Abstract
In this work, earthworm effect on the efficiency of biobeds for glyphosate degradation was studied. Three biomixtures with and without the addition of earthworms (Eisenia fetida species) were evaluated. The initial concentration of glyphosate was 1000 mg/kg biomixture. Glyphosate and biological parameters were measured as a function of time. Earthworm survival, biomass, and reproduction were evaluated as well. All biomixtures that contain earthworms reached 90% of glyphosate degradation at 90 days in comparison with the biomixtures without earthworms that reached 80% approximately at the same time. Also, within the biomixtures that contained earthworms, glyphosate degradation rate was significantly higher in the one made up with soil and wheat stubble (Ws-E) showing excellent capacity for aminomethylphosphonic acid (AMPA) degradation, the main metabolite of glyphosate degradation. In addition, a study performed after the vermiremediation process showed that E. fetida can tolerate high glyphosate concentration without modifications in its life traits. It can be concluded that the use of E. fetida within the biobeds is an excellent combination to improve glyphosate and AMPA removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vermicomposting is the process by which earthworms are employed to transform organic materials into humus-like material known as vermicompost. The vermicompost generated is a valuable plant growth medium or soil amendment, and several wastes as crop residue and pig and poultry manure have been tested in the vermicomposting process (Gupta and Garg 2009).
Rodríguez-Campos et al. (2014) reviewed the potential use of earthworms in a commonly named vermiremediation process. Certainly, earthworms can be used in remediation of contaminated soils or wastes, due to their ability to improve the removal of pollutants, such as pesticides, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, crude oil, and metals (Rorat et al. 2017; Binet et al. 2006; Contreras-Ramos et al. 2008). Earthworms have been shown to aerate soils and improve nutrient availability and fertility, which are variables known to limit bioremediation. Indigenous microorganisms have the capacity to degrade pollutants from soil, but their mobility is limited. Earthworms increase the contact between the pollutant and the soil microorganisms since desorption of contaminants can occur as the soil passes through the earthworm’s gut. Also, they promote and disperse organic contaminant-degrading microorganisms (Hickman and Reid 2008; Rodríguez-Campos et al. 2014).
Earthworm species at high densities, such as Eisenia fetida and Lumbricus terrestris, seems to be effective to remove high concentrations of herbicides when organic wastes are added (Tejada and Masciandaro 2011). The natural habitat of E. fetida is organic matter; E. fetida is a compost species that consume organic waste (Rodríguez-Campos et al. 2014). This behavior would favor their growth and reproduction in substrates made up of soil and organic wastes as straw and peat, such as biobeds.
Torstensson and del Castillo (1997) developed first biobeds in Sweden. Biobeds are low-cost technologies developed to treat wastewater containing a high concentration of pesticides. Contamination is produced during agricultural activities (pesticide formulation dilution, filling the spraying tank with pesticides, washing the spraying tank, etc.).
Biobeds employ a biologically active mixture where pesticides are removed from the wastewater by sorption and biodegradation. It was originally built, employing peat (25%), wheat straw (50%), and agricultural soil (25%), and covered by grass. Degradation occurs mainly by the microbiota developed in the biomixture (Cooper et al. 2016). In addition, the process of adsorption also takes place into the biobeds (Castillo et al. 2008).
Biobeds have been used by several European countries for approximately 20 years proven its efficiency. In Latin America, the first studies on biobeds began in Chile in 2013, continuing in other countries such as Argentina, Brazil, Costa Rica, Uruguay, and México (Dias et al. 2020). In Argentina, the first studies published were made, employing different local materials for glyphosate degradation (Lescano et al. 2018; Masin et al. 2018). It is crucial to select and prove the local materials present in each country and the pesticides used to develop these kinds of systems.
In 2014, the global agricultural use of glyphosate reached 79,000 tons (Myers et al. 2016). The genetically modified crops resistant to glyphosate in Argentina have been growing steadily, reaching 22 million ha nowadays. Around 200 million l of this herbicide is applied every year in Argentina, and its residues are often found in the environment (soil, water, and food) (Baier et al. 2017; Mac Loughlin et al. 2020). This intensive use has generated concerns about its effects on the environment, as well as on human health issues. A new concern has emerged about glyphosate, which possibly relates to carcinogenicity (Myers et al. 2016). Therefore, it is very important to have a system to treat glyphosate and aminomethylphosphonic acid (AMPA) (its main metabolite) since it generates a huge amount of wastes attributable to direct losses as spillages resulting from the filling operation, leakages of the spray equipment, etc. The implementation of biobeds is a suitable option to reduce contamination from point sources generated by these and other agrochemicals.
As we stated before, in our previous work, the degradation of high glyphosate concentration was evaluated in biomixtures containing local materials: alfalfa straw, wheat stubble, river waste, and soil. We conclude that these materials showed higher glyphosate degradation capacity compared to the soil alone. Also, the biomixture made up of soil and wheat stubble (Ws) showed the highest glyphosate degradation rate (Lescano et al. 2018). In addition to this, the innocuousness of biomixtures employed for glyphosate degradation was tested using E. fetida and the results allowed the identification of several biomixtures for good maintenance and development of E. fetida (Masin et al. 2018).
In this context, the addition of earthworms in biomixtures could be a way to improve pesticide degradation. The objective of this paper was to evaluate the glyphosate degradation, employing different biomixtures prepared with local materials in the presence of E. fetida. In all biomixtures, glyphosate degradation was followed with time and biological activity, as fluorescein diacetate (FDA) hydrolysis was also followed. In order to evaluate the performance of E. fetida in the vermiremediation assays, the following parameters were measured: earthworm survival, biomass, and reproduction. For comparison, soil alone and biomixtures without earthworms were run as controls.
Materials and methods
Earthworm culture conditions
Adult, clitellated earthworms of E. fetida (mean body weight 300 ± 25 mg) were cultured in the Ecotoxicology Laboratory of INTEC. Breeding conditions were as follows: 25 ± 2 °C, constant artificial light, moisture around 60% by mass, and weekly feeding following the methodology detailed in Masin and Rodríguez (2012).
Preparation of biomixtures for the vermiremediation process
The biomixtures used were a mixture of an agricultural soil with alfalfa straw/wheat stubble, as lignocellulosic substrates, and river waste. The soil and agricultural crop residues were collected from a field in the north of Santa Fe Province, Argentina (29° 42′ 59″ S and 60° 5′ 35″ W). Physiochemical properties of both materials are shown in Tables 1 and 2. It is very important to use the local materials where the biobed will be installed mainly because the local soil is microbiological adapted to degrade certain pesticides (De Wilde et al. 2007). In this case, the soil used has more than 20 years of continuous soybean cultivation where glyphosate was applied. The river waste is a commercial product that consists of an accumulation of plant residues and was purchased in a vivarium (Santa Isabel, Santa Fe, Argentina).
Lignocellulosic materials were chopped into a particle size of 2 to 3 cm approximately, and river waste was used without any treatment.
The biobeds were prepared in the volume relation shown in Table 3, and 15 L of biomixture was placed in boxes (24 cm × 16 cm × 9 cm). Moisture was adjusted to 60–70%. Room temperature was 25 ± 2 °C, and the illumination was constant (Organization for Economic Cooperation and Development (OECD) 2004) (with slight modifications). Soil as the only component and soil with the addition of earthworms were used as controls.
The biomixtures were maturated for 100 days. This maturation or composting period was tested through the analysis of earthworm survival in the biomixtures since raw organic waste may be harmful to these organisms (Al-Maliki and Scullion 2013; Masin et al. 2018). The commercial glyphosate was sprayed over the biomixtures at a concentration of 1000 mg glyphosate/kg dry biomixture. This concentration was selected according to the residues produced in the area on the farm related to the rinsing of empty containers and water belonging from spray tank washing (De Wilde et al. 2007; Lescano et al. 2018). Twenty E. fetida adult individuals were added to the biomixtures As-E, Ws-E, and AsRw (Table 3) and to the box that contains soil alone.
The experiment last 90 days, and samples were taken immediately after glyphosate application (day 0) and after 15 days, 30 days, 60 days, and 90 days.
AMPA and glyphosate concentration and FDA were determined as a function of time. Also, yeast, fungi, and total viable mesophilic bacteria were estimated at the beginning and at the end of the assay.
In biomixtures containing earthworms, three additional parameters were measured: survival (live adult organisms/total), adults biomass (wet weight in g), and reproduction (cocoons and juveniles number). These parameters were recorded at 0 day, 15 days, 30 days, 60 days, and 90 days.
All assays were conducted using three independent replicates. A scheme describing experimental assays and all parameters registered is shown in Fig. 1.
Reagents
Formulated glyphosate (N-(phosphonomethyl)glycine salt, 35.6% as acid active compound) was purchased from Eskoba®, Red Surcos, Argentina; p-toluenesulfonyl chloride, AMPA, and glyphosate standards (fluorescein diacetate, fluorescein sodium salt) were purchased from Sigma-Aldrich.
Extraction and pesticide analysis
Glyphosate and AMPA extraction and quantification were carried out according to Lescano et al. (2018). KH2PO4 0.1 M was used as the extracting solvent (relation biomixture-solvent 1:2). The derivatization procedure (based on Kawai et al. 1991) after AMPA and glyphosate extraction was carried out, employing p-toluenesulfonyl chloride (TsCl). After the derivatization procedure, the sample was analyzed by HPLC using an HPLC-UV Waters® chromatograph equipped with an YMC-Triart C18 column and a Waters 2489 UV–Vis detector (detection wavelength = 240 nm). Phosphate buffer (NaH2PO4 0.2 M; pH = 2.3):acetonitrile (85:15 v/v) was used as the mobile phase. AMPA and glyphosate recovery values ranged from 70 to 80%, and the limit of detection (LOD) was 10 mg/L for both compounds.
Biological activity and microbial community
FDA method was performed according to Schnürer and Rosswall (1982), including some adaptations (Lescano et al. 2018). The microbial community was estimated by the plate count method (Bórtoli et al. 2012; Ratcliff et al. 2006). More details can be found in Lescano et al. (2018).
Earthworm recovery after the vermiremediation process
After vermiremediation process, a new study was done in order to evaluate earthworm recovery and performance in the biomixtures As-E, Ws-E, and AsRw-E and in Soil-E. In this case, earthworms were fed weekly following the methodology detailed in Masin and Rodríguez (2012). The parameters studied were adult biomass (wet weight in g) and reproduction (number of cocoons). These parameters were recorded at 0 day, 15 days, 30 days, and 60 days.
Statistical analysis data
Experiments were conducted using three independent replicates. Analysis of variance (one-way ANOVA) was applied, and Duncan’s multiple range test at 95% confidence level compared the averages obtained.
Results and discussion
Removal of glyphosate in biomixtures and soil: comparison of results with and without earthworms
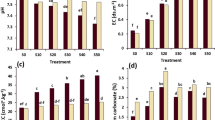
The removal of glyphosate was assayed in biomixtures with the addition of earthworms and without earthworms, and its performance was compared to the removal that takes place employing soil as a single component (Fig. 2a, b). Figure 2a shows that at 30 days of assay, only the biomixture AsRw had higher removal than the soil alone, but at 60 days, the biomixtures AsRw and Ws reached more than 60% degradation overcoming soil. At the end of the experiment (90 days) in all biomixtures, the glyphosate removal reached approximately 80% against 60% in soil. However, when one-way ANOVA was applied in analyzing the effect of “biomixtures”, this factor did not show a statistically significant effect on the response “glyphosate degradation.” There is only one homogenous group (As-Ws-Soil-AsRw). On the other hand, when one-way ANOVA was applied analyzing the effect of biomixtures that include earthworms, this factor showed a statistically significant effect on the same response (Fig. 2b). The results showed that for the factor “biomixtures,” there are two homogenous groups that are statistically different (As-E-Soil-E-AsRw-E and Ws-E). According to this, glyphosate degradation was significantly higher in Ws-E when compared with As-E, Soil-E, and AsRw-E. In Fig. 2b, it can be seen that all biomixtures show higher glyphosate removal from the beginning of the experiment in comparison with soil, reaching above the 90%. The biomixture Ws-E reported a special behavior with the highest removal rate, and glyphosate disappeared almost completely after 90 days (in agreement with the statistical study).
a Percentage of glyphosate degradation in soil and biomixtures. b Percentage of glyphosate degradation in soil and biomixtures using E. fetida. c Comparison between the percentage of glyphosate initial degradation rate with E. fetida and that without E. fetida. Different letters refer to significant differences between glyphosate degradation means (%), taking into account the factor “biomixtures” with Duncan’s test (p < 0.05)
The comparison between both experiments with and without earthworms through the glyphosate degradation removal (%) at 15 days (or initial degradation rate) is shown in Fig. 2c. Glyphosate removal was clearly enhanced in biomixtures with earthworms. All biomixtures and soil show higher glyphosate degradation at 15 days when earthworms were used (glyphosate degradation values were 16 to 60% higher in biomixtures that contain earthworms), but the difference was more significant for Ws vs. Ws-E (60% higher in Ws-E than Ws). In order to support the obtained data, the effect of biomixtures on “initial glyphosate degradation rate” was checked through one-way ANOVA, which reinforced our findings. The results showed that there are seven homogenous groups that are statistically different. The following is the order of increasing glyphosate degradation means at 15 days: (Ws) < (As) < (Soil-AsRw) < (Soil-E) < (As-E) < (AsRw-E) < (Ws-E). According to these results, glyphosate degradation was significantly higher in the biomixtures where E. fetida is present. In addition, within the biomixtures that contained earthworms, glyphosate degradation was significantly higher in Ws-E when compared with AsRw-E, As-E, and Soil-E. This higher degradation could be related with the different lignocellulosic materials used since wheat stubble has minor nitrogen content and higher lignin content (lignin is part of the raw fiber) than alfalfa straw (Table 2). It is known that the development of microbiota in materials that are richer in nitrogen as alfalfa is difficult. Also, the use of materials rich in fiber permits the selection of those microorganisms that are specialized in the degradation of materials such as lignin (a component included in raw fiber) and pesticides (Castillo et al. 2008).
These results confirm the capacity of E. fetida to improve the removal of high concentrations of glyphosate (1000 mg/kg) and the synergic effect between the earthworms and the microorganisms during the exposure where E. fetida modify the substrate structure with mucus production (Brown et al. 2000; Devliegher and Verstraete 1995). This mucus stimulates the appearance of a more active and specialized microflora for pollutant degradation (Aira and Domínguez 2008).
AMPA and glyphosate degradation in Ws-E and Soil-E
It is interesting to compare the AMPA and glyphosate degradation between Ws-E and soil during the experiment. AMPA is a metabolite of glyphosate microbial degradation in soils (Borggaard and Gimsing 2008). Figure 3a shows AMPA generation in relation to the glyphosate degradation at 30 days, after that, AMPA is slowly degraded until the end of experiment. In contrast, soil shows a different behavior since AMPA is slowly generated until 90 days (Fig. 3b). AMPA is more persistent than glyphosate to the biological degradation in soils, and many works have been published on its accumulation in soil (Grandcoin et al. 2017; Mamy et al. 2010; Souza et al. 2006). Therefore, this important result shows higher capacity of Ws-E to improve AMPA and glyphosate removal in comparison with Soil-E.
E. fetida’s growth performance and biological activity in biomixtures and soil
In this study after 90 days of exposure, the observed earthworm mortality was low for all biomixtures and soil (Table 4). In addition, coinciding with contributions by Correia and Moreira (2010), the species E. fetida tolerated high glyphosate concentrations (1000 mg/kg).
Percentage of mean individual worm biomass variation with time is depicted in Fig. 4a. The biomass increased initially in all biomixtures followed by a weight loss at the last stages of the experiment. Maximum biomass was obtained between 15 and 30 days, and after 30 days of experiment, the earthworm biomass began to decline until the end of the experiment. Also, the three biomixtures showed higher biomass than soil as a single component during the entire assay. These changes in the biomass may reflect the availability of food at the start of the study and the exhaustion of food with time. Other authors reported similar behavior related to the growth of E. fetida during the vermicomposting of different wastes (Gong et al. 2018; Sharma and Garg 2018).
The determination of biological activities such as hydrolytic activity based on the FDA (3′,6′-diacetylfluorescein) activity has been used to determine amounts of active fungi and bacteria since it is hydrolyzed by a number of different enzymes, such as proteases, lipases, and esterases (Schnürer and Rosswall 1982). Figure 4b shows the FDA evolution during the assay for all biomixtures and soil using earthworms. All biomixtures showed higher initial FDA compared to the soil. After that, the activity increases gently up to day 15, and from this time, it decreases markedly. This behavior follows the same trend that earthworm biomass changes (Fig. 4a), and it could probably be due to a depletion of the carbon resources readily available for the microorganisms in the biomixtures that occur after 15 days of assay. The same results were reported by other authors using similar composition of biomixtures for other pesticides (Tortella et al. 2012; Urrutia et al. 2013). Regarding glyphosate degradation, the rate is maximum at 15 days in concordance with the higher values obtained in the FDA test at 15 days for each substrate, showing close relationship between glyphosate degradation and FDA activity measurements (Figs. 2b and 4b). In addition, the biomixture that shows higher glyphosate degradation rate (Ws-E) is the one that exhibits higher FDA values in almost all sampling points, and at the end, it is the one that presents higher residual FDA. This tendency could be related to different enzymes or the amount of enzyme present in different biomixtures that render different FDA values and, consequently, different glyphosate degradation values.
On the other hand, the number of total viable mesophilic bacteria (around 108 CFU/g biomixture) and yeast and fungi (around 105 CFU/g biomixture) did not vary significantly throughout the experiment. In order to support the results, one-way ANOVA test was applied, showing that the factor “days” did not show statistically significant effect on the response (CFU/g for bacteria and yeast and fungi). It can be inferred that both communities were not altered due to glyphosate and AMPA concentration present in the biomixtures even though no information was collected on the prevalence of some species through their identification. In this sense, the application of genomic analysis techniques in combination with pesticide degradation is expected to provide useful insights into pesticide-microbe interactions occurring in these systems (Marinozzi et al. 2013; Holmsgaard et al. 2017; Bergsveinson et al. 2018).
E. fetida reproduction
Cocoons were first detected on day 30 only in Ws-E, but on day 60, cocoons appear in all biomixtures and soil, being Ws-E and the Soil-E the substrates with a higher number of cocoons (9 and 20, respectively) (Fig. 5a). Juvenile earthworms were only detected in Ws-E and Soil-E, and its number on day 60 was 22 and 12, respectively. After that, on day 90, the number of juveniles declines (12 in Ws-E and 3 in soil) (Fig. 5b). The high E. fetida reproduction on days 60 and 90 is coincident with the biomass loss shown in Fig. 4a. This observation agrees with the results reported by Correia and Moreira (2010), Piola et al. (2013), and Domínguez et al. (2016), indicating an inverse relationship between weight and reproduction in situations of long-term exposure to sublethal concentrations of glyphosate.
E. fetida recovery after the vermiremediation process
At the end of the vermiremediation process, a new study was done in order to evaluate earthworm recovery. Percentage of mean individual earthworm biomass variation with time is depicted in Fig. 6a. The biomass increased in all biomixtures being more marked in Soil-E and AsRw-E (298% and 227%, respectively, at the end of the assay). Cocoons were first detected on day 30 in Soil-E and only in biomixtures Ws-E and As-E, but on day 60, cocoons appear in all biomixtures, being the Soil-E, As-E, and Ws-E the substrates with a higher content of cocoons (18, 16, and 12, respectively) (Fig. 6b). This assay confirms that the weight loss at the last stages of the vermiremediation process probably was due to a depletion of the carbon resources (Fig. 4a) and was not an effect of glyphosate. In a recent study, Owagboriaye et al. (2020) showed that the earthworm species Alma millsoni, Eudrilus eugeniae, and Libyodrilus violaceus bioaccumulated certain amount of glyphosate in their tissues despite the hydrophilic nature of the herbicide. However, the increased rate of glyphosate removal from soil containing E. eugeniae and L. violaceus suggested that both earthworm species may be used to vermiremediate soil contaminated with glyphosate.
In the present work, no studies of possible glyphosate accumulation were done. However, the results obtained showed a clear recovery of E. fetida’s growth performance and could confirm that this species tolerate high glyphosate concentrations without showing alterations in its life traits.
Conclusions
In this work, the addition of earthworms to several biomixtures as a way to improve glyphosate degradation was studied.
All biomixtures with E. fetida showed higher glyphosate removal in comparison with biomixtures without earthworms. Also, the biomixture made up with wheat stubble and soil containing earthworms (Ws-E) showed the higher initial degradation rate and more capacity to improve the AMPA removal.
The species E. fetida can tolerate high glyphosate concentration without modifications in its life traits, especially when E. fetida is used to remove glyphosate in Ws-E.
The biobeds can incorporate the use of earthworms to improve the glyphosate and AMPA removal.
References
Aira M, Domínguez J (2008) Optimizing vermicomposting of animal wastes: effects of rate of manure application on carbon loss and microbial stabilization. J Environ Manag 88:1525–1529
Al-Maliki S, Scullion J (2013) Interactions between earthworms and residues of differing quality affecting aggregate stability and microbial dynamics. Appl Soil Ecol 64:56–62
Baier CJ, Gallegos CE, Raisman-Vozari R, Minetti A (2017) Behavioral impairments following repeated intranasal glyphosate-based herbicide administration in mice. Neurotoxicol Teratol 64:63–72
Bergsveinson J, Perry BJ, Sheedy C, Braul L, Reedyk S, Gossen BD, Yost CK (2018) Identifying the core bacterial and fungal communities within four agricultural biobeds used for the treatment of pesticide rinsates. J Appl Microbiol 125:1333–1342
Binet F, Kersanté A, Munier-Lamy C, Le Bayon RC, Belgy MJ, Shipitalo MJ (2006) Lumbricid macrofauna alter atrazine mineralization and sorption in a siltloam soil. Soil Biol Biochem 38:1255–1263
Borggaard O, Gimsing A (2008) Review: Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456
Bórtoli P, Verdeneli R, Conforto C, Vargas S, Meriles J (2012) Efectos del herbicida glifosato sobre la estructura y el funcionamiento de comunidades microbianas de dos suelos de plantaciones de olivo. Ecol Austral 22:33–42
Brown GG, Barois I, Lavelle P (2000) Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactions with other edaphic functional domains. Eur J Soil Biol 36:177–198
Castillo MP, Torstensson L, Stenström J (2008) Biobeds for environmental protection form pesticide use. A review. J Agric Food Chem 56:6206–6219
Contreras-Ramos SM, Álvarez-Bernal D, Dendooven L (2008) Removal of polycyclic aromatic hydrocarbons from soil amended with biosolid or vermicompost in the presence of earthworms (Eisenia fetida). Soil Biol Biochem 40:1954–1959
Cooper RJ, Fitt P, Hiscock KM, Lovett AA, Gumm L, Dugdale SJ, Rambohul J, Williamson A, Noble L, Beamish J, Hovesen P (2016) Assessing the effectiveness of a three-stage on-farm biobed in treating pesticide contaminated wastewater. J Environ Manag 181:874–882. https://doi.org/10.1016/j.jenvman.2016.06.047
Correia FV, Moreira JC (2010) Effects of glyphosate and 2,4-D on earthworms (Eisenia foetida) in laboratory tests. Bull Environ Contam Toxicol 85:264–268
De Wilde T, Spanoghe P, Debaer C, Ryckeboer J, Springael D, Jaeken P (2007) Overview of on-farm bioremediation systems to reduce the occurrence of point source contamination. Pest Manag Sci 63:111–128
Devliegher W, Verstraete W (1995) Lumbricus terrestris in a soil core experiment: nutrient-enrichment processes (NEP) and gut-associated processes (GAP) and their effect on microbial biomass and microbial activity. Soil Biol Biochem 27(12):1573–1580
Dias L, Gebler L, Niemeyer J, Itako A (2020) Destination of pesticide residues on biobeds: state of the art and future perspectives in Latin America. Chemosphere 248:126038. https://doi.org/10.1016/j.chemosphere.2020.126038
Domínguez A, Brown GG, Sautter KD, Ribas de Oliveira CM, Carvalho de Vasconcelos E, Niva CC, Bartz MLC, Bedano JC (2016) Toxicity of AMPA to the earthworm Eisenia andrei, Bouchè, 1972 in tropical artificial soil. Sci Rep 6:1–9
Gong X, Cai L, Li S, Chang S, Sun X, An Z (2018) Bamboo biochar amendment improves the growth and reproduction of Eisenia fetida and the quality of green waste vermicompost. Ecotox Environ Saf 156:197–204
Grandcoin A, Piel S, Baurés E (2017) Amino methyl phosphonic acid (AMPA) in natural waters: its sources, behavior and environmental fate. Water Res 117:187–197
Gupta R, Garg VK (2009) Vermiremediation and nutrient recovery of non-recyclable paper waste employing Eisenia fetida. J Hazard Mater 162:430–439
Hickman ZA, Reid BJ (2008) Increased microbial catabolic activity in diesel contaminated soil following addition of earthworms (Dendrobaena veneta) and compost. Soil Biol Biochem 40:2970–2976
Holmsgaard PN, Dealtry S, Dunon V, Heuer H, Hansen LH, Springael D, Smalla K, Riber L, Sørensen SJ (2017) Response of the bacterial community in an on-farm biopurification system, to which diverse pesticides are introduced over an agricultural season. Environ Pollut 229:854–862
Kawai S, Uno B, Tomita M (1991) Determination of glyphosate and its major metabolite aminomethylphosphonic acid by high-performance liquid chromatography after derivatization with p-toluenesulphonyl chloride. J Chromatogr A 540:411–415
Lescano M, Pizzul L, Castillo M, Zalazar C (2018) Glyphosate and aminomethylphosphonic acid degradation in biomixtures based on alfalfa straw, wheat stubble and river waste. J Environ Manag 228:451–457
Mac Loughlin TM, Peluso ML, Aparicio VC, Marino DJG (2020) Contribution of soluble and particulate-matter fractions to the total glyphosate and AMPA load in water bodies associated with horticulture. Sci Total Environ 703:134717
Mamy L, Benoît G, Barriuso E (2010) Comparative environmental impacts of glyphosate and conventional herbicides when used with glyphosate-tolerant and nontolerant crops. Environ Pollut 158:3172–3178. https://doi.org/10.1016/j.envpol.2010.06.036
Marinozzi M, Coppola L, Monaci E, Karpouzas DG, Papadopoulou E, Menkissoglu-Spiroudi U, Vischetti C (2013) The dissipation of three fungicides in a biobed organic substrate and their impact on the structure and activity of the microbial community. Environ Sci Pollut Res 20:2546–2555
Masin CE, Rodríguez AR (2012) Evaluación preliminar de dos tipos de dieta en la especie endogea Aporrectodea trapezoides (Dugés, 1828) (Oligochaeta: Lumbricidae). Megadrilogica. 15(10):219–226
Masin CE, Lescano MR, Rodríguez AR, Godoy JL, Zalazar CS (2018) Earthworms to assess the innocuousness of spent biomixtures employed for glyphosate degradation. J Environ Sci Health B:1–7
Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vandenberg LN, Vom Saal FS, Welshons WV, Benbrook CM (2016) Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health:15–19
Organization for Economic Cooperation and Development (OECD) (2004) Guideline for the testing of chemicals. Earthworm reproduction test (Eisenia fetida/Eisenia andrei). OECD, París, No. 222, p 18
Owagboriaye F, Dedeke G, Bamidele J, Aladesida A, Isibor P, Feyisola R, Adeleke M (2020) Biochemical response and vermiremediation assessment of three earthworm species (Alma millsoni, Eudrilus eugeniae and Libyodrilus violaceus) in soil contaminated with a glyphosate-based herbicide. Ecol Indic 108:105678
Piola L, Fuchs J, Oneto ML, Basack S, Kesten E, Casabé N (2013) Comparative toxicity of two glyphosate-based formulations to Eisenia andrei under laboratory conditions. Chemosphere 91:545–551
Ratcliff A, Busse M, Shestak C (2006) Changes in microbial community structure following herbicide (glyphosate) additions to forest soils. Appl Soil Ecol 34:114–124
Rodríguez-Campos J, Dendooven L, Álvarez-Bernal D, Contreras-Ramos SM (2014) Potential of earthworms to accelerate removal of organic contaminants from soil: a review. Appl Soil Ecol 79:10–25
Rorat A, Wloka D, Grobelak A, Grosser A, Sosnecka A, Milczarek M, Jelonek P, Vandenbulcke F, Kacprzak M (2017) Vermiremediation of polycyclic aromatic hydrocarbons and heavy metals in sewage sludge composting process. J Environ Manag 187:347–353
Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microb 6:256–1261
Sharma K, Garg VK (2018) Comparative analysis of vermicompost quality produced from rice straw and paper waste employing earthworm Eisenia fetida (Sav.). Bioresour Technol 250:708–715
Souza TA, Matta MHR, Montagner E, Abreu ABG (2006) Estudo de recuperaçao de glifosato e AMPA derivados em solo usando resinas nacionais. Quim Nova 29:1372–1376
Tejada M, Masciandaro G (2011) Application of organic wastes on a benzo(a) pyrene polluted soil. Response of soil biochemical properties and role of Eisenia fetida. Ecotox Environ Safe 74:668–674
Torstensson L, del Castillo MP (1997) Use of biobeds in Sweden to minimize environmental spillages from agricultural spray equipment. Pestic Outlook 8:24–27
Tortella G, Rubilar O, Castillo M, Cea M, Mella-Herrera R, Diez M (2012) Chlorpyrifos degradation in a biomixture of biobed at different maturity stages. Chemosphere 88:224–228
Urrutia C, Rubilar O, Tortella GR, Diez MC (2013) Degradation of pesticide mixture on modified matrix of a biopurification system with alternatives lignocellulosic wastes. Chemosphere 92:1361–1366
Acknowledgments
We thank Ing. Matías Caillat from the Cooperativa Mixta Ltda (Margarita, Santa Fe, Argentina) for providing the materials used in this work.
Funding
This work was financed by CONICET, ANPCyT, SECTeI, and UNL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Chris Lowe
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lescano, M.R., Masin, C.E., Rodríguez, A.R. et al. Earthworms to improve glyphosate degradation in biobeds. Environ Sci Pollut Res 27, 27023–27031 (2020). https://doi.org/10.1007/s11356-020-09002-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09002-w