HCL were added to acidify the sample and vortexed well. After incubation overnight on a shaking incubator, 3 mL of 0.9% NaCl were vortexed with the mixture, then centrifuged at 300×g for 3 min. The lower organic phase containing the lipid extract was piped into a pre-weighed vessel. The solvent was evaporated and the weight of lipids was measured gravimetrically, then the lipid content was calculated as a percentage of the dry weight (%dw, w/w).
Routes design
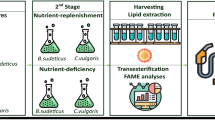
Biodiesel and bioethanol production of the most promising seaweed was evaluated by applying three different routes (Fig. 2). The first route (R1) represented biodiesel production through lipid extraction followed by transesterification. The second route (R2) represented direct fermentation of the whole biomass for bioethanol production without prior lipid extraction. The third route (R3) represented biodiesel production from the extracted lipids, followed by fermentation of the residual lipid-free biomass for bioethanol production.
Selection of the promising seaweed
Selection of the promising seaweed depends mainly on carbohydrate and lipid productivity, which are influenced by the biomass yield. However, the seaweed with high lipid productivity might be not the same one with high carbohydrate productivity. Therefore, selection based on the relative increase in energy compounds (REEC, %) including carbohydrates and lipids was calculated from a novel generated equation as follows:
where, PH represents the productivity of carbohydrates (Carb) and lipids (Lip) for the tested species, while PL represents the lowest recorded productivities among the all studied species.
Fatty acid profile and biodiesel properties
Fatty acids were converted into FAMEs using the modified method of Christie (1998) using benzene and 1% sulfuric acid. After lipid extraction as mentioned previously, about 200 mg of lipids were mixed with 4 mL benzene and 50 mL of 1% methanolic sulfuric acid. The mixture was refluxed at 90 °C for 90 min, then cooled down at room temperature. In order to separate the two phases, 20 mL of distilled water were added and the resulted esters forming the upper layer were siphoned and further washed with 10 mL benzene. The solvent was evaporated and the composition of FAMEs was analyzed using gas chromatography–mass spectrum (GC/MS, Perkin Elmer Clarus 580/560 S) manufactured with Rxi-5 Sil MS column (30 m length and 0.25 mm internal diameter). The initial oven temperature (90 °C) was maintained for 6 min and increased to 290 °C at a rate of 8 °C min−1. The temperature of the injector was adjusted at 290 °C, and 1 μL of sample was injected at a split ratio of 20:1. For biodiesel, several models have been built recently to estimate the biodiesel characteristics based on the fatty acids profile (Francisco et al. 2010; Hoekman et al. 2012; Nascimento et al. 2013; Song et al. 2013; Ma et al. 2014). Amongst, Hoekman et al. (2012) complied considerable information on the compositions and characteristics of FAMEs from different feedstocks, and investigated the relationships across many realistic biodiesel types. Therefore, biodiesel properties in the present study were estimated according to Hoekman et al. (2012) as previously described by Abomohra et al. (2018b).
Biomass hydrolysis and saccharification
The whole biomass and lipid-free biomass of the selected macroalga were investigated further for bioethanol production by a simultaneous saccharification-fermentation. About 5 g of the dried seaweed were hydrolyzed with 50 mL of 2% HCl and heated for 60 min at 120 °C and 1.5 bar. The treated biomass was then mixed vigorously with 50 mL distilled water for extraction of the soluble reducing sugars. The extract was filtered via a cheese cloth for solids separation, then the pH of the filtrate was adjusted to 7.
Bioethanol production
Angel yeast (Saccharomyces cerevisiae CECA) was purchased from the local market as dry active granules. It is isolated from cabernet sauvignon spontaneous fermentation and was reported to have a short lag phase, high alcohol tolerance, and excellent fermentation kinetics (Angel Yeast Co. 2018). The batch fermentation was carried out in 250-mL Erlenmeyer flasks using Saccharomyces cerevisiae at 1:20 inoculum:solution ratio. All flasks were tightly closed and incubated with shaking at 37 °C in a water bath for 6 days. At the start and the end of the experiments, samples were taken for glucose and ethanol determination using high performance liquid chromatography (HPLC 1100, Agilent, USA) equipped with a refractive index detector and Shimadzu Shim-pack SCR-101N column (7.8 × 300 mm). Ultra-pure water was used as a mobile phase at a flow rate 0.7 mL min−1 and the column temperature was adjusted at 50 °C.
Statistical analysis
Data were calculated as the mean ± standard deviation of three replicates. SPSS (IBM, version 20) was used to carry out the statistical analysis by applying one-way analysis of variance (ANOVA) followed by LSD test at a probability level (P) ≤ 0.05.
Results and discussion
Water characteristics
Abu Qir Bay is considered as one of the most environmentally sensitive shore areas in Egypt which is located 35 km east of Alexandria City. There are three openings (Rosetta mouth of Nile River, El-Tabia pumping station, and Lake Edku outlet) which provide the bay with various types of continental discharges, with a total of about 2130 × 106 m3 year−1 freshwater discharge rate (Khairy et al. 2012; Zakaria et al. 2019). Results in Table 1 showed the highest water salinity of 81.7‰ in autumn, which decreased to 66.2‰, 52.8‰, and 52.2‰ in winter, summer, and spring, respectively. In addition, the highest water conductivity (46.5 μS) was recorded in autumn. Water temperature fluctuated from 15 °C in winter to 32 °C in summer, where the seasonal temperature variations followed the climate conditions. Turbidity was 1.62 NTU in winter, 1.52 NTU in spring, 0.96 NTU in summer, and 1.64 in autumn. The pH value varied between a maximum of 8.52 during autumn and minimum of 7.84 during winter, whereas pH value during spring and summer was 7.94 and 8.36, respectively. COD, BOD, and DO were 1180, 0.6, and 8.2 mg L−1 in winter, and changed to 1058, 1.0, and 9.2 mg L−1 in summer, respectively. In addition, NO3–N showed the maximum value of 66.4 mg L−1 in winter, followed by 18.13 mg L−1 in summer. NH4–N showed the maximum value of 9.1 mg L−1 in winter followed by 5.1 mg L−1 in autumn. Heavy metal analysis showed no zinc or lead through the four seasons, while copper in winter and spring represented 0.04 and 0.02 mg L−1, respectively, with no recorded copper in summer and autumn. The enrichment of nitrate and ammonia is attributed to the municipal wastewater effluent in this area. The fluctuation in physicochemical characteristics of water during the four seasons resulted in significant changes in biomass production as discussed in the following section.
Biomass production and composition
A total of 22 species of seaweeds (8 chlorophyceae, 8 rhodophyceae, and 6 phaeophyceae) were collected from the studied location (Figs. S1-S3, Supplementary data). The seasonal and annual biomass yields of the studied species showed significant variations. From all the collected biomass around the year, summer showed the highest biomass for chlorophytes and phaeophytes, while autumn was the best for rhodophytes (Fig. 3). Thus, summer and autumn showed the highest seasonal biomass yield of 156.2 and 124.1 g m−2 season−1, respectively. It might be attributed to the relatively high temperature which indicates more sunny days and light intensity during these seasons. In addition, these two seasons showed relatively higher BOD and no copper content which might inhibit the growth of seaweeds at the studied area. Among all the collected species, the phaeophyte Ectocarpus siliculosus showed the lowest annual biomass yield of 1.28 g m−2 year−1; while the rhodophyte Amphiroa compressa and the chlorophyte Ulva intestinalis showed the highest annual biomass yield of 75.2 and 61.5 g m−2 year−1, respectively (Fig. 4). Proteins and carbohydrates were the major biochemical components in all studied seaweeds, while lipids represented the lowest proportion (Table 2). Chondracanthus teedei and Gelidium crinale recorded the highest carbohydrate contents of 54.8 and 48.1%dw, respectively, in summer. However, Taonia atomaria showed the highest significant lipid content of 10.2%dw in spring, followed by Ectocarpus siiculosus (8.6%dw).
Lipid and carbohydrate contents cannot be used for the selection of the promising seaweed for dual purpose of biodiesel and bioethanol production, as the areal productivity of these energy compounds will be influence by the biomass as well. Therefore, annual areal lipid productivity (ALP) and carbohydrates productivity (ACP) were calculated as g m−2 year−1 (Table 3). The highest ACP and ALP of 17.03 and 2.99 g m−2 year−1 were recorded for Ulva fasciata and Ulva intestinalis, respectively. It is attributed to the enhancement of both biomass and carbohydrate or lipid content for the same species. Merging carbohydrates and lipid productivities into consideration, REEC of Ulva intestinalis showed 14.8% higher than that of Ulva fasciata due to the higher biomass yield (Table 3). Therefore, Ulva intestinalis was selected for further studies as a promising macroalga for dual bioethanol and biodiesel production. The highest seasonal lipid content and productivity of Ulva intestinalis (6.1%dw and 1.47 g m−2 season−1, respectively) were recorded in winter (Fig. 5). However, the maximum carbohydrate content and productivity of 28.3%dw and 6.66 g m−2 season−1 were recorded in autumn and winter, respectively.
Fatty acid profile and biodiesel characteristics
As described in the previous section, Ulva intestinalis was selected for further evaluation as a promising biodiesel and bioethanol feedstock. Knothe (2009) reported that lipids containing C16–C18 fatty acids, namely 16:0, 18:0, 18:1, 18:2, and 18:3, are the most suitable biodiesel feedstock. Fatty acids of Ulva intestinalis showed a wide range of carbon chain length (C14–C20), while 16- and 18-carbon were the dominant fatty acids (Table 4). From the quality aspect, high content of unsaturated fatty acids is not preferable in order to obtain high oxidative stability and avoid the problems of cold flow (Hoekman et al. 2012; Krzemińska and Oleszek 2016). Saturated fatty acids (SAFs) showed 73.4%, with palmitic acid as the dominant fatty acid (43.8%). In addition, a relatively high monounsaturated fatty acids (MUFAs) content of 14.5% was recorded, while the lowest proportion of 12.1% was recorded for polyunsaturated fatty acids (PUFAs).
FAME characteristics were estimated in order to evaluate the suitability of Ulva intestinalis lipids as a biodiesel feedstock. The properties of FAMEs, together with the corresponding recommended values by the international standards are shown in Table 5. The average degree of unsaturation (ADU) is a crucial parameter that has a high correlation with many other biodiesel properties. For instance, the increase of ADU results in lower cetane number and poor oxidation stability while improves the biodiesel performance at low temperatures. The ADU of Ulva intestinalis FAMEs was 0.508, which shows a relatively high saturation degree. According to the ASTM D6751-08 (2008) and EN 14214 (2008) standards, CN should be higher than or equal to 47.0 and 51.0, respectively; while EN 14214 standards recommended a maximum iodine value (IV) of ≤ 120 g I2/100 g oil. ASTM D6751-08 standards recommended 0.85–0.90 kg−1 and 1.9–6.0 mm2 s−1 for specific gravity and kinematic viscosity, respectively. Table 5 confirms that the values of these parameters were in agreement with those recommended by the international standards. The biodiesel characteristics are comparable with those of Ulva intestinalis reported by Abomohra et al. (2018b). However, Ulva lactuca showed higher ADU, which resulted in lower cetane number and higher iodine value.
IV is a parameter that determines the total fatty acid unsaturation in the biodiesel (Knothe 2005). There is a positive correlation between the IV and ADU, i.e., the more unsaturation, the higher IV (Kyriakidis and Katsiloulis 2000; Knothe 2002). A maximum value of 120 g I2/100 g is recommended by the EN 14214 standards, which excluded many other lipid-rich biomass such as soybean and sunflower to be utilized as a biodiesel feedstocks (Mittelbach and Remschmidt 2004). However, the IV of Ulva intestinalis biodiesel in the present study represented 50.49 g I2/100 g oil, which comply the EN 14214 standard. The biodiesel obtained from Ulva intestinalis showed relatively high CN of 59.49, which helps the engine to start rapidly with less noise and indicates the good ignition quality with low NOx emissions (Ashokkumar et al. 2015; Sharma et al. 2016). In addition, the biodiesel higher heating value (HHV) affects the consumption of fuel by the diesel engine. The results showed that Ulva intestinalis biodiesel has HHV of 39.43 MJ kg−1, which is in agreement with those reported previously to Padina boryana and Ulva intestinalis (39.43 and 39.37 MJ kg−1, respectively) (Abomohra et al. 2018b). Higher kinematic viscosity of the biodiesel results in serious problems due to incomplete combustion (Ashokkumar et al. 2017). However, biodiesel with lower kinematic viscosity is much easier to be pumped to the engine and keeps a good state for injector (Sahoo and Das 2009). The estimated kinematic viscosity of Ulva intestinalis biodiesel was 4.89 mm2 s−1 which in accordance with the ASTM standards.
Bioethanol production
Lipids were extracted and transesterified to biodiesel as discussed in the previous section, representing the first route (R1). Further, the whole macroalgal biomass and lipid-free biomass were examined for bioethanol production (R2 and R3, respectively). During hydrolysis, cellulose and hemicellulose are converted to simple reducing sugars. The hydrolysate obtained after saccharification of Ulva intestinalis biomass in R2 and R3 contained 1.22 and 1.15 g L−1, respectively, reducing sugars (Table 6), which were utilized as a substrate for fermentation by S. cerevisiae. The lower sugar content in R3 might be attributed to the loss of some sugars during lipid extraction. The conversion of sugars into bioethanol was measured on the 6th day of fermentation. Bioethanol yield of hydrolysate from R3 was 0.081 g g−1 dw, which was 14.1% higher than that of R2 (Table 6). It is attributed to the high efficiency of sugar utilization by the yeast in R3, which showed 7.7% reduction in the final sugar content. Fermentation acceleration in R3 might be accredited to the absence of lipids in the residual biomass, which might affect the yeast growth. Moreover, lipid extraction can be proposed as a pretreatment for better degradation of cells which results in higher hydrolysis efficiency.
Gross energy output
From the energy aspect, the gross energy output was calculated for each route by multiplying the biodiesel and/or bioethanol yield by the HHV of each product. The HHV of biodiesel was 39.4 MJ kg−1 (Table 5), while that of bioethanol was considered 26.7 MJ kg−1 (Sadhukhan et al. 2019). It is noteworthy to mention that these calculations are a simplified analysis where energy input involved in harvesting, transportation, drying, and fermentation were not considered. The first route of biodiesel production (R1) showed gross energy output of 1.27 GJ ton−1 (Table 6). However, bioethanol produced from the second route (R2) showed 1.89 GJ ton−1. Sequential energy recovery through biodiesel and bioethanol production (R3) enhanced the bioethanol recovery, which resulted in 170.9% and 82.0% higher gross energy output than R1 and R2, respectively. Thus, the present study confirmed that production of bioethanol from macroalgae is more favorable than biodiesel due to the higher energy yield. In addition, sequential biofuel recovery from biomass through lipid extraction followed by fermentation of lipid-free biomass is an effective approach to enhance the total energy output.
Conclusion
The present work evaluated the potential of the naturally-grown macroalgae collected from Abu Qir Bay around the year for biodiesel and bioethanol production. Ulva intestinalis showed the highest REEC value of 6.99 × 103% and, therefore, it was used for sequential biofuel experiments. Lipid extraction prior to fermentation (R3) enhanced the hydrolysis efficiency and sugar availability which resulted in 14.1% higher bioethanol yield than that of R2. Sequential energy recovery showed 170.9% and 82.0% higher gross energy output than individual production of biodiesel or bioethanol, respectively. Although sequential conversion enhanced the energy yield, detailed life cycle assessment of the different applied routes is of great importance for future studies. In addition, studying and integration of other biofuel routes such as biogas production and thermochemical conversion into crude bio-oil are in progress.
References
Abomohra AE-F, El-Sheekh M, Hanelt D (2014) Pilot cultivation of the chlorophyte microalga Scenedesmus obliquus as a promising feedstock for biofuel. Biomass Bioenergy 64:237–244
Abomohra, Jin W, Tu R, Han S, Eid M, Eladel H (2016) Microalgal biomass production as a sustainable feedstock for biodiesel: current status and perspectives. Renew Sust Energ Rev 64:596–606
Abomohra AE-F, El-Sheekh M, Hanelt D (2017) Screening of marine microalgae isolated from the hypersaline Bardawil lagoon for biodiesel feedstock. Renew Energy 101:1266–1272
Abomohra A, Eladel H, El-Esawi M, Wang S, Wang Q, He Z, Feng Y, Shang H, Hanelt D (2018a) Effect of lipid-free microalgal biomass and waste glycerol on growth and lipid production of Scenedesmus obliquus: innovative waste recycling for extraordinary lipid production. Bioresour Technol 249:992–999
Abomohra, El-Naggar AH, Baeshen AA (2018b) Potential of macroalgae for biodiesel production: screening and evaluation studies. J Biosci Bioeng 125:231–237
Angel Yeast Co. (2018) Wine yeast CECA [WWW Document]. The source pdf at URL https://en.angelyeast.com/upload/files/2018/3/angel-wine-yet-ceca-(Saccharomyces-cerevisiae).pdf (accessed 12.8.18)
APHA (1998) Standard methods for the examination of water and waste water, 19th edn
Aresta M, Dibenedetto A, Barberio G (2005) Utilization of macro-algae for enhanced CO2 fixation and biofuels production: development of a computing software for an LCA study. Fuel Process Technol 86:1679–1693
Ashokkumar V, Salam Z, Tiwari ON, Chinnasamy S, Mohammed S, Ani F (2015) An integrated approach for biodiesel and bioethanol production from Scenedesmus bijugatus cultivated in a vertical tubular photobioreactor. Energy Convers Manag 101:778–786
Ashokkumar V, Salim MR, Salam Z, Sivakumar P, Chong CT, Elumalai S, Suresh V, Ani FN (2017) Production of liquid biofuels (biodiesel and bioethanol) from brown marine macroalgae Padina tetrastromatica. Energy Convers Manag 135:351–361
ASTM D6751-08 (2008) Standard specification for biodiesel fuel blend stock (B100) for middle distillate fuels. ASTM Int West Conshohocken
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chandra R, Ghosh UK, Nayak JK (2017) Phycoremediation potential of marine microalga Tetraselmis indica on secondary treated domestic sewage for nutrient removal and biodiesel production. Environ Sci Pollut Res 24:20868–20875
Christie WW (1998) Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids 33:343–353. https://doi.org/10.1007/s11745-998-0214-x
Cozzi L, Gould T (2011) World Energy Outlook 2011. Annex A 2:650–651
Devendra LP, Kumar MK, Pandey A (2016) Evaluation of hydrotropic pretreatment on lignocellulosic biomass. Bioresour Technol 213:350–358
Ebaid R, Wang H, Sha C, Abomohra AE, Shao W (2019) Recent trends in hyperthermophilic enzymes production and future perspectives for biofuel industry: a critical review. J Clean Prod 238:117925
EIA US (2010) EIA Annual Energy Outlook 2010 with Projections to 2035. DOE/EIA-0383. US Energy Information Administration, Washington, DC
Elsayed M, Abomohra AE-F, Ai P, Wang D, El-Mashad H, Zhang Y (2018) Biorefining of rice straw by sequential fermentation and anaerobic digestion for bioethanol and/or biomethane production: comparison of structural properties and energy output. Bioresour Technol 268:183–189
Elsayed M, Ran Y, Ai P, Azab M, Mansour A, Jin K, Zhang Y, Abomohra AE (2020) Innovative integrated approach of biofuel production from agricultural wastes by anaerobic digestion and black soldier fly larvae. J Clean Prod 263:121495
EN 14214 (2008) Automotive fuels D fatty acid methylesters (FAME) for diesel engines. requirements and test methods. Eur Comm Stand EN14214
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:495–509
Francisco EC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Gao K, McKinley KR (1994) Use of macroalgae for marine biomass production and CO2 remediation: a review. J Appl Phycol 6:45–60
Ge L, Wang P, Mou H (2011) Study on saccharification techniques of seaweed wastes for the transformation of ethanol. Renew Energy 36:84–89
Gruenspecht H (2010) International energy outlook 2011. Cent Strateg Int Stud
Hadizadeh Z, Mehrgan MS, Shekarabi SPH (2020) The potential use of stickwater from a kilka fishmeal plant in Dunaliella salina cultivation. Environ Sci Pollut Res 27:2144–2154
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16:143–169
Hu Y, Wang S, Li J, Wang Q, He Z, Feng Y, Abomohra AF, Afonaa-Mensah S, Hui C (2018) Co-pyrolysis and co-hydrothermal liquefaction of seaweeds and rice husk: comparative study towards enhanced biofuel production. J Anal Appl Pyrolysis 129:162–170
IEA O (2010) OECD and World Bank (2010), Analysis of the scope of energy subsidies and suggestions for the G-20 initiative. In: IEA, OPEC, OECD and World Bank Joint Report prepared for submission to the G-20 Summit Meeting Toronto (Canada), 26-27 June 2010
Jiang D, Xia Z, Wang S, Li H, Gong X, Yuan C, el-Fatah Abomohra A, Cao B, Hu X, He Z, Wang Q (2019) Mechanism research on catalytic pyrolysis of sulfated polysaccharide using ZSM-5 catalysts by Py-GC/MS and density functional theory studies. J Anal Appl Pyrolysis 143:104680
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193
Jung KA, Lim S-R, Kim Y, Park JM (2013) Potentials of macroalgae as feedstocks for biorefinery. Bioresour Technol 135:182–190
Khairy MAE-H, Kolb M, Mostafa AR, Anwar EF, Bahadir M (2012) Risk posed by chlorinated organic compounds in Abu Qir Bay, East Alexandria, Egypt. Environ Sci Pollut Res 19:794–811
Knothe G (2002) Structure indices in FA chemistry. How relevant is the iodine value? J Am Oil Chem Soc 79:847–854
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci 2:759–766
Kochert G (1978) Carbohydrate determination by the phenol-sulfuric acid method. Handb Phycol Methods Phycol Biochem methods 95
Krzemińska I, Oleszek M (2016) Glucose supplementation-induced changes in the Auxenochlorella protothecoides fatty acid composition suitable for biodiesel production. Bioresour Technol 218:1294–1297
Kyriakidis NB, Katsiloulis T (2000) Calculation of iodine value from measurements of fatty acid methyl esters of some oils: comparison with the relevant American oil chemists society method. J Am Oil Chem Soc 77:1235–1238
Liu S, Yang G, Fu J, Zhang G (2018) Synchronously enhancing biogas production, sludge reduction, biogas desulfurization, and digestate treatment in sludge anaerobic digestion by adding K2FeO4. Environ Sci Pollut Res 25:35154–35163
Ma Y, Wang Z, Yu C, Yin Y, Zhou G (2014) Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour Technol 167:503–509
Ma X, Zheng H, Addy M, Anderson E, Liu Y, Chen P, Ruan R (2016) Cultivation of Chlorella vulgaris in wastewater with waste glycerol: strategies for improving nutrients removal and enhancing lipid production. Bioresour Technol 207:252–261
Martínez-Ruano JA, Caballero-Galván AS, Restrepo-Serna DL, Cardona CA (2018) Techno-economic and environmental assessment of biogas production from banana peel (Musa paradisiaca) in a biorefinery concept. Environ Sci Pollut Res 25:35971–35980
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46
Mittelbach M, Remschmidt C (2004) Biodiesel thé comprehensive handbook. M Mittelbach (Publisher), Gratz, Austria
Mousavi S, Najafpour GD, Mohammadi M (2018) CO2 bio-fixation and biofuel production in an airlift photobioreactor by an isolated strain of microalgae Coelastrum sp. SM under high CO2 concentrations. Environ Sci Pollut Res 25:30139–30150
Nascimento IA, Marques SSI, Cabanelas ITD, Pereira SA, Druzian JI, de Souza CO, Vich DV, de Carvalho GC, Nascimento MA (2013) Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res 6:1–13
Novakovic J, Kontogianni N, Barampouti EM, Mai S, Moustakas K, Malamis D, Loizidou M (2020) Towards upscaling the valorization of wheat straw residues: alkaline pretreatment using sodium hydroxide, enzymatic hydrolysis and biogas production. Environ Sci Pollut Res:1–13
Østgaard K, Indergaard M, Markussen S, Knutsen SH, Jensen A (1993) Carbohydrate degradation and methane production during fermentation of Laminaria saccharina (Laminariales, Phaeophyceae). J Appl Phycol 5:333–342
Pandey A, Srivastava S, Kumar S (2020) Development and cost-benefit analysis of a novel process for biofuel production from microalgae using pre-treated high-strength fresh cheese whey wastewater. Environ Sci Pollut Res 1–18
Payne JK, Stewart JR (1988) The chemical composition of the thallus wall of Characiosiphon rivularis (Characiosiphonaceae, Chlorophyta). Phycologia 27:43–49
Russell G (1977) Vegetation on rocky shores at some north Irish Sea sites. J Ecol 65:485–495
Sadhukhan J, Martinez-Hernandez E, Amezcua-Allieri M, Aburto J (2019) Economic and environmental impact evaluation of various biomass feedstock for bioethanol production and correlations to lignocellulosic composition. Bioresour Technol Rep:100–230
Sahoo PK, Das LM (2009) Combustion analysis of Jatropha, Karanja and Polanga based biodiesel as fuel in a diesel engine. Fuel 88:994–999
Salisbury FB, Ross CW (1992) Plant physiology, 4th edn. Wadsworth Publishing Company, Belmont
Sharma AK, Sahoo PK, Singhal S, Joshi G (2016) Exploration of upstream and downstream process for microwave assisted sustainable biodiesel production from microalgae Chlorella vulgaris. Bioresour Technol 216:793–800
Somma D, Lobkowicz H, Deason JP (2010) Growing America’s fuel: an analysis of corn and cellulosic ethanol feasibility in the United States. Clean Techn Environ Policy 12:373–380
Song M, Pei H, Hu W, Ma G (2013) Evaluation of the potential of 10 microalgal strains for biodiesel production. Bioresour Technol 141:245–251
Sudhakar K, Premalatha M, Rajesh M (2014) Large-scale open pond algae biomass yield analysis in India: a case study. Int J Sustain Energy 33:304–315
Sudhakar MP, Merlyn R, Arunkumar K, Perumal K (2016) Characterization, pretreatment and saccharification of spent seaweed biomass for bioethanol production using baker’s yeast. Biomass Bioenergy 90:148–154
Tu R, Jin W, Xi T, Yang Q, Han SF, Abomohra A (2015) Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivated in municipal wastewater. Water Res 86:132–138
Ven Den Hoeck C, Mann DG, Jahns HM (1995) Algae: an introduction to phycology Cambridge University Press
Vergara-Fernández A, Vargas G, Alarcón N, Velasco A (2008) Evaluation of marine algae as a source of biogas in a two-stage anaerobic reactor system. Biomass Bioenergy 32:338–344
Wang S, Cao B, Feng Y, Sun C, Wang Q, Abomohra AEF, Afonaa-Mensah S, He Z, Zhang B, Qian L, Xu L (2019a) Co-pyrolysis and catalytic co-pyrolysis of Enteromorpha clathrata and rice husk. J Therm Anal Calorim 135:2613–2623
Wang S, Yerkebulan M, Abomohra AEF, El-Khodary S, Wang Q (2019b) Microalgae harvest influences the energy recovery: a case study on chemical flocculation of Scenedesmus obliquus for biodiesel and crude bio-oil production. Bioresour Technol 286:121371
Wei N, Quarterman J, Jin Y-S (2013) Marine macroalgae: an untapped resource for producing fuels and chemicals. Trends Biotechnol 31:70–77
Wi SG, Kim HJ, Mahadevan SA, Yang DJ, Bae H (2009) The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol 100:6658–6660
Xu S, Elsayed M, Ismail GA, Li C, Wang S, Abomohra A (2019) Evaluation of bioethanol and biodiesel production from Scenedesmus obliquus grown in biodiesel waste glycerol: a sequential integrated route for enhanced energy recovery. Energy Convers Manag 197:111907
Zakaria HY, Radwan A, Said M (2019) Zooplankton community characteristics of the different water types in Abu Qir Bay, Alexandria, Egypt. Egypt J Aquat Res 45:131–138
Acknowledgments
Authors are grateful to the help provided by Prof. Mohamed S. Abdel-Kareem, Botany and Microbiology Department, Faculty of Science, Alexandria University. Authors confirm that there is no funding to be declared.
Author information
Authors and Affiliations
Contributions
Conceived and designed the analysis: MEHO, AMA, and AA. Sampled and identified the seaweed: MOA and MEE. Wrote the paper: MOA. Revised the paper: MEHO, AMA, and AA. Journal correspondence: AA. All authors analyzed and interpreted the results.
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3135 kb)
Rights and permissions
About this article
Cite this article
Osman, M.E.H., Abo-Shady, A.M., Elshobary, M.E. et al. Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ Sci Pollut Res 27, 32481–32493 (2020). https://doi.org/10.1007/s11356-020-09534-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09534-1