Abstract
Globally, a large number of school-aged children is suffering from water-borne diseases, particularly in low-income countries. Arsenic (As) is a hazardous and potentially carcinogenic metal(loid) in drinking water. Nowadays, alarming levels of As have been reported in the groundwater of Vehari District, Punjab Pakistan. In this study, drinking water supplies for high and higher secondary schools were examined in Vehari District. A total of 164 water samples were collected from schools and subjected to heavy metal(loid) analysis (As) and basic water physicochemical parameters. The results were analyzed with respect to sampling area, school type, school education level, sources of sample collection, and the depth of the source. The results revealed that As concentration of water samples in boys’ and girls’ schools was 12.8 μg/L and 9.2 μg/L, respectively. However, when the As concentration in drinking water was evaluated at the school education level, a notable higher concentration of As was observed in the higher secondary schools than the high schools with an average of 19.5 and 9.7 μg/L, respectively. The risk assessment indices were calculated based on education level and different age groups of the children (primary, elementary, high, and higher secondary). High carcinogenic (cancer risk = 0.001) and non-carcinogenic (hazard quotient = 2.0) risks were noted for the children in higher secondary school. The current findings anticipated that the drinking water of schools in Vehari District did not meet the requirement of the World Health Organization (WHO) drinking water quality guidelines. Safe drinking water is crucial for the development and growth of children. Therefore, it is important for educational authorities to take steps for provision of As free safe drinking water to students and local inhabitants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As), a naturally occurring element, is well-known for its high toxicity and carcinogenicity (Altundoğan et al. 2000; Asmel et al. 2017; Zhang et al. 2013). Groundwater contamination by As is a global concern and threatens the health and life of millions of people, particularly in the densely populated areas (Fakhri et al. 2018). More than 100 million people worldwide ingest an excessive amount of As through contaminated water from natural resources (Natasha et al. 2020). In Asia, millions of people consume As-contaminated groundwater and use it for irrigation as well as for domestic purposes (Mondal et al. 2013; Shahid et al. 2018b; Shakoor et al. 2015). Some countries have lower As level in groundwater (< 10 μg/L), but many countries such as Bangladesh (Islam et al. 2019), China (Zhang et al. 2019), Egypt (Embaby and Redwan 2019), India (Bhattacharya et al. 2007), Indonesia (Rochaddi et al. 2019), Nepal (Mueller and Hug 2018), and the Philippines (Canencia and Walag 2016) have higher As concentration (> 50 μg/L) in their groundwater aquifers.
Arsenic presence in shallow aquifers is widely attributed to the reductive dissolution of arsenic-bearing minerals (Ali et al. 2018; Feng et al. 2018; Hernández-Flores et al. 2018). Arsenic naturally exists in > 200 different minerals (Çiftçi and Henden 2015). Arsenic is also released into the aquifers during the anthropogenic activities such as mining and smelting of metals, industrial waste discharge, fertilizers, agricultural activities, and wastewater irrigation (Asmel et al. 2017; Hernández-Flores et al. 2018).
Recent research on As contamination in Pakistan, particularly in the groundwater, has highlighted the serious situation of As contamination in the country, notably in the area that adjoins the Indus River and its tributaries (Natasha et al. 2020; Nickson et al. 2005; Rabbani et al. 2017). Approximately, 70% of groundwater and surface water reserves in Pakistan are contaminated with biological, organic, and inorganic contaminants (Shahid et al. 2018b; Shams et al. 2018). Current scenario of As contamination of the drinking water in Pakistan showed that almost 47 million people in Pakistan are living in the areas having > 50% of the well water containing As > 10 μg/L (Shahid et al. 2018b) and 17% exceeded 50 μg/L (Fatima et al. 2018). Only 26% of the people of Pakistan have access to clean water supplies (As < 10 μg/L) (Memon et al. 2011). The hazard and risk maps of As content from 1187 groundwater samples from Pakistan indicated that Indus Basin has elevated As contamination (Podgorski et al. 2017). The high As level might be attributed to oxidative desorption with a rise in evaporative concentration process with other physicochemical parameters (Fatima et al. 2018).
Drinking As-contaminated water may induce toxic effects on human health and has been reported globally, particularly in Asian countries (Rahman et al. 2009). Smith et al. (2002) reported that the risk of cancer due to As contamination is ~ 0.7 per 100 people, whereas for other carcinogens, it ranges from 0.001 to 0.012 per 100 people (US-EPA 2010). Chronic exposure to As from drinking As-contaminated water can lead to non-carcinogenic risk such as liver damage, kidney, cancer, neurological disorders, skin lesions, and stomach problems and in severe cases may also cause death (Wang et al. 2014; Yu et al. 2013).
Literature shows that Vehari District and its adjoining areas remained under consideration of researchers owing to the high As content in the groundwater. Many studies have been conducted to explore the groundwater contamination with As in Vehari District with respect to different areas such as rural and urban areas (Shahid et al. 2017), peri-urban areas (Shah et al. 2019), rivers (Fatima et al. 2018), and health facilities (Murtaza et al. 2019b). The facility of safe drinking water in schools greatly contributes to the awareness of the right to education. The learning ability of children can be restrained if the school’s authorities do not provide access to safe drinking water. Hence, it is an urgent need, especially for the developing country to ensure safe drinking water for the children and the future of the nation. Previously in Pakistan, Gul et al. (2019) evaluated the As contamination of drinking water in high schools of Multan, Pakistan. To the best of our knowledge, no such study has been conducted to estimate the water contamination status and As levels in the drinking water of schools in Vehari District.
In this study, the drinking water from high and higher secondary schools of Vehari District was monitored to ensure safe drinking water for school children. The As contamination of the drinking water of schools along with other water quality parameters was revealed. The As data was subjected to health risk assessment of children at different education levels and was compared with the drinking water standards. The findings of this study will stimulate the educational authorities to ensure the availability of As free and safe drinking water to students and local inhabitants.
Material and methods
Description of the study area
Vehari is a part of Nili Bar, situated at latitude 29.9719°N, longitude 72.4258°E, and covers approximately 4364 km2. Vehari District consists of three tehsils, namely, Vehari, Mailsi, and Burewala (Fig. 1). In the last few decades, with the rapid urbanization and increase in agricultural activities in the district, the demand for water has increased manifold. Since the groundwater is the most dynamic natural resource for water supply, it has been extensively exploited, and the whole groundwater regime in the area has been affected.
Unconsolidated alluvial sediments form the major aquifers in the area. It was reported that the underlying sedimentary complex has been previously developed by the tributaries of the Indus River and is approximately 400 m thick. The sedimentary complex contains quartz, muscovite, and some percentage of heavy minerals. The nearby rivers such as Sutlej and Chenab are considered possible sources of aquifer recharge (Shahid et al. 2017). The climate of Vehari is hot, arid and receives < 125 mm of annual rainfall.
Groundwater sampling and preservation
The drinking water samples were collected from 164 secondary and higher secondary schools from the three tehsils covering the whole school systems of the study area. The water samples were collected from different sources (electric pumps, turbines, hand pumps, and water from filters) ranging in depth from 30 to 92 m. All of the water samples were collected with their coordinates (latitude and longitude), and their locations are shown in Fig. 1. Two samples were taken from each sampling point in properly labeled high-density polyethylene bottles. Prior to sampling, each sampling source was pumped for 10 min until it purged out approximately twice the well volume to ensure a steady chemical composition at a given location. Before sampling, the bottles were washed with dilute acid followed by tap water and finally rinsed several times with deionized (DI) water (Ryan et al. 2007). All the samples were immediately brought to the laboratory and stored at 4 °C in dark and dry conditions. All the water samples were filtered through Whatman filter paper 42 prior to analysis.
One replication was used for As content measurement analysis by stabilizing pH < 2 using concentrated nitric acid (HNO3) to prevent possible metal(loid) precipitation. The other replication was used for physicochemical and biological analysis without using any preservative.
Analytical procedures
In this study, samples were analyzed physically, chemically, and biologically. All the glassware was washed with 10% HNO3 and rinsed with deionized water (DI) to avoid contamination.
Physicochemical analysis of water samples
The water samples were analyzed for various physicochemical parameters at District Water Testing Laboratory and COMSATS University Islamabad, Vehari, campus. The analyzed indices for water quality included calcium (Ca2+), magnesium (Mg2+), carbonates (CO32−), bicarbonate (HCO3−), chloride (Cl−), nitrate (NO3−), nitrite (NO2−), fluoride (F), pH, electrical conductivity (EC), total dissolved solids (TDS), total hardness (TH), turbidity, and trace elements such as As and Fe. The pH, EC, and TDS were measured at sampling site using their respective meters, while all other parameters were measured using the ICARDA manual (Ryan et al. 2007).
Determination of arsenic and iron content in water samples
The As analysis was carried out using atomic absorption spectrometer with hydride generation (AAS-HG) (PerkinElmer Atomic Absorption Spectrometer PinAAcle 900F), where argon with a flow rate of 0.3 L min−1 was used as a carrier gas and the data was recorded at an analytical wavelength of 193.7 nm. At first, the calibration curve was made using the As standard solutions of different concentrations of the certified reference materials. The electrodeless discharge lamp (EDL) was used for atomic absorption. For the As determination, 3% NaBH4 and 1.5% HCl solutions were used. For quality assurance, 10 mL calibration volume of 1.5% HCl was used as a blank. The samples and blank were prepared in the same way and diluted with 1.5% HCl where needed.
The Fe content in the water samples was measured using a direct flame (air-acetylene) by using atomic absorption spectrophotometer (AAS) (PerkinElmer Atomic Absorption Spectrometer PinAAcle 900F) at the analytical wavelength of 372.0 nm. The AAS was calibrated with standards of Fe reference material before use. A non-contaminated water sample (DI water) was used as a blank for quality assurance. Additionally, the readings were replicated thrice for each sample by AAS.
Microbiological determination
The microbial contamination was determined by total coliform. The bacteria were quantified by membrane filtration. Ampouled m-Endo Broth (2 mL) media was used for total coliform which grows as colorless to pink colonies. About 100 mL of the drinking water sample was filtered with 0.45-μm pore size membrane filter (Millipore, MA, USA). The membranes were then placed on solid media for each bacterium. Every enumeration of bacteria and total coliform was obtained at 35 ± 2 °C, and growth was examined at 18–24 h (APHA 2001, 2005).
Human health risk assessment for arsenic
In order to better understand the critical exposure concentrations of As to school children, it is highly practical to estimate the potential human health risk for children. In this study, the data used for risk assessment was separated with respect to education level of the children in schools of different age groups (primary, 4–9 years; elementary, 10–12 years; high, 13–15; higher secondary, 16–18 years), exposure duration in terms of years, and average body weight of the children (US-EPA 2015). All the parameters used for the risk assessment have been described in Supplementary Table 1.
Average daily dose (ADD)
The ADD of As via drinking water of Vehari District was estimated using Equation (1) (Ali et al. 2019; Shah et al. 2019; Tabassum et al. 2018):
where C indicates the As concentration (mg/L) in water samples; IR represents the ingestion rate of water per day (L/day) which was 1.18, 1.55, 1.83, and 2.06 L/day at primary, elementary, high, and higher secondary level, respectively; ED specifies the exposure duration for water (primary level, 6 years; elementary level, 3 years; high level, 3 years; higher secondary level, 3 years); EF shows water exposure frequency (365 days/year); and BW is used for body weight (primary level, 24.1 kg; elementary level, 41.3 kg; high level, 59.7 kg; higher secondary level, 65.6 kg), while AT signifies average life expectancy (days). All of the data regarding consumption, body weight, and exposure of children regarding age groups was obtained from US-EPA (2005b).
Calculation of hazard quotient (HQ)
The HQ of groundwater samples due to the presence of As was estimated using Equation (2) (Antoniadis et al. 2019; Shah et al. 2019):
where RfD indicates the oral reference dose of As (0.0003 mg kg−1 day−1) (US-EPA 2005a). Water samples with HQ values < 1 are considered safe for drinking purpose (Dadar et al. 2017; Razzaghi et al. 2018).
Cancer risk (CR) assessment
The CR was estimated according to US-EPA (2005a) by using Equation (3).
where CSF indicates cancer slope factor for heavy metals. The CSF used for calculating health risk is 1.5 (mg kg−1 day−1)−1 (US-EPA 2005a). If CR is > 0.0001, exposed population will be at considerable cancer risk (Rahmani et al. 2018).
Principal component analysis
The characteristics of all the groundwater samples were subjected to principal component analysis (PCA) to understand the correlations between As contents and physicochemical properties of water samples. Multivariate statistical techniques (PCA, factor analysis, and cluster) were considered useful tools to develop a correlation and identify the key factors contributing to the data structure. These techniques have been widely used previously in a number of studies (Natasha et al. 2018; Shah et al. 2019; Tabassum et al. 2018).
Spatial analysis
The inverse distance weighted (IDW) method of Arc. GIS (Version 10.4.1) was used for spatial analysis of As distribution. The IDW interpolation estimates unknown values with specific distance on the basis of closest points.
Results and discussion
Arsenic contamination status of groundwater
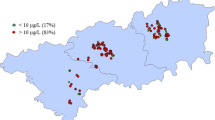
Figure 2a and b show variation in drinking water As concentration with respect to school type and school level. The mean As concentration in the water samples of schools of Vehari District was 11 μg/L, slight higher than the WHO recommended permissible level of As in drinking water (10 μg/L). The As concentration of water samples in boys’ schools was 12.8 μg/L; however, the girls’ schools showed low As concentration (9.2 μg/L) in the drinking water. Similar results of As level in groundwater (9 μg/L) have been reported by Tabassum et al. (2019) in Hasilpur (a tehsil adjacent to Vehari District).
In contrast, when the As concentration in drinking water was evaluated on the school education level, a notable higher concentration of As was observed in the higher secondary schools than the high schools with an average of 19.5 and 9.7 μg/L, respectively. Among all, the maximum As concentration (100 μg/L) was observed in the girls’ high school which was beyond the Pakistan drinking standard guideline (50 μg/L) and has been declared unfit for the children. When As concentration in these schools was evaluated at tehsil level, remarkable variation was observed with a mean As concentrations of 12.7, 15.1, and 6.6 μg/L, respectively, in Tehsil Vehari, Mailsi, and Burewala (Fig. 2).
Arsenic concentration also varies with respect to source and depth of the collected water samples (Fig. 3). Comparison of As concentration in drinking water in terms of sampling depth showed that the average As remained < 10 μg/L except for the samples collected at a depth of ≤ 100 ft showing the mean As concentration of 13 μg/L. Among the sampling sources, it was strangely noticed that the water samples collected from water supplies and filter water contained high As contents (17 μg/L) compared with the samples from electric pumps (10 μg/L) and turbines (11 μg/L). However, only one school used hand pump extracted water for drinking purpose which showed the maximum As concentration of 50 μg/L. Some previous studies in Vehari also reported variation in As level with respect to sampling source and depth (Murtaza et al. 2019a; Shahid et al. 2017; Tabassum et al. 2019).
Magnitude of water samples exceeding their respective thresholds
The As content of the collected drinking water samples was compared with the permissible limits proposed by different environmental agencies: 5 μg/L (DEP-NJ), 10 μg/L (WHO), and 50 μg/L (Pak-EPA). The percentage of water samples with respect to school types, school level, source, depth, and tehsils exceeding the permissible limits is presented in Table 1. From the gender perspective, 99% of the water samples from both girls’ and boys’ school were fit for drinking purpose having As concentration of < 50 μg/L. However, when compared with the permissible limits of the WHO and DEP-NJ, 17% and 33% of the water samples from girls’ school were unfit for drinking, respectively, while 44% and 28% water samples were unfit from boys’ schools having concentration of > 5 μg/L and 10 μg/L, respectively. For high schools, 34% samples have As contents of ≥ 5 μg/L, 18% samples have As contents of ≥ 10 μg/L, and only 1% samples have As contents of ≥ 50 μg/L. In higher secondary schools, 65% of samples contained As contents of ≥ 5 μg/L and 52% of samples showed As level of ≥ 10 μg/L, while none of the samples has As concentration of ≥ 50 μg/L. Recently, Shah et al. (2019) revealed that the As level was higher in 85%, 83%, and 42% of water samples compared with safe limits of the DEP-NJ, WHO, and Pak-EPA, respectively.
At tehsil level, 36%, 50%, and 31% of water samples from Tehsil Vehari, Mailsi, and Burewala, respectively, showed As content higher than the permissible limit of 5 μg/L (DEP-NJ). However, the water samples from the three tehsils showed 24%, 33%, and 12% higher As content than the WHO threshold limit (10 μg/L). Interestingly, only 4% of the water samples from Tehsil Vehari exceeded the threshold value of 50 μg/L, while none of the samples from Tehsil Burewala and Mailsi had As concentration of > 50 μg/L.
The As content in water samples of schools was also evaluated as function of sampling source and depth with the permissible limits (Table 1). It was found that 40%, 22%, and 1% of the water samples from electric pump showed As concentration higher than the limits of 5 μg/L, 10 μg/L, and 50 μg/L, respectively. About 30% of the water samples collected from the turbines have higher As concentration than 5 μg/L and 10 μg/L limits. However, none of the samples have As concentration higher than 50 μg/L. It was noticed that 71%, 86%, and 86% of the filter water samples showed high As concentration when compared with the limits of the DEP-NJ, WHO, and Pak-EPA, respectively. Only one water sample was extracted from hand pump, and the As concentration in this sample was <5 μg/L. None of the water samples collected from a depth of > 300 ft showed As concentration higher than the permissible limit. The concentration of As in water samples collected at a depth of < 100 ft showed that the As concentration is higher than the limits of the DEP-NJ, WHO, and Pak-EPA, respectively, by 42%, 29%, and 2%. The samples collected from the depth of 101–300 ft had As concentration of > 5 μg/L and 10 μg/L by 39% and 22%, respectively (Table 1).
Comparison with previous studies
In the recent past, As distribution in soil and water systems has gained significant attention of the researchers, policy makers, and the environmental organizations due to its high carcinogenic and persistent nature (Natasha et al. 2020; Shahid et al. 2018b). Recently, Natasha et al. (2020) reported that about 50–60 million habitants of Pakistan are drinking As-rich water and are at serious risk of As poisoning. Kazi et al. (2009) reported that in Sindh province of Pakistan, 61–73% of the population has been suffering from chronic As toxicity.
Several studies have evaluated the groundwater As contamination status of Vehari District, Pakistan. However, no study has been yet reported the drinking water contamination status of schools in the study area. In this study, we have collected and compared the As contamination of drinking water in schools on a broad level (internationally) and the groundwater contamination of the area at a local level (Vehari District) (Table 2, Supplementary Table 2).
This study first time identified the As contamination level in groundwater of high and higher secondary schools of Vehari District and it was found that the water samples exceeded the WHO safe limit. Some of the previous studies have reported the high As contamination of drinking water of the schools, globally (Table 2). The results indicated that As contamination in groundwater is a local, national, and global health problem. The CR associated with the ingestion of As-contaminated drinking water is also highlighted in Table 2 showing a range of 0.0–0.012.
Exposure assessment
A thorough understanding of As contamination of drinking water of schools is fundamental to estimate the children health risk and its possible impact on their education and learning abilities. Arsenic enters the human body through several pathways including oral intake, inhalation, and dermal contact, but oral intake via ingestion is the most common pathway (Natasha et al. 2020; Shah et al. 2019). Currently, risk assessment is highly notable due to increased awareness of ecotoxicology and associated health hazards among the local community (Li et al. 2018; Mandal et al. 2019; Natasha et al. 2020). In this regard, environmental organizations have become more concerned about ecotoxicology, food and water security, and health risks associated with As. In this study, carcinogenic and non-carcinogenic risk was calculated to trace out possible transfer of As and predict possible health hazards to the school children due to the ingestion of As-contaminated water.
Arsenic health risk assessment in drinking water of schools was evaluated in terms of education level and age groups. Four education levels are common in the study area, namely, primary, elementary, high, and higher secondary. The carcinogenic and non-carcinogenic assessment was calculated on the basis of exposure duration of the children at each level.
The values of ADD were 0.0005, 0.0004, 0.0003, and 0.0006, respectively, for primary, elementary, high, and higher secondary school education level (Table 3). Moreover, the HQ values remained > 1 for all the education levels (1.1–2.0). The cancer risk assessment indices indicated high cancer risk for all of the students at every level due to high As contamination of the drinking water. The maximum CR was observed for the higher secondary school due to the long-term exposure to As. Among the four education levels, the carcinogenic and non-carcinogenic high risk was observed for the children in higher secondary standards owing to the longest duration of their exposure. The order of risk was Higher sec. > Primary > Elementary > High schools (Table 3). Previously, many studies have highlighted the carcinogenic and non-carcinogenic risk due to the ingestion of As-contaminated drinking water in the study area (Shahid et al. 2017, 2018a). However, none of the studies indicated the risk to the school children and its impact on their nourishment and education.
Hydrogeochemistry of the study area
The values of physicochemical parameters of water samples were evaluated with respect to school type, school level, and area (Table 4, Supplementary Table 3). All the physicochemical parameters were compared with the threshold values of the WHO, and the results are presented in Supplementary Table 5.
The pH values of the water samples ranged between 6.7 and 7.98, and none of the water samples has pH value greater than permissible limit of the WHO (6.5–8.5). There was a slight variation in pH value in the water samples of girls’ and boys’ school as well as from high and higher secondary schools. The EC value of all the samples ranged between 300 and 4340 μS/cm. It was seen that 12%, 19%, 22%, and 9% water samples from girls, boys, high, and higher secondary school have EC > WHO limit value of 2000 μS/cm. The EC and TDS values are function of soluble salts and ions. About 21% of the water samples from girls’ and boys’ school have TDS value > WHO limit (1000 mg/L). Moreover, the TDS evaluation on school level basis showed that TDS value of the water samples was 39% and 26% higher than the limit value for high and higher secondary school, respectively.
The HCO3 concentration in water samples was 11%, 15%, 17%, and 20% higher than the WHO limit of 500 mg/L in girls, boys, high, and higher secondary school, respectively. The Ca concentration in the water samples ranged between 0.36 and 400 mg/L. The value of Ca in water samples should be less than 200 mg/L (WHO). Only 1% of the water samples from girls’ and boys’ school showed Ca higher than the limit value, while 13% and 7% of the water samples from high and higher secondary schools were higher in Ca concentration than 200 mg/L. The limit value of Mg set by the WHO is 150 mg/L. It was observed that none of the water samples showed Mg concentration higher than 150 mg/L.
According to the WHO (2011), the Cl− concentration in the water samples should not exceed 250 mg/L. The Cl− concentration in water samples was 12%, 8%, 35%, and 11% higher than the limit value in girls, boys, high, and higher secondary school, respectively. A few samples (4%, 2%, 4%, and 1%) from girls, boys, high, and higher secondary school have F concentration higher than the permissible limit of 1.5 mg/L (WHO), and the F concentration in water samples remained in the range of 0.0 to 2.1 mg/L. None of the water samples showed NO3− and NO2− concentration higher than the limit value of 50 mg/L and 3 mg/L, respectively (Table 5). The limit value of Fe in the water samples is 0.3 mg/L. The water samples showed the Fe concentration between 0.0 and 0.93 mg/L. The Fe concentration in water samples was 5%, 11%, and 13% higher than the limit value, respectively, from girls, boys, and higher secondary school. None of the water samples from high school showed Fe concentration > the threshold level.
A piper diagram is presented in Fig. 4 by using the chemical data of groundwater samples. The piper plots have two triangles, first for anions and the second for cations. The results indicate that groundwater aquifers were dominated by Na, Ca, and K among cations, while mainly HCO3 followed by SO4 and Cl among anions. The concentration of cations in groundwater samples presented the following trend Na < Ca < Mg < K, while the anions were in the pattern of HCO3 < SO4 < Cl < CO3. A similar trend of cations was reported by Ahmad and Bhattacharya (2018) in the Indus River Basin of Pakistan. The groundwater chemistry of schools using piper plot was Na–SO4/HCO3, Ca–SO4/Na–Mg–SO4, and Na–/SO4/CO3/Cl (saline water) type (Fig. 4). Furthermore, the groundwater of aquifer showed conservative mixing behavior regarding Na- and Ca-dominated groundwater. The sources of saline water are Na- and Ca-containing mineral phase dissolution, geochemical composition of the sediment, and shallow groundwater leakage (Luo et al. 2018). However, Abbas et al. (2015) showed the predominance of Ca–Mg–SO4–HCO3, Ca–HCO3–SO4, Ca–Mg–HCO3–SO4, Ca–Mg–HCO3–SO4–Cl, and Mg–Ca–HCO3–SO4 water types which revealed that silicate and carbonate weathering controlled the hydrochemistry of the groundwater.
The total coliform value ranged from 0 to 35 MPN/100 mL in all the samples. The standard value for total coliform in drinking water is 0 MPN/100 mL of water. About 30% of the water samples showed that the coliform values are higher than the limit value.
Principal component analysis
The principal component analysis (PCA) was applied to explore the correlation between As and other physicochemical parameters of drinking water (Shahid et al. 2018c). In PCA, all the variables were divided into four major factors (F1–F4) (Supplementary Table 6). The factor F1 showed the major contribution (62.9%) to the total variance. Factor loading graph of PCA formulated two major groups (Fig. 5). The PCA graph has combined As, EC, TDS, TH, Ca, HCO3, NO3, and F in one group indicating a strong positive correlation among these variables. The pH and As content was negatively correlated. The correlation matrix developed a strong correlation between As and other physicochemical parameters (EC, TDS, TH, Ca, Mg, Cl, F) (Supplementary Table 7). Supplementary Fig. 1 showed the eigenvalues and cumulative variability of the water samples data for the schools of Vehari District. Similar to our findings, many studies have highlighted a strong correlation of As with other water quality parameters (Shah et al. 2019; Tabassum et al. 2018). However, this may significantly vary with respect to collection area, sampling source, and sampling depth.
Conclusion
The present study has provided source and depth specific drinking water quality of high and higher secondary schools of Vehari District. The results revealed that drinking water in schools of Tehsil Mailsi, Burewala, and Vehari is not safe for children due to elevated levels of As according to the WHO guidelines. About 70% drinking water samples from 164 schools contained As concentration of > 10 μg/L. The groundwater As contamination level was high in boys school (28%), higher secondary schools (52%), Tehsil Mailsi (33%), and for filter water sources (86%). Moreover, the physicochemical parameters of water did not meet the guidelines standards of the WHO adding more to potential toxicity. Arsenic-contaminated groundwater in the schools of Vehari District can pose a severe carcinogenic health risk to the exposed children via oral consumption (CR; 0.0005–0.0009). Multivariate analysis revealed a positive correlation of groundwater As contents with EC, Ca, Mg, Cl, and F. Therefore, the school authorities and relevant government departments should pay more attention to the unsafe drinking water quality and provide an access to safe drinking water for the school children and the staff as well.
References
Abbas Z, Su C, Tahira F, Mapoma HWT, Aziz SZ (2015) Quality and hydrochemistry of groundwater used for drinking in Lahore, Pakistan: analysis of source and distributed groundwater. Environ Earth Sci 74:4281–4294
Ahmad A, Bhattacharya P (2018) Arsenic contamination of groundwater in Indus River Basin of Pakistan. In: Mukherjee A (ed) Groundwater of South Asia. Springer Singapore, Singapore, pp 393–403
Ali S, Fakhri Y, Golbini M, Thakur SK, Alinejad A, Parseh I, Shekhar S, Bhattacharya P (2019) Concentration of fluoride in groundwater of India: a systematic review, meta-analysis and risk assessment. Groundw Sustain Dev 100224
Ali W, Rasool A, Junaid M, Zhang H (2018) A comprehensive review on current status, mechanism, and possible sources of arsenic contamination in groundwater: a global perspective with prominence of Pakistan scenario. Environ Geochem Health:1–24
Altundoğan HS, Altundoğan S, TuÈmen F, Bildik M (2000) Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag 20:761–767
Antoniadis V, Shaheen S, Levizou E, Shahid M, Niazi N, Vithanage M, Ok Y, Bolan N, Rinklebe J (2019) A critical prospective analysis of the potential toxicity of trace element regulation limits in soils worldwide: are they protective concerning health risk assessment? - a review. Environ Int 127:819–847
APHA (2001) Compendium of methods for the microbiological examination of foods, 4th ed. Pouch DF, Ito K (eds) American Public Health Association, Washington, DC
APHA (American Public Health Association) (2005) Standard methods for the examination of water and wastewater. 21st ed. American Public Health Association. Washington DC
Asmel NK, Yusoff ARM, Krishna LS, Majid ZA, Salmiati S (2017) High concentration arsenic removal from aqueous solution using nano-iron ion enrich material (NIIEM) super adsorbent. Chem Eng J 317:343–355
Bhattacharya P, Welch AH, Stollenwerk KG, McLaughlin MJ, Bundschuh J, Panaullah G (2007) Arsenic in the environment: biology and chemistry. Elsevier
Canencia OP, Walag AMP (2016) Coal combustion from power plant industry in Misamis Oriental, Philippines: a potential groundwater contamination and heavy metal detection. Asian J Microbiol Biotechnol Environ Sci 18:55–59
Çiftçi TD, Henden E (2015) Nickel/nickel boride nanoparticles coated resin: a novel adsorbent for arsenic (III) and arsenic (V) removal. Powder Technol 269:470–480
Dadar M, Adel M, Nasrollahzadeh Saravi H, Fakhri Y (2017) Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Rev 36:222–227
Embaby A, Redwan M (2019) Sources and behavior of trace elements in groundwater in the South Eastern Desert, Egypt. Environ Monit Assess 191:686
Fakhri Y, Mohseni-Bandpei A, Oliveri Conti G, Ferrante M, Cristaldi A, Jeihooni AK, Karimi Dehkordi M, Alinejad A, Rasoulzadeh H, Mohseni SM, Sarkhosh M, Keramati H, Moradi B, Amanidaz N, Baninameh Z (2018) Systematic review and health risk assessment of arsenic and lead in the fished shrimps from the Persian gulf. Food Chem Toxicol 113:278–286
Fatima S, Hussain I, Rasool A, Xiao T, Farooqi A (2018) Comparison of two alluvial aquifers shows the probable role of river sediments on the release of arsenic in the groundwater of district Vehari, Punjab, Pakistan. Environ Earth Sci 77:382
Feng C, Aldrich C, Eksteen J, Arrigan D (2018) Removal of arsenic from gold cyanidation process waters by use of cerium-based magnetic adsorbents. Miner Eng 122:84–90
Gul M, Mashhadi AF, Iqbal Z, Qureshi TI (2019) Monitoring of arsenic in drinking water of high schools and assessment of carcinogenic health risk in Multan, Pakistan. Hum Ecol Risk Assess:1–13
Hernández-Flores H, Pariona N, Herrera-Trejo M, Hdz-García HM, Mtz-Enriquez AI (2018) Concrete/maghemite nanocomposites as novel adsorbents for arsenic removal. J Mol Struct 1171:9–16
Islam ME, Reza AS, Sattar GS, Ahsan MA, Akbor MA, Siddique MAB (2019) Distribution of arsenic in core sediments and groundwater in the Chapai Nawabganj district, Bangladesh. Arab J Geosci 12:99
Kazi TG, Arain MB, Baig JA, Jamali MK, Afridi HI, Jalbani N, Sarfraz RA, Shah AQ, Niaz A (2009) The correlation of arsenic levels in drinking water with the biological samples of skin disorders. Sci Total Environ 407:1019–1026
Li R, Kuo Y-M, Liu W-w, Jang C-S, Zhao E, Yao L (2018) Potential health risk assessment through ingestion and dermal contact arsenic-contaminated groundwater in Jianghan Plain, China. Environ Geochem Health:1–15
Luo W, Gao X, Zhang X (2018) Geochemical processes controlling the groundwater chemistry and fluoride contamination in the Yuncheng Basin, China—An area with complex hydrogeochemical conditions. PLoS One 13
Mandal U, Singh P, Kundu AK, Chatterjee D, Nriagu J, Bhowmick S (2019) Arsenic retention in cooked rice: effects of rice type, cooking water, and indigenous cooking methods in West Bengal, India. Sci Total Environ 648:720–727
Memon M, Soomro MS, Akhtar MS, Memon KS (2011) Drinking water quality assessment in Southern Sindh (Pakistan). Environ Monit Assess 177:39–50
Mondal P, Bhowmick S, Chatterjee D, Figoli A, Van der Bruggen B (2013) Remediation of inorganic arsenic in groundwater for safe water supply: a critical assessment of technological solutions. Chemosphere 92:157–170
Mueller B, Hug SJ (2018) Climatic variations and de-coupling between arsenic and iron in arsenic contaminated ground water in the lowlands of Nepal. Chemosphere 210:347–358
Murtaza B, Amjad M, Shahid M, Imran M, Shah NS, Abbas G, Naeem MA (2019a) Compositional and health risk assessment of drinking water from health facilities of District Vehari, Pakistan. Environ Geochem Health:1–13
Murtaza B, Natasha AM, Shahid M, Imran M, Shah NS, Abbas G, Naeem MA (2019b) Compositional and health risk assessment of drinking water from health facilities of District Vehari, Pakistan. Environ Geochem Health:1–13
Natasha SM, Dumat C, Khalid S, Rabbani F, Farooq ABU, Amjad M, Abbas G, Niazi NK (2018) Foliar uptake of arsenic nanoparticles by spinach: an assessment of physiological and human health risk implications. Environ Sci Pollut Res Int 26:20121–20131
Natasha SM, Imran M, Khalid S, Murtaza B, Niazi NK, Zhang Y, Hussain I (2020) Arsenic environmental contamination status in South Asia. In: Srivastava S (ed) Arsenic in drinking water and food. Springer Singapore, Singapore, pp 13–39
Nickson R, McArthur J, Shrestha B, Kyaw-Myint T, Lowry D (2005) Arsenic and other drinking water quality issues, Muzaffargarh District, Pakistan. Appl Geochem 20:55–68
Podgorski JE, Eqani SAMAS, Khanam T, Ullah R, Shen H, Berg M (2017) Extensive arsenic contamination in high-pH unconfined aquifers in the Indus Valley. Sci Adv 3:e1700935
Rabbani U, Mahar G, Siddique A, Fatmi Z (2017) Risk assessment for arsenic-contaminated groundwater along River Indus in Pakistan. Environ Geochem Health 39:179–190
Rahman MM, Naidu R, Bhattacharya P (2009) Arsenic contamination in groundwater in the Southeast Asia region. Environ Geochem Health 31:9–21
Rahmani J, Fakhri Y, Shahsavani A, Bahmani Z, Urbina MA, Chirumbolo S, Keramati H, Moradi B, Bay A, Bjørklund G (2018) A systematic review and meta-analysis of metal concentrations in canned tuna fish in Iran and human health risk assessment. Food Chem Toxicol 118:753–765
Razzaghi N, Ziarati P, Rastegar H, Shoeibi S, Amirahmadi M, Conti GO, Ferrante M, Fakhri Y, Mousavi Khaneghah A (2018) The concentration and probabilistic health risk assessment of pesticide residues in commercially available olive oils in Iran. Food Chem Toxicol 120:32–40
Rochaddi B, Atmodjo W, Satriadi A, Suryono CA, Irwani I, Widada S (2019) The heavy metal contamination in shallow groundwater at coastal Ares of Surabaya East Java Indonesia. Jurnal Kelautan Tropis 22:69–72
Ryan J, Estefan G, Rashid A (2007) Soil and plant analysis laboratory manual. ICARDA
Shah AH, Shahid M, Khalid S, Natasha SZ, Bakhat HF, Murtaza B, Farooq A, Akram M, Shah GM, Nasim W, Niazi NK (2019) Assessment of arsenic exposure by drinking well water and associated carcinogenic risk in peri-urban areas of Vehari, Pakistan. Environmental Geochemistry and Health
Shahid M, Khalid M, Dumat C, Khalid S, Niazi NK, Imran M, Bibi I, Ahmad I, Hammad HM, Tabassum RA (2017) Arsenic level and risk assessment of groundwater in Vehari, Punjab Province, Pakistan. Expos Health:1–11
Shahid M, Khalid M, Dumat C, Khalid S, Niazi NK, Imran M, Bibi I, Ahmad I, Hammad HM, Tabassum RA (2018a) Arsenic level and risk assessment of groundwater in Vehari, Punjab Province, Pakistan. Exposure Health 10:229–239
Shahid M, Niazi NK, Dumat C, Naidu R, Khalid S, Rahman MM, Bibi I (2018b) A meta-analysis of the distribution, sources and health risks of arsenic-contaminated groundwater in Pakistan. Environ Pollut 242:307–319
Shahid M, Pinelli E, Dumat C (2018c) Tracing trends in plant physiology and biochemistry: need of databases from genetic to kingdom level. Plant Physiol Biochem 127:630–635
Shakoor M, Niazi N, Bibi I, Rahman M, Naidu R, Dong Z, Shahid M, Arshad M (2015) Unraveling health risk and speciation of arsenic from groundwater in rural areas of Punjab, Pakistan. Int J Environ Res Public Health 12:12371–12390
Shams W, Siraj U, Rehman G, Ullah Z, Ahmad N, Miraj M, Ullah A, Niaz S, Khan K, Alam H, Gul N, Naz T, Islam S, Khan AJ, Khan K, Jamil A (2018) Physiochemical and biological properties of water of Khyber Paktun Khwa District Bannu, Pakistan 2014. Int J Photochem Photobiol 2:12–15
Smith AH, Lopipero PA, Bates MN, Steinmaus CM (2002) Arsenic epidemiology and drinking water standards. American Association for the Advancement of Science
Tabassum RA, Shahid M, Dumat C, Niazi NK, Khalid S, Shah NS, Imran M, Khalid S (2018) Health risk assessment of drinking arsenic-containing groundwater in Hasilpur, Pakistan: effect of sampling area, depth, and source. Environ Sci Pollut Res Int
Tabassum RA, Shahid M, Dumat C, Niazi NK, Khalid S, Shah NS, Imran M, Khalid S (2019) Health risk assessment of drinking arsenic-containing groundwater in Hasilpur, Pakistan: effect of sampling area, depth, and source. Environ Sci Pollut Res 26:20018–20029
US-EPA (2005a) Guidelines for carcinogen risk assessment; EPA/630/P-03/001F. Risk Assessment Forum, Washington, DC, USA
US-EPA (2005b) Guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants. Risk Assessment Forum Washington, DC., pp. 50, 397 K, November 2005, 630-P-03-003F
US-EPA (2010) Toxicological review of inorganic arsenic (Cancer), EPA/635/R-10/001
US-EPA (2015) Exposure parameters. United States Environmental Protection Agency. https://www.epa.gov/sites/production/files/2015-11/documents/exposureparams.pdf
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014) Removal of As (III) and As (V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131
WHO (2011) Guidelines for quality drinking-water. In: Organization WH (Hrsg)
Yu L, Peng X, Ni F, Li J, Wang D, Luan Z (2013) Arsenite removal from aqueous solutions by γ-Fe2O3–TiO2 magnetic nanoparticles through simultaneous photocatalytic oxidation and adsorption. J Hazard Mater 246:10–17
Zhang G, Ren Z, Zhang X, Chen J (2013) Nanostructured iron (III)-copper (II) binary oxide: a novel adsorbent for enhanced arsenic removal from aqueous solutions. Water Res 47:4022–4031
Zhang Y, Xu B, Guo Z, Han J, Li H, Jin L, Chen F, Xiong Y (2019) Human health risk assessment of groundwater arsenic contamination in Jinghui irrigation district, China. J Environ Manag 237:163–169
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 52 kb)
Rights and permissions
About this article
Cite this article
Murtaza, B., Nazeer, H., Natasha et al. Hydrogeochemical investigation of arsenic in drinking water of schools and age dependent risk assessment in Vehari District, Punjab Pakistan: a multivariate analysis. Environ Sci Pollut Res 27, 30530–30541 (2020). https://doi.org/10.1007/s11356-020-09334-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09334-7