Abstract
Mercury and selenium were assessed in Mustelus henlei, which is a carnivorous predatory shark that is important for the coastal communities of the northern Mexican Pacific (NMP). Sixty-two individuals were sampled; muscle and liver were isolated and analyzed by atomic absorption spectrophotometry. The mean Hg concentrations (wet weight) obtained for muscle (0.08 ± 0.10 μg g−1) and liver (0.09 ± 0.26 μg g−1) were below the allowed limits (< 1.0 μg g−1 Hg). The average Se concentration was 0.03 ± 0.01 μg g−1 in muscle and 0.13 ± 0.05 μg g−1 in liver. The Se/Hg molar ratio of muscle was 1.83; however, the selenium health benefit value (HBVSe) was of 0.08. We calculated that an adult man (70 kg), an adult woman (60 kg), and a child (16 kg) could consume 1595, 838, and 223 g/week of M. henlei muscle, respectively, without risks to health. In conclusion, the concentrations and molar ratio of Hg and Se in M. henlei muscle mean that consumption of this shark’s meat does not represent neither a benefit nor a public health risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) occurs in organic and inorganic forms in the marine environment. The transformation of Hg between these two forms can have major effects on the bioaccessibility, mobility, volatility, and solubility of this metal through biological or chemical processes (Storelli et al. 2002). Briefly, Hg accumulates rapidly and mainly in its methylated form (CH3Hg), which passage across cell membranes; moreover, its high affinity for the sulfhydryl groups of protein and enzymes, its half-life, and its tendency to bioaccumulate from one trophic level to the next and increasing its concentration throughout the trophic web makes it a hazardous substance to human health and the environment (Gray 2002; Storelli et al. 2002). Thus, to protect public health, a maximum permissible limit has been established in marine products for human consumption in Mexico. For fish, the level limit for total Hg (NOM-027-SSA1-1993 1995) is 1.0 μg g−1 (wet weight) and for methyl-Hg (CH3Hg), the value is 0.5 μg g−1 (NOM-242-SSA1-2009 2011).
On the other hand, selenium (Se) is a metalloid essential for metabolism; however, in elevated concentrations, Se can be harmful to human health (Plant et al. 2001). It is well known that Se can counteract Hg toxicity. Selenium does not decrease Hg concentrations, but it neutralizes the effects of Hg in the body when both elements occur together. A Se:Hg molar ratio above 1:1 is considered protective against adverse mercury effects (Kaneko and Ralston 2007; Burger et al. 2012). Selenium might contribute to the CH3Hg demethylation process in organisms through selenocysteine; Hg might be transformed to inorganic Hg that can be excreted in an easier and simpler manner through the urinary and gastrointestinal tracts (Mann and Truswell 2002; Havelková et al. 2008). There is no regulation in Mexico stating allowable Se limits; however, other countries have established limits of 6.5 μg g−1 dry weight (USA) (Skorupa et al. 1996) and 1.0 μg g−1 dry weight (Australia) (Nauen 1983) to guarantee public well-being.
The main pathway for exposure to essential and non-essential elements is through food; therefore, products that are frequently consumed by humans should be monitored. Fish have been identified as a source of Hg and Se exposure in humans (Squadrone et al. 2014), particularly fish at high trophic levels such as elasmobranchs (sharks and rays). Actually, Mexico is one of the most important countries in terms of fisheries production and of elasmobranch catches (Ramírez-Amaro et al. 2013). In addition to providing a food resource in Mexico, the elasmobranch fishery creates numerous jobs for the coastal communities of Mexico (Bizzarro et al. 2009). The Triakidae family includes the brown smooth-hound shark Mustelus henlei, an abundant species in the northern Mexican Pacific (NMP) which is distributed in temperate and tropical waters at depths ranging from the shallow intertidal to 200 m with a geographic range spanning Coos Bay, Oregon (USA), to Peru and Ecuador including the Gulf of California. However, this species is considered to occur primarily in the northern Pacific (Chabot et al. 2015). M. henlei constitutes a commercial resource in Mexico (Rodríguez-Romero et al. 2013). This shark has been classified as K-type strategists, because they display slow growth, long gestation times, low fecundity, and late age at maturity (Holden 1974). These biological characteristics make them susceptible to overfishing and to exposure to toxic elements.
Due to its toxicity, persistence, and accumulation capacity, it is important to investigate Hg and Se levels in marine ecosystems and in commercially important species such as the brown smooth-hound shark M. henlei. Moreover, Hg and Se levels should be evaluated jointly to understand in an integrated manner the antagonistic relationship between these elements, and to verify the toxicity of Hg in products consumed by humans. Hypothetically, sharks are considered long-lived top predators, and we expected to find Hg values in M. henlei above the permissible limits allowed by Official Mexican Standards (> 1.0 μg g−1), but with a molar ratio of Se:Hg ≥ 1. Actually, Hg and Se levels in M. henlei are unknown, so the purposes of the present study were to assess whether Hg and Se concentrations found in M. henlei are within allowable limits set by Mexican and international regulations, and to assess the Se (selenium health benefit value (HBVSe)) benefit to public health from consumption of this shark’s meat. In addition, Hg and Se concentrations in liver were also evaluated.
Materials and methods
Field sampling
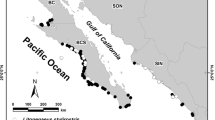
Sixty-two specimens of brown smooth-hound shark M. henlei were collected from eight sites in the northern Mexican Pacific Ocean (NMP) (Fig. 1), during 2015 (June, July, August, and October), 2016 (April, June, July, and August), and 2017 (July and August). Sharks were captured using shrimp trawl nets with 2¾-in. mesh size and bottom-set gillnets with 3.5- to 6-in. mesh size placed at selected sites in the NMP. Total length (TL) was measured before dissection, and sex and maturity state (juvenile and adult) were recorded. Males of TL > 64 cm and females of TL > 68 were considered mature (Silva-Santos 2012). Muscle (from dorsal section) and liver samples (~ 5 g) were obtained from each specimen and stored in polyethylene bags at − 20 °C until processing.
Chemical analysis
Muscle and liver samples were weighed and lyophilized at − 35 °C and 100 × 10−3 mbar during 72 h; samples were then ground and homogenized using an agate mortar and pestle. Samples (~ 0.25 g) were pre-digested overnight with 5 mL of nitric acid (HNO3) in a closed Teflon container (Savillex). Digestion was performed using hot plates (Barnstead) during 3 h at 120 °C. Digested samples were then diluted to 25 mL with Milli-Q water. Hg concentration was measured using a cold vapor spectrophotometer (CV-AAS) with stannous chloride (SnCl2) as reducing agent and a 253.5-nm cathode lamp, whereas Se concentration was measured with a 196-nm cathode lamp and hydride generation (Varian Model VGA-77), using NaBH4 and an air-acetylene flame. Before Se analysis, 1 mL of nitric acid (trace metal grade) was added to 7 mL of digested sample and placed in a polyethylene container in a water bath at 120 °C. Hg and Se concentrations are expressed as μg g−1 wet weight. Accuracy of the analyses and results was validated by using blanks and two certified reference materials (DORM-3 fish protein and DOLT-4 dogfish liver). The average recovery for Hg was 83.8% in the DORM-3 and 91.5% in the DOLT-4; and for Se, was 83.0% in the DOLT-4.
Statistical analysis
Data normality was analyzed statistically using Kolmogorov-Smirnov and Levene’s tests (Zar 1999). Hg concentrations did not display a normal distribution (muscle: K-S, d = 0.3076, p < 0.01, n = 62; liver: K-S, d = 0.22691, p < 0.01, n = 62), whereas Se concentrations showed normality (muscle: K-S, d = 0.13425, p > 0.20, n = 62; liver: K-S, d = 0.09402, p > 0.20, n = 62). Mann-Whitney’s U test and Spearman’s correlations were applied to Hg data, whereas Student’s t test and Pearson’s correlation were applied to Se data. Statistical analysis was performed with STATISTICAL software.
Se/Hg molar ratio
The Se/Hg molar ratio, mean, and standard deviation were calculated for each shark. The concentrations, measured as μg g−1, were converted to μmol kg−1 and the Se/Hg molar ratio calculated from molar mass of Hg (200.59 g mol−1) and Se (78.96 g mol−1).
Health benefits
The HBVSe was calculated as follows (Ralston et al. 2016):
This equation includes Hg and Se molar concentrations. A positive HBVSe indicates a health benefit, but a negative result of HBVSe indicates that the health risk occurs.
Health risk assessment
The maximum consumption of fish per week (MCFW) of Hg was calculated using the following formula:
where PTIW is the provisional tolerable intake per week (4.0 μg kg−1 body weight week−1 for men; 2.45 μg−1 kg−1 body weight week−1 for pregnant women, lactating women, or children; JECFA 2010) and [Hg]j is the M. henlei Hg concentration (μg g−1). MCFW is expressed in g of fish intake week−1 per capita (kg). Average weights of 70 kg (adult men), 60 kg (adult women), and 16 kg (children) were included in the analysis.
Considering that the contribution of methylmercury to total mercury is commonly 80–100% in fish, a conversion of mercury to methylmercury was realized applying the 90% (EFSA 2012). Likewise, the MCFW was established with the PTWI for methylmercury (1.6 μg kg−1 wet weight) (EFSA 2012). For women and children, a proportional PTWI (2.45 to 0.98 μg−1 kg−1 body weight week−1) was applied according to the JECFA (2010).
Results
Tissue samples (muscle and liver) from 62 sharks were obtained (12 males, 41 females, 9 uncategorized), comprising 31 juveniles, 22 adults, and 9 uncategorized. The sharks ranged in length from 43.5 to 102.7 cm (mean = 67.8 ± 14.4 cm). Males and females reach sexual maturity at 64 cm and 68 cm, respectively (Silva-Santos 2012). Unfortunately, some individuals were considered as uncategorized due to sex and size were not recorded. These individuals were not included in the specific analysis by male/female and juveniles/adults, but these data were included in the general average of Hg and Se.
Mercury and selenium in muscle and liver, influence of sex and size
The mean Hg concentration (μg g−1) for M. henlei was 0.08 ± 0.11 in muscle (0.01–0.68, n = 62) and 0.09 ± 0.26 in liver (0.01–2.02, n = 62). Se concentrations were obtained in muscle (0.03 ± 0.02; 0.01–0.06, n = 62) and liver (0.13 ± 0.05; 0.02–0.26, n = 62).
There were no statistically significant differences in Hg concentration between males (muscle = 0.11 ± 0.19; liver = 0.06 ± 0.06) and females (muscle = 0.07 ± 0.06; liver = 0.05 ± 0.04) for muscle (U = 193, p > 0.05) or liver (U = 189, p > 0.05) (Table 1). Similarly, Se values of females (muscle = 0.03 ± 0.02; liver = 0.07 ± 0.06) and males (muscle = 0.03 ± 0.01; liver = 0.14 ± 0.07) were not significantly different (muscle: t = 1.13, p > 0.05; liver: t = 1.74, p > 0.05) (Table 1).
Mean Hg concentrations of adults (0.12 ± 0.15) and juveniles (0.05 ± 0.04) were significantly different for muscle (p < 0.05) but not for liver (juveniles 0.05 ± 0.04; adults 0.07 ± 0.06; U = 224, p > 0.05). Se concentrations were not significantly different between adults and juveniles for muscle (adults 0.02 ± 0.01, juveniles 0.03 ± 0.02) or liver (adults 0.02 ± 0.01; juveniles 0.03 ± 0.02) (Table 1).
Correlations between size (TL) and Hg concentrations were significantly positive for muscle (rs = 0.35, p < 0.05) and liver (rs = 0.27, p < 0.05) (Fig. 2a). Se was not significantly (p > 0.05) associated with TL (muscle r2 = 0.01; liver r2 = 0.12) (Fig. 2b).
Toxicological and health benefit assessment
The calculated amount of shark filet that a man (70 kg) could consume safely per week was 3590 g; however, a consumption of 412 g is recommended because an individual shark presented Hg concentrations above 1.0 μg g−1. Restrictions are more rigorous for women and children, especially with the estimation of CH3Hg (Table 2).
The molar ratio of liver (Se/Hg(liver) = 7.78) was higher than that of muscle (Se/Hg(muscle) = 1.83); however, considering the HBVSe value obtained for muscle tissue, there was not a high Se benefit (Table 3), whereas hepatic tissue showed a more positive HBVSe value.
Discussion
Mercury and selenium: tissue distribution and influence of sex and size
This is the first study on the Se/Hg relationship in M. henlei in the northern Mexican Pacific. Hg concentrations found in this species were below the maximum Hg limit established by Mexican norms (NOM ≥ 1.0 μg g−1 on wet weight basis). These results are consistent with the general pattern reported for congeneric species (e.g., M. griseus, M. schimitti, M. norrisi) in different regions, including the western coast of Baja California Sur, Mexico (Espinoza-García 2016) (Table 4). This has been mainly attributed to feeding and life habits and other factors such as metabolic and growth rates of the species (Andersen and Depledge 1997). Piscivore species occupying high trophic levels show significantly higher Hg concentrations than consumers of crustaceans or cephalopods (Storelli and Marcotrigiano 2000; Escobar-Sánchez et al. 2016). Espinoza et al. (2015) found that the diets of immature individuals of M. henlei in the Pacific coast of Costa Rica consisted of larger proportions of invertebrates (e.g., shrimp, stomatopods, and polychaetes), while teleosts were more important (in terms of biomass, frequency, and abundance) for adults. Conversely, Amariles et al. (2017) in the coast of the Colombian Pacific found that this species fed almost exclusively on teleosts. However, the diet of M. henlei in Mexican waters includes crustaceans, mainly (index of relative importance, 81.4%) the pelagic red crab Pleuroncodes planipes (Rodríguez-Romero et al. 2013). Therefore, predation upon low trophic level preys such as crustaceans could lead to lower Hg levels in M. henlei that in top predators.
In some locations (e.g., Japan or South Africa), high Hg levels (≥ 1.0 μg g−1) have been observed in Mustelus species such as M. mustelus and M. manazo (Table 4); these high values were attributed to differences in size and biological differences between sexes (habits, size, etc.) (Pethybridge et al. 2010). Some authors have reported that intraspecific differences in Hg concentrations between males and females could be caused by factors such as energetic requirements, maturity conditions, Hg deposition, and Hg transfer from females to embryos (Lyle 1986; Frías-Espericueta et al. 2015). It has even been considered that in some species such as M. mustelus, the growth rate of males is slower than that of females, and this could imply that the muscle tissue of males has greater Hg concentrations than that of females (Bosch et al. 2013). However, in our study, no statistical differences were found in the Hg concentrations of the two sexes, which indicate a lack of sexual segregation; both sexes could share the same biological and ecological characteristics as habitat and sources of elements, migratory routes, feeding types, growth rates, etc. (Núñez-Nogueira et al. 1998). No differences have been found between M. henlei males and females in feeding habits or behavior in the NMP (Rodríguez-Romero et al. 2013), which could explain the similar Hg concentrations found in this study for both sexes and tissue types.
Intraspecific variations have also been associated with length, weight, age, and sexual maturity (Wheeler 1996). In this study, a significantly positive correlation was found between TL and Hg concentrations in muscle and liver, which indicates that the concentration level could increase with size, as has been reported for other sharks, such as Galeocerdo cuvier, Carcharhinus albimarginatus, C. plumbeus, and C. leucas (Endo et al. 2008). However, more adult specimens should be studied to explain this trend, as it has been reported that adults tend to have a slow metabolism and, therefore, they would need more time to metabolize Hg, which decreases excretion rate and results in greater Hg accumulation in tissues. Larger animals feed on larger preys, which would also lead to a greater quantity of Hg in consumers (de Pinho et al. 2002; Gutiérrez-Mejía et al. 2009).
Selenium concentrations found in M. henlei muscle and liver were under the allowable limits established for human consumption by the Health Department of Australia (1.0 μg g−1 ww) (Nauen 1983) and of the USA (6.5 μg g−1 dry weight) (Skorupa et al. 1996). Based on this, values obtained for M. henlei muscle and liver in the NMP do not represent a risk of intoxication from Se.
Studies on Se presence in sharks are scarce in Mexico. However, in other regions, Se concentrations have been assessed in Mustelus species, such as M. griseus (Japan) and M. mustelus (South Africa), where it was found that Se values were higher than Hg values in muscle tissue (Ueda and Takeda 1983; Zaera and Johnsen 2011). It should be mentioned that this is not a general pattern for the Mustelus genus, as it was reported that Se values were lower than Hg concentrations in M. manazo and M. mustelus (Ueda and Takeda 1983; Bosch et al. 2016); this could be due to factors such as element bioavailability in ecosystems and stage of organisms. It has been reported that there are greater quantities of Se in the liver because that is where Hg detoxification occurs (Branco et al. 2007), which would explain that in M. henlei, the greatest Se concentrations were found in liver tissue. Moreover, demethylation and Hg accumulation occur in the liver through the effect of the selenoproteins of glutathione (GSH), that contribute to eliminate Hg by excreting it in the bile as cysteine-mercury (Patrick 2002; Branco et al. 2007). That is, Se tends to be found in greater concentrations in the liver because it dominates in the competition for space in the liver; the opposite occurs in muscle, where Hg is more dominant due to its affinity with muscular tissue and with the thiol groups of proteins (Lacerda et al. 2000; Raymond and Ralston 2004). Despite the possibility of this affinity and even though Se concentrations in muscle are lower than those of liver, in this study, there were no significant differences of Se concentrations between the two tissues. There were no significant differences in Se values between males and females, or between juveniles and adults for the two analyzed tissues, which could be due to the same intraspecific factors that influenced Hg concentrations, such as similar type of food, habitat, and probably the same availability of both elements.
Toxicological and health benefit assessment—Se:Hg molar ratio
According the concentrations found here of Hg in M. henlei, such Hg values should not represent a risk to human health (NOM-031-SSA1-1993; NOM-0242-SSA1-2009). However, as a precautionary measure, we recommend that an adult man (70 kg) should consume only up to 183 g per week of M. henlei meat to reduce possible risks; a specimen with Hg concentration below 1.0 μg g−1 was found in this study. Women (60 kg) can consume up to 96 g of M. henlei meat, and children (16 kg) can consume up to 26 g per week. Although these recommendations can appear strict, the US-EPA (United States Environmental Protection Agency) has stated that rigorous restrictions are meant to protect human health. However, concentrations as well as the time of exposure should be taken into account, to establish whether exposure was acute or chronic.
The Se benefit value measured through the HBVSe index was very low in muscle compare with other predators as the silky shark Carcharhinus falciformis (HBVSe = 52.3; Bodin et al. 2017) or dolphinfish Coryphaena hippurus (HBVSe = 1.77; Vega-Sánchez et al. 2019) because Hg concentrations were higher than Se concentrations. The opposite (Se > Hg) was found in M. henlei liver tissue, indicating a benefit provided by a greater quantity of Se compared with Hg in the liver tissue. This was also previously reported for the shark Prionace glauca, based on the Se/Hg molar ratio (Escobar-Sánchez et al. 2011). Some shark species such as Sphyrna zygaena have been reported to display a greater Se molar proportion compared with Hg (Escobar-Sánchez et al. 2010), which in addition to neutralizing the effects of Hg could allow the animal to have enough Se for physiological processes. Considering the Hg and Se values obtained in the present study, which are below established regulations, there is no risk of intoxication caused by these elements from the consumption of M. henlei muscle, but given the low Se values found, there is no benefit to health from the consumption of this marine product.
Conclusions
This is the first study on the relationship between Hg and Se in M. henlei in the world. Low Hg concentrations in M. henlei could be attributed to feeding habits and own specific parameters of the species such as a relatively low metabolic and growth rates or life habits (e.g., reproductive mode, habitats). A positive and significant (p < 0.05) correlation was observed between Hg in muscle and total length, with Hg concentration increasing with size. There were no significant differences for the Hg and Se concentrations between males and females, so the two sexes could be sharing the same habitat, feeding on the same resources, using the same migratory routes, with no relevant differences influenced by the dissimilarities in the growth rate, metabolism, or reproduction between sexes.
References
Adams DH, McMichael RH Jr, Henderson GE (2003) Mercury levels in marine and estuarine fishes of Florida 1989–2001. Florida Marine Research Institute Technical Report TR-9. 2nd ed. rev. pp 57
Amariles DF, Navia AF, Giraldo A (2017) Food resource partitioning of the Mustelus lunulatus and Mustelus henlei (Elasmobranchii: Carcharhiniformes). Environ Biol Fish 100:717–732. https://doi.org/10.1007/s10641-017-0598-x
Andersen JL, Depledge MH (1997) A survey of total mercury and methylmercury in edible fish and invertebrates from Azorean waters. Mar Environ Res 44(3):331–350. https://doi.org/10.1016/S0141-1136(97)00011-1
Bizzarro J, Smith WD, Castillo-Géniz L, Ocampo-Torres L, Márquez-Farías F, Hueter R (2009) The seasonal importance of small coastal sharks and rays in the artisanal elasmobranch fishery of Sinaloa, Mexico. Pan-Am J Aquat Sci 4(4):513–531
Bloom H, Ayling GM (1977) Heavy metals in the Derwent estuary. J Environ Geol 2(1):3–22. https://doi.org/10.1007/BF02430661
Bodin N, Lesperance D, Albert R, Hollanda S, Michaud P, Degroote M, Churlaud C, Bustamante P (2017) Trace elements in oceanic pelagic communities in the western Indian Ocean. Chemosphere. 174:354–362
Bosch AC, Sigge GO, Kerwath SE, Cawthorn DM, Hoffman LC (2013) The effects of gender, size and life-cycle stage on the chemical composition of smoothhound shark (Mustelus mustelus) meat. J Sci Food Agric 93(10):2384–2392. https://doi.org/10.1002/jsfa.6100
Bosch A, O’Neill B, Sigge GO, Kerwath SE, Hoffman LC (2016) Heavy metal accumulation and toxicity in smoothhound (Mustelus mustelus) shark from Langebaan Lagoon, South Africa. Food Chem 190:871–878. https://doi.org/10.1016/j.foodchem.2015.06.034
Branco V, Vale C, Canário J, dos Santos MN (2007) Mercury and selenium in blue shark (Prionace glauca, K. 1758) and swordfish (Xiphias gladius, L. 1758) from two areas of the Atlantic Ocean. Environ Pollut 150:373–380. https://doi.org/10.1016/j.envpol.2007.01.040
Burger J, Gochfeld M, Jeitner C, Donio M, Pittfield T (2012) Interspecific and intraspecific variation in selenium:mercury molar ratios in saltwater fish from the Aleutians: potential protection on mercury toxicity by selenium. Sci Total Environ 431:46–56. https://doi.org/10.1016/j.scitotenv.2012.05.024
Chabot CL, Espinoza M, Mascarenas-Osorio I, Rocha-Olivares A (2015) The effect of biogeographic and phylogeographic barriers on gene flow in the brown smoothhound shark, Mustelus henlei, in the northeastern Pacific. Ecol Evol 5(8):1585–1600. https://doi.org/10.1002/ece3.1458
Cumont G, Gilles G, Bernard F, Bryand MB, Stephan G, Ramonda G, Guillon G (1975) Bilan de la contamination de Poissons de mer par le mercure à l’occasion d’un contrôle portant sur 3 années. Ann Hyg L Fr Med Nut 11:17–25
De Marco SG, Botté SE, Marcovecchio JE (2006) Mercury distribution in abiotic and biological compartments within several estuarine systems from Argentina: 1980–2005 period. Chemosphere 65(2):213–223. https://doi.org/10.1016/j.chemosphere.2006.02.059
de Pinho PA, Guimarães Davée JR, Martins A, Costa PAS, Olavo G, Valentin J (2002) Total mercury in muscle tissue of five shark species from Brazilian offshore waters: effects of feeding habit, sex, and length. Environ Res 89(3):250–258. https://doi.org/10.1006/enrs.2002.4365
Domi N, Bouquegneau JM, Das K (2005) Feeding ecology of five commercial shark species of the Celtic Sea through stable isotope and trace metal analysis. Mar Environ Res 60(5):551–569. https://doi.org/10.1016/j.marenvres.2005.03.001
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) (2012) Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J 10(12):2985
Endo T, Hisamichi Y, Haraguchi K, Kato Y, Ohta C, Koga N (2008) Hg, Zn and Cu levels in the muscle and liver of tiger sharks (Galeocerdo cuvier) from the coast of Ishigaki Island, Japan: relationship between metal concentrations and body length. Mar Pollut Bull 56(10):1774–1780. https://doi.org/10.1016/j.marpolbul.2008.06.003
Endo T, Hisamichi Y, Kimura O, Ogasawara H, Ohta C, Koga N, Kato Y, Haraguchi K (2013) Levels of mercury in muscle and liver of star-spotted dogfish (Mustelus manazo) from the northern region of Japan: a comparison with spiny dogfish (Squalus acanthias). Arch Environ Contam Toxicol 64(3):467–474. https://doi.org/10.1007/s00244-012-9858-0
Endo T, Kimura O, Terasaki M, Fujii Y, Haraguchi K, Ohta C, Kato Y (2017) Growth-related changes in non-essential and essential metals in the liver of star-spotted smooth-hounds (dogfish) Mustelus manazo from the northern region of Japan. Mar Environ Res 131:156–161. https://doi.org/10.1016/j.marenvres.2017.09.009
Escobar-Sánchez O, Galván-Magaña F, Rosíles-Martínez R (2010) Mercury and selenium bioaccumulation in smooth hammerhead shark Sphyrna zygaena Linnaeus from the Mexican Pacific Ocean. Bull Environ Contam Toxicol 84(4):488–491. https://doi.org/10.1007/s00128-010-9966-3
Escobar-Sánchez O, Galván-Magaña F, Rosíles-Martínez R (2011) Biomagnification of mercury and selenium in blue shark Prionace glauca from the Pacific Ocean off Mexico. Biol Trace Elem Res 144:550–559. https://doi.org/10.1007/s12011-011-9040-y
Escobar-Sánchez O, Ruelas-Inzunza J, Moreno-Sánchez XG, Romo-Piñera AK, Frías-Espericueta MG (2016) Mercury concentrations in Pacific angel sharks (Squatina californica) and prey fishes from Southern Gulf of California, Mexico. Bull Environ Contam Toxicol 96(1):15–19. https://doi.org/10.1007/s00128-015-1708-0
Espinoza M, Samantha Munroe SEM, Clarke TM, Fisk AT, Wehrtmann IS (2015) Feeding ecology of common demersal elasmobranch species in the Pacific coast of Costa Rica inferred from stable isotope and stomach content analyses. J Exp Mar Biol Ecol 470:12–25. https://doi.org/10.1016/j.jembe.2015.04.021
Espinoza-García S (2016) Bioacumulación y biomagnificación de Cd, Hg y Pb en Mustelus henlei (Gill 1863) de la costa suroccidental de Baja California Sur. Dissertation, CICIMAR-IPN
Frías-Espericueta MG, Zamora-Sarabia FKG, Márquez-Farías F, Osuna-López JI, Ruelas-Inzunza J, Voltolina D (2015) Total mercury in female Pacific sharpnose shark Rhizoprionodon longurio and their embryos. Lat Am J Aquat Res 43(3):534–538. https://doi.org/10.3856/vol43-issue3-fulltext-14
García-Hernández J, Cadena-Cárdenas L, Betancourt-Lozano M, García-De-La-Parra LM, García-Rico M-FL, Márquez-Farías F (2007) Total mercury content found in edible tissues of top predator fish from the Gulf of California, Mexico. Toxicol Environ Chem 89(3):507–522. https://doi.org/10.1080/02772240601165594
Glover JW (1979) Concentrations of arsenic, selenium and ten heavy metals in school shark, Galeorhinus australis (Macleay), and gummy shark, Mustelus antarcticus Günther, from South-eastern Australian waters. Aust J Mar Freshwat Res 30(4):505–510. https://doi.org/10.1071/MF9790505
Gray JS (2002) Biomagnification in the marine systems: the perspective of an ecologist. Mar Pollut Bull 45:46–52. https://doi.org/10.1016/S0025-326X(01)00323-X
Gutiérrez-Mejía E, Lares ML, Sosa-Nishizaki O (2009) Mercury and arsenic in muscle and liver of the golden cownose ray, Rhinoptera steindachneri, Evermann and Jenkins, 1891, from the Upper Gulf of California, Mexico. Bull Environ Contam Toxicol 83(2):230–234. https://doi.org/10.1007/s00128-009-9730-8
Havelková M, Dušek L, Némethová D, Poleszczuk G, Svobodová Z (2008) Comparison of mercury distribution between liver and muscle—a biomonitoring of fish from lightly and heavily contaminated localities. Sensors 8(7):4095–4109. https://doi.org/10.3390/s8074095
Holden MJ (1974) Problems in the rational exploitation of elasmobranch populations and some suggested solutions. In: FRH J (ed) Sea fisheries research. Wiley, New York, pp 117–137
Hurtado-Banda R, Gómez-Álvarez A, Márquez-Farías F, Córdoba-Figueroa M, Navarro-García G, Medina-Juárez L (2012) Total mercury in liver and muscle tissue of two coastal sharks from the northwest of Mexico. Bull Environ Contam Toxicol 88:971–975. https://doi.org/10.1007/s00128-012-0623-x
JECFA (Joint FAO/WHO Expert Committee on Food Additives) (2010) Joint FAO/WHO Food Standards Programme, Committee of the Codex Alimentarius Commission, thirty-third Session. www.fsis.usda.gov/PDF/2010-CAC/cac33_15e.pdf. Accessed 13 june 2018
Kaneko JJ, Ralston NVC (2007) Selenium and mercury in pelagic fish in the central north Pacific near Hawaii. Biol Trace Elem Res 119:242–254. https://doi.org/10.1007/s12011-007-8004-8
Kousteni V, Megalofonou P, Dassenakis M, Stathopoulou E (2006) Total mercury concentrations in edible tissues of two elasmobranch species from Crete (eastern Mediterranean Sea). Cybium 30(4):102–108
Lacerda LD, Paraquetti HHM, Marins RV, Rezende CE, Zalmon IR, Gomes MP, Farias V (2000) Mercury content in shark species from the south-eastern Brazilian coast. Braz J Biol 60(4):571–576. https://doi.org/10.1590/S0034-71082000000400005
Lyle JM (1986) Mercury and selenium concentrations in sharks from northern Australian waters. Mar Freshw Res 37(3):309–321. https://doi.org/10.1071/MF9860309
Mann J, Truswell A (2002) Essentials of human nutrition. Oxford University Press, Oxford
Marcovecchio JE, Moreno VJ, Pérez A (1986) Bio-magnification of total mercury in Bahia Blanca Estuary shark. Mar Pollut Bull 17(6):276–278. https://doi.org/10.1016/0025-326X(86)90064-0
Marcovecchio JE, Moreno VJ, Pérez A (1991) Metal accumulation in tissues of sharks from the Bahia Blanca Estuary, Argentina. Mar Environ Res 31(4):263–274. https://doi.org/10.1016/0141-1136(91)90016-2
Mol JH, Ramlal JS, Lietar C, Verloo M (2001) Mercury contamination in freshwater, estuarine, and marine fishes in relation to small-scale gold mining in Suriname, South America. Environ Res 86(2):183–197. https://doi.org/10.1006/enrs.2001.4256
Nauen C (1983) Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fish Circ 764:1–102
NOM-027-SSA1-1993. Diario Oficial de la Federación (1995). NOM-027-SSA1-1993. Bienes y servicios. Productos de la Pesca. Pescados frescos-refrigerados y congelados. Especificaciones sanitarias. Diario Oficial de la Federación. Mexico. 3 de marzo de (1995)
NOM-242-SSA1-2009. Diario Oficial de la Federación (2011) NOM-242-SSA1-2009 Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. pp 17
Núñez-Nogueira G, Bautista-Ordoñez J, Rosiles-Martínez R (1998) Concentración y distribución de mercurio en tejidos del cazón (Rhizoprionodon terraenovae) del Golfo de Mexico. Vet Mex 29(1):15–21
Patrick L (2002) Mercury toxicity and antioxidants: part 1: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Altern Med Rev 7(6):456–471
Perez A, Moreno VJ, Moreno JEA, Malaspina A (1985) Distribución de mercurio total en organismos del Mar Argentino. Rev Invest Des Pesq 6:103–115
Pethybridge H, Cossa D, Butler E (2010) Mercury in 16 demersal sharks from southeast Australia: biotic and abiotic sources of variation and consumer health implications. Mar Environ Res 69:18–26. https://doi.org/10.1016/j.marenvres.2009.07.006
Plant J, Smith D, Smith B, Williams L (2001) Environmental geochemistry at the global scale. Appl Geochem 16:1291–1308. https://doi.org/10.1016/S0883-2927(01)00036-1
Ralston NV, Ralston CR, Raymond LJ (2016) Selenium health benefit values: updated criteria for mercury risk assessments. Biol Trace Elem Res 171(2):262–269. https://doi.org/10.1007/s12011-015-0516-z
Ramírez-Amaro SR, Cartamil D, Galván-Magaña F, Gonzalez-Barba G, Graham JB, Carrera-Fernandez M, Escobar-Sanchez O, Sosa-Nishizaki O, Rochin-Alamillo A (2013) The artisanal elasmobranch fishery of the Pacific coast of Baja California Sur, Mexico, management implications. Sci Mar 77:473–487. https://doi.org/10.3989/scimar.03817.05A
Ratkowsky DA, Dix TG, Wilson KC (1975) Mercury in fish in the Derwent Estuary, Tasmania, and its relation to the position of the fish in the food chain. Mar Freshw Res 26(2):223–231. https://doi.org/10.1071/MF9750223
Raymond L, Ralston N (2004) Mercury: selenium interactions and health implications. Seychelles Med Dent J 7(1):72–77
Rodríguez-Romero J, Álvarez-Bauman E, Ochoa-Díaz MR, López-Martínez J, Maldonado-García M (2013) Feeding habits of Mustelus henlei on the western coast of Baja California Sur, Mexico. Rev Biol Mar Oceanogr 48(2):261–271. https://doi.org/10.4067/S0718-19572013000200006
Sandoval-Herrera NI, Vargas-Soto JS, Espinoza M, Clarke TM, Fisk AT, Wehrtmann IS (2016) Mercury levels in muscle tissue of four common elasmobranch species from the Pacific coast of Costa Rica, Central American. Reg Stud Mar Sci 3:254–261. https://doi.org/10.1016/j.rsma.2015.11.011
Scapini EM, Andrade S, Marcovecchio JE (1993) Total mercury distribution in two shark species from Buenos Aires province coastal waters, in Argentina. Proceedings International Conferences of Heavy Metals in the Environment, Toronto, 1:82-85
Silva-Santos JR (2012) Biología reproductiva del tiburón mamón pardo Mustelus henlei (Gill, 1983) en la costa occidental de Baja California Sur, Mexico. Dissertation, CICIMAR-IPN
Skorupa JP, Morman SP, Sefchick-Edwards JS (1996) Guidelines for interpreting selenium exposures of biota associated with nonmarine aquatic habitats. U.S. Fish and Wildlife Service. The National Irrigation Water Quality Program, Sacramento, p 74
Squadrone S, Ciccotelli V, Favaro L, Scanzio T, Prearo M, Abete MC (2014) Fish consumption as a source of human exposure to perfluorinated alkyl substances in Italy: analysis of two edible fish from Lake Maggiore. Chemosphere 114:181–186. https://doi.org/10.1016/j.chemosphere.2014.04.085
Storelli MM, Marcotrigiano GO (2000) Total, organic and inorganic arsenic and mercury in crustaceans (Squilla mantis). Ital J Food Sci 12(3):365–370
Storelli MM, Giacominelli-Stuffler R, Marcotrigiano G (2002) Mercury accumulation and speciation in muscle tissue of different species of sharks from Mediterranean Sea, Italy. Bull Environ Contam Toxicol 68:201–210. https://doi.org/10.1007/s001280239
Storelli MM, Cuttone G, Marcotrigiano GO (2011) Distribution of trace elements in the tissues of smooth hound Mustelus mustelus (Linnaeus, 1758) from the southern–eastern waters of Mediterranean Sea (Italy). Environ Monit Assess 174(1–4):271–281. https://doi.org/10.1007/s10661-010-1456-x
Taylor DL, Kutil NJ, Malek AJ, Collie JS (2014) Mercury bioaccumulation in cartilaginous fishes from southern New England coastal waters: contamination from a trophic ecology and human health perspective. Mar Environ Res 99:20–33. https://doi.org/10.1016/j.marenvres.2014.05.009
Ueda T, Takeda M (1983) Mercury and selenium level in two species of sharks. Bull Jpn Soc Sci Fish 49(11):1731–1735. https://doi.org/10.2331/suisan.49.1731
Vega-Sánchez B, Ortega-García S, Ruelas-Inzunza J, Frías-Espericueta M, Escobar-Sánchez O, Jara-Marini M (2019) Selenium and mercury in dolphinfish (Coryphaena hippurus) from the Gulf of California: inter-annual variations and selenium health benefit value. Environ Sci Pollut Res 27:2311–2318. https://doi.org/10.1007/s11356-019-06795-3
Walker TI (1976) Effects of the species, sex, length, and locality on the mercury content of school shark Galeorhinus australis (Macleay) and gummy shark Mustelus antarticus (Gunther) from south-eastern Australian waters. Aust J Mar Freshwat Res 270:603–616. https://doi.org/10.1071/MF9760603
Wheeler M (1996) Measuring mercury. Environ Health Perspect 104:826–830
Zaera D, Johnsen E (2011) Foetal deformities in a smooth-hound shark, Mustelus mustelus, from an oil exploited area in Angola. Cybium 35(3):231–236
Zar JH (1999) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgments
The authors are grateful to all who collaborated in the laboratory, to Humberto Bojórquez and Karla Guadalupe Sánchez-Osuna. Brenda Medina Bañuelos and Gustavo Andrade Domínguez participated in the biological sampling in the CRIAP.
Funding
This work was supported by CONACYT through the project Ciencia Básica (no. 255687) and Problemas Nacionales (no. 248708). OES thanks CONACYT for the project Cátedras CONACYT (no. 2137) and for financial support through SNI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Medina-Morales, S.A., Corro-Espinosa, D., Escobar-Sánchez, O. et al. Mercury (Hg) and selenium (Se) content in the shark Mustelus henlei (Triakidae) in the northern Mexican Pacific. Environ Sci Pollut Res 27, 16774–16783 (2020). https://doi.org/10.1007/s11356-020-08198-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08198-1