Abstract
Polyethyleneimine modified activated carbon (PEI-AC) was prepared through a treatment of immersion, and used for the adsorption of formaldehyde. The adsorption capacity of formaldehyde by unmodified AC is 190.1 mg g-1, and the adsorption capacity of formaldehyde can reach to 317.6 mg g-1 after 10 g L-1 of PEI modified, being about 1.67 times than unmodified activated carbon (AC: 191.2 mg g-1). And the 10 g L-1 of PEI modified AC (PAC-30) has the highest adsorption capacity of formaldehyde, reached to 650 mg g-1, with an increasing magnitude of 240% in comparison with that without modified AC. This is mainly due to changes in the pore structure and surface functional groups after modification. However, as the PEI concentration increases, the adsorption performance is inhibited. Through kinetic studies, it was found that all adsorption curves follow the second-order kinetics, and the breakthrough curves follow the Boltzmann model, and the adsorption process can also be described by the intraparticle diffusion model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indoor air purification is vital to human health because people currently spend more than 80% of their time in homes, offices, and cars. Volatile organic compounds (VOCs) are one of the most common indoor air pollutants (Yu et al. 2013). Among the VOCs, formaldehyde (HCHO) is regarded as the chief culprit of indoor air pollution due to its widely coming from indoor decoration materials, such as paints, wood-based panels, laminate floors, and furniture (Nie et al. 2013). High concentrations of formaldehyde have profound effects on the human nervous system, respiratory system, and immune system. Long-term exposure to indoor air containing formaldehyde can make an adverse impact on human health even at a very low concentration. Therefore, there is an urgent need to further develop a more efficient and environmentally friendly method of removing formaldehyde.
To date, some physical and chemical techniques have been developed to remove formaldehyde, including adsorption (Huang & Han 2011, Wang et al. 2018, Wang 2012, Wen et al. 2011), photocatalytic oxidization (Yang et al. 2000), electrochemical oxidation (Yi et al. 2011), thermal catalytic oxidization (Shen et al. 2008), plasma technology (Wan et al. 2011), and biological methods (Lu et al. 2012). Among these methods, adsorption has been widely applied due to its low cost, easy operation, and high efficiency. Activated carbon (AC), as one of the most promising adsorbents, has attracted more and more attention due to its high surface area, large pore volume, and good thermal stability (Aygün et al. 2003). However, due to the specificity of the molecular structure of formaldehyde and the limitation of adsorbent’s adsorption capacity, removal of formaldehyde is still a challenge. Therefore, it is considered that the modified activated carbon to improve its adsorption selectivity and adsorption ability for formaldehyde is an effective method for eliminating formaldehyde release in a certain short period.
The aims of modified activated carbon are to improve the adsorption performance by changing the functional group and internal void structure on activated carbon via some physical or chemical methods. At present, the activated carbon modification methods reported in the literatures include: acid modification (Kim et al. 2006), alkali modification (Nie et al. 2013), and ozonation modification (Chiang et al. 2002). The purpose of these modification methods is to increase the oxygen-containing functional groups on the surface of activated carbon (Wu & Chen 2001), such as carboxyl, oxime, carbonyl, lactone, hydroxyl, and carboxylic anhydrides (Yin et al. 2007b). And there are many other methods to modify activated carbon on internal void structure, such as microwave modification (Alslaibi et al. 2013), heat treatment modification (Chingombe et al. 2006), and hot steam treatment modification (Rangel-Mendez & Cannon 2005). Comparison to these techniques, it is recognized that the adsorptive capacity of activated carbon can be greatly boosted by impregnation with appropriate chemicals (Owlad et al. 2010). Polyethyleneimine (PEI) is such an ideal impregnating compound, because it contains a large number of hydrophilic groups, such as amino groups, hydroxyl groups, and carboxyl groups (Amara & Kerdjoudj 2003, Mao et al. 2011), and nitrogen, and oxygen-containing functional groups may be contributed to the adsorption and enhance the efficiency of the adsorbent. Most importantly, there are no studies on PEI impregnated activated carbon to enhance the adsorption of VOCs.
Herein, polyethyleneimine modified activated carbon (PEI-AC) was successfully prepared through a simple impregnation process, and thus used to the adsorption of formaldehyde in a fixed bed. As expected, the adsorption capacity of formaldehyde has been improved significantly compared with the unmodified activated carbon. Through characterization, the enhanced adsorption capacity was found to be attributed to the improvement of internal void structure and introduction of surface hydrophilic functional groups. The modification of activated carbon by PEI may be a useful technique for purifying indoor air containing formaldehyde.
Experimental section
Materials
HCl, NaOH, and formaldehyde were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Polyethyleneimine (PEI) (<1000 g mol-1) was purchased from Aldrich (Shanghai, China). The anthracite coal based activated carbon was purchased from Ningxia Huahui Activated Carbon Co., Ltd. (Yinchuan, China). The size of granular activated carbon was selected from 1 to 1.5 mm. All the reagents were of analytical purity and were used as received.
Modification of activated carbon
Preparation of polyethyleneimine modified activated carbon (PEI-AC) was synthesized by an impregnation process. First, preparation of different concentrations of PEI solution: 0 g, 0.50 g, 1.00 g, 1.50 g, and 2.50 g of PEI were separately added to 50 mL of distilled water in five conical flasks to form 0, 10, 20, 30, and 50 g L-1 PEI, respectively. Second, 10 g of activated carbon was separately added into each flask and sealed. The flasks were then placed in an oscillator and shaken at 180 rpm for 72 h at 25 °C. Finally, the PEI-AC was washed three times with distilled water and dried at 80 °C for 12 h. They were marked as PEI-AC, PEI-AC10, PEI-AC20, PEI-AC30, and PEI-AC50, respectively.
Experimental apparatus and procedure
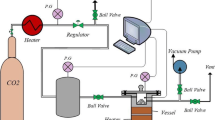
The column adsorption studies of PEI-AC for formaldehyde was mainly achieved by the following apparatus, which have been reported in our previous work (Zhang et al. 2018). As shown in Fig. 1, the experimental apparatus consists of two functional sections, one for the preparation of gas-VOCs vapor mixture and the other for the adsorption process in the fixed bed. In the former section, formaldehyde solution was placed in the gas generator (1), and nitrogen was used as the carrier gas. The flow rate of gas stream was controlled by a valve (3) and measured by a flow meter (2), and the initial concentration of formaldehyde was controlled at 50 mg L-1. The combination of infrared carbon fiber heating and quartz spiral tubes (4) were used for thermal desorption process. The nitrogen temperature inside the enclosure was measured by a digital thermometer (5). The part of fixed bed mainly contained of an adsorption column (7) and a digital balance (11). The glass column was packed with the 50 g of adsorbent. The gas mixture was fed from the bottom of the column and flows upward through the adsorbent fixed bed (inner diameter 5.2 cm, height 28 cm) until the adsorbent was saturated. The temperature of the gas mixture in the column was measured by a digital thermometer (6). The digital balance (11) was used to measure the amount of formaldehyde adsorbed based on the weight change of PEI-AC before and after the adsorption process. These data were output by a computer. The outlet concentrations of formaldehyde were measured by a VOCs analyzer (PGM-7300, RAE Systems) with UV lamp detector (8). The equipment was fitted in an enclosure with teflon tube (13) (14), which was used for the insulation.
Characterization
X-ray diffraction (XRD) patterns of PEI-AC were conducted on a Bruker D8 Advance X-ray diffractometer with Cu Ka (λ = 1.5406 Å) radiation, operated at 40 mA and 40 kV. The morphology of AC and PEI-AC was investigated by scanning electron microscopy (SEM, ZEISS Sigma HD, Germany) with an acceleration voltage of 50 kV. The Brunauer-Emmett-Teller (BET) specific surface areas of the samples were determined by nitrogen adsorption at 77 K on Autosorb-iQ (Quantachrome, USA) after vacuum degassing process at 300 °C overnight. Fourier transform infrared (FTIR) spectra were obtained on a Nexus470 (Thermo Fisher Scientific).
Dynamic adsorption study
The adsorption performance of AC and PEI-AC for formaldehyde was evaluated through the dynamic adsorption in the fixed bed. In the studies, the PEI-AC (50 g) was added into glass column, and the initial concentration of formaldehyde was controlled at 50 mg L-1 (300 mL), and the flow rate of the gas mixture was maintained at 200 mL/min. The change of adsorption amount was recorded by the digital balance, and the concentration changes were detected through a VOCs analyzer. All experiments were repeated three times and the results were reproducible within experimental error (± 5%).
Results and discussion
Characterization of PEI-AC
Fig. 2 showed the XRD patterns of unmodified AC and PEI-AC of different ratio. All samples have a strong peak at 2θ = 24.8° and a weak peak at about 43.3°, which means that the main component of these samples was still g-C3N4. However, the intensity of peak at 24.8° decreases with the increase of PEI concentration, and the similar situation can also be seen at 43.3°. These results indicate that the internal structures and crystal forms of PEI-AC may have been changed (Owlad et al. 2010).
The functional groups of AC and PEI-AC samples were analyzed by FTIR spectra, as shown in Fig. 3, PEI-AC samples exhibited typical IR patterns which is similar to AC, illustrating that PEI-AC still maintains the basic structure of AC. In the FTIR spectra of samples, all samples generally show prominent absorbances at approximately 1250 cm-1 which can be assigned to carboxylic acid groups (-C-O) (Rios et al. 2003). With the increasing concentration of PEI, the absorbance of the band at 1380 cm-1, 3200–3500 cm-1, and 1700–1500 cm-1 increased, which are assigned to N-H (Swiatkowski et al. 2004), and its increase in absorbance for PEI-AC is expected since PEI contains high amounts of amine-based groups. After modification, the nitrogen functional groups may be contributed to the adsorption and enhance the efficiency of the adsorbent (Saleh et al. 2017). The stretching vibrations at about 3450–4000 cm-1 may be due to the presence of surface hydroxyl groups and chemisorbed water (Yin et al. 2007a). These results indicate that PEI introduces many hydrophilic amine groups and oxygen-containing groups on the AC surface, which will facilitate the adsorption of formaldehyde.
In the subsequent adsorption studies, PEI-AC30 showed the best adsorption performance, so PEI-AC30 was chosen as the object of characterization. The morphology of PEI-AC-30 sample was observed by SEM in comparison with AC. As seen in Fig. 4 (a) and (b), in general, PEI-AC30 displays a similar morphology with AC, which contains a lot of pores. However, the morphology of AC surface (Fig. 4a) is smooth, the surface of PEI-AC30 is rougher and has numerous of pores in the interior and surface (Fig. 4b), which might be due to the changes in the internal pore structure.
The structural properties of the as-prepared samples were investigated by the N2 adsorption-desorption isotherms. As shown in Fig. 5 (a), all samples display type I curves according to the IUPAC classification (Zhang et al. 2018), a remarkable uptake at a relative low pressure (P/P0) was found in all the samples, indicating there are abundant micropores. It is interesting to note that the volume of nitrogen adsorbed on all PEI-AC samples is higher than that on unmodified AC. The BET surface area gradually increased from 983.89 m2 g-1 of AC to 1267.42 m2 g-1 of PEI-AC30, the enhancement of BET surface area might be originated from the PEI molecules may have adsorbed predominantly on macro- and mesopores of the AC and as a result, expands its internal structure. This subsequently results in creation of additional meso- and micropores which explains the higher volume of adsorbed nitrogen (Aroua et al. 2008). However, as the PEI concentration was further increased to 50 g L-1, the specific surface area of PEI-AC50 dropped to 1109.49 m2 g-1, this may be due to the collapse of the tunnel and the destruction of the internal structure during the high concentration PEI impregnation process (Cao et al. 2019a, Cao et al. 2019b, Mao et al. 2015, Zhao et al. 2018). The density functional theory (DFT) analysis (Fig. 5b) showed the pore diameter of PEI-AC samples also increased as the concentration of PEI increased, and decreased when the concentration of PEI reached 50 g L-1. The textural characteristics of PEI-AC are listed in Table 1. PEI-AC30 has a maximum BET surface area of 1267.42 m2 g-1, pore volume of 0.73 m3 g-1, and pore diameter of 8.74 nm, all of these characteristics in PEI-AC are beneficial for the adsorption of the formaldehyde.
The adsorption performance of PEI-AC
The adsorption curve of PEI-AC for formaldehyde is shown in Fig. 6. It can be seen that the PEI-AC-30 has the maximum adsorption capacity for formaldehyde (650 mg g-1), and the adsorption capacity of unmodified AC is only 190.1 mg. g-1. The adsorption capacities of PEI-AC10 and PEI-AC20 are 317.6 mg g-1 and 420 mg g-1, respectively. The adsorption capacity of PEI-AC50 does not continue to increase, but decreases to 503.6 mg g-1. The main reason may be because PEI brings a large number of polar groups like amine group to the surface of AC, which has similar properties to polar formaldehyde, so it can promote the formaldehyde adsorbed on PEI-AC. However, as the concentration of PEI increases, the excess PEI may block the pores and cause a decrease in pore utilization.
The experimental data of PAC adsorbed formaldehyde was fitted by pseudo-second-order model and the results were shown in Table 2. It can be seen that all of these adsorption curves are well-fitted by pseudo-second-order kinetic model. The pseudo-second-order adsorption rate constants of PEI-AC are different, which is in the order of kPEI-AC30 > kPEI-AC50 > kPEI-AC20 > kPEI-AC10 > kAC.
Figure 7(a) shows penetration curves at different intake flow rates of PEI-AC30. Obviously, as the intake nitrogen flow gradually increases, the penetration time becomes shorter. Considering the adsorption performance and economic cost, we finally choose the 200 mL min-1 as the dynamic adsorption’s intake flow rate. Fig. 7(b) shows the penetration curves fitted by Boltzmann model, and fitted parameters are shown in Table 3. The penetration curves of formaldehyde dynamic adsorbed on all samples are typical S-shaped. The fitting results show that the above five dynamic adsorption curves can be well fitted using the Boltzmann model. The PEI-AC30 has the highest value of fitted parameter 1/dx (9.1413), meaning that the mass transfer resistance between PEI-AC30 and formaldehyde is smallest, and the shorter mass transfer zone usually leads to higher utilization efficiency of the adsorbent in the fixed bed system.
The fitting results of the intragranular diffusion model of formaldehyde adsorbed by PEI-AC are shown in Fig. 8. The diffusion curve of formaldehyde on modified and unmodified AC can be divided into three sections, the first stage is external in the surface adsorption phase, the second stage is the slow adsorption process, and the third stage is the final equilibrium phase. The adsorption capacity of PEI-AC30 was the largest, and the unmodified AC adsorption performance was the lowest among the five adsorbents. According to the fitting results in Table 4, the adsorption rates of the various stages are also different. In the surface adsorption process, kPEI-AC30 > kPEI-AC50 > kPEI-AC20 > kPEI-AC10 > kAC, and the second stage slow adsorption stage kPEI-AC50 > kPE-AC20 > kPEI-AC30 > kPEI-AC10 > kAC, the third stage final equilibrium stage kPEI-AC30 > kPEI-AC50 > kAC > kPEI-AC20 > kPEI-AC10. The reason for this may be that the pore structure is different from the original one after PEI modification, and the hydrophilic of the activated carbon surface is enhanced, resulting in large differences in the properties of the respective surfaces.
The adsorption steps were repeated five times. As shown in Fig. 9(a), the formaldehyde removal by PEI-AC30 was still about 95% after the fifth run, indicating the excellent stability of PEI-AC30. The adsorption of formaldehyde by PEI-AC30 also did not change markedly after five cycles. In addition, the 5-run experiments did not induce obvious change of XRD characteristic peaks of PEI-AC30 (Fig. 9b), suggesting that PEI-AC30 were stable.
Moreover, the adsorption performance of PEI-AC30 for formaldehyde was also compared with other adsorbents reported in the literatures (Boonamnuayvitaya et al. 2005, Salman et al. 2012, Shin & Song 2011, Srisuda & Virote 2008, Wen et al. 2011, Yang et al. 2017). As seen in Table 5, the PEI-AC30 showed better adsorption performance than most of reported AC and modified AC adsorbents. The reason for this is mainly related to the simultaneous change of pore structure and surface functional groups after PEI modification.
Conclusions
A simple method of immersion is used to modify activated carbon and to adsorb formaldehyde. Through studies, we found that all modified activated carbon with PEI have good formaldehyde adsorption performance, but the adsorption performance will be influenced by the excessive increase of PEI dosage. The improvement of adsorption performance is mainly attributed to the increase of specific surface area and the increase of surface amine content after modification. After kinetic fitting, all the adsorption curves were consistent with the second-order kinetics, and all the breakthrough curves could be described by using the Boltzmann model. And we also find the cycling performance of PAC30 is really excellent. Therefore, we believe that PEI modified activated carbon can be used as a useful technology for the essence of VOCs.
References
Alslaibi TM, Abustan I, Ahmad MA, Foul AA (2013) A review: production of activated carbon from agricultural byproducts via conventional and microwave heating. Chem Technol Biotechnol 88:1183–1190
Amara M, Kerdjoudj H (2003) Modification of the cation exchange resin properties by impregnation in polyethyleneimine solutions: application to the separation of metallic ions. Talanta 60:991–1001
Aroua MK, Daud WMAW, Yin CY, Adinata DJS (2008) Adsorption capacities of carbon dioxide, oxygen, nitrogen and methane on carbon molecular basket derived from polyethyleneimine impregnation on microporous palm shell activated carbon. Sep Purif Technol 62:609–613
Aygün A, Yenisoy-Karakaş S, Duman I, (2003): Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater 66, 189–195
Boonamnuayvitaya V, Sae-ung S, Tanthapanichakoon W (2005) Preparation of activated carbons from coffee residue for the adsorption of formaldehyde. Sep Purif Technol 42:159–168
Cao J, Nie W, Huang L, Ding Y, Lv K, Tang H (2019a) Photocatalytic activation of sulfite by nitrogen vacancy modified graphitic carbon nitride for efficient degradation of carbamazepine. Appl Catal B Environ 241:18–27
Cao J, Pan C, Ding Y, Li W, Lv K, Tang H (2019b) Constructing nitrogen vacancy introduced g-C3N4 pn homojunction for enhanced photocatalytic activity. J Environ Chem Eng 7:102984
Chiang H-L, Chiang P, Huang CP (2002) Ozonation of activated carbon and its effects on the adsorption of VOCs exemplified by methylethylketone and benzene. Chemosphere 47:267–275
Chingombe P, Saha B, Wakeman RJ (2006) Effect of surface modification of an engineered activated carbon on the sorption of 2, 4-dichlorophenoxy acetic acid and benazolin from water. J Colloid Interface Sci 297:434–442
Huang Y, Han M (2011) The influence of α-Al2O3 addition on microstructure, mechanical and formaldehyde adsorption properties of fly ash-based geopolymer products. J Hazard Mater 193:90–94
Kim K-J, Kang C-S, You Y-J, Chung M-C, Woo M-W, Jeong W-J, Park N-C, Ahn H-G (2006) Adsorption–desorption characteristics of VOCs over impregnated activated carbons. Catal Today 111:223–228
Lu N, Pei J, Zhao Y, Qi R, Liu J (2012) Performance of a biological degradation method for indoor formaldehyde removal. Build Environ 57:253–258
Mao J, Kwak I-S, Sathishkumar M, Sneha K, Yun YS (2011) Preparation of PEI-coated bacterial biosorbent in water solution: optimization of manufacturing conditions using response surface methodology. Bioresour Technol 102:1462–1467
Mao J, Kim S, Wu XH, Kwak I-S, Zhou T, Yun Y-S (2015) A sustainable cationic chitosan/E. coli fiber biosorbent for Pt (IV) removal and recovery in batch and column systems. Sep Purif Technol 143:32–39
Nie L, Yu J, Li X, Cheng B, Liu G, Jaroniec M (2013) Enhanced performance of NaOH-modified Pt/TiO2 toward room temperature selective oxidation of formaldehyde. Environ Sci Technol 47:2777–2783
Owlad M, Aroua MK, Daud WMAW (2010) Hexavalent chromium adsorption on impregnated palm shell activated carbon with polyethyleneimine. Bioresour Technol 101:5098–5103
Rangel-Mendez J, Cannon FS (2005) Improved activated carbon by thermal treatment in methane and steam: physicochemical influences on MIB sorption capacity. Carbon 43:467–479
Rios RRA, Alves DE, Dalmázio I, Bento SFV, Donnici CL, Lago RM (2003) Tailoring activated carbon by surface chemical modification with O, S, and N containing molecules. Mater Res 6:129–135
Saleh TA, Tuzen M, Sarı A (2017) Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem Eng Res Des 117:218–227
Salman M, Athar M, Shafique U, Rehman R, Ameer S, Ali SZ, Azeem M (2012) Removal of formaldehyde from aqueous solution by adsorption on kaolin and bentonite: a comparative study. Turk J Eng Environ Sci 36:263–270
Shen Y, Yang X, Wang Y, Zhang Y, Zhu H, Gao L, Jia M (2008) The states of gold species in CeO2 supported gold catalyst for formaldehyde oxidation. Appl Catal B Environ 79:142–148
Shin S, Song J (2011) Modeling and simulations of the removal of formaldehyde using silver nano-particles attached to granular activated carbon. J Hazard Mater 194:385–392
SRISUDA S, VIROTE B (2008) Adsorption of formaldehyde vapor by amine-functionalized mesoporous silica materials. J Environ Sci 20:379–384
Swiatkowski A, Pakula M, Biniak S, Walczyk M (2004) Influence of the surface chemistry of modified activated carbon on its electrochemical behaviour in the presence of lead (II) ions. Carbon 42:3057–3069
Wan Y, Fan X, Zhu T (2011) Removal of low-concentration formaldehyde in air by DC corona discharge plasma. Chem Eng J 171:314–319
Wang D, Wu G, Zhao Y, Cui L, Shin C-H, Ryu M-H, Cai J (2018) Study on the copper (II)-doped MIL-101 (Cr) and its performance in VOCs adsorption. Environ Sci Pollut Res Int 25:28109–28119
Wang L (2012) Adsorption of formaldehyde (HCOH) molecule on the SiC sheet: A first-principles study. Appl Surf Sci 258:6688–6691
Wen Q, Li C, Cai Z, Zhang W, Gao H, Chen L, Zeng G, Shu X, Zhao Y (2011) Study on activated carbon derived from sewage sludge for adsorption of gaseous formaldehyde. Bioresour Technol 102:942–947
Wu S, Chen P (2001): Modification of a commercial activated carbon for metal adsorption by several approaches, proceedings of the 2001 International Containment & Remediation Technology Conference and Exhibition, Orlando, FL, USA
Yang J, Li D, Zhang Z, Li Q, Wang H (2000) A study of the photocatalytic oxidation of formaldehyde on Pt/Fe2O3/TiO2. J Photochem Photobiol A Chem 137:197–202
Yang S, Zhu Z, Wei F, Yang X (2017) Enhancement of formaldehyde removal by activated carbon fiber via in situ growth of carbon nanotubes. Build Environ 126:27–33
Yi Q, Niu F, Yu W (2011) Pd-modified TiO2 electrode for electrochemical oxidation of hydrazine, formaldehyde and glucose. Thin Solid Films 519:3155–3161
Yin CY, Aroua MK, Daud WMAW (2007a) Impregnation of palm shell activated carbon with polyethyleneimine and its effects on Cd2+ adsorption. Colloids Surf A Physicochem Eng Asp 307:128–136
Yin CY, Aroua MK, Daud WMAW (2007b) Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep Purif Technol 52:403–415
Yu J, Li X, Xu Z, Xiao W (2013) NaOH-modified ceramic honeycomb with enhanced formaldehyde adsorption and removal performance. Environ Sci Technol 47:9928–9933
Zhang D, Cao J, Wu G, Cui L (2018) Dynamic adsorption model fitting studies of typical VOCs using commercial activated carbon in a fixed bed. Water Air Soil Pollut 229:178
Zhao Y, Cho C-W, Cui L, Wei W, Cai J, Wu G, Yun Y-S (2018) Adsorptive removal of endocrine-disrupting compounds and a pharmaceutical using activated charcoal from aqueous solution: kinetics, equilibrium, and mechanism studies. Environ Sci Pollut Res Int 26:33897–33905
Acknowledgments
The authors are sincerely grateful to Environmental Protection Research Project of Hubei Province for the financial grant for this research (project number 2018HB12).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, D., Zhang, M., Ding, F. et al. Efficient removal of formaldehyde by polyethyleneimine modified activated carbon in a fixed bed. Environ Sci Pollut Res 27, 18109–18116 (2020). https://doi.org/10.1007/s11356-020-08019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08019-5